Abstract
Arousal from sleep is associated with transient stimulation of ventilation above normal waking levels that predisposes to subsequent breathing instability and central apnoea. The transient hyperpnoea at arousal is normally explained by differences in arterial partial pressure of CO2 (Pa,CO2) between sleep and wakefulness, with a higher Pa,CO2 in sleep leading to stimulation of ventilation at arousal according to the awake ventilatory response to CO2. Surprisingly, however, the validity of this current model in fully explaining the increased ventilation at arousal from sleep has not been directly tested.
This study tests the hypothesis that the level of ventilation at arousal from non-rapid eye movement (non-REM) sleep is greater than that produced by elevating Pa,CO2 in wakefulness to the sleeping level, i.e. the ventilation predicted by the current model.
Studies were performed in five dogs. Inspired CO2 was used to increase end-tidal partial pressure of CO2 (PET,CO2) in wakefulness and measure the ventilatory response. The same PET,CO2 was then maintained in non-REM sleep. Ventilation was measured for 10 breaths before and after arousal from non-REM sleep induced by a 72 dB tone.
Arousal from sleep produced a transient surge in ventilation of 1.42 ± 0.35 l min−1 (P= 0.005). This increased ventilation was due to arousal from sleep per se as the tone alone produced no change in awake ventilation. In support of the hypothesis, ventilation at wake onset from sleep was greater by 0.83 ± 0.28 l min−1 (P= 0.031) than the ventilation elicited in wakefulness by raising PET,CO2 to the sleeping level.
The results show that > 50% of the increase in ventilation at wake onset from sleep is not attributable to the awake ventilatory response to the elevated Pa,CO2 that was previously present in sleep. This result leads to important modifications of the physiological model currently used to explain the ventilatory consequences of arousal from sleep.
Arousals from sleep are associated with transient stimulation of ventilation above normal waking levels that can lead to subsequent reductions in arterial partial pressure of CO2 (Pa,CO2), breathing instability and central sleep apnoea (Phillipson & Bowes, 1986; Xie et al. 1994; Bradley & Phillipson, 2000). The transient hyperpnoea at arousal from sleep is normally attributed to homeostatic mechanisms, i.e. differences in set points for Pa,CO2 between sleep and wakefulness. In this model, the higher Pa,CO2 in sleep leads to transient stimulation of ventilation at wake onset in accordance with the awake ventilatory response to CO2 (Phillipson & Bowes, 1986; Bradley & Phillipson, 2000). As such, the stimulation of ventilation at wake onset is currently explained purely in terms of the ventilatory response in wakefulness to the elevated Pa,CO2 that was previously present in sleep.
Large transient increases in ventilation have commonly been observed at arousal from sleep in several studies (for example see Trinder et al. 1992; Carley et al. 1997; for review see Phillipson & Bowes, 1986), as have surges in heart rate and blood pressure (for example see Horner et al. 1995; Morgan et al. 1996). However, the physiological control mechanisms mediating these phenomenological observations have not been fully determined. This is particularly true for the mechanisms underlying the transient hyperpnoea at arousal from sleep where, surprisingly, the validity of the current model in explaining this response based on differences in Pa,CO2 has been assumed, but not directly tested. Some recent observations suggest, albeit indirectly, that a component of the drive to breathe at wake onset may be unrelated to Pa,CO2. For example, in a study in sleeping dogs undergoing constant mechanical ventilation to reduce Pa,CO2 and abolish spontaneous breathing, arousal from sleep was noted to transiently stimulate breathing despite the hypocapnia (Horner et al. 1995). However, this study focused on heart rate responses to arousal, and effects on ventilation were observed only qualitatively. Similar observations using mechanical ventilation have also been made in humans (Trinder et al. 2001). However, since mechanical ventilation per se inhibits respiratory activity (Simon et al. 1991), the degree to which these studies can be interpreted with respect to respiratory control mechanisms at arousal is limited. Carley et al. (1997) observed that peak ventilation following arousal from sleep is increased by hypercapnia but the absolute change in ventilation from sleep to wakefulness was similar to room air. This result implied that the hyperpnoea was not entirely due to state-dependent increases in chemosensitivity, although that study did not measure ventilation elicited by the same CO2 in wakefulness to directly test the relative role of CO2 in the hyperpnoea at arousal.
Overall, these observations imply that a component of the increased ventilation at wake onset from sleep may be unrelated to Pa,CO2, although this hypothesis has not been specifically addressed, nor systematically studied, in previous investigations. Nor has the hypothesized component unrelated to Pa,CO2 been quantified. This issue is of importance in understanding the hyperpnoea at arousal in clinical disorders such as central and obstructive sleep apnoea, as well as understanding basic respiratory control mechanisms and the influences of wakefulness. Indeed, since recent evidence suggests that arousal from sleep leads to spontaneous activation of a transiently heightened awake state compared with subsequent waking (Horner et al. 1997), arousal mechanisms per se may independently contribute to the ventilatory response at wake onset. Accordingly, the present study tested the hypothesis that wake onset leads to a degree of ventilatory stimulation over and above that which can be attributed to CO2. If valid, the hypothesis would lead to important modifications of the models currently used to explain the ventilatory consequences of arousal from sleep.
METHODS
Animals and surgical preparation
Studies were performed on five adult dogs (25.2-38.5 kg, three female) trained to lie quietly and sleep in the laboratory. Each dog lay on one side and this body position was adopted in all studies. The Animal Care Committee of the University of Toronto approved all procedures. For monitoring ventilation, each dog was prepared with a permanent side-hole tracheotomy at least 24 months before the studies (Phillipson et al. 1970). Surgery was performed under general anaesthesia and aseptic conditions. The dogs were premedicated with atropine (0.02-0.05 mg kg−1i.m.) and penicillin (15 000-20 000 units kg−1i.m.). Anaesthesia was induced with a short-acting barbiturate (thiamyal sodium, 10-20 mg kg−1i.v.) and maintained with a long-acting barbiturate (pentobarbitone, titrated to effect, typically 30 mg kg−1i.v.). After surgery the dogs were kept warm and monitored until they were recovered from anaesthesia and eating and drinking normally. The dogs were then returned to their normal housing in the animal facility. At the time of the experiments all animals were healthy, were eating and behaving normally, and were receiving no medication. After the experiments they were returned to their normal housing.
Recordings
During the experiments the dogs breathed via a cuffed endotracheal tube (10 mm internal diameter) inserted through the chronic tracheotomy. The dogs were attached to a breathing circuit via this endotracheal tube. Airflow was measured using a heated pneumotachograph (Fleisch No. 2, P. K. Morgan Ltd, Rainham, Kent, UK) and integrated to obtain tidal volume. Airflow was also analysed by computer for breath-by-breath tidal volume, inspiratory and expiratory times, respiratory rate and ventilation (Kimoff et al. 1997). Airway pressure (Statham P23Db transducer, Gould Inc., Oxnard, CA, USA) and CO2 levels (LB-2, Beckman Instruments Inc., Fullerton, CA, USA) were also measured and end-tidal PCO2 (PET,CO2) was calculated. The electroencephalogram (EEG), neck electromyogram (EMG) and electrocardiogram (ECG) were monitored via pairs of subdermal needle electrodes (Type E2, Grass Instrument Co.) placed in the scalp, neck and chest. Sleep-wake states were documented according to EEG and neck EMG criteria (Horner et al. 1995). In addition to the raw ECG signal, the instantaneous heart rate was also derived (Cardiotachometer Coupler, Beckman, type 9857).
Control of CO2 levels and application of auditory stimuli
The dogs were studied under four separate experimental conditions in spontaneous periods of wakefulness and sleep.
Condition 1: spontaneous room air breathing
This condition established the normal changes in PET,CO2 between wakefulness and non-REM sleep.
Condition 2: addition of CO2 in wakefulness to raise the awake PET,CO2 to sleeping levels
The aim of this condition was to measure the ventilatory response in wakefulness to the increased CO2 levels encountered in sleep. Following changes in inspired CO2, at least 6 min was allowed to elapse to achieve a steady state.
Condition 3: keeping CO2 levels in sleep the same as those produced in wakefulness
In this condition the CO2 that was added in wakefulness was withdrawn when the dogs went into non-REM sleep to keep PET,CO2 constant. Runs were rejected if PET,CO2 deviated by > 1 mmHg from condition 2. In practice, the mean difference in PET,CO2 levels between conditions 2 and 3 was 0.1 ± 0.2 mmHg (see Results).
Condition 4: application of auditory tones to induce arousal from sleep
In this condition auditory tones (72 dB, 0.5 s duration) were applied to induce arousal from non-REM sleep. Auditory tones, rather than spontaneous awakenings, were used so that a consistent stimulus to arousal from sleep could be applied at a consistent phase of the breathing cycle (end expiration) and at similar stages of non-REM sleep as judged by visual inspection. A sound level of ≈70 dB was chosen as this was loud enough to consistently produce arousal from sleep, but quiet enough to avoid startle as judged by the absence of sudden, brief postural muscle activation identified visually and recorded in the neck EMG (Horner et al. 1997). The purpose of this condition was to measure ventilation at wake onset from sleep and determine if it exceeded the ventilation produced by the same CO2 stimulus when applied in established, steady-state wakefulness in condition 2, i.e. as predicted by the hypothesis. To determine that the tones per se caused no change in ventilation (i.e. that the ventilatory responses were due to arousal from sleep and not the tone), further studies were performed in which the same auditory stimuli were applied in steady-state wakefulness at the same PET,CO2 levels as when the tones were applied in sleep.
Multiple observations and interventions were performed awake and asleep in each dog. At matched PET,CO2 levels an average of 8.3 ± 0.5 auditory stimuli (range, 7-10) were applied to induce arousal from non-REM sleep, and 9.4 ± 0.5 stimuli (range, 7-11) were applied in established wakefulness. Tones were separated by an average of 2.2 ± 0.2 min. Two of the five dogs were studied twice on separate days. However, data from each day in these dogs were averaged thereby providing a single mean value for each dog (i.e. n = 5 for all comparisons).
Analyses and statistics
Analyses were performed to answer the following two major questions. (1) Was the level of ventilation elicited at wake onset from non-REM sleep greater than the ventilation elicited by elevating Pa,CO2 in steady-state wakefulness to the same level as in sleep? (2) What was the time course of the increase in ventilation following arousal from non-REM sleep?
Breath-by-breath respiratory variables were measured for the 10 breaths immediately before the tone (i.e. in the two conditions of non-REM sleep and established wakefulness) and the 10 breaths immediately after the tone (i.e. at wake onset from non-REM sleep and continuing established wakefulness). The respiratory variables were then averaged across interventions in each dog before and after the tone. Following arousal from non-REM sleep, only those breaths in which the dogs were in documented wakefulness as judged by EEG and EMG criteria were included. The average and peak ventilation before and after the stimuli in each condition were then used for statistical analyses. To normalise for differences in baseline ventilation between dogs, breath-by-breath ventilation awake and asleep was also calculated as the percentage of the mean ventilation measured in established wakefulness.
For the second analysis, the 10 breaths that followed the auditory tone were time-aligned for each intervention in each dog. The breath-by-breath ventilation, expressed as a percentage of the mean ventilation in established wakefulness, and the PET,CO2 levels, were calculated for these breaths. Breath-by-breath ventilation was also calculated for the 10 control breaths immediately before application of the auditory stimuli. Analyses were then performed to determine the breath numbers when ventilation at wake onset exceeded the 95 % confidence interval of the control non-REM values.
For all comparisons, differences were considered significant if the null hypothesis was rejected at P < 0.05 using Student's two-tailed t test. Post hoc tests to determine potential differences in ventilatory parameters between conditions were performed only after the initial analysis of variance (ANOVA) with repeated measures showed a significant effect (P < 0.05). Bonferroni's method for multiple comparisons was used as appropriate. Analyses were performed using SigmaStat software (SPSS Inc., Chicago, IL, USA). Data are presented as means ±s.e.m. unless indicated otherwise.
RESULTS
Ventilation at wake onset vs. ventilation at the same PET,CO2 in established wakefulness
Figure 1 shows an example of the changes in ventilation and PET,CO2 levels across experimental conditions. Note the normal decreases in ventilation and increases in PET,CO2 from wakefulness to non-REM sleep during room air breathing. Also note the stimulation of ventilation by raising inspired CO2 in wakefulness to achieve the same PET,CO2 as in sleep. Isocapnia is then maintained in non-REM sleep, and at wake onset there is a surge in ventilation produced by increases in respiratory rate and tidal volume. Importantly, the overall ventilation observed at wake onset from sleep in this example was greater than the ventilation produced in steady-state wakefulness for the same PET,CO2. Figure 1 also shows an example of the changes in heart rate across sleep-wake states and between experimental conditions. Note the surge in heart rate at arousal from sleep accompanying the increased ventilation.
Figure 1. Example of the changes in ventilation and PET,CO2 levels across experimental conditions.
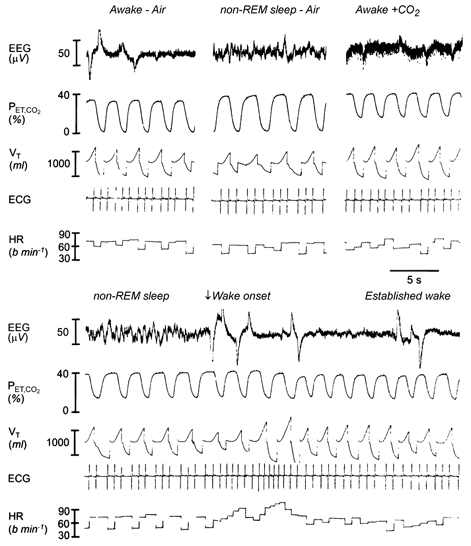
The left and middle traces in the upper panel show the normal decreases in lung ventilation and increases in PET,CO2 from wakefulness to non-REM sleep during room air breathing. Also note the stimulation of ventilation by CO2 added in wakefulness to raise PET,CO2 to the same level as previously observed in non-REM sleep (upper panel, right). The lower panel traces show that this same PET,CO2 is maintained in subsequent non-REM sleep by manipulating inspired CO2, and that wake onset is associated with a surge in ventilation produced by increased respiratory rate and tidal volume (VT). The overall level of ventilation observed at wake onset from non-REM sleep was greater than the ventilation produced in steady-state wakefulness for the same PET,CO2. The traces also show changes in the electrocardiogram (ECG) and instantaneous heart rate (HR) across sleep-wake states and between experimental conditions. Note the surge in heart rate at arousal from sleep, and the oscillations in heart rate with breathing due to sinus arrythmia. The large voltage artifacts on the EEG signal in wakefulness are due to eye movements.
During normal room air breathing in condition 1, the transition from non-REM sleep to wakefulness was associated with increases in ventilation (7.97 ± 1.16 to 9.68 ± 1.44 l min−1, P = 0.05). In condition 2 when CO2 was added in wakefulness (mean increase in PET,CO2= 2.60± 0.27 mmHg), ventilation increased in only four of the five dogs (7.82 ± 1.31 to 8.75 ± 0.99 l min−1, P = 0.069). The absence of a ventilatory response to CO2 in the one dog was confirmed in subsequent experiments over a wider range of CO2 concentrations. Nevertheless, the results from this dog are included with the others in the analyses.
By manipulating inspired CO2 levels in condition 3 (see Methods) we were able to keep the PET,CO2 levels in sleep at the same levels as those in established wakefulness (mean PET,CO2 awake = 37.9 ± 0.7 vs. 37.8 ± 0.8 mmHg in sleep, P = 0.564). ANOVA showed that application of tones at these matched PET,CO2 levels produced effects on ventilation that were different depending on whether the tones were applied in established wakefulness or non-REM sleep (F1,4= 14.31, P = 0.019). Post hoc t tests showed that ventilation increased significantly from non-REM sleep to wakefulness and averaged 1.42 ± 0.35 l min−1 (8.49 ± 0.81 to 9.91 ± 0.65 l min−1, P = 0.005, Fig. 2). However, the ventilation at wake onset from sleep after the tone was significantly larger than the ventilation in steady-state wakefulness at the same PET,CO2 (mean difference was 0.83 ± 0.28 l min−1, P = 0.031, Fig. 2, 9.91 ± 0.65 vs. 9.08 ± 0.91 l min−1). The relatively large error bars in Fig. 2 are due to differences in mean ventilation between dogs rather than variability within dogs. When the data were normalised between dogs by expressing ventilation as a percentage of that in established wakefulness, the consistency of responses is more apparent (Fig. 2B). As with the raw data, there still was a larger increase in ventilation at wake onset compared with the ventilation in established wakefulness at matched starting PET,CO2 values (Fig. 2B, F1,4= 11.554, P = 0.027 from ANOVA, P = 0.031 from post hoc t test).
Figure 2. Ventilation at wake onset from non-REM sleep exceeds the ventilation in established wakefulness at matched PET,CO2 levels.
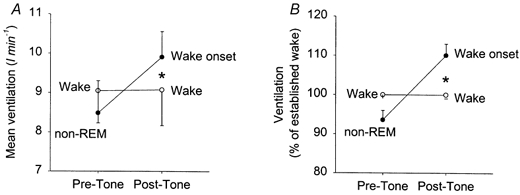
A, mean ventilation before and after application of the auditory tone in non-REM sleep (•) and established wakefulness (○) at matched PET,CO2 levels between conditions. Note that after the tone the ventilation at wake onset from sleep is larger than the ventilation in continuing established wakefulness (*P < 0.05). See text for further details. Data are plotted as means ±s.e.m. The size of the error bars reflects differences in mean values between animals (see Fig. 3). This is exemplified in B, which shows mean ventilation normalised in each dog for the average ventilation measured in established wakefulness.
This overall higher ventilation at wake onset from sleep compared with established wakefulness was due to faster respiratory rates (F1,4= 28.23, P = 0.006 from ANOVA, P = 0.042 from post hoc t test) rather than effects on tidal volume (F1,4 = 3.09, P = 0.154 from ANOVA). Application of the tone alone in established wakefulness produced no change in ventilation (mean difference = 0.03 ± 0.09 l min−1, Fig. 2).
Responses in individual dogs
Figure 3 shows individual data for each dog and illustrates that mean and peak ventilation were significantly larger at wake onset compared with established wakefulness at matched PET,CO2 levels. Even in the dog without a measured ventilatory response to CO2 (symbol ♦) there is a clear increase in ventilation at arousal from sleep (0.72 l min−1), and a larger mean and peak ventilation at wake onset compared with established wakefulness.
Figure 3. Increased ventilation at wake onset from non-REM sleep at a PET,CO2 level matched with steady-state wakefulness.
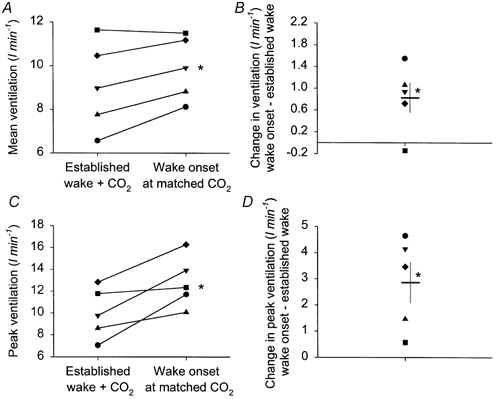
A, mean ventilation over 10 breaths produced by CO2 added in established wakefulness compared with the mean ventilation at wake onset from sleep at matched PET,CO2 levels. Paired data points are shown for each dog represented by a different symbol. B, difference in mean ventilation between established wakefulness and wake onset at matched PET,CO2 levels, i.e. the extra ventilation produced by arousal from sleep per se and not attributed to stimulation by CO2 according to current model. C and D, the same relationships for peak ventilation elicited in the 10 breaths following application of the tone in established wakefulness and wake onset at matched PET,CO2 levels. The means ±s.e.m. are also shown, *P < 0.05.
Time course of changes in ventilation at arousal from sleep
Figure 4A shows the breath-by-breath data illustrating the time course of the increased ventilation at arousal from sleep. There was a significant effect of breath number on lung ventilation following the tone (F10,39 = 2.34, P = 0.028, one-way ANOVA with repeated measures). Ventilation following arousal from sleep increased above the 95 % confidence interval of the preceding sleeping values on breaths 2-9 after the auditory stimulus. Breath 1 after the stimulus was slightly lower than the 95 % confidence interval of preceding sleeping breaths. Despite these significant changes in lung ventilation with breath number, there was no statistically significant effect of breath number on PET,CO2 (F10,39 = 1.88, P = 0.077, one-way ANOVA with repeated measures). Figure 4B shows that there was no statistically significant effect of breath number on lung ventilation or PET,CO2 when the tones were applied in continuous wakefulness (F10,39 > 2.03, both P > 0.05). These breath-by-breath data also showed that mean ventilation for breaths 2-9 after arousal from sleep were above the means for the corresponding breaths after the tone in established wakefulness (Fig. 4, P = 0.003, paired t test).
Figure 4. Time course of the increased ventilation at wake onset from sleep.
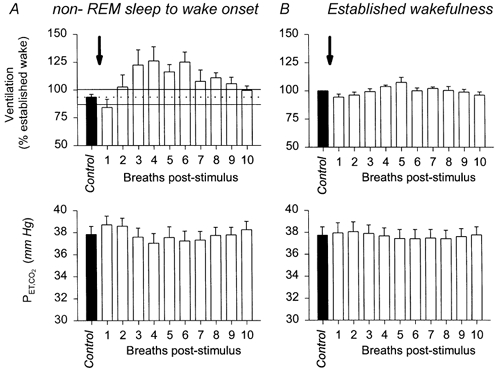
This figure shows group data (means ±s.e.m.) from five dogs. Ventilation is normalised in each dog to the average ventilation measured in established wakefulness (hence the absence of an error bar on the control values in B). A, transition from non-REM sleep to wake onset is associated with increased ventilation and transient reductions in Pa,CO2. The arrow shows the application of the auditory tone producing arousal from sleep. Filled columns indicate the grand mean of the 10 control breaths before the stimulus, and the open columns show the 10 experimental breaths following the auditory tone. The continuous and dotted lines show the mean ± 95 % confidence interval for the ventilation in non-REM sleep before arousal. Ventilation increases above sleeping levels on the second breath after wake onset. Also note that compared with established wakefulness the ventilation at wake onset from non-REM sleep exceeds the ventilation elicited by the same PET,CO2 levels when applied in steady-state established wakefulness (compare ventilation and PET,CO2 levels in A vs. B). B shows that application of the tone alone in established wakefulness caused no change in average ventilation and PET,CO2. See text for further details.
Effect of arousal from sleep on heart rate
During normal room air breathing in condition 1, the transition from non-REM sleep to wakefulness was associated with increases in heart rate (80.5 ± 8.1 to 90.5 ± 8.3 beats min−1, P = 0.039). The addition of CO2 in wakefulness in condition 2 did not significantly affect heart rate compared with room air (76.3 ± 5.3 vs. 83.6 ± 8.4 beats min−1, P = 0.372). ANOVA showed that application of tones at the matched PET,CO2 levels produced significant effects on heart rate depending on wakefulness or sleep (F1,4 = 63.991, P = 0.001). Post hoc t tests showed that heart rate increased following arousal from sleep (mean = 73.7 ± 4.8 to 88.4 ± 6.6 beats min−1, P = 0.0004), but did not change when the tone was applied in continuous wakefulness (76.3 ± 5.3 to 77.4 ± 5.5 beats min−1, P = 0.490). Heart rate after arousal from sleep was greater than in established wakefulness (P = 0.002).
DISCUSSION
An essential feature of the current model to explain the transient hyperpnoea at arousal from sleep is that the period of wakefulness immediately after wake onset is assumed to be functionally similar to subsequent wakefulness, with CO2 mechanisms being responsible for the hyperpnoea (Fig. 5). The results of the present study, however, show that the raised Pa,CO2 in sleep contributed less than 50 % of the increased ventilation elicited at wake onset, suggesting that mechanisms associated with arousal from sleep per se lead to independent stimulation of ventilation. There is accumulating evidence for a state of heightened brainstem arousal spontaneously generated at awakening from sleep which is then withdrawn in subsequent wakefulness (Aston-Jones & Bloom, 1981; Jacobs & Azmitia, 1992; Horner et al. 1997). Neuronal processes associated with arousal are thought to provide significant excitatory drives to the respiratory system and be the source of the ‘wakefulness stimulus’ to breathing (Phillipson & Bowes, 1986; Orem, 1994). As such, a component of the transient stimulation of ventilation at wake onset may result from heightened arousal processes producing transient facilitation of breathing. In this case, such increased excitatory drive to the respiratory system at wake onset would act in addition to the ventilatory stimulation elicited by differences in Pa,CO2. The dominant role played by CO2-independent mechanisms in stimulating ventilation at arousal from sleep highlights a fundamental, but necessary, revision of the previous model to explain the transient hyperpnoea at wake onset (Fig. 5), and has potentially important clinical implications for understanding the pathophysiology of breathing instability in sleep.
Figure 5. Previous and revised models to explain the transient hyperpnoea at wake onset from sleep.
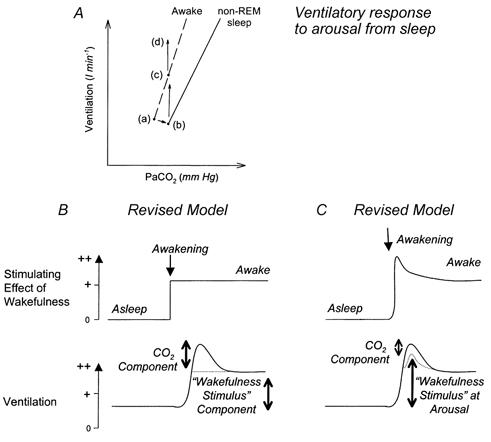
A, previous model used to explain the surge in ventilation upon awakening from sleep and revisions based on the results of the present study. Points a and b indicate the changes in Pa,CO2 and ventilation between wakefulness and non-REM sleep, respectively, and the dashed and continuous lines represent the ventilatory responses to CO2 in these two states. Upon awakening from sleep (at point b), the prevailing Pa,CO2 is initially hypercapnic for the levels normally encountered in wakefulness, and this discrepancy drives ventilation in accordance with the waking hypercapnic ventilatory response (point c). The results of the present study show that this sleeping CO2 level actually contributes < 50 % of the total ventilation elicited at wake onset from sleep (point d). B and C show alternative, but not mutually exclusive, conceptual models further illustrating the modifications to the current model to explain the transient hyperpnoea at awakening from sleep. In B, it is the difference in physiological control mechanisms between sleep and wakefulness, with an inappropriate level of CO2 at wake onset that produces the surge in ventilation at wake onset. In this scheme, the stimulatory effect of wakefulness (i.e. the wakefulness stimulus) is the same at wake onset compared with subsequent wakefulness. In C, it is the property of the awake state at wake onset that importantly contributes to respiratory activation by providing a transiently increased wakefulness stimulus. See text for further discussion. The magnitude and time constant of the depicted changes are arbitrary, and were chosen simply to highlight the differences between the two models.
It may be argued that chemoreflex sensitivity to CO2 may be increased at wake onset compared with subsequent wakefulness to account for the increased ventilation. Even if this were the case, however, to accept this possibility one still has to reject the major assumption of the current model that the period of wakefulness immediately after wake onset is similar to subsequent established wakefulness. That one dog showed no ventilatory response to inhaled CO2, but showed a clear hyperpnoea at arousal from sleep (symbol ♦ in Fig. 3), further argues against ventilatory responses to CO2 as being overly important in the transient hyperpnoea at wake onset.
Time course of ventilatory response to arousal from sleep
Ventilation increased above the 95 % confidence interval of the preceding sleeping values on the second breath after the point of awakening (Fig. 4). This time lag corresponds to approximately 2.4-4.8 s in this study, which also corresponds to the time to peak and the duration of the heart rate and blood pressure responses to arousal (Horner et al. 1995). Although the tones were well below the threshold to elicit startle (see Methods) they may have elicited an alerting or orienting response that could have transiently suppressed ventilation on the first breath (Anderssen et al. 1993) thereby delaying the hyperpnoea until the second breath. Such a slight decrease in ventilation was also observed when the tone was applied in continuous established wakefulness, although overall mean ventilation and heart rate did not change at this time. We do not think, however, that this first breath effect detracts from the main result because such an effect would bias against observing significant hyperpnoea at wake onset. Such an effect would also probably have led to an underestimation of the true hyperpnoea that would have occurred at awakening. Nevertheless, this brief hypoventilation may have produced a transient hypercapnic stimulus that could have subsequently stimulated ventilation and produced larger respiratory responses. However, such a hypercapnic stimulus could not account for the subsequent undershoot in Pa,CO2 (Fig. 4A) which must have resulted from a non-chemical drive to breathe. Nevertheless, that the tone itself can transiently modify breathing, albeit slightly, highlights the need to control for tone application and to not limit stimuli application just to sleeping periods as in previous studies (for example see Carlson et al. 1994; Morgan et al. 1996; Carley et al. 1997). Moreover, applying the stimuli in wakefulness at matched PET,CO2 levels was a requirement in our study to determine the causes of hyperpnoea at arousal and the underlying contribution of CO2 mechanisms.
Induced arousals from sleep vs. spontaneous arousals
Tones were used to elicit the arousals from sleep rather than waiting for spontaneous awakenings to occur so that a consistent stimulus to arousal from sleep could be applied at a consistent phase of the breathing cycle and at similar stages of non-REM sleep. Moreover, a required comparison in this study was that ventilation at wake onset be compared with periods of established wakefulness. Our use of auditory tones permitted time locking of the stimuli in these two states for such comparisons. Using stimuli that were time locked to ventilation also allowed for effects on a breath-breath basis to be analysed for subsequent comparisons within and between animals. Nevertheless, spontaneous awakenings from sleep may be different from arousals elicited by auditory tones. When recordings have been performed in cell groups involved in arousal, however, the only major difference appears to be that these neurons show a surge of activity following the induced arousal rather than preceding it as in spontaneous awakenings (Trulson & Jacobs, 1979; Aston-Jones & Bloom, 1981).
Previous studies have shown that the magnitude of the autonomic responses to awakening from sleep are related to the degree of arousal based on EEG and EMG criteria (Davies et al. 1993; Xie et al. 1994; Morgan et al. 1996). In the present study, the auditory stimuli consistently produced arousal from sleep with the EEG and EMG changes being of similar appearance between trials. As such, we did not have a sufficient range of arousal responses within a dog to correlate the potential degree of arousal with the ventilatory response. This consistency of arousal response was probably related to application of stimuli at a particular phase of the breathing cycle and at similar stages of non-REM sleep. However, comparisons of average and peak ventilation before and after the tones applied in both wakefulness and sleep was appropriate to test the main hypothesis of this study. Moreover, the consistencies in arousal responses following the tones in our study were advantageous in reducing intra- and intersubject variability to better control the experiment and improve statistical power. Following spontaneous arousals from sleep there was a similarly large increase in ventilation of 1.71 l min−1 compared with 1.42 l min−1 with induced arousals. However, it is uncertain with spontaneous awakenings if the internally generated stimulus to arousal is reproducible, whereas we are confident with tone-induced arousals that the stimulus is consistent. The less homogenous nature of spontaneous arousals from sleep made the change in ventilation more variable and of borderline statistical significance (P = 0.05) whereas the changes in ventilation with induced arousals were of similar magnitude but less variable.
Potential influences of the upper airway
Changes in upper airway resistance are important in mediating a component of the change in ventilation across sleep-wake states (Skatrud & Dempsey, 1985; Henke et al. 1992). Changes in upper airway resistance can also affect intrathoracic pressure changes associated with breathing, and lead to modulation of respiratory-related heart rate and blood pressure fluctuations (for example Daly, 1986; Novak et al. 1993). These effects of upper airway resistance on haemodynamics and ventilation can also themselves produce reflex alterations of both cardiovascular autonomic outflow and breathing (for example Daly, 1986; Seals et al. 1990). In the present study, however, the potentially confounding effects of changes in upper airway resistance at arousal from sleep were avoided because all experiments were performed with the upper airway bypassed and the dogs breathing via an endotracheal tube. In studies in subjects with intact upper airways such confounding influences affecting state-specific changes in ventilation cannot be discounted. Nevertheless, it is possible that the smaller difference in intrathoracic pressure swings between states of sleep and wakefulness due to the tracheotomy may have altered the haemodynamic changes between these two states and modified the measured ventilatory responses. Even if this were the case, however, this effect of tracheotomy would be consistent across all experimental conditions, and would probably not explain the different ventilation measured in both states of wakefulness that were the primary focus of this study, i.e. wake onset versus established wakefulness.
In our study, the endotracheal tube also provided direct attachment to the pneumotachograph for measurements of tidal volume. We chose not to use a facemask for these studies to avoid the potentially disturbing effects of a mask on the pattern of breathing and to avoid potential problems of leaks. Nevertheless, it is possible that by avoiding airflow and chemoreceptors in the upper airway we may have altered the measured ventilatory responses to CO2 (Boushey & Richardson, 1973; Sant'Ambrogio et al. 1983). However, we do not think that this possibility has significant effects on the present results because we have shown previously that upper airway receptors in adult dogs have little role in ventilatory control with or without added CO2 (Stradling et al. 1987). We have also shown that the minimal effect of the upper airway on ventilatory control was not due to long-term tracheotomy since ventilatory responses were not affected by the time post tracheotomy and upper airway responses to irritants were also unaffected (Stradling et al. 1987).
Acknowledgments
This work was supported by an operating grant (MT-4606) from the Medical Research Council (MRC) of Canada. R.L.H. is a recipient of an MRC of Canada Scholarship. The authors thank Dr Richard Stephenson (Department of Physiology, University of Toronto) for critical review of this manuscript.
References
- Anderssen SH, Nicolaisen RB, Gabrielsen GW. Autonomic response to auditory stimulation. Acta Paediatrica. 1993;82:913–918. doi: 10.1111/j.1651-2227.1993.tb12598.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. Journal of Neuroscience. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushey HA, Richardson PS. The reflex effects of intralaryngeal carbon dioxide on the pattern of breathing. Journal of Physiology. 1973;228:181–191. doi: 10.1113/jphysiol.1973.sp010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley TD, Phillipson EA. Sleep disorders. In: Murray JF, Nadel JA, editors. Textbook of Respiratory Medicine. Philadelphia, PA, USA: W. B. Saunders; 2000. pp. 2153–2169. [Google Scholar]
- Carley DW, Applebaum R, Basner RC, Önal E, Lopata M. Respiratory and arousal responses to acoustic stimulation. Chest. 1997;112:1567–1571. doi: 10.1378/chest.112.6.1567. [DOI] [PubMed] [Google Scholar]
- Carlson DM, Carley DW, Önal E, Lopata M, Basner RC. Acoustically induced cortical arousal increases phasic pharyngeal muscle and diaphragmatic EMG in NREM sleep. Journal of Applied Physiology. 1994;76:1553–1559. doi: 10.1152/jappl.1994.76.4.1553. [DOI] [PubMed] [Google Scholar]
- Daly M de Burgh. Interactions between respiration and circulation. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, section 3The Respiratory System, Control of Breathing. part 2. Vol. 2. Bethesda, MD, USA: American Physiological Society; 1986. pp. 529–594. [Google Scholar]
- Davies RJO, Belt PJ, Roberts SJ, Ali NJ, Stradling JR. Arterial blood pressure responses to graded transient arousal from sleep in normal humans. Journal of Applied Physiology. 1993;74:1123–1130. doi: 10.1152/jappl.1993.74.3.1123. [DOI] [PubMed] [Google Scholar]
- Henke KG, Badr MS, Skatrud JB, Dempsey JA. Load compensation and respiratory muscle function during sleep. Journal of Applied Physiology. 1992;72:1221–1234. doi: 10.1152/jappl.1992.72.4.1221. [DOI] [PubMed] [Google Scholar]
- Horner RL, Brooks D, Kozar LF, Tse S, Phillipson EA. Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. Journal of Applied Physiology. 1995;79:151–162. doi: 10.1152/jappl.1995.79.1.151. [DOI] [PubMed] [Google Scholar]
- Horner RL, Sanford LD, Pack AI, Morrison AR. Activation of a distinct arousal state immediately after spontaneous awakening from sleep. Brain Research. 1997;778:126–133. doi: 10.1016/s0006-8993(97)01045-7. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiological Reviews. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Kimoff RJ, Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Champagne V, Mayer P, Phillipson EA. Ventilatory and arousal responses to hypoxia and hypercapnia in a canine model of obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine. 1997;156:886–894. doi: 10.1164/ajrccm.156.3.9610060. [DOI] [PubMed] [Google Scholar]
- Morgan BJ, Crabtree DC, Puleo DS, Badr MS, Toiber F, Skatrud JB. Neurocirculatory consequences of abrupt change in sleep-state in humans. Journal of Applied Physiology. 1996;80:1627–1636. doi: 10.1152/jappl.1996.80.5.1627. [DOI] [PubMed] [Google Scholar]
- Novak V, Novak P, De Champlain J, Le Blanc AR, Martin R, Nadeau R. Influence of respiration on heart rate and blood pressure fluctuations. Journal of Applied Physiology. 1993;74:617–626. doi: 10.1152/jappl.1993.74.2.617. [DOI] [PubMed] [Google Scholar]
- Orem J. Respiratory neurons and sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia, PA, USA: W. B. Saunders; 1994. pp. 177–193. [Google Scholar]
- Phillipson EA, Bowes G. Control of breathing during sleep. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, section 3 The Respiratory System, Control of Breathing. part 2. Vol. 2. Bethesda, MD, USA: American Physiological Society; 1986. pp. 649–689. [Google Scholar]
- Phillipson EA, Hickey RF, Bainton CR, Nadel JA. Effect of vagal blockade on regulation of breathing in conscious dogs. Journal of Applied Physiology. 1970;29:475–479. doi: 10.1152/jappl.1970.29.4.475. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio G, Mathew OP, Fisher JT, Sant'Ambrogio FB. Laryngeal receptors responding to transmural pressure, airflow and local muscle activity. Respiration Physiology. 1983;54:317–330. doi: 10.1016/0034-5687(83)90075-0. [DOI] [PubMed] [Google Scholar]
- Seals DR, Suwarno NO, Dempsey JA. Influence of lung volume on sympathetic nerve discharge in normal humans. Circulation Research. 1990;67:130–141. doi: 10.1161/01.res.67.1.130. [DOI] [PubMed] [Google Scholar]
- Simon PM, Skatrud JB, Badr MS, Griffin DM, Iber C, Dempsey JA. Role of airway mechanoreceptors in the inhibition of inspiration during mechanical ventilation in humans. American Review of Respiratory Disease. 1991;144:1033–1041. doi: 10.1164/ajrccm/144.5.1033. [DOI] [PubMed] [Google Scholar]
- Skatrud JB, Dempsey JA. Airway resistance and respiratory muscle function in snorers during NREM sleep. Journal of Applied Physiology. 1985;59:328–335. doi: 10.1152/jappl.1985.59.2.328. [DOI] [PubMed] [Google Scholar]
- Stradling JR, England SJ, Harding R, Kozar LF, Andrey S, Phillipson EA. Role of upper airway in ventilatory control in awake and sleeping dogs. Journal of Applied Physiology. 1987;62:1167–1173. doi: 10.1152/jappl.1987.62.3.1167. [DOI] [PubMed] [Google Scholar]
- Trinder J, Padula M, Berlowitz D, Kleiman J, Breen S, Rochford P, Worsnop C, Thompson B, Pierce R. Cardiac and respiratory activity at arousal from sleep under controlled ventilation conditions. Journal of Applied Physiology. 2001;90:1455–1463. doi: 10.1152/jappl.2001.90.4.1455. [DOI] [PubMed] [Google Scholar]
- Trinder J, Whitworth F, Kay A, Wilkin P. Respiratory instability during sleep onset. Journal of Applied Physiology. 1992;73:2462–2469. doi: 10.1152/jappl.1992.73.6.2462. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Research. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Xie A, Wong B, Phillipson EA, Slutsky AS, Bradley TD. Interaction of hyperventilation and arousal in the pathogenesis of idiopathic central sleep apnea. American Journal of Respiratory and Critical Care Medicine. 1994;150:489–495. doi: 10.1164/ajrccm.150.2.8049835. [DOI] [PubMed] [Google Scholar]


