Abstract
This study addressed the hypothesis that ventrolateral medullary respiratory neurones participate in the control of laryngeal motoneurones during both eupnoea and coughing.
Data were obtained from 28 mid-collicular decerebrated, artificially ventilated cats. Cough-like motor patterns (fictive cough) in phrenic, lumbar and recurrent laryngeal nerves were elicited by mechanical stimulation of the intrathoracic trachea. Microelectrode arrays were used to monitor simultaneously several neurones in the ventral respiratory group, including the Bötzinger and pre-Bötzinger complexes. Spike trains were evaluated for responses during fictive cough and evidence of functional connectivity with spike-triggered averages of efferent recurrent laryngeal nerve activity.
Primary features were observed in averages triggered by 94 of 332 (28 %) neurones. An offset biphasic wave with a positive time lag was present in the unrectified average for 10 inspiratory and 13 expiratory neurones. These trigger neurones were respectively identified as inspiratory laryngeal motoneurones with augmenting, decrementing, plateau and ‘other’ discharge patterns, and expiratory laryngeal motoneurones with decrementing firing patterns.
Rectified averages triggered by inspiratory neurones included 37 offset peaks, 11 central peaks and one offset trough. Averages triggered by expiratory neurones had 12 offset peaks, six central peaks and four offset troughs. Relationships inferred from these features included premotor actions of inspiratory neurones with augmenting, decrementing, plateau and ‘other’ patterns on inspiratory laryngeal motoneurones, and premotor actions of decrementing and ‘other’ expiratory neurones on expiratory laryngeal motoneurones. Corresponding changes in neuronal firing patterns during fictive cough supported these inferences.
The data confirm and extend previous results on the control of laryngeal motoneurones during eupnoea and support the hypothesis that the same premotor neurones help to shape motoneurone firing patterns during both eupnoea and coughing.
The generation of a cough requires control of the glottis by a coordinated sequence of activity in the intrinsic laryngeal muscles (for reviews, see Widdicombe, 1964, 1986; Korpas & Tomori, 1979; Sant'Ambrogio et al. 1997). The glottis is actively opened, closed and reopened during the inspiratory, compressive and expulsive phases of cough, respectively.
The motoneurones of the intrinsic laryngeal muscles are located in the nucleus ambiguus, which extends from the retrofacial nucleus to a region caudal to the obex. The motoneurones are close to bulbospinal and propriobulbar neurones of the ventral respiratory group, and their axons travel in the recurrent laryngeal nerve and external branch of the superior laryngeal nerve (Iscoe, 1988; Bianchi et al. 1995; Yoshida et al. 1998). The respiratory modulated discharge patterns of laryngeal motoneurones during eupnoea result primarily from inhibitory and excitatory synaptic influences of other neurones in the ventrolateral medullary respiratory network (Richter et al. 1979; Barillot et al. 1990; Ezure, 1990).
It has been proposed that neurones controlling the discharge pattern of laryngeal motoneurones during eupnoea also influence the pattern during cough (Gestreau et al. 2000). However, presynaptic neurones specifically involved in producing the cough pattern have not been identified. We have previously reported evidence for the participation of ventrolateral medullary respiratory neurones in the generation of the cough motor patterns of respiratory pump muscles (Shannon et al. 1998, 2000). The objective of the present study was to address the hypothesis that such neurones also contribute to the control of laryngeal motoneurones during cough. Spike trains of simultaneously recorded neurones were evaluated for responses during fictive cough and for evidence of functional connectivity using spike-triggered averages of efferent recurrent laryngeal nerve activities. Preliminary accounts of the results have been reported (Shannon et al. 1996, 1999).
METHODS
General methods
Most of the methods have been described in detail elsewhere (Shannon et al. 1998, 2000). Experiments were performed under protocols approved by the University of South Florida's Animal Care and Use Committee. Data were obtained from 28 adult cats (2.5–4.1 kg) of either sex. Animals were initially anaesthetized with intravenous sodium thiopental (22.0 mg kg−1) and later decerebrated using the technique of Kirsten & St John (1978).
Before surgery, atropine (0.5 mg kg−1, i.m.) was administered to reduce mucus secretion in the airways, and dexamethasone (2.0 mg kg−1, i.v.) was given to help prevent hypotension and minimize brainstem swelling. Femoral arteries and veins were catheterized for monitoring arterial blood pressure, acquisition of arterial blood samples, and administration of intravenous fluids and drugs. Throughout the experiment, arterial blood samples were periodically analysed for PO2, PCO2, pH and [HCO3−]; these parameters were maintained within normal limits. Solutions of 5 % dextrose in 0.45 % NaCl, 5 % dextran, or lactated Ringer solution were administered intravenously as needed to maintain a mean blood pressure of at least 100 mmHg. Until decerebration was completed (to render the animal insentient), the level of anaesthesia was assessed periodically by toe pinch. If the withdrawal reflex occurred or there was an increase in blood pressure or respiration, additional anaesthesia was given until the response was absent.
For decerebration, the external carotid arteries were ligated rostral to the lingual arteries bilaterally. A craniotomy was performed in the parietal bones. The brainstem was transected at the midcollicular level and nervous tissue rostral to the transection was aspirated. During the decerebration and thereafter, animals were continuously infused intravenously with the neuromuscular blocker gallamine triethiodide (4.0 mg kg−1 h−1), and artificially ventilated through a tracheal cannula with a phrenically driven respirator. End-tidal CO2 was maintained at 4.0–5.0 %. A bilateral thoracotomy was performed to minimize brainstem movement that could result from changes in thoracic pressure during positive-pressure ventilation. When necessary, the fraction of inspired O2 was increased to prevent hypoxaemia, which often occurs during long-term experiments because of ventilation-perfusion mismatching resulting from the open chest. The functional residual capacity of the lungs in thoracotomized animals was maintained within a normal range by adjustment of end-expiratory pressure. Periodically, the trachea was suctioned and the lungs were hyperinflated. Rectal temperature was maintained at 38.0 ± 0.5 °C. Animals were placed prone in a stereotaxic frame. At the end of the experiments, cats were killed with an overdose of sodium pentobarbital and then potassium chloride.
Nerve recordings
The right lumbar iliohypogastric (L1) and left phrenic (C5) nerves were desheathed, cut and their efferent activities recorded with bipolar silver electrodes in pools of mineral oil. The right recurrent laryngeal nerve was desheathed and cut close to the larynx in order to leave the tracheal innervation as intact as possible; efferent activity was recorded with bipolar silver electrodes covered with cotton pledgets saturated with mineral oil. Nerve signals were amplified and filtered (bandpass 0.1–5 kHz). Phrenic, lumbar and recurrent laryngeal nerve discharges were integrated with a leaky resistor-capacitor circuit (0.2 s time constant) and recorded on a polygraph to monitor the effectiveness of the stimuli to elicit cough.
Neurone recordings
An occipital craniotomy was performed and portions of the caudal cerebellum were removed by suction to expose the medulla. Medullary respiratory neurones were monitored with two independently controlled planar arrays of tungsten microelectrodes (10–12 MΩ) positioned on the right side of the medulla. Each array consisted of six to eight microelectrodes. In the initial experiments, individual electrodes in an array were fixed to each other. In later experiments, the depth of each electrode was adjusted individually with micromotor controllers. Signals were amplified and filtered (bandpass 0.1–5 kHz). The medullary surface was covered with a pool of warm mineral oil.
Regions of the ventral lateral medulla searched for respiratory modulated neurones included the following. (a) The rostral ventral respiratory group, which contains the Bötzinger and pre-Bötzinger complexes; 3.0–5.5 mm rostral to obex, 3.0–4.5 mm lateral to midline, 3.0–5.5 mm below the dorsal surface. (b) The intermediate-caudal ventral respiratory group; 2.0 mm rostral to 4.0 mm caudal to obex, 3.0–4.5 mm lateral to midline, 2.5–4.5 mm below the dorsal surface (Lindsey et al. 1987; Ezure, 1990; Bianchi et al. 1995; Schwarzacher et al. 1995; Shannon et al. 1998).
Evoking fictive cough
Mechanical stimulation of the intrathoracic trachea has been used in spontaneously breathing animals to elicit cough and in artificially ventilated animals (under neuromuscular block) to produce cough-like patterns (fictive cough) in respiratory motor nerve activities (Korpas & Tomori, 1979; Shannon et al. 1998, 2000). See Fig. 1 in Shannon et al. (2000) for a schematic illustration of the stimulator and electronic controller. Fictive coughing was elicited by stimulating sections of the intrathoracic trachea (mid-cervical to carinal region) with two loops of polyethylene tubing configured as ellipses and attached to a thin wire inserted through a port in the tracheal cannula. Movement of the stimulator into and out of the trachea, its rotation rate and the region of stimulation were controlled electronically. The same parameters were used in each cough series. Following each stimulus period, the stimulator was retracted into the cannula. When more than one cough occurred in a trial, only the first was analysed. Cough was characterized by a large increase in phrenic nerve activity immediately followed by a large increase in lumbar nerve activity. At least five separate episodes of cough were produced in each recording. Each stimulus trial was separated by at least 1 min. This interval was sufficient for a return to control inspiratory and expiratory motor patterns. A phrenically driven ventilator was used to allow matching of pulmonary stretch receptor activity with central inspiratory and expiratory activity.
Figure 1. Spike-triggered averages with features identifying inspiratory neurones as laryngeal motoneurones and putative premotor neurones.
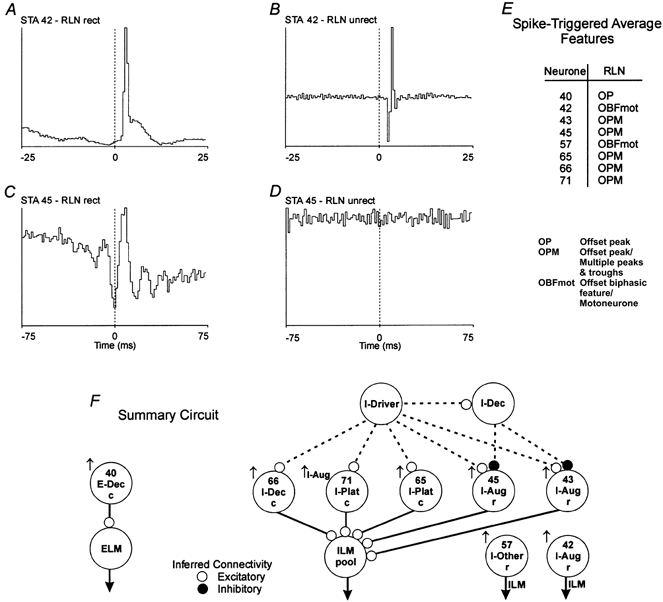
An offset biphasic feature in an unrectified (unrect) average of recurrent laryngeal nerve (RLN) efferent activity indicates the trigger neurone was a motoneurone (B). An offset peak (primary feature) in a full-wave rectified (rect) average (C) and a flat unrectified average (D) suggests the neurone was excitatory to motoneurones. A, spike-triggered average (STA); offset peak with a lag of 2.0 ms; half-width, 1.0 ms; number of trigger events, 16763. B, STA; offset biphasic feature. C, STA; offset peak with a lag of 3.0 ms; half-width, 4.5 ms; number of trigger events, 41235. D, STA; flat. E, spike-triggered average feature table summarizing primary features in spike-triggered average histograms in the data set. F, summary circuit showing inferred functional connections detected in the data set in Fig. 2. Changes in peak firing rate (↑, increase) and pattern (Aug, augmenting) during cough. c, intermediate-caudal VRG; r, rostral VRG, which contains the Bötzinger and pre-Bötzinger complexes; ELM, expiratory laryngeal motoneurone; ILM, inspiratory laryngeal motoneurone. Continuous lines represent inferred connectivity from the STA. Dotted lines are hypothesized connections.
Data acquisition, entry and preprocessing
During the experiments, signals were monitored on oscilloscopes, a polygraph and audio monitors and recorded on magnetic tape for off-line analysis. These data included signals from the microelectrode arrays, efferent nerve activities, arterial blood pressure, tracheal pressure and stimulus timing signals. Multifibre efferent phrenic, lumbar and recurrent laryngeal nerve activities were integrated to obtain a moving time average of activity in the nerves. These analog signals, together with arterial blood pressure, tracheal pressure, stimulus timing signals, and signals from each microelectrode, were digitized and stored on hard drives. Time stamps were derived from the integrated phrenic signal to indicate the onset of each inspiratory and expiratory phase. Action potentials of single neurones were converted to times of occurrence with spike-sorting software (Datawave Tech. Corp.). Data files were transferred to Hewlett-Packard 9000/735 and c160 computers for subsequent processing and analysis. The signals of efferent multiunit nerve activities were high-pass filtered (40 Hz, 3 dB cut-off) and, along with the common synchronization timing pulses, were digitized (5 kHz) with a 16-bit ADC488/16 analog-to digital converter hosted by a Hewlett-Packard 9000/380 computer. These files were subsequently analysed with the corresponding spike files for spike-triggered averaging as described below. The program Xscope (Lindsey et al. 1992) provided a graphical representation of the times of action potentials and other digital and analog signals. The program allowed additional event codes to be added and graphically confirmed the selection of data segments to be written as separate files for later analysis.
Spike train analysis methods for each single neurone
The following measures were computed from a 5 min control period that preceded the beginning of the cough trials. Spike trains were subjected to two statistical evaluations of respiratory modulation and a measure of respiratory modulation, η2, was calculated (Orem & Dick, 1983; Morris et al. 1996). Cycle-triggered histograms were used to classify cells with statistically significant respiratory modulation according to the phase (inspiratory (I) or expiratory (E)) in which they were more active. Neurones with peak firing rates in the first half of the phase were classified as decrementing (Dec), whereas those cells with peak firing rates in the second half of the phase were denoted as augmenting (Aug). Cells with a relatively constant discharge rate throughout a respiratory phase were classified as plateau (Plat). Neurones, generally with low firing rates, that could not be placed in one of these major classifications were denoted as I-Other or E-Other. Cells that were silent during control cycles and were elicited during cough were denoted as Recruit (e.g. peak activity during the expiratory phase of cough, E-Recruit).
Autocorrelograms were computed for each spike train to ensure that it represented the activity of one neurone. A recording with action potentials from two or more neurones would include short intervals not constrained by refractoriness. Spike trains were also evaluated statistically for changes in peak discharge rate and duration of firing during cough. These values during the first cough cycle, averaged over five trials, had to be significantly different from the mean for five control cycles preceding the coughs (P < 0.05, Student's t test).
Spike-triggered averaging of recurrent laryngeal efferent nerve signals
Each average was computed over the entire recording period. Occurrence times of action potentials in each neurone defined intervals of the digitized recurrent laryngeal nerve activity that were used to generate corresponding ‘triggered’ averages of both unrectified and full-wave rectified signals. A short-latency (1.5–3.5 ms; Christakos et al. 1994), short-duration biphasic feature in the unrectified recurrent laryngeal nerve average provided evidence that the recorded neurone was a laryngeal motoneurone (Botteron & Cheney, 1989; Maier et al. 1998; Perlmutter et al. 1998). The biphasic feature represents ‘motoneurone’ action potentials. The presence of an offset positive feature in the rectified but not the unrectified signal suggested that the trigger neurone was (1) a premotor neurone that projected to the monitored motoneurone pool, or (2) a neurone that was tightly synchronized with premotor neurones by functionally antecedent shared inputs (Davies et al. 1985; Botteron & Cheney, 1989; Maier et al. 1998; Perlmutter et al. 1998). The presence of an offset trough in a rectified average was consistent with functional inhibition, i.e. any mono- or paucisynaptic process that reduces motoneurone firing probability. A central peak could be attributed to shared inputs with similar actions and time lags. Feature onset latencies and half-widths (width at half-peak height) were determined with 0.5 ms bin widths.
RESULTS
Averages of recurrent laryngeal nerve efferent activity, triggered by spikes in each simultaneously recorded neurone, were screened for features that identified the cell as either a laryngeal motoneurone or a neurone with functional links to motoneurones. Changes in neurone discharge patterns during fictive cough also aided placement of the neurones in a functional context. Each recording was approximately 45 min and included the spike trains of 6–20 single neurones. The figures presented subsequently do not include the complete data sets of neurones recorded simultaneously. Rather, they include primarily motoneurones and those cells with inferred functional associations. Other spike trains in the data set were omitted in order to focus attention on these specific neurones. The neural correlate of the compressive phase of cough in the figures was estimated from the duration of the peak activity in E-Dec neurones, E-Recruit neurones or integrated recurrent laryngeal nerve activity.
In 28 cats, the spike trains of 332 respiratory modulated neurones were recorded in the rostral (including Bötzinger and pre-Bötzinger regions), intermediate and caudal ventral respiratory group (VRG). Table 1 presents a summary of control discharge patterns and changes in peak firing rate during fictive cough of neurones with primary features in their recurrent laryngeal nerve averages. Other results from these neuronal recordings were included in previous reports (Shannon et al. 1998, 2000). Primary features were evident in nerve averages triggered by 59 inspiratory and 35 expiratory neurones. Inspiratory neurone averages included 10 offset biphasic features, 37 offset peaks, 11 central peaks and one offset trough. Expiratory neurone averages included 13 offset biphasic features, 12 offset peaks, six central peaks and four offset troughs.
Table 1.
Primary feaatures in spike-triggered ayerages of recurrent laryngeal nerve activity
| n | OBFmot | OP | CP | OT | |
|---|---|---|---|---|---|
| I-Aug | 27/81 | 4↑ | 18↑ | 3↑, 2→ | — |
| I-Dec | 16/49 | 2↑ | 7↑ 1↓, 2→ | 4↑ | — |
| I-Plat | 8/15 | 1↑ | 4↑ 1↓, 2→ | — | — |
| I-Other | 8/54 | 3↑ | 2↑ | 2→ | 1↑ |
| E-Aug | 5/39 | — | 1↓ | 1→ | 3↑ |
| E-Dec | 24/52 | 7↑, 3→ | 8↑ | 4↑, 1→ | 1↑ |
| E-Plat | 1/5 | — | 1↑ | — | — |
| E-Recruit | 3/5 | 3↑ | — | — | — |
| E-Other | 2/32 | — | 2↑ | — | — |
| Total | 94/332 |
Neurones were classified as expiratory (E) or inspiratory (I), and augmenting (Aug), decrementing (Dec), Other, plateau (Plat), or recruited (Recruit). n, number of neurones (numerator is the number of neurones with primary features in the spike-triggered average; denominator is the total number of neurones used as trigger neurones); OBFmot, offset biphasic feature in unrectified average indicating that the neurone was a motoneurone; CP, central peak; OP, offset peak; OT, offset trough; Direction of change in peakfiring rate during cough: ↑, increase; ↓, decrease; →, no change. The medullary domains in whichrecordings were made are fully defined in the text.
Other averages, triggered by 55 inspiratory neurones and two expiratory neurones, had multiple peaks and troughs but no primary feature and are not included Table 1. These averages with high and medium frequency oscillations (Christakos et al. 1994; Huang & Cohen, 2000) and those with primary central peaks, included in Table 1, are not considered further; they presumably reflected inputs shared by the individual trigger neurones and laryngeal motoneurones.
Inspiratory laryngeal motoneurones
Ten cells with phasic inspiratory modulated firing rates were identified as laryngeal motoneurones. The averages of recurrent laryngeal nerve activity triggered by neurone 42 (Fig. 1A and B) show representative offset features with positive time lags. A short-onset latency, short-duration peak was found in the rectified average (Fig. 1A). The unrectified average had a biphasic feature (Fig. 1B) indicative of spikes arising from a motoneurone with an axon in the recorded nerve (Botteron & Cheney, 1989; Maier et al. 1998; Perlmutter et al. 1998). The mean and standard deviation of the peak onset latencies and half-widths in the rectified averages of the 10 motoneurones were 3.0 ± 1.4 and 2.8 ± 1.4 ms, respectively.
The control discharge patterns of identified inspiratory motoneurones included four with augmenting firing rates, two with decrementing patterns, and one plateau neurone with a relatively uniform average firing rate. Three other motoneurones had irregular discharge patterns and were characterized as I-Other. Figure 2 shows integrated activities of an augmenting inspiratory motoneurone together with five other inspiratory neurones, one expiratory neurone, phrenic, lumbar and recurrent laryngeal nerves, and tracheal pressure during control and cough cycles. During control intervals, motoneurone 42 showed an augmenting discharge pattern that ended when phrenic nerve inspiratory activity stopped. During cough cycles, the firing rate increased and the active phase was prolonged, ending concurrently with phrenic nerve activity. Augmenting inspiratory motoneurones in other recordings had similar discharge patterns; the activity of one extended into the expiratory phase of cough.
Figure 2. Simultaneous responses of inspiratory laryngeal motoneurones and putative premotor inspiratory neurones during fictive cough.
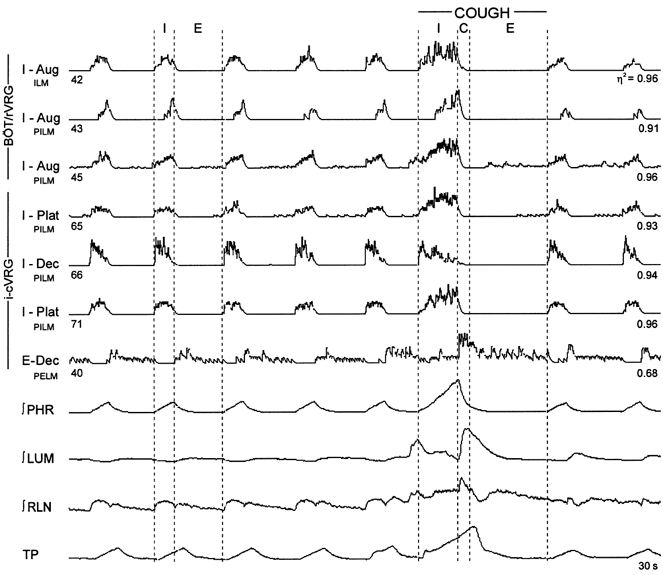
Integrated activities of six inspiratory and one expiratory neurone, phrenic (∫PHR), lumbar (∫LUM) and recurrent laryngeal (∫RLN) nerves, and tracheal pressure (TP). C, estimated neural correlate of the compressive phase; E, expiratory phase; I, inspiratory phase; BÖT/rVRG, rostral VRG, which contains the Bötzinger and pre-Bötzinger complexes; i-cVRG, intermediate-caudal VRG; η2, statistical measure of respiratory modulation; PELM, premotor to expiratory laryngeal motoneurones; ILM, inspiratory laryngeal motoneurone; PILM, premotor to inspiratory laryngeal motoneurones.
The integrated record of a motoneurone with a decrementing inspiratory pattern during control cycles and the corresponding cycle-triggered histogram are shown in Fig. 3A and B, respectively. During cough, the neurone designated 41 had an accentuated augmenting discharge pattern (increased peak and duration of activity) that ceased at the end of the inspiratory phase (Fig. 3A). An I-Dec motoneurone from a different animal had a similar activity pattern that extended into the expiratory phase. During cough cycles, an inspiratory motoneurone with a plateau discharge pattern (similar to I-Plat neurones in Fig. 2) also exhibited an increased peak firing rate in the latter part of the inspiratory phase.
Figure 3. Laryngeal expiratory and inspiratory motoneurones.
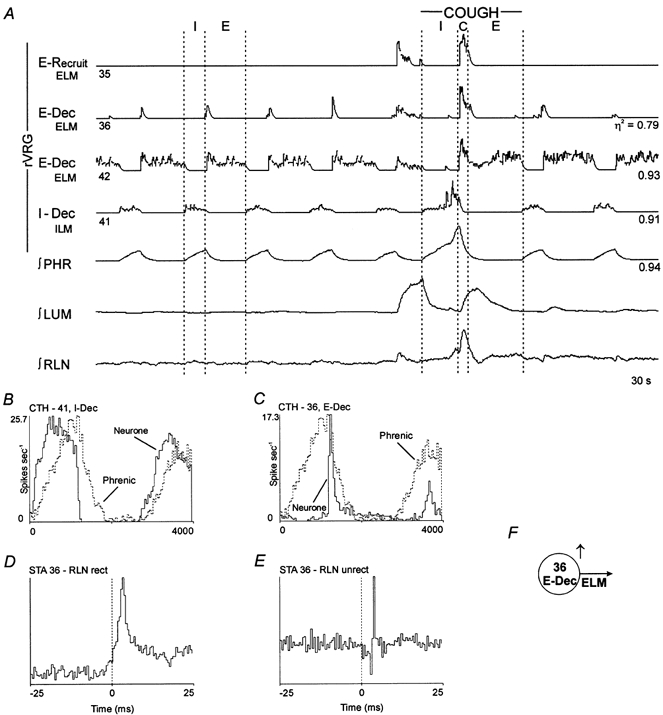
Spike-triggered averages and concurrent responses during the neural compressive phase of fictive cough were consistent with E-Recruit neurone 35 and E-Dec neurones 36 and 42 being motoneurones; note the similarity with the ∫RLN. The I-Dec cell is another example of an inspiratory motoneurone. A, simltaneous activity of expiratory and inspiratory laryngeal motoneurones. B and C, cycle-triggered histograms (CTHs); 100 cycles averaged. D, STA; offset peak with a lag of 1.5 ms; half-width, 1.5 ms; number of trigger events, 5291. E, STA; offset biphasic feature. F, model of inference from STA.
Expiratory laryngeal motoneurones
Thirteen expiratory neurones were identified as laryngeal motoneurones (e.g. Fig. 3E and F). The mean and standard deviations of the peak onset latencies and half-widths for the rectified averages (Fig. 3D) of the 13 motoneurones were 3.0 ± 1.3 and 1.6 ± 0.6 ms, respectively. Ten of the motoneurones had decrementing patterns during both control and cough cycles. Three other motoneurones were silent during control cycles and recruited during cough. The integrated activities of two decrementing expiratory motoneurones (36 and 42) and one motoneurone (35) recruited during cough are shown in Fig. 3A. During control cycles, neurone 36 discharged briefly in the early expiratory phase (Fig. 3A and C) whilst neurone 42 reached peak firing rate in early expiration and maintained a relatively constant rate throughout the remainder of the phase (Fig. 3A). During cough, there was a brief increase in the firing rates of the three motoneurones at the transition between the inspiratory and expiratory phases, marking the neural correlate of the compressive phase. Neurones 35 and 36 were silent following the neural compressive period. Neurone 42 continued activity throughout the remainder of the expiratory phase; a similar response was apparent in the integrated tracing of recurrent laryngeal nerve activity. Another record of decrementing expiratory motoneurone activity is included in Fig. 4A (neurone 78). The peak firing rates of three other decrementing expiratory motoneurones during cough cycles were similar to those during control periods, although the duration of their active phase was prolonged (not shown).
Figure 4. Responses during fictive cough and functional connectivity of a set of decrementing expiratory neurones.
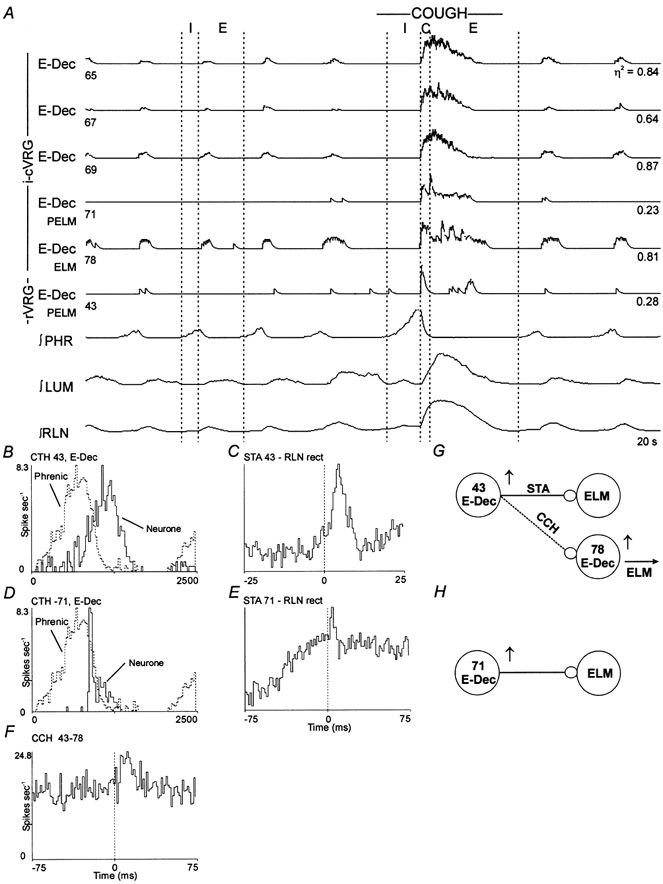
A, simultaneous activity of six E-Dec neurones. Data were consistent with E-Dec neurones 43 and 71 exciting expiratory motoneurones. B and D, CTHs; 100 cycles averaged. C, STA; offset peak with a lag of 2.0 ms; half-width, 4.0 ms; number of trigger events, 1771. E, STA; offset peak with a lag of 1.5 ms; half-width, 3.0 ms; number of trigger events, 885. F, cross-correlogram (CCH); offset peak with a lag of 7.5 ms; half-width, 10.0 ms; detectability index, 5.6; correlation strength, 0.58; 2424 reference and 10591 target spikes. G and H, summary models representing inferred connectivity.
Discharge patterns of neurones with functional links to inspiratory laryngeal motoneurones
Offset peaks were present in the rectified, but not unrectified, averages of recurrent laryngeal nerve activity triggered by spikes in 37 inspiratory neurones. Trigger neurones included 18 augmenting inspiratory neurones, 10 decrementing inspiratory neurones, seven neurones with plateau patterns, and two with low or irregular firing rates (I-Other; Table 1). The mean and standard deviation of peak onset latencies and half-widths for the 37 neurones were 3.2 ± 1.6 and 6.3 ± 2.7 ms, respectively. In one data set, five rectified averages triggered by simultaneously recorded inspiratory neurones each had an offset peak with secondary multiple peaks and troughs. One of these averages is shown (Fig. 1C) together with the corresponding unrectified average (Fig. 1D). This and other primary features detected in averages from this recording are summarized in Fig. 1E. Integrated records from the represented neurones, for both control respiratory cycles and cough, are shown in Fig. 2.
The circuit in Fig. 1F represents simple parsimonious functional connections inferred from the primary features in the averages. The presence of an offset peak exclusively in the rectified average is consistent with the trigger neurone being a premotor neurone or otherwise functionally antecedent to the motoneurone pool, rather than a motoneurone. This and subsequent summary diagrams are placed adjacent to corresponding results to facilitate further consideration in the Discussion.
During cough, all augmenting inspiratory neurones with functional links to inspiratory laryngeal motoneurones had accentuated augmenting firing patterns; examples of this altered activity are shown in Fig. 2 (neurones 43 and 45) and Fig. 5A (neurone 52). Of the 10 decrementing inspiratory cells, seven responded during cough with accentuated decrementing patterns similar to neurone 83 in Fig. 5A, whilst two others showed no change in peak rate but exhibited a prolonged active phase (e.g. Fig. 2, neurone 66). There was a reduction in firing rate in one neurone (not shown). During cough, two inspiratory plateau neurones had prolonged active phases but no change in peak rate (e.g. see Fig. 3A in Shannon et al. 1998), four others had enhanced augmenting patterns (e.g. Fig. 2, neurones 65 and 71), and the firing rate of one decreased. All but two of the inspiratory neurones ceased activity at the termination of the inspiratory phase of cough.
Figure 5. Connectivity among inspiratory neurones and an expiratory motoneurone, and discharge patterns during fictive cough.
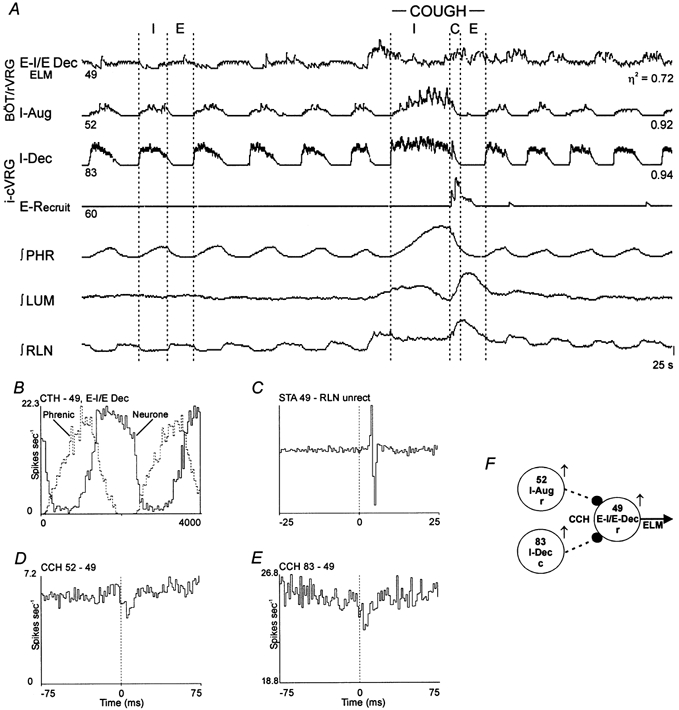
A, discharge patterns of two inspiratory and two expiratory neurones. Results suggest I-Aug neurone 52 and I-Dec neurone 83 inhibit expiratory motoneurone 49. B, CTH; 100 cycles averaged. C, STA; offset biphasic feature with a lag of 3.0 ms; number of trigger events, 39081. D, CCH; offset trough with a lag of 6.0 ms; half-width, 3.0 ms; detectability index, 5.2; correlation strength, 0.27; 39098 reference and 38274 target spikes. E, CCH; offset trough with a lag of 6.0 ms; half-width 4.5 ms; detectability index, 3.7; correlation strength, 0.14; 18590 reference and 63998 target spikes. Correlogram was scaled-up to show significant primary trough by subtraction of 70 % of the counts in the minimum bin from each bin. F, summary circuit of inferred connectivity.
Discharge patterns of neurones with functional links to expiratory laryngeal motoneurones
Rectified averages of recurrent laryngeal nerve activity triggered by eight decrementing, one augmenting, one plateau and two ‘other’ expiratory neurones had offset peaks suggesting functional associations between the trigger neurones and expiratory laryngeal motoneurones. The mean and standard deviation of peak onset latencies and half-widths in the rectified averages triggered by these 12 neurones were 2.0 ± 1.1 and 3.8 ± 2.5 ms, respectively.
Averages triggered by two decrementing expiratory neurones (designated 43 and 71) are shown in Fig. 4C and E. Neither neurone discharged in every control cycle (Fig. 4A). However, the average firing rates of both neurones in control respiratory cycles had decrementing patterns (Fig. 4B and D). During a fictive cough, the two neurones had step increases in activity associated with the neural compressive phase, followed by declining activity as the expiratory phase progressed (Fig. 4A). The six other decrementing expiratory neurones of this group had comparable response patterns during cough (e.g. neurone 40 in Fig. 2A). Note that this pattern was similar to the activity profile of motoneurone 78 (Fig. 4A). There were no features in the averages triggered by the other decrementing expiratory neurones in Fig. 4A, designated 65, 67 and 69. However, the firing patterns of these neurones were similar to the activity profile of the recurrent laryngeal nerve during cough.
In previous work on network mechanisms that control respiratory pump muscles during cough (Shannon et al. 2000), results pertinent to the present study were detected in two recordings that constituted part of a common database. Cross-correlation analysis of pairs of spike trains indicated functional associations between VRG neurones and single expiratory laryngeal motoneurones. One pair (Fig. 4) included a decrementing expiratory neurone (43) and an expiratory laryngeal motoneurone (78). When the motoneurone spikes were used as target events, the primary feature in the cross-correlogram was an offset peak with a positive lag (Fig. 4F). The firing rates of both neurones increased during cough (Fig. 4A).
Another recording (Fig. 5A) included inspiratory neurones with augmenting and decrementing discharge patterns and an expiratory laryngeal motoneurone (Fig. 5C) with an E–I/E phase-spanning decrementing discharge pattern (see CTH in Fig. 5B). When each inspiratory neurone served as the reference cell and the motoneurone served as the target, an offset trough with a positive time lag was the primary feature in each correlogram (Fig. 5D and E). The firing rate of the motoneurone declined when the inspiratory neurones were active (Fig. 5A).
Offset troughs in spike-triggered averages
Offset troughs were also observed in rectified averages of recurrent laryngeal nerve activity triggered by spikes in four expiratory neurones. The mean and standard deviation of the onset latencies and half-widths were 6.6 ± 4.9 and 2.8 ± 0.5 ms, respectively. In two averages triggered by augmenting expiratory neurones (Fig. 6B and C), troughs indicative of decreased nerve activity following the trigger spikes were superimposed on a broader activity profile associated with the respiratory phase transition. Neurone 44 showed an enhanced augmenting pattern during cough (Fig. 6A), whilst neurone 56, and one other E-Aug neurone (different animal, not shown), showed little change in discharge pattern. One average triggered by a decrementing expiratory neurone had an offset trough (not shown). The discharge pattern of the neurone during fictive cough was similar to those of other decrementing neurones.
Figure 6. Changes in firing pattern during fictive cough and functional connectivity of augmenting expiratory neurones.
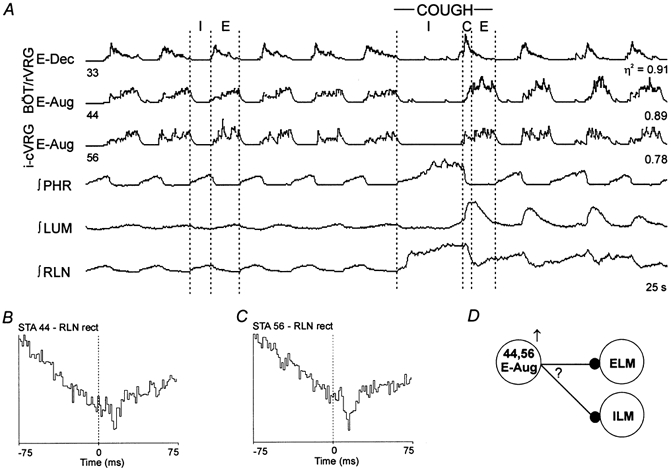
A, discharge patterns of the three expiratory neurones. Results suggest E-Aug neurones 44 and 56 inhibited either inspiratory or expiratory laryngeal motoneurones. B, STA; offset trough with a lag of 12.0 ms; half-width, 3.0 ms; number of trigger events, 29626. C, STA; offset trough with a lag of 9.0 ms; half-width, 6.0 ms: number of trigger events, 19798. D, summary model representing possible inferred connectivity. Question mark indicates that the troughs in the averages could represent inhibition of ELM or ILM (see text for further explanation).
DISCUSSION
This study is the first to identify neurones in the ventrolateral medullary respiratory network with putative roles in the modulation of laryngeal motoneurone discharge during eupnoea and cough. When considered together with our previous reports (Shannon et al. 1998, 2000), the data support the hypothesis that neurones which shape laryngeal motoneurone activity during cough are reconfigured elements of the eupnoeic respiratory rhythm/pattern-generating network (Barillot et al. 1990; Ezure, 1990). The results are consistent with the concept of ‘functional plasticity’ (multifunctional nature) of the respiratory network and laryngeal motoneurones (Grélot et al. 1995; Shiba et al. 1999; Gestreau et al. 2000).
Inspiratory laryngeal motoneurones
Inspiratory laryngeal motoneurones displayed three identifiable discharge patterns during control respiratory cycles: augmenting, decrementing and plateau. Similar types of eupnoeic pattern have been reported previously (for references, see Iscoe, 1988; Barillot et al. 1990; Gestreau et al. 2000), and may be related to distinct movements produced by different fibre bundles of the abducting posterior cricoarytenoid muscles innervated by these motoneurones (Barillot & Bianchi, 1971; Shiba et al. 1999). During fictive cough, all inspiratory motoneurones identified in this study had prolonged active phases and increased peak firing rates, particularly late in inspiration; two continued discharging into the expiratory phase. These results would be consistent with increased abduction of the glottis during the inspiratory phase and little activity in the abducting muscles during the expiratory phase of mechanical cough.
There have been three other reports describing the responses of inspiratory laryngeal motoneurones during mechanical or fictive coughing. In one study using spontaneously breathing decerebrate cats, the intra-thoracic trachea was probed to elicit mechanical coughing (Dawid-Milner et al. 1993). All inspiratory laryngeal motoneurones had increased peak firing rates during the inspiratory phase of the cough, and activity continued during the subsequent compressive and expulsive phases. In another study, electrical stimulation of the superior laryngeal nerve was used to evoke fictive coughing in decerebrate cats under neuromuscular block (Shiba et al. 1999). Inspiratory laryngeal motoneurones, recorded with intracellular electrodes, depolarized more during the inspiratory phase of the cough cycle than during control inspiration, hyperpolarized briefly at the inspiratory to expiratory phase transition (compressive phase), and depolarized and then gradually repolarized during the expiratory phase. Using similar methods to Shiba et al. (1999), Gestreau et al. (2000) reported no change in either the mean or peak firing rates of motoneurones during the inspiratory phase of fictive cough. All inspiratory motoneurones were silent during the expiratory phase. The different results in these reports presumably reflect the different experimental conditions. Distinct cough motor patterns can be evoked from the larynx and tracheobronchial tree; responses also depend on the type, intensity and duration of stimulation (Korpas & Tomori, 1979; Tatar et al. 1994).
Expiratory laryngeal motoneurones
All spontaneously active cells identified as expiratory laryngeal motoneurones had decrementing discharge patterns during control cycles. Some neurones discharged only briefly in early expiration, whilst others continued to discharge into the late expiratory phase. These control patterns are consistent with other reports (Barillot & Dussardier, 1976; Barillot et al. 1984, 1990; Iscoe, 1988; Gestreau et al. 2000). Three ‘silent’ cells identified as motoneurones were recruited during fictive cough; these neurones also exhibited a decrementing expiratory discharge pattern. The majority of the spontaneously active motoneurones and those recruited during fictive cough had transient increases in peak firing rate near the inspiratory to expiratory phase transition. These fluctuations in activity presumably represent the neural correlate of the compressive phase of mechanical cough when the glottis would be closed. Most spontaneously active motoneurones also had prolonged active phases during cough; the two exceptions only discharged during the neural compressive period.
Some decrementing expiratory neurones, without features in their recurrent laryngeal nerve averages, showed discharge profiles strikingly similar to that of the recurrent laryngeal nerve during cough (neurones 65, 67 and 69 in Fig. 4). These cells could have been premotor or motoneurones for the cricothyroid (CT) muscles of the larynx, which are active during the expiratory phase of cough (Sant'Ambrogio et al. 1997). Cricothyroid motoneurone axons are in the external branch of the superior laryngeal nerve and thus would not have been identified in this study.
The discharge patterns of expiratory motoneurones found in this study were similar, with minor exceptions, to those in previous studies of fictive cough (Shiba et al. 1999; Gestreau et al. 2000). The majority of the expiratory laryngeal motoneurones were active during both the neural compressive and expiratory phases of cough. These results suggest two hypotheses regarding mechanical coughing in spontaneously breathing animals. First, the initial transient increase in expiratory motoneurone activity causes a maximal level of glottis adduction during the compressive phase of coughing. Second, a lower level of glottis adduction persists throughout the subsequent expulsive phase due to the maintained activity. Sustained narrowing of the airways during the expiratory phase of cough could improve cough effectiveness by increasing the linear velocity of the gas (Macklem, 1974). The latter hypothesis does not agree with the general consensus that adductor muscles (thyroarytenoid and arytenoideus) completely relax during the expulsive phase of cough, a view based on studies that measured laryngeal muscle electromyographic activity in anaesthetized cats and dogs (for references see Korpas & Tomori, 1979; Sant'Ambrogio et al. 1997). These differences presumably reflect the use of anaesthesia in the electromyographic studies; laryngeal motoneurone activity is readily measured in unanaesthetized (decerebrated) cats, as in the present work. Anaesthesia is known to depress the activity of laryngeal motoneurones, particularly expiratory motoneurones (Iscoe, 1988; Bartlett, 1989). The absence of sensory feedback from the larynx during fictive cough in paralysed animals could also contribute to the observed differences. However, Sant'Ambrogio et al. (1997) reported that laryngeal muscle responses were independent of laryngeal sensory feedback during coughing in unparalysed dogs.
The mechanisms leading to the glottis opening during the expulsive phase of cough remain poorly understood. It is generally accepted that the opening is sudden, maintained and results from relaxation of the adducting muscles, contraction of the abducting muscles and increased airway pressure. Data from this study and Gestreau et al. (2000) do not support significant activity by abducting muscles during the expulsive phase of cough, whereas other studies support this view (Korpas & Tomori, 1979; Dawid-Milner et al. 1993; Sant'Ambrogio et al. 1997; Shiba et al. 1999). Resolving the questions of inspiratory and expiratory laryngeal motoneurone/ muscle activity during the expulsive phase of mechanical cough will require measurement of laryngeal motoneurone or muscle activity during coughing in unanaesthetized, unparalysed animals.
Evidence for functional associations of premotor neurones
Spike-triggered averaging of neurograms is widely recognized as an effective means of identifying motoneurones and detecting putative relationships between motoneurones and functionally antecedent neurones. The evidence for neuronal interactions is based upon measured time-locked fluctuations in the recruitment and firing rates of motoneurones due to direct, indirect or parallel paths. Advantages and limitations of these methods have been considered elsewhere (Cohen et al. 1974; Davies et al. 1985; Botteron & Cheney, 1989; Maier et al. 1998; Perlmutter et al. 1998). An additional limitation to feature interpretation had to be considered in this study. Because the recurrent laryngeal nerve contains both inspiratory and expiratory laryngeal motoneurone axons, either of which may be active in both phases of respiration, the presumptive target motoneurone for any inferred connection may be inspiratory, expiratory, or both. However, because there is no evidence in the literature for excitatory connections between phasic inspiratory and expiratory modulated neurones in the VRG (Ezure, 1990; Balis et al. 1994; Bianchi et al. 1995), the simplest interpretation of an offset peak is that it represents a functional excitatory connection between a premotor neurone and motoneurones having the same phase of respiratory modulation. On the other hand, there is evidence for inhibitory connections between inspiratory and expiratory neurones; thus, an offset trough in a recurrent laryngeal nerve average could represent functional inhibition by the trigger cell of either inspiratory or expiratory laryngeal motoneurones. These considerations have been taken into account in the following sections. The inferred connections discussed in the following paragraphs represent simple interpretations of the spike-triggered averages that also take concurrent changes in activity during cough into account.
Putative premotor neurone-inspiratory motoneurone interactions
Offset primary peaks in rectified averages of recurrent laryngeal nerve activity triggered by neurones with augmenting, decrementing and plateau inspiratory discharge patterns are consistent with a model in which these ‘types’ of neurone have excitatory premotor actions on inspiratory laryngeal motoneurones.
Offset primary peaks with short time lags, with and without secondary peaks and troughs, were observed in the averages (e.g. Fig. 1C). A simple model inferred from these results includes neurones 43, 45, 65, 66 and 71 as inspiratory premotor neurones (Fig. 1F). The primary peak in each average reflects the joint parallel influences of the particular trigger neurone and other ‘observed’ and ‘unobserved’ premotor neurones that projected to the monitored motoneurone pool. The peaks could also reflect indirect actions; with the synchrony of the trigger neurones a consequence of functionally antecedent inputs shared with the motoneurones (Davies et al. 1985; Botteron & Cheney, 1989; Maier et al. 1998; Perlmutter et al. 1998).
The plausibility of these inferences is supported by the similarities of discharge patterns of these neurones and inspiratory laryngeal motoneurones during eupnoea, and previously reported spike-triggered averages of neurone membrane potentials indicative of excitation of inspiratory modulated vagal motoneurones (i.e. inspiratory laryngeal motoneurones) by rostral VRG augmenting and plateau (‘continuous’) inspiratory neurones (Ezure & Manabe, 1989; Ezure et al. 1989). In general, results from this study support the conclusion of Barillot et al. (1990): ‘In terms of underlying mechanisms responsible for variations in membrane potential during eupnoea, there appears to be no fundamental difference between vagal (laryngeal) and non-vagal inspiratory neurones.‘
Secondary peaks and troughs in the averages also match the predicted consequences of the parallel and serial interactions in our model (Fig. 1F). These features may be explained by the shared influence of I-Driver neurones on the premotor neurones and on decrementing inspiratory neurones that, in turn, have inhibitory actions on the premotor neurons; the decrementing neurones contribute to the troughs (reduced firing probability of motoneurones) through their inhibitory actions on the premotor neurones. Some inhibitory relationships are included in Fig. 1F to illustrate this aspect of the model.
The present work is the first to provide evidence for the hypothesis that inspiratory neurones with various discharge patterns drive inspiratory laryngeal motoneurones during cough. Most of the putative premotor augmenting, plateau and decrementing inspiratory neurones evaluated in this study increased activity during fictive cough, in a manner similar to motoneurones with like discharge patterns. A subset of the decrementing and plateau neurones had decreased firing rates and durations of their active phase. Others had prolonged periods of activity with no change in peak firing rate. These observations suggest distinct subpopulations of premotor neurones with different functions. For example, inspiratory plateau neurones with prolonged activity but no change in peak firing rate during cough may correspond to I-Driver neurones described previously and have a role in phase timing (Ezure & Manabe, 1989; Balis et al. 1994; Morris et al. 1996; Shannon et al. 1998; see Fig. 1). Considered together, these observations support the conclusion that premotor neurones involved in shaping inspiratory laryngeal motoneurone discharge patterns during eupnoea are also involved in configuring the patterns during cough (Gestreau et al. 2000).
Putative interactions with expiratory motoneurones
The offset peaks observed in spike-triggered averages of recurrent laryngeal nerve activity and cross-correlograms, both triggered by decrementing expiratory neurons, support excitatory premotor actions of such cells on expiratory laryngeal motoneurones (Fig. 4G and H). These inferences were consistent with the similarity of the activity profiles of these neurones and expiratory laryngeal motoneurones during both eupnoea and fictive cough.
Offset troughs with positive time lags in two cross-correlograms calculated using expiratory motoneurone action potentials as target events suggest that inhibitory actions of inspiratory neurones also help to shape the activity of expiratory laryngeal motoneurones (Fig. 5F). Increased activities of augmenting and decrementing inspiratory neurones during fictive cough were associated with decreased expiratory laryngeal motoneurone activity, supporting these inferences.
These results support aspects of current models of the control of expiratory laryngeal motoneurones (Richter et al. 1979; Barillot et al. 1990) and the hypothesis that the same neurones influence expiratory laryngeal motoneurone discharge patterns during both eupnoea and cough.
Other functional associations
Previous results based on spike-triggered averaging and other analyses of intracellular membrane potentials have suggested that both inspiratory (Richter et al. 1979; Barillot et al. 1990) and expiratory (Ezure & Manabe, 1988; Jiang & Lipski, 1990) laryngeal motoneurones receive inhibitory inputs from decrementing and augmenting expiratory neurones, although a recent study by Tian et al. (1999) reported that Bötzinger augmenting expiratory neurones do not inhibit those motoneurones in rats. In the present study, offset troughs were observed in averages triggered by spikes of augmenting and decrementing expiratory neurones. Because the recurrent laryngeal nerve contains axons of both inspiratory and expiratory motoneurones, the signal recorded during the expiratory phase may have included ‘residual’ inspiratory activity. Therefore, the troughs could represent transient declines in inspiratory or expiratory motoneurone activity, or both. For this reason, possible inhibitory actions of augmenting and decrementing expiratory neurones are labelled by question marks in the schematic summary in Fig. 6D and in the network cough model shown in Fig. 7 (described in the next section). Resolution of the ambiguity in this study will require recordings that include single inspiratory or expiratory laryngeal motoneurone axon spike trains.
Figure 7. Network model for the control of laryngeal motoneurones.
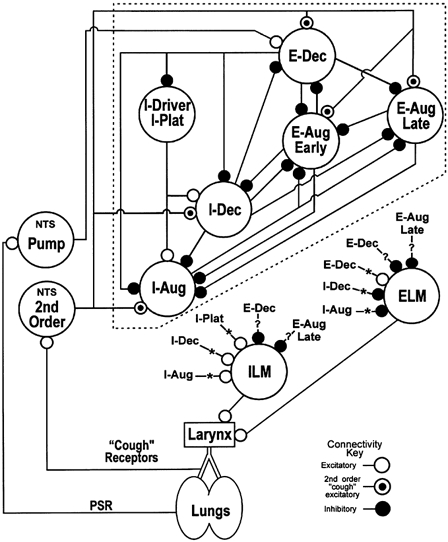
Schematic diagram of ventrolateral medullary respiratory neuronal network connections and hypothesized inputs from nucleus tractus solitarius (NTS) cough receptor second-order neurones and pulmonary stretch receptor (PSR) Pump cells. Neurone connections onto ILM and ELM arise from the ‘core’ network (enclosed by dashed-line box). E-Aug Early and E-Aug Late, neurones that begin discharging prior to and during the latter part of the expiratory phase, respectively. I-Driver, inspiratory neurone also active before the expiratory-inspiratory phase transition and with a relatively constant discharge rate throughout the inspiratory phase (I-Plat); definition specifically limited to BÖT/rVRG neurones with previously identified excitatory functional links to other inspiratory neurones (Balis et al. 1994). Other abbreviations are described in detail in the text. For a detailed description of the ‘core’ (excluding ILM and ELM) of the model, see Shannon et al. (1998, 2000). The connections with asterisks and question marks represent those inferred from the results of this study.
In the present study, offset peaks were observed only once in averages triggered by augmenting or plateau expiratory neurons. Although these results, included for the sake of completeness, are suggestive of excitation of expiratory laryngeal motoneurones, there is, to our knowledge, no other evidence in the literature to support this inference.
A network model for the control of laryngeal motoneurones during cough
A schematic summary of a network model for the control of laryngeal motoneurone activity is shown in Fig. 7. Previously reported simulations based on the ‘core’ of the model (neurones enclosed by dashed-line box) and cross-correlation data support the hypothesis that the medullary network involved in the generation of the eupnoeic respiratory rhythm also participates in the production of the cough motor pattern expressed in inspiratory and expiratory pump muscles (Balis et al. 1994; Shannon et al. 1998, 2000). The relationships between the core network and the inspiratory and expiratory laryngeal motoneurones suggested by the present results and other work cited in the preceding sections are indicated by the connections marked with asterisks and question marks. The discharge patterns observed in inferred premotor neurones and identified laryngeal motoneurones are in general agreement with predictions from cough patterns in laryngeal motoneurones observed in network simulations (Fig. 2 in Shannon et al. 1998). The following paragraphs summarize the neuronal responses and interactions during a cough; specific actions supported by data from the present study are in italics.
Inspiratory phase of cough
Cough afferents in the airways excite second-order neurones in the nucleus tractus solitarius that, in turn, increase (directly or indirectly) the firing rates of propriobulbar inspiratory neurones, including premotor neurones with augmenting, decrementing and plateau discharge patterns. These actions result in increased firing rates of inspiratory laryngeal motoneurones, leading to enlargement of the glottis opening and, through the activity of I-Driver neurones and inhibitory decrementing inspiratory neurones, prolongation of the inspiratory phase. Inspiratory neurone activity is terminated by enhanced inhibitory actions of propriobulbar decrementing expiratory cells.
Compressive phase of cough
A brief, large increase in the firing rate of expiratory laryngeal motoneurones occurs at the end of the inspiratory phase leading to closure of the glottis. This enhanced activity is due to excitation by premotor decrementing expiratory neurones, whose discharge is enhanced by ‘cough’ and pulmonary stretch receptors. Other factors that promote this burst of activity include enhanced post-inhibitory rebound resulting from the cessation of actions from decrementing and augmenting inspiratory neurones. During the compressive, and expulsive phases, inspiratory laryngeal motoneurones are inhibited by decrementing and augmenting expiratory neurones.
Expulsive phase of cough
The glottis is opened quickly due to decreasing activity in expiratory motoneurones. The rapid decline in expiratory motoneurone firing rate is a consequence of reduced excitation (disfacilitation) from premotor decrementing expiratory neurones. As the expiratory phase progresses, active expiratory motoneurones continue to discharge with a decrementing pattern due to declining excitation by premotor decrementing expiratory neurones and inhibition by late augmenting expiratory neurones. Expiratory activity is terminated by enhanced activity in decrementing and augmenting inspiratory neurones after they are released from inhibition by expiratory neurones.
Acknowledgments
This research was supported by grant HL49813 from the National Heart, Lung and Blood Institute. We thank Rebecca McGowan, Kim Ruff, Jan Gilliland and Peter Barnhill for excellent technical support.
References
- Balis UJ, Morris KF, Koleski J, Lindsey BG. Simulation of ventrolateral medullary neural network for respiratory rhythmogenesis inferred from spike train cross-correlation. Biological Cybernetics. 1994;70:311–327. doi: 10.1007/BF00200329. [DOI] [PubMed] [Google Scholar]
- Barillot JC, Bianchi AL. Activité des motoneurones laryngés pendant le réflexe de Hering-Breuer. Journal de Physiologie. 1971;63:783–792. [PubMed] [Google Scholar]
- Barillot JC, Bianchi AL, Gogan P. Laryngeal expiratory motoneurones: morphology and electrophysiological evidence of separate sites for excitatory and inhibitory synaptic events. Neuroscience Letters. 1984;47:107–112. doi: 10.1016/0304-3940(84)90414-2. [DOI] [PubMed] [Google Scholar]
- Barillot JC, Dussardier M. Activité des motoneurones laryngés expiratoires. Journal de Physiologie. 1976;72:311–343. [PubMed] [Google Scholar]
- Barillot JC, Grélot L, Reddad S, Bianchi AL. Discharge patterns of laryngeal motoneurones in the cat: an intracellular study. Brain Research. 1990;509:99–106. doi: 10.1016/0006-8993(90)90314-2. [DOI] [PubMed] [Google Scholar]
- Bartlett D., Jr Respiratory functions of the larynx. Physiological Reviews. 1989;69:33–57. doi: 10.1152/physrev.1989.69.1.33. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: Neuronal circuitry, membrane properties, and neurotransmitters. Physiological Reviews. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Botteron GW, Cheney PD. Corticomotoneuronal postspike effects in averages of unrectified EMG activity. Journal of Neurophysiology. 1989;52:1127–1139. doi: 10.1152/jn.1989.62.5.1127. [DOI] [PubMed] [Google Scholar]
- Christakos CN, Cohen MI, Sica AL, Huang W-X, See , W R, Barnhardt R. Analysis of recurrent laryngeal inspiratory discharges in relation to fast rhythms. Journal of Neurophysiology. 1994;72:1304–1316. doi: 10.1152/jn.1994.72.3.1304. [DOI] [PubMed] [Google Scholar]
- Cohen MI. Phrenic and recurrent laryngeal discharge patterns and the Hering-Breuer reflex. American Journal of Physiology. 1975;228:1489–1496. doi: 10.1152/ajplegacy.1975.228.5.1489. [DOI] [PubMed] [Google Scholar]
- Cohen MI, Piercey MF, Gootman PM, Wolotsky P. Synaptic connections between medullary inspiratory neurons and phrenic motoneurons as revealed by cross-correlation. Brain Research. 1974;81:319–324. doi: 10.1016/0006-8993(74)90946-9. [DOI] [PubMed] [Google Scholar]
- Davies JGMcF, Kirkwood PA, Sears TA. The detection of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. Journal of Physiology. 1985;368:33–62. doi: 10.1113/jphysiol.1985.sp015845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid-Milner MS, Lara JP, Milan A, Gonzalez-Baron S. Activity of inspiratory neurones of the ambiguus complex during cough in the spontaneously breathing decerebrate cat. Experimental Physiology. 1993;78:835–838. doi: 10.1113/expphysiol.1993.sp003730. [DOI] [PubMed] [Google Scholar]
- Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Progress in Neurobiology. 1990;35:429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- Ezure K, Manabe M. Decrementing expiratory neurons of the Bötzinger complex. II. Direct inhibitory synaptic linkage with ventral respiratory group neurons. Experimental Brain Research. 1988;72:159–166. doi: 10.1007/BF00248511. [DOI] [PubMed] [Google Scholar]
- Ezure K, Manabe M. Monosynaptic excitation of medullary inspiratory neurons by bulbospinal inspiratory neurons of the ventral respiratory group in the cat. Experimental Brain Research. 1989;74:501–511. doi: 10.1007/BF00247352. [DOI] [PubMed] [Google Scholar]
- Ezure K, Manabe M, Otake K. Excitation and inhibition of medullary respiratory neurons by two types of burst inspiratory neurons in the cat. Neuroscience Letters. 1989;104:303–308. doi: 10.1016/0304-3940(89)90593-4. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Grélot L, Bianchi AL. Activity of respiratory laryngeal motoneurons during fictive coughing and swallowing. Experimental Brain Research. 2000;130:27–34. doi: 10.1007/s002210050003. [DOI] [PubMed] [Google Scholar]
- Grélot L, Milano S, Gestreau C, Bianchi AL. Are medullary respiratory neurones multipurpose neurones? In: Bostock H, Kirkwood PA, Pullen AH, editors. Neurobiology of Disease: Contributions from Neuroscience to Clinical Neurology. NY, USA: Cambridge University Press; 1995. pp. 299–308. [Google Scholar]
- Huang W-X, Cohen MI. Population and unit synchrony of fast rhythms in expiratory recurrent laryngeal discharges. Journal of Neurophysiology. 2000;84:1098–1102. doi: 10.1152/jn.2000.84.2.1098. [DOI] [PubMed] [Google Scholar]
- Iscoe SD. Central control of the upper airways. In: Mathew OP, Sant'Ambrogio G, editors. Respiratory Function of the Upper Airway, Lung Biology in Health and Disease. Vol. 35. New York, NY, USA: Dekker; 1988. pp. 125–192. [Google Scholar]
- Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Bötzinger complex in the cat. Experimental Brain Research. 1990;81:639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- Kirsten EB, St John WM. A feline decerebration technique with low mortality and long-term homeostasis. Journal of Pharmacological Methods. 1978;1:263–268. [Google Scholar]
- Korpas J, Tomori Z. Cough and other respiratory reflexes. Progress in Respiration Research. 1979;12:39–46. [Google Scholar]
- Lindsey BG, Hernandez YM, Morris KF, Shannon R, Gerstein GL. Dynamic reconfiguration of brainstem neural assemblies: Respiratory phase-dependent synchrony vs. modulation of firing rates. Journal of Neurophysiology. 1992;67:923–930. doi: 10.1152/jn.1992.67.4.923. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Segers LS, Shannon R. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. II. Evidence for inhibitory actions of expiratory neurons. Journal of Neurophysiology. 1987;57:1101–1117. doi: 10.1152/jn.1987.57.4.1101. [DOI] [PubMed] [Google Scholar]
- Macklem PT. Physiology of cough. Annals of Otolaryngology. 1974;83:761–768. [Google Scholar]
- Maier MA, Perlmutter SI, Fetz EE. Response patterns and force relations of monkey spinal interneurons during active wrist movement. Journal of Neurophysiology. 1998;80:2495–2513. doi: 10.1152/jn.1998.80.5.2495. [DOI] [PubMed] [Google Scholar]
- Morris KF, Arata A, Shannon R, Lindsey BG. Inspiratory drive and phase duration during carotid chemoreceptor stimulation: medullary neurone correlations. Journal of Physiology. 1996;491:241–259. doi: 10.1113/jphysiol.1996.sp021212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orem J, Dick T. Consistency and signal strength of respiratory neuron activity. Journal of Neurophysiology. 1983;50:1098–1107. doi: 10.1152/jn.1983.50.5.1098. [DOI] [PubMed] [Google Scholar]
- Perlmutter SI, Maier MA, Fetz EE. Activity of spinal interneurons and their effects on forearm muscles during voluntary wrist movements in the monkey. Journal of Neurophysiology. 1998;80:2475–2494. doi: 10.1152/jn.1998.80.5.2475. [DOI] [PubMed] [Google Scholar]
- Richter DW, Camerer H, Messmann M, Rohring N. Studies on the synaptic interconnection between bulbar respiratory neurones of cats. Pflügers Archiv. 1979;380:245–257. doi: 10.1007/BF00582903. [DOI] [PubMed] [Google Scholar]
- Sant‘Ambrogio G, Kuna TK, Vanoye CR, Sant‘Ambrogio FB. Activation of intrinsic laryngeal muscles during cough. American Journal of Respiration and Critical Care Medicine. 1997;155:637–641. doi: 10.1164/ajrccm.155.2.9032206. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. Journal of Neurophysiology. 1995;73:1452–1461. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Li Z, Lindsey BG. Medullary expiratory neuron influence on expiratory laryngeal motoneuron activity during fictive cough. Federation of American Societies for Experimental Biology Journal Abstracts. 1999;13:A1016. [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Li Z, Lindsey BG. Functional connectivity among ventrolateral medullary respiratory neurones and responses during fictive cough in the cat. Journal of Physiology. 2000;525:207–224. doi: 10.1111/j.1469-7793.2000.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. Journal of Applied Physiology. 1998;84:2020–2035. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- Shannon R, Morris KF, Baekey DM, Li Z, Lindsey BG. Medullary neurons that shape laryngeal motoneuron activity during fictive cough: Evidence from spike triggered averaging. The Physiologist. 1996;39:174. [Google Scholar]
- Shiba K, Satoh I, Kobayashi N, Hayashi F. Multifunctional laryngeal motoneurons: an intracellular study in the cat. Journal of Neuroscience. 1999;19:2717–2727. doi: 10.1523/JNEUROSCI.19-07-02717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Sant‘Ambrogio G, Sant‘Ambrogio F. Laryngeal and tracheobronchial cough in anesthetized dogs. Journal of Applied Physiology. 1994;76:2672–2679. doi: 10.1152/jappl.1994.76.6.2672. [DOI] [PubMed] [Google Scholar]
- Tian G-F, Peever JH, Duffin J. Bötzinger-complex, bulbospinal expiratory neurones monosynaptically inhibit ventral-group respiratory neurones in the decerebrate rats. Experimental Brain Research. 1999;124:173–180. doi: 10.1007/s002210050612. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG. Respiratory reflexes. In: Fenn WO, Rahn H, editors. Handbook of Physiology. I. Washington, DC, USA: American Physiological Society; 1964. pp. 585–630. section 3, Respiration chap. 24. [Google Scholar]
- Widdicombe JG. Reflexes from the upper respiratory tract. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology. II. Bethesda, MD, USA: American Physiological Society; 1986. pp. 363–394. section 3 The Respiratory System Control of Breathing, part I, chap. 11. [Google Scholar]
- Yoshida Y, Yatake K, Tanaka Y, Imamura R, Fukunaga H, Nakashima T, Hirano M. Morphological observation of laryngeal motoneurons by means of cholera toxin B subunit tracing technique. Acta Otolaryngology. 1998;539(suppl.):98–105. doi: 10.1080/00016489850182242. [DOI] [PubMed] [Google Scholar]


