Abstract
Localized calcium release events (calcium sparks) were studied in voltage-clamped cut twitch fibres of Rana temporaria.
A histogram of thousands of spontaneous sparks displayed a monotonically decreasing amplitude distribution from the low to the high limit of > 7 ΔF/F0 units.
Several effects of low micromolar concentrations of ryanodine (0.4–2 μm) on spontaneous sparks, reproducing the agent's effects on single ryanodine receptor channel current in bilayers, were observed collectively for the first time in live fibres, namely (a) increases in spark frequency followed by (b) conversions of sparks into steady glows lasting tens of seconds, (c) occasional interruptions of the glows by brief gaps of darkness, and (d) abolition of sparks at the locations of the glows. The glow could reflect the incessant Ca2+ flux through a single (or a few) calcium release channel locked in the semi-open state, which was allowed to make occasional transitions to the closed state but not to the fully open state.
Higher concentrations of ryanodine (≥ 20 μm) suppressed the spontaneous sparks effectively and permanently, presumably by deactivating the ryanodine receptors.
Depolarization-evoked sparks elicited with small pulses had higher frequencies and larger amplitudes than spontaneous sparks and were abolished by both concentrations of ryanodine.
With 1–2 μm ryanodine, however, a uniform non-sparking calcium release persisted during the pulse, with the globally averaged increase in fluorescence intensity being about half that of the control. A possible origin of this non-sparking release may be related to the structural coupling between the voltage sensors and the ryanodine receptors that can exist only in live fibres but not in the bilayer preparation.
Contraction in striated muscle is regulated by Ca2+ released from the sarcoplasmic reticulum (SR) through calcium release channels (CRCs). For many years, Ca2+ flux through individual CRCs could be studied only in lipid bilayers; in live fibres, the calcium release signal had to be studied globally in a region covering > 10 sarcomeres. With commercially available laser scanning confocal microscopes in recent years, extremely localized calcium release events, also called calcium sparks, have been detected in cardiac muscle (Cheng et al. 1993; Lopez-Lopez et al. 1994) and in skeletal muscle (Tsugorka et al. 1995; Klein et al. 1996). This presents the opportunity of gaining information about Ca2+ flux through a calcium release unit (CRU) consisting of a single, or a few, CRCs in live fibres.
The macromolecule housing a CRC is also called a ryanodine receptor (RyR) because of the high specificity of the plant alkaloid ryanodine (Ry) on this protein (reviewed by Sutko et al. 1997). Low [Ry] activates and high [Ry] deactivates the RyRs, thereby opening and closing the CRCs, respectively. These opposing effects have been attributed to the co-existence of activating and deactivating binding sites on the RyRs having different affinities for Ry (Humerickhouse et al. 1993, 1994; Bidasee & Besch, 1998). In the presence of an activating [Ry], skeletal muscle undergoes an irreversible contracture, which can be explained by the ability of Ry to modify and ‘lock’ the CRCs in a semi-open state (Rousseau et al. 1987), causing a maintained Ca2+ flux into the myoplasm until the SR Ca2+ store is depleted. If a calcium spark is generated by the transient Ca2+ flux through one CRU, then after the CRC(s) in the CRU is locked in the semi-open state by Ry, the steady Ca2+ flux should be detectable. The success of Cheng et al. (1993) in capturing one (and only one) such event in cardiac muscle prompted us to search for the same event in skeletal muscle, which was the first aim of this paper. It will be seen that our attempts were successful.
A related question is, when a CRC is modified by Ry to the semi-open state, whether it is locked rigidly in that conformation. The maintained contracture suggested that the modified CRC cannot close unless a higher [Ry] is applied, but the possibility that it can be opened further by depolarization remains. If it can be opened further, then it is not rigidly locked. On the other hand, if it cannot, then it should be incapable of releasing any additional Ca2+ when the fibre is depolarized. This question cannot be answered by studies in bilayers because the CRCs in such preparations are no longer linked to the voltage sensors. Thus, the second aim of this paper was to try to search for an answer with live fibres. It will be seen that the opening of a CRC from the semi-open state to a higher conductance state by depolarization is a distinct possibility. A preliminary report of these findings has been published (Hui et al. 1998).
METHODS
Composition of solutions
Solution A (relaxing solution): 120 mm potassium glutamate, 1 mm MgSO4, 0.1 mm K2-EGTA, 5 mm K2-Pipes; pH 7.0. Solution B (internal solution): 92.5 mm caesium glutamate, 20 mm Na2-creatine phosphate, 0.1 mm Cs2-EGTA, 3 mm MgCl2, 5 mm Na2ATP, 5 mm glucose, 5 mm Cs-MOPS, 0.2 mm fluo-3; pH 7.0. Solution C (external solution): 117 mm TEA-CH3SO3, 5 mm Cs-Mops, 2 mm CaCl2, 1 μm tetrodotoxin; pH 7.1.
Fibre preparation
Experiments were performed on cut fibres from semitendinosus muscles of English frogs, Rana temporaria, cold adapted in a refrigerator. In accordance with a procedure approved by the Institutional Animal Care and Use Committee, the animals were killed by decapitation and destruction of the brain and spinal cord. Cut fibre segments were dissected in solution A. A stretched fibre segment (sarcomere length 3.5–4.0 μm) was mounted in a double Vaseline-gap chamber designed for an inverted microscope (DiFranco et al. 1999). After saponin treatment (Irving et al. 1987), the two end pools were filled with solution B and the centre pool with solution C. The membrane potential was usually clamped at −90 mV. All experiments were performed at room temperature (21–24 °C).
Confocal imaging
The fluorescence (F) signal was monitored with a Nikon PCM2000 system consisting of a scan head mounted on a Nikon Diaphot 300 inverted microscope and an argon laser. The images presented here were recorded in the line-scan mode using a Nikon Plan Apochromat × 60 1.2 n.a. water-immersion objective. The image resolution was set at 1024 x-pixels by 1024 t-pixels. Each x-pixel covered 0.070 μm at a zoom factor of 3 and each t-pixel occupied 2.5 ms. Thus, all the images shown in this report have a full scale of 71.6 μm by 2.56 s, except for the cropped ones. The scanning operation and data acquisition were executed with SimplePCI software (Compix Inc.). Subsequent off-line data analysis was performed with programs written in IDL (Research Systems Inc.).
Statistical tests of significance
The difference between the mean values of two sets of results, whether paired or unpaired, was assessed with Student's two-tailed t test. The difference was considered to be significant if P < 0.05.
RESULTS
Spontaneous calcium sparks in polarized cut fibres
Figure 1a shows a longitudinal line-scan image from a fibre held at −90 mV, displaying an alternation of bright and dark bands. The bright bands were confirmed in other fibres to coincide with the A-bands in the bright field images and agreed with the finding that fluo-3 molecules could be localized in the A-bands (Harkins et al. 1993). Spontaneous calcium sparks were visualized lying between the bright bands. They appeared to originate at the Z-lines where the triads in amphibians are located. To correct for the inhomogeneous localization of fluo-3 and to facilitate the visualization of the sparks, the F change at every location and every instant, ΔF(x,t), was normalized by the mean background F, F0(x), which was obtained by averaging all the pixels at that location over time.
Figure 1. Spontaneous calcium sparks in a cut fibre.
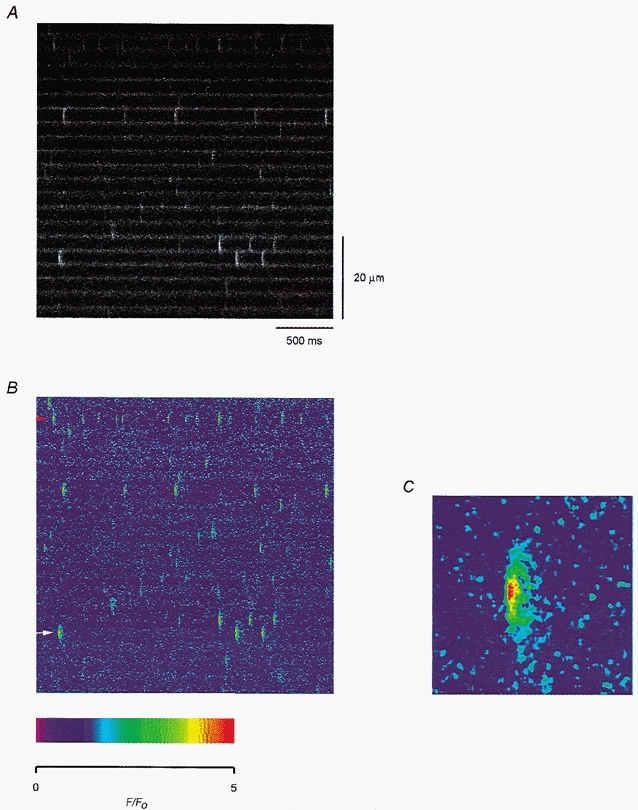
A, fluorescence change recorded from a line placed longitudinally along a resting fibre. The time and distance scales are applicable to all the line-scan images in this report. B, pseudocolour image obtained after smoothing and executing the F-ratio procedure, as described in the text. The colour calibration is indicated by the scale below the image. C, magnified image of the bright spark (indicated by the white arrow) in the lower left corner of B. Fibre 1. Holding potential −90 mV. Sarcomere length 3.5 μm.
After such normalization and smoothing with a 3-by-3 boxcar algorithm, the image is shown again in pseudocolour in Fig. 1B. In the absence of the bright bands, the sparks could be visualized more vividly. The spark frequency was higher in this fibre than some others (e.g. the one in Fig. 6a). Averaging over the whole image, the frequency was 0.2 s−1 per sarcomere if only the bright sparks were counted, and 1.3 s−1 per sarcomere if all the visually resolvable sparks were included. The brightness of the sparks also varied from sarcomere to sarcomere and sometimes within the same sarcomere. Part of the variation can be attributed to the contributions from out-of-focus CRUs.
Figure 6. Depolarization-evoked calcium sparks in a cut fibre.
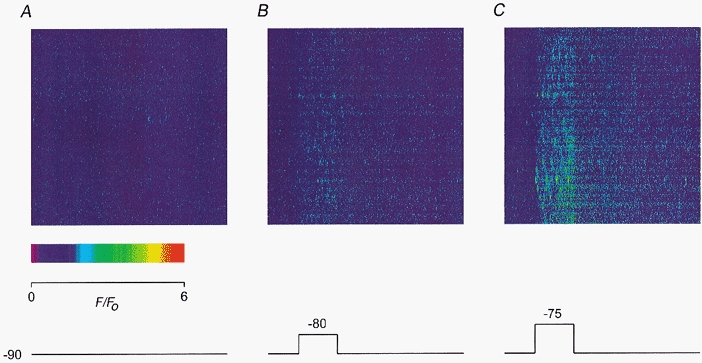
A, line-scan image recorded at rest. B, line-scan image recorded during a 500 ms pulse to −80 mV. C, line-scan image recorded during a 500 ms pulse to −75 mV. The colour calibration is indicated by the scale in A. The pulse protocol is shown below the images. Fibre 13. Holding potential −90 mV. Sarcomere length 3.4 μm.
The spark indicated by the white arrow at the lower left corner of Fig. 1B was among the most prominent ones in the image. It is magnified ∼7 times and shown in Fig. 1C. Its peak intensity had a ΔF/F0 value of 4.3, which is somewhat larger than the values published in some earlier studies (e.g. Klein et al. 1996) but within the range of values obtained by Shirokova et al. (1999) and Wier et al. (1999). Its full-width-half-maximum (FWHM) was 1.5 μm, which is not much different from the values in the literature. The Nikon PCM2000 confocal microscope system does not provide as fine kinetic resolution as some other video rate systems (Lacampagne et al. 1999; Brum et al. 2000). Nonetheless, the image in Fig. 1C shows that the rising edge of the spark was very sharp, rising from the basal value to the peak value within three t-pixels, i.e. 7.5 ms, and spread to both sides very rapidly. The trailing edge was much more blurred. The full-duration-half-maximum (FDHM) of the spark was 20 ms.
As more experiments were completed, the number of line-scan images increased enormously. It became obvious that it is impossible to analyse all the sparks manually in the same way as was done in Fig. 1C. A program was written to detect and analyse the sparks automatically in each image. The only human input was to set the criteria to discriminate between a spark and a deflection due to noise. That turned out to be quite challenging because if the threshold was set too high, then some true sparks would be missed. On the other hand, if the threshold was set too low, then some false sparks would be included.
After some careful consideration, a set of criteria was established to select only events with parameters having the following lower limits: (a) ΔF/F0 > 0.6, (b) FWHM > 0.7 μm and (c) FDHM > 6 ms. Additionally, in order to avoid counting double sparks that are not distinctly separated temporally or spatially, the upper limits of (d) FWHM < 2.5 μm and (e) FDHM < 30 ms were also imposed. The sparks in Fig. 1B (and some other images not shown) were first analysed manually and re-analysed with this program. The program missed just a few sparks that could be marginally detected by eye and picked up instead a few other sparks that could easily be overlooked by eye. The spark frequency so determined in the image of Fig. 1B was 1.4 s−1 per sarcomere, as compared with 1.3 s−1 per sarcomere obtained by manual detection (see above).
The analysis program also revealed that the visually prominent sparks in Fig. 1B, including the one marked by the white arrow, did not have the largest peak amplitude. The spark in the upper left corner marked by the red arrowhead had an even larger peak ΔF/F0 value of 4.9, but its much narrower width and shorter duration made it appear less prominent. This value of ΔF/F0 is plotted in Fig. 2a as the first filled circle. The number 1 indicates that the value was obtained from the first location of the fibre. Averaged over all the sparks in Fig. 1B, the mean amplitude was 2.1. This value is plotted as the first filled circle in Fig. 2B. Two other images were scanned at location 1 at 1 min interval and the largest spark amplitude (or mean spark amplitude) from each image is plotted as an open circle following the filled circle in Fig. 2a (or B).
Figure 2. Temporal and spatial variations of the amplitude of spontaneous calcium sparks.
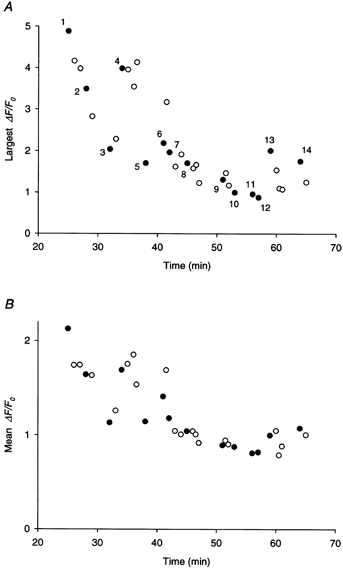
A, amplitude of the brightest calcium spark in each line-scan image plotted against time. Zero in the time scale corresponds to the instant the membrane potential was clamped at −90 mV. Each filled circle represents the value obtained from the first image scanned at a new location (indicated by the number) and the open circles represent those from the subsequent images scanned at the same location as the filled circle immediately preceding them. The filled circle with the number 1 corresponds to the spark marked by the red arrowhead in Fig. 1B. B, mean amplitude of all the detectable calcium sparks in each line-scan image plotted against time of the experiment. Each point in A corresponds to a point in B with an identical value in time. Same fibre as in Fig. 1.
Subsequently, images were scanned at 13 other locations of the fibre. Values of the largest amplitude and mean amplitude are also plotted in Fig. 2a and B. For each location, both values from the first scan are represented by filled circles whereas the values from later scans, if they existed, are represented by open circles. At location 1, both values from the later scans were smaller than the values from the first scan. However, this pattern was not consistently reproduced at other locations, otherwise the pattern would imply that repetitive scanning at the same location might cause some photo-damage to the calcium release process or photo-bleaching of the fluo-3 molecules. On the other hand, a parallel trend over time is apparent for the two quantities, namely a slow progressive diminution from the beginning of the experiment to the end. These changes were probably not caused by any gross deterioration of fibre condition, as the structure of the sarcomeres appeared crisp under bright field observation even towards the end of the experiment and the holding current only changed slightly from −44 nA at the start to −55 nA at the end. The fibre was one of the most stable ones used in our inverted double Vaseline-gap chamber to date. The reduction of the two quantities over time could be a consequence of a slow depletion of the calcium store. An alert of a progressive change in the physiological state of the cut fibre preparation over time was issued by Maylie et al. (1987). Moreover, the 0.1 mm[EGTA]i used in the internal solution is not as ideal as a high concentration, such as 20 mm, in stabilizing and maintaining the health of cut fibres, as remarked by Hui & Chen (1997).
The data in Fig. 2a and B also showed that the amplitude of the calcium spark varied tremendously from one location of the fibre to another. The largest amplitude and the mean amplitude both increased substantially from location 3 to location 4 and, to a lesser extent, from location 12 to location 13 and from location 5 to location 6. This variation could imply that the brightest spark at location 4, for example, might have originated from a CRU more in focus than the one at location 3. Alternatively, if the spark is not a unitary event, then the amplitude of the spark could vary according to the number of channels in the CRU being recruited.
Spontaneous calcium sparks were recorded in a total of 91 fibres. Surprisingly, sparks even brighter than the brightest one in Fig. 1B were observed. In three fibres, the brightest sparks had ΔF/F0 > 7. On the other hand, in nine fibres, the brightest sparks had ΔF/F0 < 1.5. The reason behind this diversity is unknown, except for the fact that the larger brightest sparks appeared to come from certain batches of frogs. A histogram of the amplitudes of the brightest sparks, one from each fibre, is shown in Fig. 3a. The mean value of the largest amplitude is 2.9 (s.e.m. = 0.1).
Figure 3. Ensemble distribution of spontaneous calcium spark amplitude.
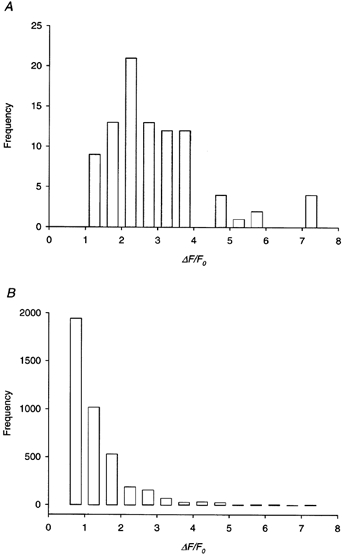
A, histogram of the amplitudes of the brightest calcium sparks. One value was taken from each of 91 cut fibres held at −90 mV. B, histogram of the amplitudes of all calcium sparks from the same fibres as in A. In each fibre, only the calcium sparks from the line-scan image containing the brightest spark in A were included in the calculation. The total number of calcium sparks was 4001.
A histogram of the amplitudes of calcium sparks of all sizes from all fibres is shown in Fig. 3B. In each fibre, only the sparks in the image containing the brightest spark are included in the analysis. The image was not necessarily the first one in each experiment, but was invariably an early one. The reasons for including only one image from each fibre are twofold: first, choosing one image from each fibre will ensure that each fibre receives the same weighting in the histogram; second, if sparks from all the images are included, the size of the data bank is enormously huge. The figure also shows that, with increasing spark amplitude, the number of occurrences decreased monotonically, which is in agreement with the conclusion drawn by Cheng et al. (1999) that the distribution of sparks should not be modal.
Ry converts spontaneous calcium sparks to steady glows
Figure 4 shows some intriguing observations in a resting fibre exposed to 2 μm Ry. Each of the line-scan images was cropped to show only three adjacent triadic regions bordered by four bright bands. The usual F-ratio normalization procedure was not applied to these images for the reason explained below. Attention should be paid to the middle triad of each image. The image in A was scanned 2 min after the fibre was exposed to the agent. The middle triad was quiescent initially. About half-way through the scan, a CRU in the triad began to liberate sparks spontaneously at a frequency of 11 s−1. After another minute, the image in B was recorded. By now, the sparks had been converted to a uniform steady glow, which persisted through the whole duration of the image and into the next image (not shown). The transition from spark to glow apparently occurred in the interval between the two scans and was not captured. While these events occurred, the two neighbouring triads were relatively calm.
Figure 4. Effect of 2 μm Ry on spontaneous calcium sparks.
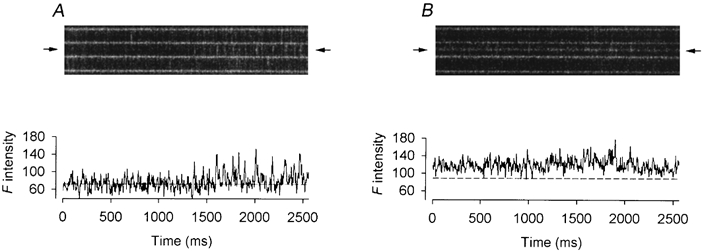
The upper panels of A and B show two successive line-scan images cropped to display the calcium events in three neighbouring triadic regions. The fluorescence intensity profile at the location of each image (marked by the pair of arrows) is plotted, as a function of time, below the image. A, increase in frequency of spontaneous sparks in the middle triadic region. B, appearance of a steady glow in the middle triadic region. Fibre 2. Holding potential −90 mV. Sarcomere length 3.7 μm.
Since the F intensity was uniform during the glow, normalization of all the pixels by the mean F0(x) at that location would nullify ΔF(x,t)/F0(x) and thus eliminate the glow. This is the reason why the F-ratio procedure was not applied and the images are not displayed in pseudocolour. To facilitate the visualization of the sparks in A and the glow in B, the F intensity profile at the location marked in the image by a pair of arrows is shown below each image. The second half of the trace in A displays frequent large fluctuations due to the repetitive spark activities. The dashed line represents the value of F0 which was estimated by averaging the points in the first half of the trace during which the CRU at that location was quiescent. The mean amplitude of the sparks in the second half of the trace was estimated to be 1.18. The trace in B showing the F intensity profile of the glow appears to be quite noisy. The dashed line represents the assumed value of F0 which was calculated by adopting the value of the dashed line in A. To account for the slight diffusional increase in myoplasmic [fluo-3], the value in A was scaled up according to the ratio of the F0 values in the neighbouring triadic regions in the image of B to those in the image of A. Based on this calculated dashed line in B, the mean value of ΔF/F0 during the glow was estimated to be 0.43. Thus, the ratio of the amplitude of the glow to that of the precursory spark was 0.36. These values of amplitudes and ratio are listed in columns 3−5 in the first row of Table 1.
Table 1.
Intensities of Ry-activated steady glows that were preceded by sparks
| Fibre no. | [Ry] (μM) | Amplitude of glow (δF/F0) | Amplitude of spark (δF/F0) | Ratio of amplitudes | Conversion captured |
|---|---|---|---|---|---|
| 2 | 2 | 0.43 | 1.18 | 0.36 | no |
| 0.65 | 1.39 | 0.47 | no | ||
| 3 | 2 | 0.27 | 0.62 | 0.43 | yes |
| 4 | 2 | 0.28 | 1.54 | 0.18 | yes |
| 0.37 | 1.40 | 0.26 | no | ||
| 5 | 2 | 0.14 | 0.83 | 0.17 | no |
| 6 | 2 | 0.32 | 1.48 | 0.22 | no |
| 0.23 | 0.83 | 0.28 | no | ||
| 7 | 0.4 | 0.17 | 0.73 | 0.24 | yes |
| 8 | 1 | 0.28 | 1.19 | 0.23 | yes |
| 9 | 1 | 0.27 | 0.94 | 0.28 | yes |
| 10 | 2 | 0.21 | 0.74 | 0.28 | no |
| 11 | 1 | 0.27 | 0.98 | 0.27 | no |
| 12 | 2 | 0.25 | 0.80 | 0.31 | no |
| Mean | — | 0.30 | 1.05 | 0.28 | — |
| s.e.m | — | 0.03 | 0.08 | 0.02 | — |
The third column shows the amplitudes of the glows estimated by two different methods depending on whether the glow lasted the whole (as in Fig. 4B), or part of the (as in Fig. 5B), time course. The fourth column shows the amplitudes of the precursory sparks leading to the glows. Each ratio in the fifth column was obtained by dividing the corresponding value in the third column by the value in the fourth column. The sixth column indicates whether the transition from a spark to a glow was captured (as in Fig. 5B), or not (as in Fig. 4B), in the same scan image.
Shortly afterwards, another glow was observed in another image (not shown), but the transition from spark to glow was missed again. In this case, the mean amplitude of the precursory sparks was estimated to be 1.39 and the mean amplitude of the glow was estimated to be 0.65. Thus, the ratio of the amplitude of the glow to that of the precursory spark was 0.47. These values of amplitudes and ratio are listed in columns 3–5 in the second row of Table 1.
Figure 5 shows a more exciting outcome in another fibre when both the high frequency sparks and the transition from the sparks to a sustained glow were captured in the same scan. The image in A was recorded 9 min after exposing the fibre to 2 μm Ry and the middle triad generated only two sparks. In the image in B recorded 1 min later, the frequency of the sparks had been greatly increased at first. At about 0.75 and 1 s, two bright sparks were generated. Almost immediately after the second bright spark, a steady glow appeared with two short interruptions at 1.5 and 2.4 s. Again, while these were occurring, the two neighbouring triads were calm. All the sparks, glow and interruptions were revealed more clearly by the F intensity profiles below the images. The spark immediately preceding the glow had a peak ΔF/F0 value of 0.62 whereas the glow (excluding the gaps) had a mean ΔF/F0 value of 0.27. Thus, the ratio of the amplitude of the glow to that of the precursory spark was 0.43. These values are listed in the third row of Table 1.
Figure 5. Effect of 2 μm Ry on spontaneous calcium sparks.
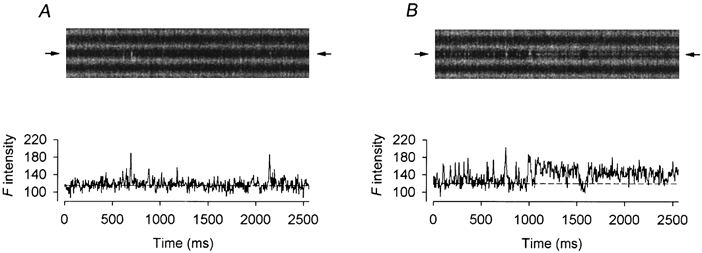
The upper panels of A and B show two successive line-scan images cropped to display the calcium events in three neighbouring triadic regions. The fluorescence intensity profile at the location of each image (marked by the pair of arrows) is plotted, as a function of time, below the image. A, infrequent spontaneous sparks in the middle triadic region. B, high frequency spontaneous sparks followed by a steady glow in the middle triadic region. Fibre 3. Holding potential −90 mV. Sarcomere length 3.8 μm.
Eleven more glows were observed in nine other fibres and the values of amplitudes are all listed in Table 1. In four images, the transition from spark to glow was captured, as in Fig. 5B, whereas in the remainder, the transition from spark to glow was missed, as in Fig. 4B. Averaged over all 14 occurrences, the ratio of the glow amplitude to the spark amplitude was 0.28 (s.e.m. = 0.02).
It should be noted that a glow was included in Table 1 only if its precursory spark(s) was captured. Many other glows were observed in the first scan image at a new location but not included in the analysis because of three reasons. First, it is impossible to estimate the value of F0 at the location of the glow to provide a value of ΔF/F0. Second, without any precursory spark, it is impossible to determine the relative amplitude of the glow with respect to the spark. Third, it is well known that sarcomeres in a fibre can be out of register among neighbouring myofibrils. Without witnessing a spark being converted into a glow, there is no way to rule out that the glow is not in fact an out-of-focus bright band in a myofibril located above or below the plane of observation. Such false glows had been observed, and when they occurred, there were usually several of them in the same image.
Depolarization-evoked calcium sparks in cut fibres
Figure 6a shows a line-scan image of a resting fibre (at −90 mV) containing spontaneous calcium sparks much less bright and less frequent compared with those in Fig. 1B. The brightest spark in this image had a ΔF/F0 value of 1.9. The mean amplitude of the sparks was 1.1 and the frequency of the sparks was 0.7 s−1 per sarcomere. The image shown in Fig. 6B was scanned during the application of a 500 ms depolarizing pulse to −80 mV, as indicated by the trace below the image. The sparks became brighter and occurred more frequently during depolarization. To apply the F-ratio procedure to an image associated with depolarization, only the t-pixels before the onset of the pulse were used to calculate F0 at each x-location. At −80 mV, the largest amplitude, the mean amplitude and the frequency of the sparks were 3.0, 1.6 and 2.8 s−1 per sarcomere, respectively, thereby increasing the values of the three quantities to 1.6, 1.4 and 4.2 times the resting values.
Figure 6C shows an image scanned during a depolarization of the same duration to −75 mV. The sparks became even brighter and occurred much more frequently during this larger depolarization. At −75 mV, the largest amplitude, the mean amplitude and the frequency of the sparks were 4.5, 2.2 and 10.1 s−1 per sarcomere, respectively, thereby increasing the values of the three quantities further to 2.3, 2.0 and 15.1 times the resting values. Also, the depolarization-evoked sparks had larger spatial and temporal dimensions than the spontaneous sparks before and after depolarization in the same image. Since spontaneous sparks occurred without any voltage change, they must be calcium activated. This is in agreement with the existence of two distinct classes of sparks, as suggested by some investigators (Klein et al. 1996).
Similar experiments were performed on 47 other fibres, but not all fibres were exposed to all the depolarizations. The number of fibres included at -65 mV was exceptionally small (see legend of Fig. 7), because either such a pulse was not applied to minimize damage to the fibres or an image was scanned in some fibres but was excluded from the analysis when any of the following complications existed. First, fibre contraction caused movement artifacts. Second, the sparks occurred at such a high frequency as to preclude distinct resolution. Third, even when the peaks of the sparks could be temporally separated, the background F during depolarization was so elevated above the baseline F0 that preceded the onset of the pulse that the normalization with F0 was inaccurate. The calculation of this background F at each location is difficult because of its spatial inhomogeneity. These complications sometimes occurred even at −75 or −70 mV. An example of the third complication can be found in the image shown in Fig. 9a, which was excluded from the present analysis.
Figure 7. Comparison of spontaneous and depolarization-evoked calcium sparks.
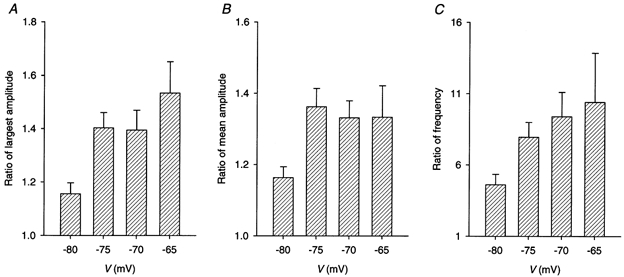
A pair of line-scan images were recorded at the same location at rest and during a 500 ms pulse to −80, −75, −70 or −65 mV in a total of 48 cut fibres. All fibres were held at −90 mV. Some fibres were exposed to more than one depolarizing potential. The mean values of the ratio of the amplitude of the brightest depolarization-evoked calcium spark to the amplitude of the brightest spontaneous calcium spark are plotted in A. The mean values of the ratio of the mean amplitude of the depolarization-evoked calcium sparks to the mean amplitude of the spontaneous calcium sparks are plotted in B. The mean values of the ratio of the frequency of the depolarization-evoked calcium sparks to the frequency of the spontaneous calcium sparks are plotted in C. n = 27, 32, 19 and 6 at V = −80, −75, −70 and −65 mV, respectively, in all three panels. The error bars represent s.e.m.s.
Figure 9. Effect of 2 μm Ry on depolarization-evoked calcium sparks and global calcium transients.
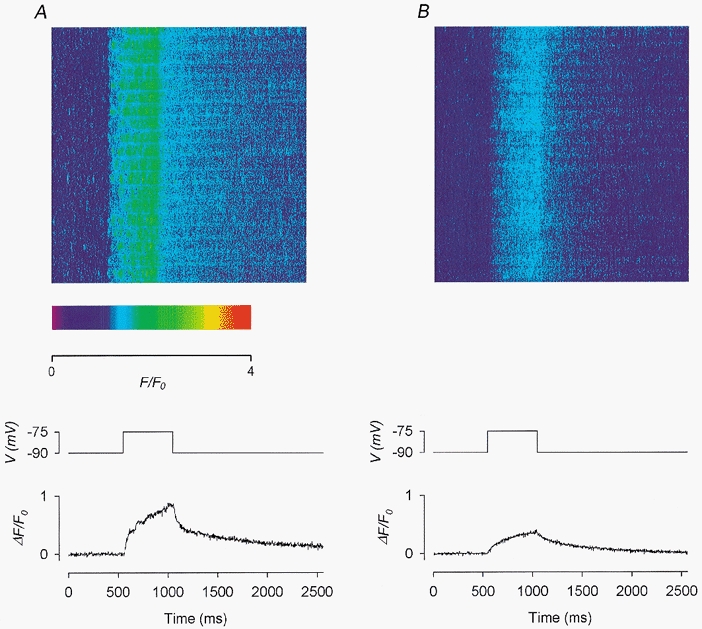
A, control before the application of Ry. B, in the presence of 2 μm Ry. Each panel shows: top, a line-scan image; middle, the depolarizing pulse used to trigger the calcium release; bottom, the global calcium transient trace obtained by compressing the 2-D image vertically into a 1-D array. Fibre 15. Holding potential −90 mV. Sarcomere length 3.9 μm.
The results from all 48 experiments are pooled in the three bar charts in Fig. 7. Each bar represents the mean value of the ratio of either the largest amplitude (in A), the mean amplitude (in B) or the frequency (in C) at a certain potential to the control value at −90 mV. The mean values of all the ratios at every potential are greater than 1 and the differences are statistically significant, implying that depolarization-evoked sparks have larger amplitudes and higher frequencies than spontaneous sparks. In each chart, the mean value at −75 mV is greater than that at −80 mV, and the difference is statistically significant, suggesting a voltage-dependent increase of the three quantities. However, the mean amplitude appeared to have saturated at −75 mV, as its mean value remained unchanged when voltage was changed from −75 to −65 mV (in B). Both the largest amplitude and the frequency appeared to continue their voltage-dependent increases beyond −75 mV (in A and C), but the increases were not statistically significant, primarily due to the limited number of observations available.
Complete suppression of depolarization-evoked calcium release by high [Ry]
In the fibre shown in Fig. 8, spontaneous sparks occurred only infrequently at a holding potential of −90 mV. Changing the holding potential to −80 mV increased the frequency of spontaneous sparks to 0.9 s−1 per sarcomere (image not shown). The line-scan image shown in the upper panel of Fig. 8a was recorded during the application of a 500 ms pulse to −70 mV (indicated by the trace immediately below the image). This depolarization activated many CRUs in most of the sarcomeres, thereby increasing the spark frequency to 7 s−1 per sarcomere. Interestingly, many sarcomeres showed sparks of variable brightness within the same sarcomere.
Figure 8. Effect of 20 μm Ry on depolarization-evoked calcium sparks and global calcium transients.
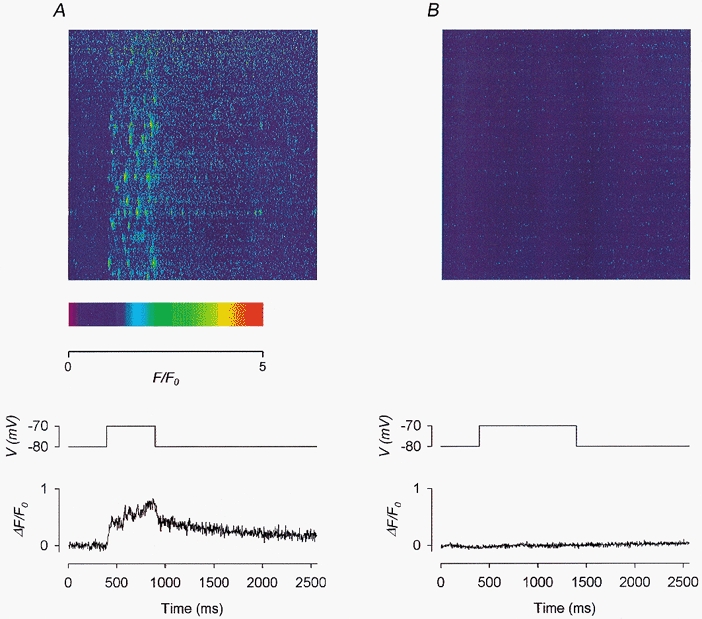
A, control before the application of Ry. B, in the presence of 20 μm Ry. Each panel shows: top, a line-scan image; middle, the depolarizing pulse used to trigger the calcium release; bottom, the global calcium transient trace obtained by compressing the 2-D image vertically into a 1-D array. Fibre 14. Holding potential −80 mV. Sarcomere length 3.7 μm.
The image shown in Fig. 8B was recorded 7 min after the application of 20 μm Ry to the centre pool. Not only were spontaneous sparks completely suppressed, but a depolarizing pulse of double duration to the same potential, as indicated by the middle trace, did not evoke any F signal. This was not surprising as a high [Ry] deactivates the RyRs, leading to the closure of the CRCs. The situation was quite different when a lower [Ry] was used, as will be shown in Fig. 9.
Each of the bottom traces in Fig. 8a and B plots the global ΔF(t)/F0versus t. Each point in a trace was obtained by averaging ΔF(x,t)/F0(x) over the whole range of x in the image for a particular t, equivalent to compressing the 2-dimensional image vertically to one dimension. In the control trace (in A), ΔF(t)/F0 rose rapidly on depolarization to about 0.4 and then gradually through the end of the pulse. On repolarization, the decay also showed a fast and then an extremely slow phase. By contrast, the test trace (in B) was practically flat. The value of ΔF/F0 after 500 ms of depolarization will be referred to as ΔF500/F0. It represents the amplitude of the calcium transient at that instant and was calculated by averaging the 181–200th point after the onset of the pulse. Its value was 0.73 in the control trace and 0.02 in the test trace. Furthermore, the presence of calcium sparks made the control trace much noisier, particularly during depolarization, than the test trace.
Partial suppression of depolarization-evoked calcium release by low [Ry]
In the experiment shown in Fig. 9, the fibre was held at −90 mV. A few faint spontaneous sparks occurred prior to depolarization in the image shown at the top of Fig. 9a. During a 500 ms depolarization to −75 mV, brighter sparks were evoked very frequently but not as bright as those in Fig. 6C or 8A (note the difference in pseudocolour scale). In fact, the frequency of the sparks was so high that some of the sparks could not be resolved individually. Thus, depolarization-evoked sparks show a large degree of variability in brightness among fibres as much as spontaneous sparks do (see Fig. 3a and associated text).
The image shown at the top of Fig. 9B was recorded 8 min after the application of 2 μm Ry to the centre pool. Spontaneous sparks were absent in this image prior to depolarization. The 500 ms depolarization to −75 mV did not elicit any spark either but elevated the fluorescence intensity progressively and uniformly throughout the depolarization time period. The absence of spontaneous and depolarization-evoked sparks here is consistent with the ability of this lower [Ry] to modify RyRs to a semi-open state. The increase in frequency of spontaneous sparks preceding modification, as previously shown in Fig. 4 and Fig. 5, must have occurred in this experiment before the image in Fig. 9B was scanned. As shown in Fig. 4, this increase in spark frequency could occur as early as 2 min after the application of ryanodine. Interestingly, in the original image from which the image of Fig. 9B was generated, an unmistakable glow and three fainter glows could be observed in four triadic regions. These glows were eliminated in the processed image shown here by the F-ratio procedure, as explained in the text associated with Fig. 4. In the rest of the triadic regions, the glows were probably too faint to be detected. Thus, the increase in F intensity during depolarization in this image was contributed to by additional calcium release on top of the steady calcium leak through modified RyRs. However, if the RyRs were ‘locked’ rigidly in the modified state, they should not be opened any further by a membrane potential change. Thus, this elevation in F intensity indeed presented a surprise (see Discussion for possible explanations).
The pair of traces shown at the bottom of Fig. 9 were obtained in the same way as those in Fig. 8. Again, the control trace in A was noisier than the test trace in B. In the control trace, the amplitude of the calcium transient was 0.84. Thus, although the depolarization-evoked sparks in this fibre were not as bright as those in Fig. 8a, the higher frequency of occurrence of the sparks rendered the amplitude of the calcium transient at −75 mV in this fibre slightly larger than the counterpart (0.73) in Fig. 8a. In the test trace, the amplitude of the calcium transient was 0.35, implying that 2 μm Ry suppressed the amplitude of the calcium transient to about 42 % of its control value. This value of residual amplitude is listed in the fifth row of Table 2.
Table 2.
Effect of Ry on global calcium transient
| Fibre no. | [Ry] (μM) | Residual amplitude (% of control) |
|---|---|---|
| 2 | 2 | 36 |
| 5 | 2 | 50 |
| 10 | 2 | 51 |
| 12 | 2 | 47 |
| 15 | 2 | 42 |
| 16 | 1 | 63 |
| 17 | 2 | 45 |
| 18 | 2 | 66 |
| 19 | 2 | 72 |
| Mean | — | 52 |
| s.e.m | — | 4 |
The global calcium transients were obtained by the procedure shown in Fig. 9. The amplitude of each transient was estimated by averaging the values of the last 20 points during the pulse(referred to as δF500/F0). The residual amplitudes in the third column were obtained by dividing the test δF500/F0 by the control δF500/F0 and then multiplying by 100.
The same measurement was repeated in another fibre to which depolarizations were applied at four potentials (images not shown). The control global ΔF(t)/F0 traces at different potentials are shown in Fig. 10a whereas the traces in the presence of 2 μm Ry are shown in Fig. 10B. The amplitudes of the calcium transients from the traces of Fig. 10A are plotted in Fig. 10C as filled circles whereas those from Fig. 10B are plotted as open circles. The residual amplitudes estimated from the ratios of the amplitudes at the four potentials were quite similar and had a mean value of 51 %. This value is listed in the third row of Table 2.
Figure 10. Effect of 2 μm Ry on global calcium transients.
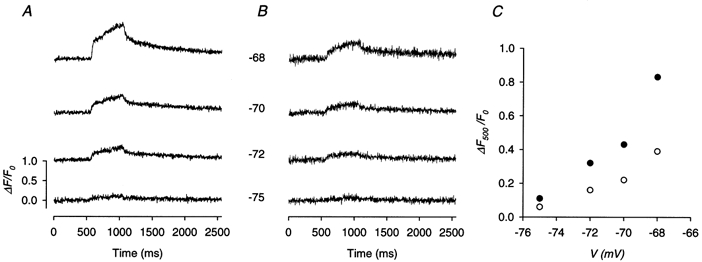
A, control global calcium transient traces before the application of Ry. B, global calcium transient traces in the presence of 2 μm Ry. Each number between a pair of control and test traces indicates the potential (in mV) during the pulses used to evoke both calcium release. C, the global value of ΔF/F0 at the end of the 500 ms depolarizing pulse plotted against the potential during the pulse. •, control values; ^, test values. Fibre 10. Holding potential −90 mV. Sarcomere length 3.7 μm.
Similar experiments were carried out in seven other fibres in the potential range between −75 and −68 mV and the results are also listed in Table 2. Averaged over all the fibres, the residual amplitude in the presence of 1–2 μm Ry is 52 ± 4 %. A general conclusion of this section is that this low [Ry] abolishes the calcium sparks completely and reduces the global calcium transient to about half of the control.
DISCUSSION
The results presented in this report demonstrate successful capture of robust spontaneous and depolarization-evoked calcium sparks in cut fibres using a Nikon PCM 2000 system and fluo-3 as an indicator. The effects of Ry reported here on spontaneous sparks establish, in the live skeletal muscle fibre preparation, several sequential effects of Ry previously reported for single CRCs in bilayers. Application of a relatively low [Ry] (0.4–2 μm) induced an increase in sparking frequency. Within a short time thereafter, some of the repetitive sparks were converted to long-lasting glows. During the persistence of a glow, short interruptions sometimes occurred, but a glow was never followed by a spark at the same location. A higher [Ry] (≥20 μm) effectively suppressed spontaneous sparks and glows, as well as entirely eliminated depolarization-evoked calcium sparks and global calcium transients. Experiments with depolarizing pulses provided new information that cannot be obtained with the bilayer preparation. At 1–2 μm, Ry effectively suppressed depolarization-evoked sparks; yet, global calcium transients persisted but were reduced in amplitude by half. The latter results show that CRCs locked half open by Ry are deprived of the ability to generate sparks but nevertheless can allow additional Ca2+ flux in response to the triggering signal from the voltage sensors.
Distribution of spontaneous calcium sparks
Calcium sparks occurred spontaneously in resting fibres. Their amplitude varied from one image to another in the same fibre (Fig. 2) and from fibre to fibre (Fig. 3). The largest amplitude in an image occurred invariably in the early stage of each experiment and it ranged from 1.0 to 7.4 in ΔF/F0 units (Fig. 3a). A histogram of thousands of early sparks from all the fibres showed that the number of occurrences decreases monotonically with increasing spark amplitude (Fig. 3B). The mean amplitude of all the sparks was 2.1, but this value is not absolutely meaningful as it must be greatly influenced by the threshold chosen in the program used to detect the sparks. The number of sparks at the lower limit is much larger than the number at the upper limit (Fig. 3B). Had it been possible to improve the noise level in the line-scan image, a lower threshold could have been chosen, resulting in a smaller mean amplitude. On the other hand, had it been possible to improve the confocality of the microscope, out-of-focus sparks could have been discriminated more stringently. If the smaller sparks in an image were primarily out of focus, that would result in a larger mean amplitude. The same argument applies to the estimate of spark frequency as it too depends on the criteria with which the sparks are selected.
Properties of depolarization-evoked calcium sparks
Whether depolarization-evoked calcium sparks share similar properties with spontaneous sparks has been discussed previously (Schneider, 1999). It stated that early results obtained by Klein et al. (1996) showed that one population of depolarization-evoked sparks had an amplitude double that of the spontaneous sparks, but later results obtained by the same group (Lacampagne et al. 1996; Klein et al. 1997) showed that depolarization affected only the frequency but not the amplitude of the sparks. In our experiments, it is obvious that, even within the same image, depolarization-evoked sparks are brighter than the pre- and post-depolarization spontaneous sparks (Figs 6B and C, and 8A). Many pair-wise comparisons were carried out in which an image was scanned at rest followed by another one synchronized with a depolarizing pulse. Statistical analysis showed that the ratios of the amplitudes (largest or mean) of depolarization-evoked sparks to those of spontaneous sparks were greater than unity and the differences were highly significant (Fig. 7a and B). This seems plausible because spontaneous sparks should be associated with the openings of calcium-gated CRCs whereas depolarization-evoked sparks might involve concerted openings of both voltage-gated and calcium-gated CRCs in some undetermined proportion. Additionally, the amplitudes (Fig. 7a and B) and the frequency (Fig. 7C) of sparks appeared to increase with increasing depolarization. The increase in spark frequency should be related to the more frequent activation of CRUs at less negative potentials whereas the increases in spark amplitudes might reflect the opening of more voltage-gated CRCs in the active CRUs. All these arguments support the idea that a spark is not a unitary event associated with a single CRC.
Effects of Ry on localized and global calcium release
A high [Ry] (≥ 20 μm) suppresses both classes of calcium sparks, presumably by occupying the deactivating sites on the RyRs. At low [Ry] (0.4–2 μm), the sparks were also suppressed but we observed four additional exciting effects. When Ry began to act on the RyRs, there was usually a period of intense activity during which the sparks fired repetitively at a high frequency (Figs. 4A and 5B). This might be due to the ability of a very low [Ry] to increase the open probability of the CRCs, as has been observed in bilayers (Bull et al. 1989). Sometimes, these high frequency sparks showed two or more levels of brightness. Since all other triads in the same scan were quiescent, it is unlikely that the generation of these sparks involved two or more CRUs at different focal planes. The second action of Ry was the apparent conversion of spontaneous sparks into glows having, on average, an intensity about one-quarter of that of the precursory sparks and lasting tens of seconds (Figs. 4B and 5B). Third, the steady glow was occasionally interrupted by short gaps (Fig. 5B) but was never followed by sparks. Finally, when the fibre was depolarized, the modified RyRs were still capable of releasing Ca2+, not in the form of sparks, but steadily and uniformly (Fig. 9B). The possible mechanisms underlying these actions will be discussed in the next two sections.
It should be noted that the low and high concentrations of Ry are not meant to provide a precise dose-response relationship of the action of Ry. Many more experiments need to be performed to establish any such quantitative relationship. Also, it is difficult to compare these concentrations directly with those used in bilayer experiments because of a difference in the preparations employed, a difference in the ionic environments between the two preparations and a possible difference in the degree of purity of the Ry samples used.
Origin of the Ry-activated steady glow
It has been shown in bilayers that Ry is capable of locking a CRC in a semi-open state (Rousseau et al. 1987; Buck et al. 1992; Tinker et al. 1996). It is then exciting to imagine that the glow in a live fibre is a manifestation of calcium release through a semi-open CRC, reminiscent of the observation by Cheng et al. (1993) in cardiac muscle. The non-existence of a reversion of the glow back to a spark and the occasional interruptions of the glow are both consistent with observations in bilayers.
Whether a calcium spark involves calcium release through a single or multiple CRC(s) has been debated actively among investigators. This issue has significant bearing on the origin of the glow. In bilayers, the semi-open state of a modified cardiac CRC has a conductance ∼40 % of that of the fully open state of an unmodified CRC, according to Rousseau et al. (1987), or ∼57 %, according to Tinker et al. (1996). The corresponding value for a modified skeletal CRC is ∼48 % (Buck et al. 1992; see also Lai et al. 1992). If a spark reflects calcium release through a fully open CRC and the glow reflects calcium release through a semi-open CRC, then the ratio of the amplitude of the glow to that of the spark should correspond to the fractional conductance of a semi-open CRC. Figure 3B of Cheng et al. (1993) showed that the amplitude of the steady glow was about half of the peak of the precursory spark, which can be easily explained by the modification of a single CRC. On the other hand, if a spark involves the opening of more than one channel and the glow also involves all the channels, then these channels must be tightly coupled in a way that they open simultaneously to generate the spark and undergo transitions to the sub-conductance state simultaneously to generate the glow, perhaps in a way related to the concerted gating of neighbouring CRCs observed by Marx et al. (1998).
Table 1 (column 5) shows that the ratio of the amplitude of the glow to the amplitude of the precursory spark in our experiments varied between 0.17 and 0.47. Although these numbers are subject to some degree of uncertainty due to the limitation in measurement precision, it is safe to conclude tentatively that the ratio is less than one-half. If RyRs in live fibres behave in the same way as in bilayers in response to the action of Ry, i.e. the modified conductance is half the full unmodified conductance, then it is safe to infer from the present results that a spark probably involves more than one CRC. Perhaps m CRCs in a CRU are involved in generating a spark and, after modification by Ry, only n CRCs are locked in the semi-open state, with m > n. Then the ratio of glow amplitude to spark amplitude should be given by n/2m. For example, if m = 2 and n = 1, the ratio is 1/4 which is close to the mean value in the fifth column of Table 1. One can even extend this idea to include other combinations of pairs of values for m and n, thus allowing a variability in the ratio of glow amplitude to spark amplitude, as observed.
Table 1 (column 4) also shows that the amplitudes of the sparks leading to glows were ≤ 1.54. These values are much smaller than the amplitudes of the brightest sparks (Fig. 3A). If these dimmer sparks are not all out-of-focus but involve the opening of a smaller number of CRCs, then the results suggest that, for some reason yet to be understood, when a group of CRCs in a CRU are activated, the number of CRCs has to be small in order that Ry can modify them into the semi-open state. Thus, there is a good chance the glow might reflect Ca2+ flux through a very small number of (or even a single) modified CRCs.
Our observation of steady glows with Ry is parallel to the situation with imperatoxin A (IpTXa). While Tripathy et al. (1998) reported that IpTXa is capable of locking cardiac and skeletal CRCs in bilayers in a semi-open state, Shtifman et al. (2000) and Gonzalez et al. (2000) observed prolonged local calcium transients in live fibres that looked somewhat like the steady glows reported here, although their events did not last as long. Interestingly, although both groups suggested that their IpTXa events could originate from a single CRC, Shtifman et al. (2000) estimated that the spark leading to a glow involved at most four CRCs while Gonzalez et al. (2000) estimated the number to be at least six. Gonzalez et al. (2000) also observed a decay of spark to a steady level of fluorescence lasting several hundred milliseconds in the presence of Ry. We feel that the events they observed were distinctly different from the glows reported here because the time courses were vastly different. Also, the concentrations of Ry used were quite different: 50 nm in their case versus 0.4–2 μm in ours. According to the information provided by bilayers experiments (Tinker et al. 1996), the modification of RyRs by Ry is practically an irreversible phenomenon and so should be long lasting. Moreover, modified CRCs cannot make transitions to the full open state and so should not be capable of generating sparks.
The steady glows that we observed are rare events. Since the action of Ry is essentially irreversible, each CRC has only one chance to be modified to the semi-open state. As the Ca2+ content of the SR is limited, although the CRCs may be opened partially forever, the release of Ca2+ through the CRCs does not last indefinitely. If the transition from a spark to glow occurs between two scans, the event will be missed. Thus, the capture of the transition is very chancy; one has to look at the right location at the right time. So far, we have been able to capture only one or two glows in each fibre (see Table 1). As mentioned in Results, other glows were captured, but without any knowledge of the precursory sparks, those events had to be excluded from our analysis.
origin of the voltage-activated uniform calcium release in the presence of 2 μm Ry
In bilayers, a CRC modified by Ry to the semi-open state never made transitions to the fully open state and only occasionally made transitions to the closed state (Rousseau et al. 1987; Tinker et al. 1996). This led researchers to believe that when a RyR is modified to that state, it is locked rigidly in the semi-open conformation. However, in a live fibre in which the RyRs can be linked to the voltage sensors, depolarization appeared to release Ca2+ steadily (Fig. 9B), in addition to the presumed voltage-independent leak through the semi-open channels. There is no doubt that Ry had acted on the RyRs by the time that image was scanned because sparks were completely abolished. So how can more Ca2+ be released during depolarization?
At least three explanations can be postulated. (a) Although a CRC in the semi-open state cannot open spontaneously to generate sparks, it can be pulled to open further by a voltage sensor, but this modified opening has altered kinetics so that no spark can be generated. If this is true, the gate of a CRC in the modified state is not held rigidly in the semi-open position. (b) There are two populations of voltage-gated RyRs, one more sensitive to Ry and capable of generating sparks and the other less sensitive to Ry and incapable of generating sparks but capable of generating unresolvable events (Shirokova & Rios, 1997), such as quarks (Lipp & Niggli, 1996). A low [Ry] can deactivate the former population but not the latter. It takes ≥ 20 μm Ry to completely deactivate the latter. To our knowledge, such diversity has not been verified in single channel recording with bilayers. (c) After Ry binding, a RyR not linked to a dihydropyridine receptor is free to make a transition to the semi-open state to generate a glow but a linked one is held in the closed state by the dihydropyridine receptor and goes to the semi-open state only during depolarization to generate unresolvable events. Other possibilities might also exist. Our current research efforts are directed towards gathering additional information to determine if any of these is the correct explanation. The results we have obtained thus far with the help of other ryanoids point to the possibility that (a) could be the correct one. If this is indeed the case, then experiments in live fibres are capable of providing information about the action of Ry not achievable with studies in bilayers.
Acknowledgments
We thank Drs Julio Vergara and Mike Klein for providing the details of their designs of the inverted chamber. We also acknowledge the Biomedical Confocal Systems Division of Nikon Inc. for technical support on the PCM2000 hardware and Compix Inc. for technical support on the SimplePCI software. This project was supported by a grant from the National Institutes of Health (NS21955) to C.S.H. and a grant from the Showalter Trust to H.R.B.Jr.
References
- Bidasee KR, Besch HR., Jr Structure-function relationships among ryanodine derivatives. Pyridyl ryanodine definitively separates activation potency from high affinity. Journal of Biological Chemistry. 1998;273:12176–12186. doi: 10.1074/jbc.273.20.12176. [DOI] [PubMed] [Google Scholar]
- Brum G, Gonzalez A, Rengifo J, Shirokova N, Rios E. Fast imaging in two dimensions resolves extensive sources of Ca2+ sparks in frog skeletal muscle. Journal of Physiology. 2000;528:419–433. doi: 10.1111/j.1469-7793.2000.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck E, Zimanyi I, Abramson JJ, Pesah IN. Ryanodine stabilizes multiple conformational states of the skeletal muscle calcium release channel. Journal of Biological Chemistry. 1992;267:23560–23567. [PubMed] [Google Scholar]
- Bull R, Marengo JJ, Suarez-Isla BA, Donoso P, Sutko JL, Hidalgo C. Activation of calcium channels in sarcoplasmic reticulum from frog muscle by nanomolar concentrations of ryanodine. Biophysical Journal. 1989;56:749–756. doi: 10.1016/S0006-3495(89)82722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Ríos E, Stern MD. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophysical Journal. 1999;76:606–617. doi: 10.1016/S0006-3495(99)77229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difranco M, Quinonez M, Digregorio DA, Kim AM, Pacheco R, Vergara JL. Inverted double-gap isolation chamber for high-resolution calcium fluorimetry in skeletal muscle fibers. Pflugers Archiv. 1999;438:412–418. doi: 10.1007/s004240050929. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Kirsch WG, Shirokova N, Pizarro G, Brum G, Pessah IN, Stern MD, Cheng H, Rios E. Involvement of multiple intracellular release channels in calcium sparks of skeletal muscle. Proceedings of the National Academy of Sciences of the USA. 2000;97:4380–4385. doi: 10.1073/pnas.070056497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins AB, Kurebayashi N, Baylor SM. Resting myoplasmic free calcium in frog skeletal muscle fibers estimated with fluo-3. Biophysical Journal. 1993;65:865–881. doi: 10.1016/S0006-3495(93)81112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui CS, Bidasee KR, Besch HR., Jr Effect of ryanodine (Ry) on calcium sparks in frog skeletal muscle fibers. Biophysical Journal. 1998;74:A235. [Google Scholar]
- Hui CS, Chen W. Charge movement in cut twitch fibres of Rana temporaria containing 0. 1 mm EGTA. Journal of Physiology. 1997;503:563–570. doi: 10.1111/j.1469-7793.1997.563bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humerickhouse RA, Besch, H R Jr, Gerzon K, Ruest L, Sutko JL, Emmick JT. Differential activating and deactivating effects of natural ryanodine cogeners on the calcium release channel of sarcoplasmic reticulum: evidence for separation of effects at functionally distinct sites. Molecular Pharmacology. 1993;44:412–421. [PubMed] [Google Scholar]
- Humerickhouse RA, Bidasee KR, Gerzon K, Emmick JT, Kwon S, Sutko JL, Ruest L, Besch HR., Jr High affinity C10-Oeq ester derivatives of ryanodine. Activator-selective agonists of the sarcoplasmic reticulum calcium release channel. Journal of Biological Chemistry. 1994;269:30243–30253. [PubMed] [Google Scholar]
- Irving M, Maylie J, Sizto NL, Chandler WK. Intrinsic optical and passive electrical properties of cut frog twitch fibers. Journal of General Physiology. 1987;89:1–40. doi: 10.1085/jgp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MG, Cheng H, Santana LF, Jiang Y-H, Lederer WJ, Schneider MF. Two mechanisms of quantized calcium release in skeletal muscle. Nature. 1996;379:455–458. doi: 10.1038/379455a0. [DOI] [PubMed] [Google Scholar]
- Klein MG, Lacampagne A, Schneider MF. Voltage dependence of the pattern and frequency of discrete Ca2+ release events after brief repriming in frog skeletal muscle. Proceedings of the National Academy of Sciences of the USA. 1997;94:11061–11066. doi: 10.1073/pnas.94.20.11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacampagne A, Lederer WJ, Schneider MF, Klein MG. Repriming and activation alter the frequency of stereotyped discrete Ca2+ release events in frog skeletal muscle. Journal of Physiology. 1996;497:581–588. doi: 10.1113/jphysiol.1996.sp021791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacampagne A, Ward CW, Klein MG, Schneider MF. Time course of individual Ca2+ sparks in frog skeletal muscle recorded at high time resolution. Journal of General Physiology. 1999;113:187–198. doi: 10.1085/jgp.113.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai FA, Liu Q-Y, Xu L, El-Hashem A, Kramarcy NR, Sealock R, Meissner G. Amphibian ryanodine receptor isoforms are related to those of mammalian skeletal or cardiac muscle. American Journal of Physiology. 1992;263:C365–372. doi: 10.1152/ajpcell.1992.263.2.C365. [DOI] [PubMed] [Google Scholar]
- Lipp P, Niggli E. Submicroscopic calcium signals as fundamental events of excitation-contraction coupling in guinea-pig cardiac myocytes. Journal of Physiology. 1996;492:31–38. doi: 10.1113/jphysiol.1996.sp021286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez JR, Shacklock PS, Balke CW, Wier WG. Local, stochastic release of Ca2+ in voltage-clamped rat heart cells: visualization with confocal microscopy. Journal of Physiology. 1994;480:21–29. doi: 10.1113/jphysiol.1994.sp020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Ondrias K, Marks AR. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors) Science. 1998;281:818–821. doi: 10.1126/science.281.5378.818. [DOI] [PubMed] [Google Scholar]
- Maylie J, Irving M, Sizto NL, Chandler WK. Comparison of arsenazo III optical signals in intact and cut frog twitch fibers. Journal of General Physiology. 1987;89:41–81. doi: 10.1085/jgp.89.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E, Smith JS, Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. American Journal of Physiology. 1987;253:C364–368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Schneider MF. Ca2+ sparks in frog skeletal muscle: Generation by one, some, or many SR Ca2+ release channels? Journal of General Physiology. 1999;113:365–372. doi: 10.1085/jgp.113.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N, Gonzalez A, Kirsch WG, Rios E, Pizarro G, Stern MD, Cheng H. Calcium sparks: Release packets of uncertain origin and fundamental role. Journal of General Physiology. 1999;113:377–384. doi: 10.1085/jgp.113.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N, Rios E. Small event Ca2+ release: a probable precursor of Ca2+ sparks in frog skeletal muscle. Journal of Physiology. 1997;502:3–11. doi: 10.1111/j.1469-7793.1997.003bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtifman A, Ward CW, Wang J, Valdivia HH, Schneider MF. Effects of imperatoxin A on local sarcoplasmic reticulum Ca2+ release in frog skeletal muscle. Biophysical Journal. 2000;79:814–827. doi: 10.1016/S0006-3495(00)76338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko JL, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacological Review. 1997;49:53–98. [PubMed] [Google Scholar]
- Tinker A, Sutko JL, Ruest L, Deslongchamps P, Welch W, Airey JA, Gerzon K, Bidasee KR, Besch HR, Jr, Williams AJ. Electrophysiological effects of ryanodine derivatives on the sheep cardiac sarcoplasmic reticulum calcium-release channel. Biophysical Journal. 1996;70:2110–2119. doi: 10.1016/S0006-3495(96)79777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy A, Resch W, Xu L, Valdivia HH, Meissner G. Imperatoxin A induces subconductance states in Ca2+ release channels (ryanodine receptors) of cardiac and skeletal muscle. Journal of General Physiology. 1998;111:679–690. doi: 10.1085/jgp.111.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugorka A, Rios E, Blatter LA. Imaging elementary events of calcium release in skeletal muscle cells. Science. 1995;269:1723–1726. doi: 10.1126/science.7569901. [DOI] [PubMed] [Google Scholar]
- Wier WG, Mauban JRH, Miriel V, Blaustein MP. Ca2+ sparks in smooth muscle cells of pressurized arteries. Biophysical Journal. 1999;76:A291. [Google Scholar]


