Abstract
Sympathetic regulation of the motility of guinea-pig ileum was investigated using mesenteric nerve (MN) stimulation to inhibit motility reflexes, in vitro.
Transmural electrical stimulation (5 Hz, 1 s) in intact intestinal segments, or inflation of a balloon against the mucosa in opened segments, evoked contractions of the circular and longitudinal muscles oral to the stimulus.
MN stimulation (10 Hz, 5 s) usually abolished contractions of the longitudinal and circular muscles evoked by either electrical or mechanical stimuli.
The inhibition was mimicked by UK14,304 (70–100 nm) and abolished by idazoxan (100 nm), revealing an enhancement of circular muscle contractions. There was no evidence for α2-receptors on the muscle, suggesting sympathetic inhibition was via the myenteric plexus.
Possible sites of action of noradrenaline released from sympathetic nerves were investigated using intracellular recordings from the circular muscle in a multichambered organ bath.
When in the stimulation chamber, UK14,304 depressed (by 50 %) excitatory junction potentials (EJPs) recorded oral to a distension stimulus, but did not affect inhibitory junction potentials (IJPs) recorded anal to the stimulus. When added to a chamber between the stimulus and recording chambers, UK14,304 depressed EJPs by 40 %, but did not alter IJPs. When in the recording chamber, UK14,304 depressed EJPs by 20 %, but had no effect on IJPs. IJPs were inhibited, however, when UK14,304 was applied to the whole bath.
It is concluded that sympathetic activity inhibits intestinal motility mainly via α2-adrenoceptors on ascending interneurons and intrinsic sensory neurons of the orally directed reflex pathway.
Stimulation of the sympathetic nerves inhibits movements of the intestine (Furness & Costa, 1987). However, in guinea-pig ileum, and indeed in the human small intestine, the muscle layers receive very little direct innervation from sympathetic neurons (Furness & Costa, 1974). Instead the inhibition is probably indirect, via the motility-controlling neurons within the myenteric plexus.
The effects of MN stimulation on contractions of intestinal longitudinal muscle have been studied by several workers (Finkleman, 1939; De Luca & Rand, 1990). It is clear from these experiments that noradrenaline released from sympathetic nerves inhibits the action of excitatory motor neurons on this muscle layer. However, although the circular muscle is more important than the longitudinal muscle in the intestinal behaviours of peristalsis and segmentation (Cannon, 1912; Kosterlitz & Lees, 1964), the effects of sympathetic stimulation on the former are not well understood. In vivo studies in dog and cat small intestine, using balloon recordings or recordings of intraluminal pressure, have shown that MN stimulation inhibits the activity of the circular muscle (Bayliss & Starling, 1899; Barry, 1932; Kock, 1959). There is some evidence that this may be via an effect on α2-adrenoceptors (Mizutani et al. 1990). Studies of the responses of the guinea-pig small intestine to sustained liquid distensions also indicate that MN stimulation inhibits propulsive contractions of the circular muscle (Lee, 1960; Kreulen et al. 1983). This idea is supported by a recent study of the distal colon, which concluded that sympathetic nerves inhibit interneurons that carry reflex activity along the intestine (Spencer et al. 1999a). However, this study did not address the question of whether the activity of intrinsic sensory neurons or of motor neurons was modified by sympathetic stimulation. Furthermore, the neural circuitry in the distal colon differs from that of the ileum (Lomax & Furness, 2000). Thus, the site, or sites, at which sympathetic neurons may act on motility reflex pathways in the small intestine are unclear.
Exogenously applied adrenergic agonists inhibit myenteric neurons in the guinea-pig ileum via both presynaptic (Nishi & North, 1973) and postsynaptic α2-adrenoceptors (Surprenant & North, 1985; Galligan & North, 1991). Activation of presynaptic α2-adrenoceptors can suppress both fast and slow synaptic transmission (Morita & North, 1981; Galligan & North, 1991), with compound fast excitatory synaptic potentials being inhibited by an average of 70 %. Furthermore, activation of postsynaptic α2-adrenoceptors hyperpolarizes some myenteric neurons (Surprenant & North, 1985; Galligan & North, 1991), including many intrinsic sensory neurons and other neurons that may be interneurons or motor neurons. As many functional neuron types and reflex pathways co-exist within the myenteric plexus, sympathetic nerves may only inhibit specific subsets of myenteric neurons and/or synapses. Indeed this has been reported to be the case in the myenteric plexus of the guinea-pig antrum (Tack & Wood, 1992).
The aim of the present experiments was to investigate the sites of action of the sympathetic nerves and α2-agonists in inhibiting intestinal motility. To this end, a preparation was developed in which the contractile activities of both the circular and longitudinal muscle layers were monitored simultaneously in an intact tube of intestine. Ascending excitatory pathways were activated either via electrical stimulation or by distension with an intraluminal balloon and the effects of sympathetic nerve stimulation on these pathways were observed. A related preparation allowed the effects of opening the intestinal tube (a procedure needed to allow intracellular recordings to be made from myenteric neurons) on the efficacy of transmission from sympathetic nerves to the myenteric plexus to be observed. We used intracellular recording from the circular muscle to examine reflex responses to distension as these methods are better suited to analysis of inhibition than studies of muscle contractility. These last experiments used a divided organ bath that allowed the selective application of drugs to different components of the local reflex pathways in the intestine (Tonini & Costa, 1990; Yuan et al. 1994). This allowed the analysis of the sites of action of a selective α2-adrenoceptor agonist, UK14,304, on the ascending excitatory and, in particular, the descending inhibitory reflex pathways.
METHODS
Guinea-pigs of either sex were killed by being stunned, then decapitated. Segments of small intestine were removed with, or without, the mesentery attached, depending on the experiments to be performed. These were then placed in physiological saline solution (mm): NaCl, 118; KCl, 4.77; NaH2PO4, 1.0; NaHCO3, 25; MgSO4, 1.2; d-glucose, 11.1; CaCl2, 2.5; bubbled with 95 % O2-5 % CO2. All procedures were approved by the University of Melbourne Animal Experimentation Ethics Committee.
As suggested by earlier studies (Finkleman, 1939; Takayanagi et al. 1977), it was found that lateral stress placed upon the entry points of the mesenteric nerves was associated with failure of electrical stimulation of these nerves to inhibit motility. Accordingly, care was taken to choose a segment of intestine that could be straightened without producing excessive shear forces. The anatomy of the mesentery in the guinea-pig is such that it is not possible to fully straighten more than a few centimetres of intestine without putting strain on the mesenteric attachment. In some experiments, the gut was bathed in physiological saline containing 200 μm procaine before removal from the animal and during dissection; this appeared to protect the preparation from the effects of straightening the intestinal segment (see Results).
Contractility studies
To study the relationship between responses in circular and longitudinal muscles, an ∼8 cm segment of intestine was cut so that the main mesenteric vessel reached the wall of the intestine near its centre. This segment was placed in an organ bath that was continually perfused with saline of the above composition at 35–37 °C. A Foley catheter (10 French gauge) filled with distilled water was inserted into the oral end of the segment and attached to a pressure transducer via a fluid-filled tube (see Fig. 1A). The pressure in this catheter was taken as a measure of circular muscle contractile activity. In some experiments, the shaft of the recording catheter was bent and placed inside the intestinal lumen with the mesenteric border at its inner curvature, thus minimizing the strain on the mesenteric attachment. Two sutures were placed in the wall of the intestine approximately 10 mm apart at either end of the balloon section of the catheter. One suture was attached to the bottom of the organ bath and the other to an isotonic force transducer via a pulley, so that the contractions of the longitudinal muscle could be measured together with the mechanical activity of the underlying circular muscle.
Figure 1. Diagram of the three types of preparation used to study the effects of sympathetic nerves on intestinal motility.

A, apparatus used to simultaneously record longitudinal and circular muscle contractions in intact tubes of intestine. A balloon catheter recorded circular muscle activity (as pressure changes, oral end). An isotonic transducer connected to the intestinal wall via a thread and pulley recorded longitudinal muscle length in the same region. Electrodes were used to stimulate both enteric nerves (transmural stimulation) and the perivascular mesenteric nerves. In some experiments an anally placed balloon catheter was used to provide a distension stimulus. B, preparation used to record ascending excitatory reflexes in flat sheets of intestine. A hemispherical balloon set into the base of the bath recorded circular muscle activity (oral end). Distensions were applied via another balloon at the anal end and an electrode used to stimulate the mesenteric nerves. C, apparatus to record ascending EJPs and descending IJPs in the circular muscle. Distensions were applied to the gut wall at two sites in each of two stimulation chambers and reflex responses recorded at the opposite end of the preparation. When the preparation was oriented so that recordings were made at the oral end, ascending EJPs were recorded, and in the opposite orientation the responses seen were IJPs.
Because the recording balloon was filled with liquid it was effectively incompressible and so its volume did not change. Thus, the balloon recordings were equivalent to isometric recordings of muscle force. By contrast, the measurement of muscle movement in the longitudinal direction was isotonic and so no force change was allowed. This means that it is unlikely that there was any interaction between the two recording systems. This supposition was confirmed as follows. Baseline recordings of spontaneous longitudinal movements revealed slow changes that were not reflected in the pressure recordings (Fig. 2A). Even when the onset of longitudinal length changes and balloon pressure changes virtually coincided, the time courses of these contractile events were often distinctly different (Fig. 2B). If there were passive interactions between the two recording systems, these would be reflected in a reduction in balloon pressure during rapid longitudinal shortening and this was not seen in any experiment. Thus, longitudinal shortening was taken to reflect isotonic contraction of the longitudinal muscle, while increases in balloon pressure were assumed to reflect isometric contraction of the circular muscle at the same location.
Figure 2. Spontaneous and evoked contractions recorded simultaneously in both muscle layers in intact segments of intestine.

A, ongoing activity in both the circular (CM, balloon pressure change, mmHg) and longitudinal (LM, transducer output in volts) muscle layers. The circular muscle remained quiescent for long periods, whereas a slowly changing baseline activity was seen in the longitudinal muscle. Occasional larger and faster contractions occurred at random intervals (asterisk), and most often involved both muscle layers. B, contraction seen at the asterisk in A on an expanded time scale. C, contraction evoked in both muscle layers by transmural electrical stimulation (onset at arrow, 1 ms, 100 V, 5 Hz, 1 s train) applied at the anal end of a different segment of intestine. Note differences in the configurations of responses in circular and longitudinal recordings. Pressure and voltage scale bars in C apply to all panels. Time scale in C applies to panels B and C.
This preparation was stimulated in two ways. In most experiments, coaxial stimulating electrodes measuring 10–15 mm in length were placed at the anal end of the preparation about 25 mm from the nearest edge of the recording balloon. These were used to stimulate enteric neural pathways to evoke ascending contractile responses in both the longitudinal and circular muscles. The stimuli were applied as trains of pulses (5 Hz for 1 s, duration 0.5 ms, strength 50–150 V); single pulses did not give consistent responses. This was termed transmural (TM) stimulation. TM stimulation was applied at intervals of 5 min to avoid rundown of the responses. In other experiments, a second Foley catheter was inserted into the anal end of the segment and inflated to distend the intestine, thereby evoking an ascending excitatory reflex.
In a second series of experiments, 6 cm segments of small intestine with attached mesentery were opened along the antimesenteric border and pinned flat, mucosal surface down, in a organ bath with two hemispherical balloons (diameter 5 mm, centres 2.5 cm apart) set into its base (Fig. 1B). The preparation was arranged so that mesenteric arteries and extrinsic nerves supplying the segment reached the gut wall about halfway between the two balloons. The balloon at the oral end was attached, via a tube filled with distilled water, to a pressure transducer and was used to measure the contraction of the circular muscle. The anal balloon was connected, via a water-filled tube, to a syringe that was used to inflate the balloon and distend the wall of the gut. A displacement transducer attached to the syringe plunger allowed the timing and duration of the distension to be recorded (Smith et al. 1990). The gut over the balloons was pinned at its cut edges on either circumferential side of the balloons, and was left very slack between the pins in the longitudinal direction. This was done to avoid damage to the mesenteric border during distensions and to ensure that contractions of the longitudinal muscle did not increase the pressure in the recording balloon, so that only circular muscle contractions would be recorded. We observed that spontaneous contractions of the longitudinal muscle moved the intestine back and forth across the balloon without changing the recorded pressure.
Effects of MN stimulation
To investigate the effects of sympathetic nerve stimulation on ascending excitatory pathways, the mesenteric artery, together with its paravascular nerves, was drawn into a stimulating electrode consisting of two loops of silver wire inside a short length of tubing. The paravascular nerves were electrically stimulated (1 ms pulses, 10 Hz) for 20–30 s, and either TM stimulation or a 5 s duration distension (volume 0.08–0.13 ml) was applied 5 s after the start of this paravascular stimulus train. The frequency of MN stimulation was chosen because it has little effect on the membrane potential of circular muscle cells, yet effectively inhibits reflexes in intestinal preparations (Weisenthal et al. 1971; Hirst & McKirdy, 1974). The effects of MN stimulation were examined using a test cycle consisting of three responses recorded at 5 min intervals. These were: a control ascending response evoked by either TM stimulation or distension, followed by a test response during MN stimulation, and then a recovery response in the absence of MN stimulation. Each test response was compared to a control value that was the mean of the amplitudes of its control and recovery responses.
Drugs to be tested were introduced into the perfusate and allowed to equilibrate for 15 min before at least two test cycles were performed. The bath was then emptied and refilled with pre-warmed saline three times at 2 min intervals. At least 10 min was allowed before further responses were recorded.
Electrophysiological analysis of the sites of α2-adrenoceptor actions in motility reflex pathways
Segments of ileum with the mesentery removed were opened along their antimesenteric borders and pinned flat, mucosal surface down, in an organ bath divided into three separately perfused chambers (see Yuan et al. 1994; Johnson et al. 1996). Hemispherical balloons, identical to those used in the contractility experiments, were set in the base of two adjacent chambers. The third chamber was used to make conventional intracellular recordings from the circular muscle (Fig. 1C). Each chamber was perfused with physiological saline at 36–37 °C containing nifedipine (3 μm) to reduce muscle contractions and allow stable recordings during reflexes. Staple-shaped pins were placed around each stimulating balloon to hold the intestine in place during distensions.
Ascending excitatory reflexes were examined by recordings from circular muscle cells in preparations oriented with the recording chamber at the oral end. The recording techniques used, and criteria for determining satisfactory impalements into circular muscle cells, were as described by Smith & Furness (1988) Distensions were applied in the two more anal chambers. Most ascending interneurons project orally for 4–8 mm (Brookes et al. 1997), and hence few interneurons with cell bodies in the most anal chamber would project across the intermediate chamber (width 10 mm) directly into the recording chamber. Thus, any reflex conducted from the far chamber to the recording site probably included a synapse between interneurons in the intermediate chamber. Previous experiments in the same apparatus have confirmed that blockade of synaptic transmission to the intermediate chamber completely blocks conduction of ascending reflexes through it (Johnson et al. 1996).
To study descending inhibitory reflexes, recordings were made from smooth muscle cells at the anal end of the preparation, while the distension balloons were situated more orally. In this case, the intermediate chamber was 15 mm in width, because interneurons in the descending pathway were substantially longer than those of the ascending pathway (Costa et al. 1996). When transmission within the intermediate chamber is blocked, descending responses conducted through it are reduced to less than 10 % of their control amplitudes, while reflexes evoked by stimuli in this chamber are reduced to about 40 % of control (Johnson et al. 1996, 1998). Thus, drugs in this chamber will act on synapses between interneurons if the stimulus is applied in the most oral chamber, and on synapses between sensory neurons and interneurons if the stimulus is applied in the intermediate chamber.
In both ascending and descending pathway experiments, distension stimuli (volume 0.13 ml) were applied to the intestine for 3–5 s, at intervals of 4 min. Drugs were applied to one or more of the chambers by superfusion, and at least 15 min allowed for equilibration of the drug before its effects on reflex responses were examined. Control solution was then superfused for at least 30 min before washout responses were recorded.
Drugs used were UK14,304, idazoxan HCl and nifedipine (Research Biomedical International, Natick, MA, USA), hexamethonium bromide, carbachol chloride (Sigma, St Louis, MO, USA), and procaine HCl (Ajax chemicals, Sydney, Australia).
Statistical comparisons were performed using Student's paired t tests.
RESULTS
Contractility studies
When recordings were made from an intact intestinal tube at rest, the longitudinal muscle over the recording balloon often exhibited slow spontaneous contractions that were not accompanied by pressure changes at the same site (Fig. 2A). While sporadic, faster spontaneous longitudinal contractions were sometimes accompanied by pressure increases, the intraluminal pressure was normally constant in the absence of stimulation. The spontaneous fast contractions recorded by both methods had similar onset times, but typically differed in time course (Fig. 2B). No spontaneous pressure increases were recorded in the opened segments at rest.
Electrical stimulation of enteric nerves at the anal end of an intact segment evoked stimulus-locked contractions of both muscle layers, and these usually differed in form from each other (Fig. 2C. Contractions were often complex with two or more discrete peaks; however, the amplitudes and latencies of the first peaks were relatively constant within any one preparation. Accordingly, the amplitudes of responses to electrical stimulation were taken to be those of the first peaks within the responses. By contrast, the amplitudes and latencies of later response peaks varied between, and within, preparations.
Under control conditions, the amplitudes of the initial contractions evoked by electrical stimulation did not vary with increasing stimulus intensity. As the TM stimulus was increased from zero, the first contraction seen was large, but intermittent, with further increases in stimulus intensity increasing the probability of seeing a contraction rather than increasing its amplitude.
Distension of the intestinal wall evoked stimulus locked contractions of both muscle layers in the intact intestinal segments and of the circular muscle in opened segments. The responses evoked by the relatively small distensions used in this study appeared to be all-or-nothing, in that if a contraction was observed its amplitude was very similar to those evoked by larger stimuli (Fig. 3). The effect of increasing the stimulus strength was to increase the probability of seeing a contraction rather than increasing the contraction amplitude. It was only when the distending volume was increased to levels (> 0.3 ml) well above those required to evoke consistent contractions (0.15 ml) that the amplitudes of these contractions in the circular muscle became graded with stimulus strength.
Figure 3. Variation in the ascending excitatory reflex evoked by distensions of different volumes.
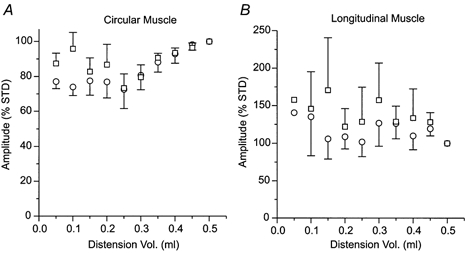
Graphs show the amplitude of the first (^) and tallest (□) components of responses to balloon distension in A, the circular muscle (n = 6 preparations, n = 3 at lowest distension volume) and B, the longitudinal muscle (n = 5, n = 1 at lowest volume). Responses were expressed as a percentage of a standard response to a 0.5 ml distension (% STD). Error bars indicate s.e.m.
The ascending contractions evoked in each layer by electrical stimulation were reversibly abolished by hexamethonium (100 μm, n = 4;200 μm, n = 2; Fig. 4A) indicating that these contractions were neurogenic.
Figure 4. Effects of hexamethonium and UK14,304 applied to the organ bath on the amplitudes of ascending contractile responses.
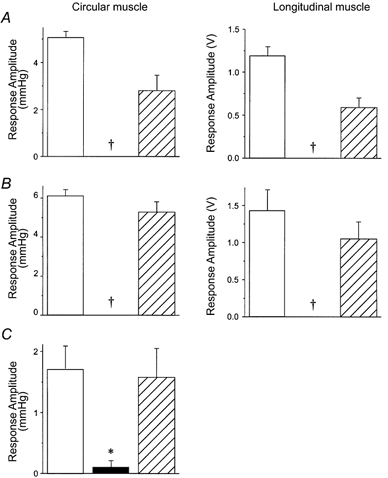
□, control responses; ▪, responses in the presence of a drug;  , responses following washout. As for all subsequent column graphs, column heights represent mean response amplitudes while error bars indicate s.e.m. Effect of hexamethonium (A; 100 μm, n = 4) and UK14,304 (B; 100 nm, n = 7) on ascending responses to transmural stimulation in both the longitudinal and circular muscle layers in intact segments of intestine. Responses in both layers were abolished by both drugs. † Significant difference from control, P < 0.005. C, effect of UK14,304 on ascending contractions of the circular muscle evoked by distension in the opened intestinal preparation. Reflex responses were virtually abolished in the presence of the drug (n = 4, *P < 0.02).
, responses following washout. As for all subsequent column graphs, column heights represent mean response amplitudes while error bars indicate s.e.m. Effect of hexamethonium (A; 100 μm, n = 4) and UK14,304 (B; 100 nm, n = 7) on ascending responses to transmural stimulation in both the longitudinal and circular muscle layers in intact segments of intestine. Responses in both layers were abolished by both drugs. † Significant difference from control, P < 0.005. C, effect of UK14,304 on ascending contractions of the circular muscle evoked by distension in the opened intestinal preparation. Reflex responses were virtually abolished in the presence of the drug (n = 4, *P < 0.02).
The selective α2-adrenoceptor agonist UK14,304 abolished the ascending contractions evoked by electrical stimulation of the intact intestinal tube (70 nm, n = 2; 100 nm, n = 7; 1 μm, n = 3; Fig. 4B) or by distension of the opened intestine (100 nm, n = 4; Fig. 4C). The effect of UK14,304 was maintained for more than 50 min when the agonist was left in the bath. This compound did not affect contractions of either muscle layer evoked by carbachol (1 μm) in the presence or absence of tetrodotoxin (TTX, n = 4, P > 0.5). This indicates that there are no α2-adrenoceptors on either muscle layer and that the effects of the agonist are mediated exclusively via neurons.
Effects of MN stimulation
MN stimulation evoked an initial contraction (‘on’ contraction) of both muscle layers in 31 of 56 intact intestinal tube preparations (the ‘on’ contraction was only seen with high stimulus strengths in 9 cases) and of the circular muscle in 7 of 30 opened preparations (Fig. 5). An ‘off’ contraction was observed in both muscle layers in 40 intact tube preparations at the end of the MN stimulus train, regardless of its duration.
Figure 5. Contractile responses to MN stimulation in both intestinal muscle layers.
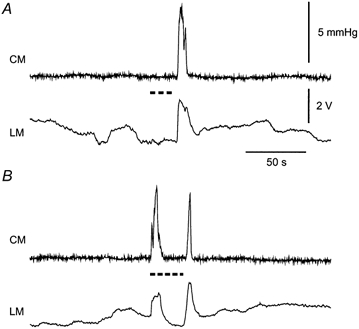
A, response to stimulation of the MN stimulation (dashed line) in one preparation at 65 V. No contractions occurred during the stimulus, but there was an ‘off’ contraction at the end of the train. MN stimulation at this voltage fully blocked the ascending contraction generated by TM stimulation. B, MN stimulation at 75 V in the same preparation caused an ‘on’ contraction of both muscle layers at a short delay from the onset of the stimulus train.
Effects of MN stimulation were only assessed in preparations that did not exhibit on contractions, as such contractions obscured responses to TM stimulation or distension. Accordingly, the effects of MN stimulation on electrically evoked ascending contractions were examined in 34 intact tube preparations (including the 9 in which ‘on’ contractions were seen only with higher stimulus strengths). Inhibitory effects were accepted if the amplitudes of test responses to TM stimulation during MN stimulation were less than both control and recovery responses in each of three separate consecutive test cycles (see Fig. 6). Such inhibitions were seen in 20 preparations. The response to TM stimulation was virtually abolished in 17 preparations, whereas in the other three there was a partial inhibition. This effect was labile in some experiments; that is, the percentage inhibition gradually decreased until there was no effect of the MN stimulation, or an ‘on’ contraction was seen. The chances of observing an inhibition appeared to be improved by measures to prevent stretching of the intestine at the mesenteric border, such as the use of the bent recording catheter described above.
Figure 6. Effect of MN stimulation on ascending excitatory responses.
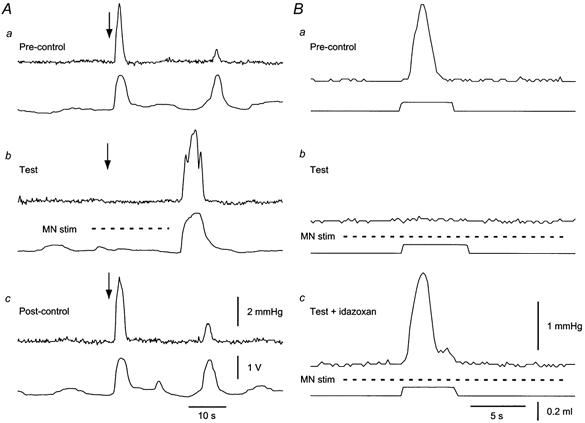
A, recordings in an intact tube preparation of ascending responses evoked by transmural stimulation (arrows). Upper traces, circular muscle contractions; lower traces, longitudinal muscle activity. One test cycle is shown, consisting of a control response (Pre-control), a test response (Test) during MN stimulation (dashed line) and a recovery response (Post-control), each separated by 5 min intervals. Note the ‘off’ contraction following the MN stimulation train in each case. B, recordings in a flattened preparation. Responses were evoked by distension of an anally placed balloon (0.08 ml). Upper traces, circular muscle contractions; lower traces, distension. a, control response; b, response during MN stimulation (dashed line); c, response during MN stimulation in the presence of 100 nm idazoxan.
At first, MN stimulation was found to be effective in only 3 of 12 opened preparations, in which it inhibited ascending excitation evoked by distension. However, when procaine was used in the bathing solution during dissections and then washed out prior to recording, inhibition of ascending contractions was seen in 13 of 25 preparations (Fig. 6) with ‘on’ contractions seen in seven others. Reproducible inhibitions could be evoked over 5–8 h in three preparations, but in three other cases the inhibitory effect of MN stimulation could not be reproduced after 1 h.
The α2-adrenoceptor antagonist idazoxan (100 nm) abolished the inhibitory effects of MN stimulation on ascending excitations evoked by TM stimulation in 10 of 12 preparations. In the other two preparations, idazoxan uncovered an ‘on’ contraction in response to MN stimulation. The effect of idazoxan was not readily reversible and recovery of the inhibition evoked by MN stimulation after washout of the drug was seen in five preparations, thereby confirming that the decrease in inhibition was not due to passage of time. Results from these five preparations were analysed quantitatively. Idazoxan abolished the inhibition by MN stimulation of TM stimulation-evoked longitudinal and circular muscle contractions (Fig. 7A and B). Indeed, MN stimulation in the presence of idazoxan enhanced the circular muscle contractions evoked by TM stimulation (n = 5, P < 0.005; Fig. 7A).
Figure 7. Effect of idazoxan (100 nm) on the inhibition by MN stimulation of ascending contractions.
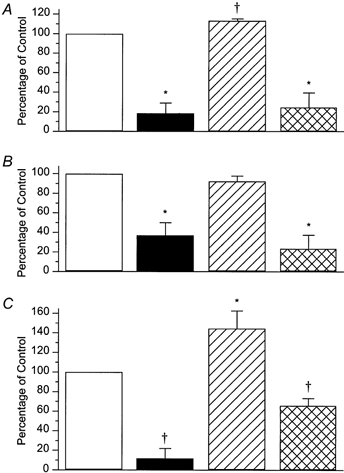
Response amplitudes during MN stimulation are expressed as a percentage of their control values in the absence of MN stimulation. □, control responses; ▪, responses during MN stimulation;  , responses during MN stimulation in the presence of 100 nm idazoxan;
, responses during MN stimulation in the presence of 100 nm idazoxan;  , responses after washout during MN stimulation. A, circular muscle, intact tube preparation; B, longitudinal muscle, intact tube preparation; C, circular muscle, flattened preparation. In all cases, the inhibition of ascending contractions during MN stimulation was abolished by idazoxan. In addition, idazoxan revealed a significant facilitation by MN stimulation of circular muscle responses in both preparations. Symbols indicate significant differences from control, *P < 0.05; †P < 0.01; n = 5 in all cases.
, responses after washout during MN stimulation. A, circular muscle, intact tube preparation; B, longitudinal muscle, intact tube preparation; C, circular muscle, flattened preparation. In all cases, the inhibition of ascending contractions during MN stimulation was abolished by idazoxan. In addition, idazoxan revealed a significant facilitation by MN stimulation of circular muscle responses in both preparations. Symbols indicate significant differences from control, *P < 0.05; †P < 0.01; n = 5 in all cases.
Idazoxan (100 nm) also reversed the inhibition by MN stimulation of ascending excitations evoked by distension in the opened intestinal tube in six of seven preparations (Fig. 7). In the remaining preparation, idazoxan revealed an ‘on’ contraction to MN stimulation. The effects of idazoxan washed out in five preparations, which were included in the quantitative analysis. As in the intact preparation, MN stimulation in the presence of the α2-adrenoceptor antagonist enhanced, rather than inhibited, the ascending circular muscle contraction evoked, in this case, by distension (Fig. 7C.
The on-contractions evoked by MN stimulation in intact tube preparations were blocked by hexamethonium at 100 μm(n = 4, n = 2 with washout), by UK14,304 at 1 μm(n = 2) and by 100 nm UK14,304 in three of four cases.
Sites of action of α2-agonist UK14,304 in the reflex pathways
Intracellular recordings made from circular muscle cells oral to a distending balloon (see Methods) showed that distension evoked excitatory junction potentials (EJPs, see Fig. 8A) in these cells. In some instances, the EJPs evoked action potentials and contractions that dislodged the recording electrode, despite the presence of nifedipine. This generally occurred after the peak of the EJP, so the maximum amplitude of the EJP could be measured. In those preparations in which recordings were made anal to the distending balloons, the circular muscle cells responded with inhibitory junction potentials (IJPs, see Fig. 8B). In each case, these responses were similar to those reported by Smith et al. (1991).
Figure 8. Distension-evoked responses in circular muscle recorded intracellularly.
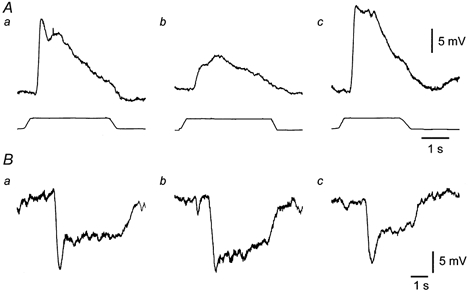
EJPs recorded at the oral end of one preparation (A) and IJPs recorded at the anal end of another preparation (B) when distensions were applied in the chamber adjacent to the recording chamber. Traces between A and B show onset and duration of distension stimuli. a, control responses; b, responses while UK14,304 (100 nm) was present in the stimulus chamber; and c, responses after wash out of the drug. Whereas ascending EJPs evoked in this way were depressed by UK14,304, IJPs recorded at a similar distance, but anally from the stimulation site, were not.
Application of UK14,304 (100 nm) to the intermediate chamber in the ascending excitatory reflex pathway significantly depressed reflexes conducted through this chamber (to 60 % of control, n = 4, P < 0.02; Fig. 9A, right panel). EJPs evoked by distensions applied within this chamber were also significantly depressed (residual response 50 % of control, n = 4, P < 0.02; Fig. 8Ab, Fig. 9A, left panel). Addition of the α2-agonist to both the intermediate and recording chambers depressed EJPs evoked by distensions in the intermediate chamber by little more than UK14,304 in the intermediate chamber alone (residual response 40 %, n = 4, P < 0.001; Fig. 9B). This suggested that there may be little effect of the agonist in the recording chamber. However, UK14,304 in the recording chamber alone caused small, but significant, reductions in the EJPs evoked by distensions in either the intermediate (residual response 80 %, n = 5, P < 0.03) or distant chambers (residual response 70 %, n = 5, P < 0.03; Fig. 9C).
Figure 9. Effects of UK14,304 applied to various chambers on the amplitudes of ascending and descending reflex responses recorded in circular muscle.
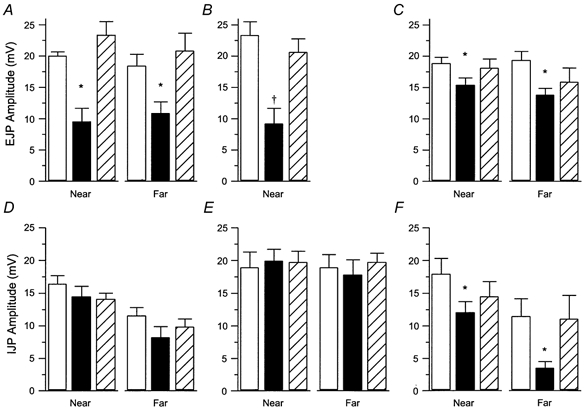
Responses were recorded in the three-chambered organ bath at one end of the preparation and distensions applied either to the adjacent chamber (Near) or to the more distant one (Far). A, effect of the drug applied to the near chamber on excitatory reflexes (EJPs) evoked in it (near distension) or conducted through it (far distension) (n = 4 in both cases). B, effects of UK14,304 when applied to the whole bath on EJPs elicited by distension in the near chamber (n = 9). C, effects of UK14,304 applied to the recording chamber on EJPs (n = 5 for both near and far chamber distensions). D, effect of UK14,304 when applied to the near chamber on descending reflexes evoked in the near and far chambers (n = 5 in both cases). E, effect of UK14,304, when applied to the recording chamber, on descending reflexes (n = 5 in both cases). F, effect of UK14,304 when applied to the whole bath on IJPs, (near chamber distension, n = 6; far chamber distension, n = 5). Symbols indicate a significant difference from control, *P < 0.03, †P < 0.001.
Application of UK14,304 to the intermediate chamber in the descending pathway had no significant effect on the IJPs evoked by distensions applied in this chamber (n = 5, P > 0.27; Fig. 8Bb, Fig. 9D, left panel) or in the more distant chamber (n = 5, P > 0.07, Fig. 9D, right panel). Addition of this α2-agonist to the recording chamber also had no significant effect on the IJPs evoked in either the intermediate or the distant chambers (n = 5, P > 0.45, P > 0.25, respectively; Fig. 9E). However, when UK14,304 was applied to the whole bath, there was a significant inhibition of the IJPs evoked by distension in either stimulation chamber with the reduction in reflexes conducted over longer distances being substantially larger (intermediate stimulation: residual response 70 %, n = 6, P < 0.02; distant stimulation: residual response 30 %, n = 5, P < 0.03, Fig. 9F).
When the effect of UK14,304 on reflexes conducted over the shorter distances was compared for both the ascending and descending pathways, the inhibition of the ascending pathway was found to be significantly greater (Wilcoxon-Mann-Whitney rank sum test for unpaired data, P < 0.05).
DISCUSSION
The results of this study indicate that the sympathetic nerves inhibit intestinal motility reflexes by an action on α2-adrenoceptors located on several functionally distinct types of neurons in both the ascending and descending motor pathways of the guinea-pig small intestine. Whether these effects are presynaptic, postsynaptic, or both remains to be determined. It was also shown that, when the actions of noradrenaline are blocked, stimulation of mesenteric nerves can enhance ascending motor activity, perhaps via activation of collaterals of extrinsic primary afferent neurons.
Properties of the ascending excitatory pathway
Ascending contractions evoked by either electrical stimulation or distension in intact tubes of intestine occurred almost simultaneously in the circular and longitudinal muscles. Bayliss & Starling (1899) first observed that a contraction of the longitudinal muscle occurs above a physiological stimulus applied to the intestinal wall. It has since been argued that ascending pathways relax this layer in response to physiological stimulation (Kottegoda, 1969; Wood, 1994). However, the present observation, that ascending excitation of the circular muscle is accompanied by excitation of the longitudinal muscle, is paralleled by recent studies of reflexes in the distal colon (Smith & Robertson, 1998) and ileum (Spencer et al. 1999b).
The contractile responses recorded in this study appeared to be all-or-nothing and not graded. This contrasts with studies of ascending excitatory reflexes, which have found that such reflexes are graded according to the magnitude of the stimulus (Tonini et al. 1996; Spencer et al. 1999b). By contrast, the peristaltic reflex evoked in vitro by stretching the intestinal wall is an all-or-nothing phenomenon that is typically not preceded by a graded contraction of the circular muscle (Brookes et al. 1999). In both the present study (Fig. 3) and in studies of the peristaltic reflex, distending stimuli well above those required to evoke the initial all-or-nothing contraction evoke larger circular muscle contractions. The most likely explanation for the all-or-nothing responses is that the recording method stimulates local enteric circuits even in the absence of other stimuli applied more anally. The recording balloon needs to be slightly inflated to measure pressure, either in the intact intestinal tube or in the opened intestine. Thus, there is a basal level of tension in the circular muscle and an ongoing mechanical stimulus to the mucosa. Rapid reflex responses in the circular muscle to sustained stimuli quickly decline to baseline levels (Smith et al. 1991), but the intrinsic sensory neurons continue to fire in response to such stimuli (Smith et al. 1991; Kunze et al. 1998). Furthermore, this firing is accompanied by slow excitatory synaptic potentials (EPSPs) that partially suppress the characteristic after-hyperpolarizing potentials (AHPs) in the intrinsic sensory neurons (Kunze et al. 1998). Thus, intrinsic sensory neurons at the recording site may be in a state of tonic excitation prior to activation of the ascending neural pathways. The output of the intrinsic sensory neurons to local motor neurons also includes slow EPSPs. Thus, motor neurons near the recording balloon will be tonically excited and primed to produce an all-or-nothing propulsive contraction in the same way that the oral end of a slowly distended segment of ileum is primed to produce a similar contraction with no graded pre-contraction (Brookes et al. 1999).
Computer simulation of the networks of intrinsic sensory neurons indicates that, if AHPs are not completely suppressed by slow EPSPs, the firing of the network is graded according to stimulus intensity (Thomas et al. 2000). Furthermore, ongoing stimulation of the mucosa greatly enhances responses to rapid changes in muscle length (Smith et al. 1991). Thus, input to the network of intrinsic sensory neurons may set its gain, so that a small stimulus produces positive feedback and an all-or-nothing response. This suggests that, like the peristaltic reflex, the all-or-nothing contractions seen in this study arise from activation of a complex motor pattern that can be clearly differentiated from the ascending excitatory reflex (Tonini et al. 1996).
Properties of the inhibition evoked by MN stimulation
The present results indicate that MN stimulation inhibits the ascending excitatory motor pattern by releasing noradrenaline from sympathetic nerve terminals onto α2-adrenoceptors on neurons of this motor pathway. The inhibition was blocked by the α2-antagonist idazoxan, and mimicked by the α2-agonist UK14,304. As the latter had no effect on contractions of the circular muscle in the presence of tetrodotoxin, it is unlikely that there are functional α2-adrenoceptors on the muscle and the sympathetic nerves must inhibit contractions indirectly, presumably via the myenteric plexus. Indeed, α2-adrenoceptor agonists produce presynaptic inhibition of transmitter release and hyperpolarize some neurons within the myenteric plexus (Surprenant & North, 1985; Galligan & North, 1991). Although an early report suggested that sympathetic stimulation causes presynaptic inhibition (Hirst & McKirdy, 1974), this has yet to be directly confirmed. In this case, the nerves stimulated did not directly innervate the recording site, and the inhibition seen was complete, suggesting the inhibition may have occurred at a remote synapse.
Excitatory effects of MN stimulation
The immediate contraction, or ‘on’ contraction, evoked in some preparations by MN stimulation is probably due to antidromic activation of extrinsic primary afferent axons innervating the intestine. After sympathetic effects are eliminated, MN stimulation causes a contraction that is abolished by capsaicin and is probably due to the release of either substance P or calcitonin gene-related peptide (Barth´ & Holzer, 1985; De Luca & Rand, 1990). These nerves do not appear to innervate the muscle layers directly (Gibbins et al. 1985; Timmermans et al. 1992). Furthermore, contractions of the circular muscle due to capsaicin-induced transmitter release from extrinsic primary afferent nerves are abolished by TTX (Barth´et al. 1994). The present results are consistent with the ‘on’ contractions being mediated entirely via enteric neurons, because they were completely blocked by hexamethonium. This has been previously described for longitudinal muscle contractions evoked by MN stimulation (Day & Rand, 1961; De Luca & Rand, 1990). This effect of hexamethonium suggests that the extrinsic primary afferents act on interneurons or intrinsic sensory neurons, rather than directly on excitatory motor neurons, which is consistent with an earlier observation that MN stimulation depolarizes some intrinsic sensory neurons in the guinea-pig ileum (Takaki & Nakayama, 1988).
The facilitation of the ascending excitatory motor pattern produced by MN stimulation in the presence of an α2-adrenoceptor antagonist is probably also due to the stimulation of extrinsic sensory axons. Release of mediators into the plexus by these nerves may ‘prime’ the local circuitry, and in particular the excitatory motor neurons by depolarizing them closer to their thresholds and so the pattern-generating circuit produces a greater response when activated.
Sites of action of the α2-agonist in motor pathways
Adrenergic agonists have effects at multiple sites within the motor pathways. The results from the addition of UK14,304 to different chambers of the divided organ bath indicate that the major sites of action of this drug, and hence the sympathetic nerves, are the intrinsic sensory neurons and interneurons of the ascending motor circuit. By contrast, transmission from interneurons to excitatory motor neurons is weakly inhibited and the descending reflex pathway is relatively resistant to α2-adrenoceptor blockade.
The inhibition of excitatory responses seen with UK14,304 in the stimulus chamber indicates that it inhibits the firing of intrinsic sensory neurons or synaptic activation of ascending interneurons by these neurons, or both. The intrinsic sensory neurons have been shown, by direct electrophysiological recordings during reflex stimulation (Kunze et al. 1995, 1998), to be of the AH electrophysiological type (displaying a prolonged after-hyperpolorization) and the Dogiel type II morphological class. Many AH-neurons are hyperpolarized by α2-agonists (Galligan & North, 1991), so a reduction in the excitability of intrinsic sensory neurons may partially account for the inhibition seen here. The intrinsic sensory neurons transmit to other intrinsic sensory neurons, to interneurons and to motor neurons via both slow EPSPs (Kunze et al. 1993) and fast EPSPs (Stebbing & Bornstein, 1996). By contrast, ascending interneurons communicate with other ascending interneurons via fast EPSPs (Brookes et al. 1997). Both fast and slow EPSPs can be inhibited by α2-agonists (Morita & North, 1981; Galligan & North, 1991), although the sources of the specific responses inhibited are not yet identified. Thus, UK14,304 may depress transmission in the pathway by an action on the release of neurotransmitter at synapses between intrinsic sensory neurons and interneurons and between interneurons, as well as depressing the activity of the sensory neurons. The weaker effect of α2-adrenoceptor activation in the recording chamber may be due to an action on synapses between ascending interneurons and excitatory motor neurons, as transmission at these synapses is via fast EPSPs (Bornstein et al. 1991). UK14,304 may also act pre-junctionally to inhibit output from motor terminals, as twitch contractions of the longitudinal muscle are inhibited by both sympathetic stimulation and α-agonists in the presence of hexamethonium (Watt, 1971). Further, UK14,304 may inhibit ongoing activity in the network of intrinsic sensory neurons at the recording site, thereby interrupting the positive feedback and preventing initiation of the excitatory motor pattern.
By contrast with the ascending motor pathway, activation of α2-adrenoceptors in any one chamber of the divided organ bath had virtually no effect on IJPs evoked by distension. This suggests that the activation of α2-adrenoceptors at any one point in the pathway has very little effect. However, when UK14,304 was added to the entire descending pathway, descending inhibitory reflexes were significantly depressed. Thus depression of transmission and intrinsic sensory neuron excitability at many points within the pathway apparently has a significant cumulative effect, although the individual effects are very small.
These results imply that UK14,304 does not affect the sensory neurons in the descending inhibitory reflex pathway as strongly as those in the ascending pathway. Only a subpopulation of the intrinsic sensory neurons are hyperpolarized via α-adrenoceptors (Galligan & North, 1991), so the neurons that initiate descending inhibitory reflexes may differ from those that initiate ascending motor patterns. This is consistent with other studies indicating that transmission from intrinsic sensory neurons differs between the ascending and descending reflex pathways (Smith et al. 1990; Johnson et al. 1996, 1998).
Implications for the action of sympathetic nerves in the intestine
This study shows that that activation of α2-adrenoceptors is necessary for sympathetic inhibition in the small intestine. There are many sites within the myenteric plexus at which neurally released noradrenaline might act, but whether all these sites are innervated remains to be determined. Nevertheless, it seems likely that the primary sites of action of the sympathetic nerves are within the ascending excitatory motor pathway.
Even if the α2-adrenoceptors on the neurons of the descending inhibitory pathways are innervated by sympathetic axons, it appears that these pathways are less susceptible to sympathetic activity than short excitatory reflexes. Sparing short inhibitory reflex pathways might permit such processes as receptive relaxation (Greenwood et al. 1987), while peristalsis, which depends on the ascending pathways, is inhibited. This would allow the extrinsic innervation to play a significant role in the selection of the motor programme controlling intestinal motility.
Acknowledgments
This work was supported by a Program Grant (963213) of the National Health and Medical Research Council of Australia.
References
- Barry DT. The functions of the great splanchnic nerves. Journal of Physiology. 1932;75:480–490. doi: 10.1113/jphysiol.1932.sp002905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthó L, Holzer P. Search for a physiological role of substance P in gastrointestinal motility. Neuroscience. 1985;16:1–32. doi: 10.1016/0306-4522(85)90043-0. [DOI] [PubMed] [Google Scholar]
- Barthó L, Maggi CA, Wilhelm M, Patacchini R. Tachykinin NK1 and NK2 receptors mediate atropine-resistant ileal circular muscle contractions evoked by capsaicin. European Journal of Pharmacology. 1994;259:187–193. doi: 10.1016/0014-2999(94)90509-6. [DOI] [PubMed] [Google Scholar]
- Bayliss WM, Starling EH. The movements and innervation of the small intestine. Journal of Physiology. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Smith TK, Trussell DC. Synaptic responses evoked by mechanical stimulation of the mucosa in morphologically characterized myenteric neurons of the guinea pig ileum. Journal of Neuroscience. 1991;11:505–518. doi: 10.1523/JNEUROSCI.11-02-00505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJH, Chen BN, Costa M, Humphreys CMS. Initiation of peristalsis by circumferential stretch of flat sheets of guinea-pig ileum. Journal of Physiology. 1999;516:525–538. doi: 10.1111/j.1469-7793.1999.0525v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJH, Meedeniya ACB, Jobling P, Costa M. Orally projecting interneurones in the guinea-pig small intestine. Journal of Physiology. 1997;505:473–491. doi: 10.1111/j.1469-7793.1997.473bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. Peristalsis, segmentation and the myenteric reflex. American Journal of Physiology. 1912;30:114–128. [Google Scholar]
- Costa M, Brookes SJH, Steele PA, Gibbins IL, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- Day MD, Rand MJ. Effect of guanethidine in revealing sympathetic cholinergic fibres. British Journal of Pharmacology. 1961;17:245–260. doi: 10.1111/j.1476-5381.1961.tb01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Rand MJ. Responses of rat isolated intestinal segments to stimulation of perivascular mesenteric nerve fibres. Journal of Autonomic Pharmacology. 1990;10:323–331. doi: 10.1111/j.1474-8673.1990.tb00032.x. [DOI] [PubMed] [Google Scholar]
- Finkleman B. On the nature of inhibition in the intestine. Journal of Physiology. 1930;70:145–157. doi: 10.1113/jphysiol.1930.sp002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB, Costa M. The adrenergic innervation of the gastrointestinal tract. Ergebnisse der Physiologie. 1974;69:1–51. [PubMed] [Google Scholar]
- Furness JB, Costa M. The Enteric Nervous System. Edinburgh, London, Melbourne, New York: Churchill Livingstone; 1987. [Google Scholar]
- Galligan JJ, North RA. Opioid, 5-HT1A and α2 receptors localized to subsets of guinea-pig myenteric neurons. Journal of the Autonomic Nervous System. 1991;32:1–12. doi: 10.1016/0165-1838(91)90229-v. [DOI] [PubMed] [Google Scholar]
- Gibbins IL, Furness JB, Costa M, MacIntyre I, Hillyard CJ, Girgis S. Co-localization of calcitonin gene related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea-pigs. Neuroscience Letters. 1985;57:125–130. doi: 10.1016/0304-3940(85)90050-3. [DOI] [PubMed] [Google Scholar]
- Greenwood B, Tremblay L, Davison J. Sympathetic control of motility, fluid transport, and transmural potential difference in the rabbit ileum. American Journal of Physiology. 1987;253:G726–729. doi: 10.1152/ajpgi.1987.253.6.G726. [DOI] [PubMed] [Google Scholar]
- Hirst GD S, McKirdy HC. Presynaptic inhibition at a mammalian peripheral synapse? Nature. 1974;250:430–431. doi: 10.1038/250430a0. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Yuan SY, Bornstein JC, Furness JB. Analysis of contributions of acetylcholine and tachykinins to neuro-neuronal transmission in motility reflexes in the guinea-pig ileum. British Journal of Pharmacology. 1996;118:973–983. doi: 10.1111/j.1476-5381.1996.tb15495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock NG. An experimental analysis of mechanisms engaged in reflex inhibition of intestinal motility. Acta Physiologica Scandinavica Supplementum. 1959;164:1–54. doi: 10.1111/j.1748-1716.1959.tb01824.x. [DOI] [PubMed] [Google Scholar]
- Kosterlitz HW, Lees GM. Pharmacological analysis of intrinsic intestinal reflexes. Pharmacological Reviews. 1964;16:301–339. [PubMed] [Google Scholar]
- Kottegoda SR. An analysis of possible nervous mechanisms involved in the peristaltic reflex. Journal of Physiology. 1969;200:687–712. doi: 10.1113/jphysiol.1969.sp008717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreulen DL, Muir TC, Szurszewski JH. Peripheral sympathetic pathways to gastroduodenal region of the guinea pig. American Journal of Physiology. 1983;245:369–375. doi: 10.1152/ajpgi.1983.245.3.G369. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Bornstein JC, Furness JB. Physiological identification of sensory nerve cells in a peripheral organ (the intestine) of a mammal. Neuroscience. 1995;66:1–4. doi: 10.1016/0306-4522(95)00067-s. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Furness JB, Bertrand PP, Bornstein JC. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. Journal of Physiology. 1998;506:827–842. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WAA, Furness JB, Bornstein JC. Simultaneous intracellular recordings from enteric neurons reveals that myenteric AH neurons transmit via slow excitatory post-synaptic potentials. Neuroscience. 1993;55:685–694. doi: 10.1016/0306-4522(93)90434-h. [DOI] [PubMed] [Google Scholar]
- Lee CY. The effect of stimulation of extrinsic nerves on peristalsis and on the release of 5-hydroxytryptamine in the large intestine of the guinea-pig and of the rabbit. Journal of Physiology. 1960;152:405–418. doi: 10.1113/jphysiol.1960.sp006496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax AEG, Furness JB. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell and Tissue Research. 2000;302:59–72. doi: 10.1007/s004410000260. [DOI] [PubMed] [Google Scholar]
- Mizutani M, Neya T, Nakayama S. Capsaicin-sensitive afferents activate a sympathetic intestinointestinal inhibitory reflex in dogs. Journal of Physiology. 1990;425:133–144. doi: 10.1113/jphysiol.1990.sp018096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, North RA. Clonidine activates membrane potassium conductance in myenteric neurones. British Journal of Pharmacology. 1981;74:419–428. doi: 10.1111/j.1476-5381.1981.tb09987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S, North RA. Presynaptic action of noradrenaline in the myenteric plexus. Journal of Physiology. 1973;231:29–30P. [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Distension-evoked ascending and descending reflexes in the circular muscle of guinea-pig ileum: an intracellular study. Journal of the Autonomic Nervous System. 1990;29:203–213. doi: 10.1016/0165-1838(90)90146-a. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Interactions between reflexes evoked by distension and mucosal stimulation: electrophysiological studies of guinea-pig ileum. Journal of the Autonomic Nervous System. 1991;34:69–76. doi: 10.1016/0165-1838(91)90009-r. [DOI] [PubMed] [Google Scholar]
- Smith TK, Furness JB. Reflex changes in circular muscle activity elicited by stroking the mucosa: An electrophysiological analysis in the isolated guinea-pig. Journal of the Autonomic Nervous System. 1988;25:205–218. doi: 10.1016/0165-1838(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Smith TK, Robertson WJ. Synchronous movements of the longitudinal and circular muscle during peristalsis in the isolated guinea-pig distal colon. Journal of Physiology. 1998;506:563–577. doi: 10.1111/j.1469-7793.1998.563bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N, McCarron SL, Smith TK. Sympathetic inhibition of ascending and descending interneurones during the peristaltic reflex in the isolated guinea-pig distal colon. Journal of Physiology. 1999a;519:539–550. doi: 10.1111/j.1469-7793.1999.0539m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N, Walsh M, Smith TK. Does the guinea-pig ileum obey the ‘law of the intestine’? Journal of Physiology. 1999b;517:889–898. doi: 10.1111/j.1469-7793.1999.0889s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing MJ, Bornstein JC. Electrophysiological mapping of fast synaptic inouts to morphologically and chemically characterised myenteric neurons of guinea-pig small intestine. Neuroscience. 1985;16:425–430. doi: 10.1016/0306-4522(96)00121-2. [DOI] [PubMed] [Google Scholar]
- Surprenant A, North RA. μ-Opioid receptors and α2-adrenoceptors coexist on myenteric but not on submucous neurones. Neuroscience. 1985;16:425–430. doi: 10.1016/0306-4522(85)90014-4. [DOI] [PubMed] [Google Scholar]
- Tack JF, Wood JD. Actions of noradrenaline on myenteric neurons in the guinea pig gastric antrum. Journal of the Autonomic Nervous System. 1992;41:67–77. doi: 10.1016/0165-1838(92)90128-4. [DOI] [PubMed] [Google Scholar]
- Takaki M, Nakayama S. Effects of mesenteric nerve stimulation on the electrical activity of myenteric neurons in the guinea-pig ileum. Brain Research. 1988;442:351–353. doi: 10.1016/0006-8993(88)91524-7. [DOI] [PubMed] [Google Scholar]
- Takayanagi I, Sato T, Takagi K. Effects of sympathetic nerve stimulation on electrical activity of Auerbach's plexus and intestinal smooth muscle tone. Journal of Pharmacy and Pharmacology. 1977;29:376–377. doi: 10.1111/j.2042-7158.1977.tb11343.x. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Bertrand PP, Bornstein JC. A computer simulation of recurrent, excitatory networks of sensory neurons of the gut in guinea-pig. Neuroscience Letters. 2000;287:137–140. doi: 10.1016/s0304-3940(00)01182-4. [DOI] [PubMed] [Google Scholar]
- Timmermans J-P, Scheuermann DW, Barbiers M, Adriaensen D, Stach W, Van Hee R, De Groodt-Lasseel MH A. Calcitonin gene-related peptide-like immunoreactivity in the human small intestine. Acta Anatomica. 1992;143:48–53. doi: 10.1159/000147227. [DOI] [PubMed] [Google Scholar]
- Tonini M, Costa M. A pharmacological analysis of the neuronal circuitry involved in distension-evoked enteric excitatory reflex. Neuroscience. 1990;38:787–795. doi: 10.1016/0306-4522(90)90071-b. [DOI] [PubMed] [Google Scholar]
- Tonini M, Costa M, Brookes SJH, Humphreys CMS. Dissociation of the ascending excitatory reflex from peristalsis in the guinea-pig small intestine. Neuroscience. 1996;73:287–297. doi: 10.1016/0306-4522(96)00040-1. [DOI] [PubMed] [Google Scholar]
- Watt AJ. The inhibitory effect of perivascular stimulation on the response of the guinea-pig isolated ileum. Journal of Physiology. 1971;219:38–39P. [PubMed] [Google Scholar]
- Weisenthal LM, Hug CC, Weisbrodt NW, Bass P. Adrenergic mechanisms in the relaxation of guinea-pig taenia coli in vitro. Journal of Pharmacology and Experimental Therapeutics. 1971;178:497–508. [PubMed] [Google Scholar]
- Wood JD. Physiology of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 423–482. [Google Scholar]
- Yuan SY, Bornstein JC, Furness JB. Investigation of the role of 5-HT3 and 5-HT4 receptors in ascending and descending reflexes to the circular muscle of guinea-pig small intestine. British Journal of Pharmacology. 1994;112:1095–1100. doi: 10.1111/j.1476-5381.1994.tb13196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


