Abstract
In 67 single motor units, the mechanical properties, the recruitment and derecruitment thresholds, and the discharge rates were recorded concurrently in the first dorsal interosseus (FDI) of human subjects during intermittent fatiguing contractions. The task consisted of isometric ramp-and-hold contractions performed at 50% of the maximal voluntary contraction (MVC). The purpose of this study was to examine the influence of fatigue on the behaviour of motor units with a wide range of activation thresholds.
For low-threshold (< 25% MVC) motor units, the mean twitch force increased with fatigue and the recruitment threshold either did not change or increased. In contrast, the twitch force and the activation threshold decreased for the high-threshold (> 25% MVC) units. The observation that in low-threshold motor units a quick stretch of the muscle at the end of the test reset the unit force and recruitment threshold to the prefatigue value suggests a significant role for fatigue-related changes in muscle stiffness but not twitch potentiation or motor unit synchronization.
Although the central drive intensified during the fatigue test, as indicated by an increase in surface electromyogram (EMG), the discharge rate of the motor units during the hold phase of each contraction decreased progressively over the course of the task for motor units that were recruited at the beginning of the test, especially the low-threshold units. In contrast, the discharge rates of newly activated units first increased and then decreased.
Such divergent behaviour of low- and high-threshold motor units could not be individually controlled by the central drive to the motoneurone pool. Rather, the different behaviours must be the consequence of variable contributions from motoneurone adaptation and afferent feedback from the muscle during the fatiguing contraction.
Changes in the mechanical properties of human motor units due to fatigue have been studied in the medial gastrocnemius with intramuscular microstimulation (Garnett et al. 1979) and in the triceps brachii (Thomas, 1995) and finger muscles (Thomas et al. 1991; Fuglevand et al. 1999) with intraneural stimulation. Other experiments have used the ‘spike-triggered averaging’ method (Milner-Brown et al. 1973) to record the mechanical motor unit response during low-force voluntary contractions in the first dorsal interosseus (FDI) and in the masseter (Nordstrom & Miles, 1990). Whereas some studies (Stephens & Usherwood, 1977; Garnett et al. 1979) showed that low-threshold motor units are more fatigue resistant than high-threshold ones, more recent studies have not found such a correlation (Nordstrom & Miles, 1990; Thomas et al. 1991; Fuglevand et al. 1999).
In addition to alterations in the contractile properties of motor units during fatigue, changes in muscle force may result from a variation in the number of motor units recruited or in the modulation of their firing rates. This is particularly true for submaximal contractions during which the muscle force can be maintained at a constant level. This occurs either by a change in the discharge frequency of the active units or by the recruitment of additional motor units, despite the progressive contractile alteration of the activated motor units. Despite a consensus in the literature that motor units are progressively recruited during sustained submaximal contractions (Person & Kudina, 1972; Bigland-Ritchie et al. 1986a; Maton & Gamet, 1989; Enoka et al. 1989; Dorfman et al. 1990; Christova & Kossev, 1998), the modulation of discharge rate appears to be variable and to depend upon the intensity and duration of the fatiguing contraction. Motor unit discharge frequency has been observed to decrease (Person & Kudina, 1972; Gantchev et al. 1986; Gatev et al. 1986; Garland et al. 1994), to increase (Bigland-Ritchie et al. 1986a; Dorfman et al. 1990), and to remain constant (Maton & Gamet, 1989).
Another mechanism that contributes to the loss of force during fatiguing contractions is the derecruitment of motor units. The inability of some motor units to discharge continuously in the presence of sustained central drive has been observed during maximal contractions (Peters & Fuglevand, 1999) and has been suggested to occur during submaximal contractions (Enoka et al. 1989; Garland et al. 1994). However, this effect probably only occurs in high-threshold motor units (Kernell & Monster, 1982b).
The absence of consistent observations on motor unit activity during fatiguing contractions appears to be due to two factors: an inadequate sample size (Thomas, 1995; Fuglevand et al. 1999; Enoka & Fuglevand, 2001) and a failure to consider concurrent changes in motor unit behaviour and contractile function. The present investigation was designed to study the behaviour (the recruitment and derecruitment thresholds and the discharge rate) and contractile function of single motor units with a wide range of recruitment thresholds, during fatigue induced by intermittent voluntary contractions at 50 % MVC in the human FDI muscle.
METHODS
Subjects and experimental set-up
Thirty-seven experiments were performed on eight healthy subjects (4 female and 4 male) aged between 21 and 41 years. The subjects were tested on several occasions (from 3 to 11) separated by at least 1 week. Most were staff members and were very familiar with the experimental procedure. This study was approved by the University Ethics Committee and the subjects gave informed consent prior to participation in the investigation. All the experimental procedures were performed in accordance with the Declaration of Helsinki.
Each subject was seated on a chair with the elbow abducted to a horizontal plane and with the palmar side of the left hand turned downwards. The elbow joint was flexed at 90 deg and the upper arm was in a frontal plane. The hand and forearm rested on a support and were immobilized by several restraints with the thumb fully abducted, the last three fingers strapped together with a velcro band, and the proximal interphalangeal joint of the index finger aligned with the force transducer.
EMG and mechanical recordings
Motor unit action potentials were recorded by a selective electrode made up of 50 μm diamel-coated nichrome wires inserted into the muscle by means of a hypodermic needle (Duchateau & Hainaut, 1990). The electrode was inserted through the skin in the middle part of the muscle belly and located at different depths from session to session. The signals were amplified by a custom-made differential amplifier and filtered (100 Hz to 10 kHz) before being displayed on a Tektronix TAS 455 oscilloscope. The surface EMG was recorded by means of two silver disk electrodes (8 mm in diameter) placed 2 cm apart on either side of the needle electrode. The ground electrode (silver plate of 2 cm × 3 cm) was located on the dorsal side of the wrist. The surface EMG signal was amplified (× 1000) and filtered (10 Hz to 5 kHz) by a custom-made differential amplifier.
The isometric abduction force produced by the FDI was measured with a strain-gauge transducer (TC 2000-50, Kulite, Basingstoke, UK) and the signal was amplified (AM 502, Tektronix, Beaverton, OR, USA; bandwith DC to 300 Hz). The sensitivity of the force transducer was 55 mV N−1 (linear range, 0-220 N). The mechanical recording of the single motor units was assessed by the spike-triggered averaging method (Milner-Brown et al. 1973). Briefly, this method consists of triggering the sweep of an averager with the action potential of the selected unit during a steady contraction, and recording the corresponding filtered (0.1 Hz to 100 Hz) isometric force. The contribution of the motor unit to the net force was then extracted by means of averaging. Because a low steady firing rate of the motor unit was necessary to avoid summation of the mechanical responses (Milner-Brown et al. 1973), the subjects were provided with visual and auditory feedback. A hardware rate limiter was also used to average the mechanical responses from the same motor unit, a process that separated the individual responses by at least 100 ms (Duchateau & Hainaut, 1990).
Experimental procedure and data analysis
The fatigue protocol consisted of intermittent isometric contractions at 50 % MVC, a level at which nearly all the motor units of the FDI are activated (Milner-Brown et al. 1973; De Luca et al. 1996). To determine the maximal voluntary torque produced by the muscle, each experimental session began with the subject performing three MVCs of 4-5 s duration separated by 2-3 min pauses. The motor units were subsequently identified by means of a template-matching algorithm program (see below) from records obtained during two or three isometric ramp contractions. Once motor unit action potential(s) had been clearly identified (usually 1-3), the subject maintained for 0.5-1 min a level of torque for each motor unit that was just sufficient to sustain the discharge frequency at a constant low rate (7-10 Hz). In the high-threshold motor units, the contraction was interrupted by one or two small pauses to minimize fatigue. These data were collected for the purpose of mechanical recording of single motor units by the spike-triggered averaging.
After the prefatigue recordings, the fatigue task was performed. It consisted of the subjects following a target on the screen of an oscilloscope and reaching 50 % MVC in 3 s, maintaining this level for 10 s, and returning slowly to the baseline in 3 s. A rest period of 4 s was allowed between two successive contractions. This protocol, which was very similar to the one proposed by Enoka et al. (1989), was repeated 3 times min−1 until the subject was unable to maintain the required level of force for three successive contractions (the endurance limit). When a motor unit had been successfully tracked throughout the fatigue test, its discharge during sustained threshold contraction was again collected using the same procedure and for the same purpose as in the prefatigue condition. For some motor units, the recovery from fatigue was tested 15 and 30 min after the fatigue test by recording its discharge during sustained threshold contraction and recruitment threshold during ramp contractions. In other experiments, the muscle was stretched by maximal adduction of the index finger for 15 s after the post-fatigue recordings, and the discharge during sustained threshold contraction and recruitment threshold of the motor unit were again recorded.
Data processing was performed off-line from taped records (Vetter 620, Rebersburg, PA, USA or Sony PCM-DAT, DTR 800, Bio-Logic, Claix, France). The signals recorded during the fatigue tests were digitized at a frequency of 10 kHz and analysed with AcqKnowledge 3.2.4 software (BIOPAC Systems Inc., Santa Barabara, CA, USA). The recruitment and derecruitment thresholds were determined during each ramp contraction for the selected motor units. The recruitment threshold was defined as the torque at the which the motor unit began to fire during the increasing phase of the contraction, whereas the derecruitment threshold was defined as the torque level corresponding to the last pulse of this unit during the decreasing phase of the contraction. In addition, the mean discharge frequency of each unit was measured during the force plateau and only units firing continuously with a minimum of 20 pulses were analysed. Motor unit discrimination was accomplished using a computer-based, template-matching algorithm (Signal Processing systems, SPS 8701, Malvern, Victoria, Australia). Action potentials of single motor units were identified on the basis of amplitude and waveform shape. Only the motor units that were clearly identifiable for the entire course of the fatigue test were included in the analysis. The mechanical recording of a single motor unit was performed by spike-triggered averaging with a digital oscilloscope (model 4094c, Nicolet, Madison, WI, USA, sampling rate: 20 kHz). Usually, 50-200 sweeps were averaged, depending upon motor unit size. For large units (recruitment threshold > 25 % MVC), 50 sweeps were often sufficient. The peak amplitude, the time to peak, and the time to half-relaxation of the mechanical response were measured for each unit.
The significance of the changes in the different parameters during fatigue and recovery was assessed either using Student's paired t test or repeated-measures analysis of variance (ANOVA). When significant main effects were found with an ANOVA, the Newman-Keuls test was used to locate the significant differences between the means. In the text, values are given as means ±s.e.m. and a significance level of P < 0.05 was used for all statistical comparisons.
RESULTS
The behaviour of single motor units was tracked during a fatigue task consisting of a subject performing a ramp-and-hold isometric contraction at 50 % MVC until exhaustion. The average abduction force during the task was 26.8 ± 1.7 N (mean ±s.e.m.) and the endurance limit ranged from 6 to 16 min, with a mean value of 8.38 ± 3.55 min. A typical performance of a subject during the fatigue task is illustrated by Fig. 1. The main observation is that while the subject attempted to maintain a constant force during the successive contractions, the task was accomplished with the recruitment of additional motor units and changes in the activation pattern of the motor unit (recruitment threshold and discharge rate). These adaptations were accompanied by a 31.7 % (P < 0.05) increase in the surface EMG during the fatigue test (419 ± 158 and 583 ± 220 μV at the onset and end of the fatigue test, respectively). Nonetheless, at the endurance limit the EMG was still significantly lower (mean deficit: 39.1 %; P < 0.05) compared with that recorded during the prefatigue MVC (934 ± 353 μV).
Figure 1. A typical performance by one subject during the intermittent fatiguing contraction.
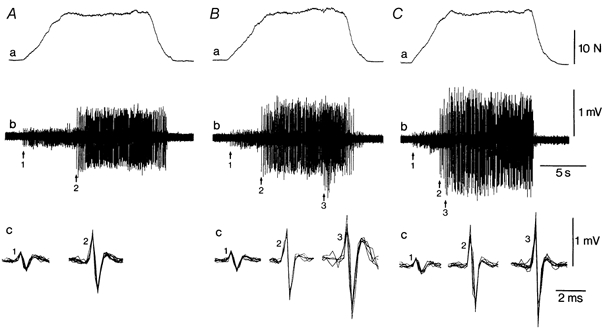
Force (a) and intramuscular EMG (b and c) are shown for the first (A), 16th (B) and 43rd (C) contractions of the test (endurance limit, 48 contractions). In trace c, 5-10 discharges of the identified units are superimposed with an expanded display. Two different units (1 and 2) were identified during the first contraction (A) and three units were discriminated during the 16th and 43rd contractions (1, 2 and 3). The recruitment threshold of unit 2 was earlier in the 16th contraction (B) compared with the first contraction (A). Similarly, unit 3 was recruited earlier in the 43rd contraction (C) compared with the 16th contraction.
Recruitment and derecruitment threshold forces
Sixty-seven motor units with recruitment thresholds ranging from 0.5 to 55.2 % MVC (26.2 ± 2.0 % MVC; 7.1 ± 0.7 N) were analysed during the fatigue task. Recruitment order was unchanged during the fatiguing contractions, but the average recruitment thresholds in absolute values declined by a mean value of 9.6 ± 1.3 % (P < 0.001). However, as illustrated in Fig. 2A for one subject, the decrease in recruitment threshold was greater for high-threshold units compared with low-threshold ones (see also Table 1). Across all subjects, eight motor units that had a low recruitment threshold (4.9 ± 1.9 % MVC) showed a small threshold increase (+3.9 ± 1.5 %; P < 0.05; Fig. 2B). This behaviour is shown in Fig. 2A for one low-threshold motor unit (•) but not for another (⋄).
Figure 2. Change in the recruitment thresholds of motor units during the fatigue task and the recovery period.
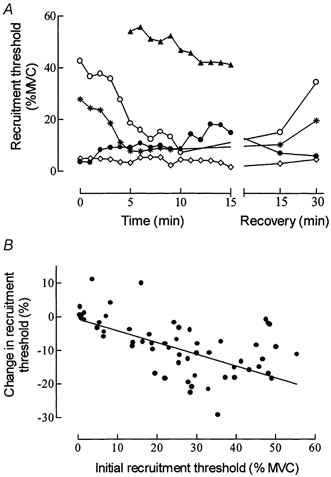
A, behaviour of 5 units with different recruitment thresholds in one subject, expressed in % MVC. Note that one unit (▴) was not recruited until 5 min into the contraction, whereas another unit (○) stopped discharging after 10 min until the recovery period. B, relationship for all subjects between the relative change in recruitment threshold (percentage of prefatigue values) as a function of the initial recruitment threshold (% MVC). The equation for the regression line is y = -0.35x - 0.61 (r = -0.57; n = 60; P < 0.001).
Table 1.
Motor unit mechanical properties, recruitment threshold and discharge rate in control and fatigue conditions
| Recruitment threshold < 25% MVC | Recruitment threshold 25-−50% MVC | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Fatigue | n | P | Control | Fatigue | n | P | |
| Force (mN) | 24.6 ± 4.7 | 32.7 ± 4.4 | 39 | 0.05 | 56.2 ± 10.0 | 31.4 ± 5.0 | 17 | 0.01 |
| (1−124) | (3−131) | (9−158) | (7−92) | |||||
| Time to peak (ms) | 43.8 ± 2.1 | 53.1 ± 3.2 | 39 | 0.01 | 52.5 ± 3.1 | 42.9 ± 2.4 | 17 | 0.01 |
| (25−78) | (29−105) | (32−75) | (30−62) | |||||
| Time to half-relaxation (ms) | 35.3 ± 4.0 | 39.5 ± 3.0 | 29 | n.s. | 43.2 ± 4.8 | 35.0 ± 3.0 | 10 | 0.06 |
| (10−70) | (17−62) | (22−58) | (28−48) | |||||
| Recruitment threshold (% MVC) | 11.6 ± 1.6 | 8.0 ± 1.3 | 33 | 0.01 | 35.4 ± 1.4 | 19.6 ± 2.2 | 27 | 0.001 |
| (0.5−25.0) | (0.5−22.7) | (25.5−48.2) | (4.8−33.2) | |||||
| Discharge rate (Hz) | 24.7 ± 1.7 | 18.6 ± 1.4 | 16 | 0.01 | 18.0 ± 1.4 | 15.4 ± 1.6 | 15 | n.s. |
| (17.7−32.1) | (11.1−26.7) | (7.4−28.1) | (8.7−24.8) | |||||
Values are means ±s.e.m. with the range given in parentheses.
The derecruitment threshold measured during the ramp-down phase of the contraction (Fig. 1) was studied in 56 motor units and showed an average increase of 7.1 ± 1.6 % (P < 0.001) by the end of the fatigue test. Because recruitment and derecruitment thresholds are influenced by the rate of contraction (Desmedt & Godaux, 1978), we compared the rates of force increase and decrease at the beginning and end of the fatiguing contraction (mean of 3 contractions). Whereas the rate of force development did not differ (P > 0.05) across all the experiments (14.4 ± 0.6 % MVC s−1 at the beginning compared with 15.5 ± 0.7 % MVC s−1 at the end of the test), the rate of force decrease was slightly (P < 0.05) faster at the end of the fatigue test (21.4 ± 1.1 compared with 16.5 ± 1.2 % MVC s−1).
We were not able to track all the single motor units during the entire fatiguing contraction. Although some motor units were probably lost due to electrode movement, others were derecruited because they reappeared during the recovery period (see Fig. 2A). Most of these units had high recruitment thresholds that declined substantially during the fatiguing contraction. Conversely, other motor units were recruited during the fatigue test (Fig. 2A). Across all experiments, we clearly identified seven high-threshold units with mean recruitment thresholds of 46.1 ± 3.7 % MVC that were not activated at the beginning of the task, but were recruited as the fatiguing contraction progressed. These units were activated at 33.9 ± 6.4 % (range 8-53 %) of the endurance limit and showed a systematic and progressive decrease in recruitment thresholds (Fig. 2A).
In 18 motor units, the recovery from fatigue was tested during ramp contractions at the same rate of force development as during the fatigue task. As illustrated in Fig. 2A, the recruitment thresholds of these units returned to initial values within 15-30 min.
Motor unit discharge pattern
The discharge pattern of single motor units was analysed during the force plateau of the intermittent contractions. Thirty-one motor units were tracked accurately throughout the fatigue test. Some units were not analysed because they were only activated during the ramp contractions and either stopped discharging during the holding phase of the contraction (n = 6) or discharged too irregularly (n = 12). Figure 3 shows the typical behaviour of three motor units with different recruitment thresholds over the entire fatigue task. Low- and high-threshold units that were recruited at the beginning of the test showed a systematic and progressive decrease in discharge rate, but this was only significant (P < 0.05) for the low-threshold motor units (Fig. 3A and Fig. 4; Table 1). Conversely, the motor units that were recruited later in the test (recruitment threshold > 50 % MVC) displayed an initial increase (P < 0.05) in the discharge rate followed by a decrease (Fig. 3B and Fig. 4C). Once involved in the fatiguing contraction, however, these units started to fire progressively earlier in successive contractions (Fig. 1).
Figure 3. Change in the recruitment threshold and discharge rate of motor units recruited at different force levels during the fatigue task.
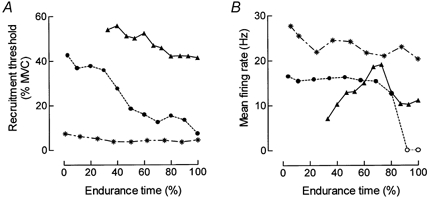
A, changes in recruitment threshold (% MVC) as a function of the endurance limit for 3 units with different activation thresholds: *, 7.4 %; •, 42 %; and ▴, 54.1 % of MVC. B, change in the discharge pattern of the same 3 units as a function of the endurance limit. Discharge rate corresponds to the average during the force plateau and each value indicates the mean value of three successive contractions. The circles (○) indicate that the unit was only activate during the ramp-up phase of the contraction and not during the plateau.
Figure 4. Changes in discharge rates in motor units with different recruitment thresholds.
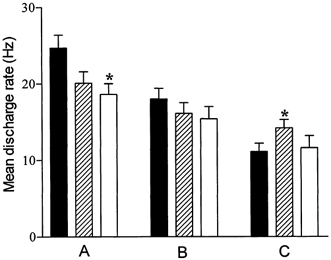
The average (mean ±s.e.m.) discharge rates during the force plateau are reported during contractions at the onset (▪), in the middle ( ) and at the end (□) of the fatigue task, for motor units with a recruitment threshold < 25 % MVC (A) and 25-50 % MVC (B). For motor units recruited during the course of the fatigue task (C; recruitment threshold > 50 % MVC), the discharge rates correspond successively to those recorded at the onset of activation, the highest rate observed during the test, and that recorded at the end of the fatigue task. * Significant difference compared with prefatigue condition (P < 0.05).
) and at the end (□) of the fatigue task, for motor units with a recruitment threshold < 25 % MVC (A) and 25-50 % MVC (B). For motor units recruited during the course of the fatigue task (C; recruitment threshold > 50 % MVC), the discharge rates correspond successively to those recorded at the onset of activation, the highest rate observed during the test, and that recorded at the end of the fatigue task. * Significant difference compared with prefatigue condition (P < 0.05).
Motor unit mechanical properties
The mechanical responses for all the motor units (n = 56) did not differ (P > 0.05) before or after the fatiguing contraction. The mean amplitude of the mechanical responses was 34.2 ± 4.9 mN (range, 1-158 mN) before and 32.3 ± 3.4 mN (range, 3-131 mN) after the fatigue task. The time to peak force (46.6 ± 1.8 and 49.7 ± 2.4 ms, respectively) and the time to half-relaxation (37.2 ± 3.3 and 38.4 ± 2.5 ms, respectively) were also unchanged (P > 0.05) for the whole population of motor units.
However, motor units with recruitment thresholds < 25 % of prefatigue MVC showed a significant (P < 0.05) increase in force whereas those with recruitment thresholds > 25 % MVC showed a significant (P < 0.01) decrease in force (Fig. 5 and Table 1). There was a linear relationship (r = 0.52; P < 0.01) between the relative change in force and the recruitment threshold (Fig. 6). These changes in force were associated with changes in the time to peak force but not the time to half-relaxation. For the low-threshold units, the time to peak force increased (P < 0.01) with fatigue whereas it decreased significantly for the high-threshold units (Table 1). All these changes were obtained without any difference (P > 0.05) in the mean motor unit discharge rate during spike-triggered averaging before (8.5 ± 0.5 Hz) and after (8.4 ± 0.4 Hz) the fatigue test.
Figure 5. Mechanical responses recorded by spike-triggered averaging, before and after fatigue.
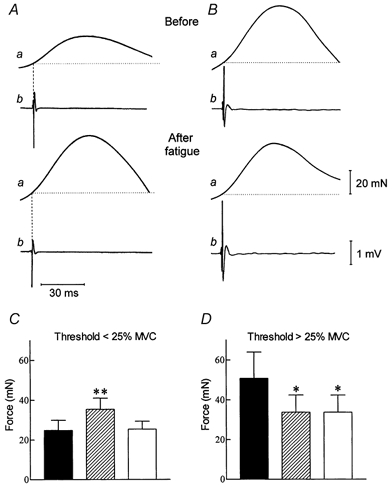
Comparison of the mechanical (a) and electrical (b) responses of 2 motor units with different recruitment thresholds (A: 9 % MVC and B: 41 % MVC), recorded before and after the fatigue task in one subject. Each trace represents 110-140 averaged sweeps. C and D, histograms showing the mean (±s.e.m.) motor unit force, before (▪) and immediately after the fatigue test ( ), and after the muscle has been stretched in the post-fatigue condition (□). * and ** denote significant differences with the prefatigue condition at P < 0.05 and P < 0.01, respectively.
), and after the muscle has been stretched in the post-fatigue condition (□). * and ** denote significant differences with the prefatigue condition at P < 0.05 and P < 0.01, respectively.
Figure 6. Relationship between the relative change in force and in recruitment threshold (both expressed as a percentage of prefatigue values) of motor units at the end of the fatigue task.
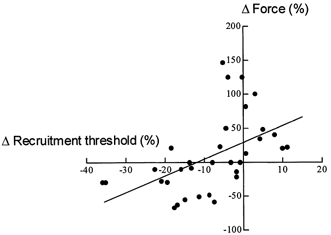
The regression line (y = 2.47x+ 29.4) indicates a significant association between the changes in force and recruitment threshold of motor units during fatigue (r = 0.52; P < 0.01).
Effects of muscle stretch after the fatiguing contraction
For 39 motor units, the muscle was passively stretched by adduction of the index finger for about 15 s after the post-fatigue recording of their mechanical properties (Fig. 5C-D). Immediately after the stretching manoeuvre, the motor unit recruitment threshold and the mechanical properties were again tested. For the motor units with a recruitment threshold < 25 % MVC (n = 30), muscle stretch induced a significant (P < 0.05) decrease in motor unit force from 35.4 ± 5.6 to 25.4 ± 4.0 mN (Fig. 5C), whereas there was no significant change for the motor units (n = 9) recruited > 25 % MVC (33.8 ± 8.6 vs. 33.8 ± 8.5 mN; Fig. 5D). In addition, muscle stretch reset the recruitment threshold of low-threshold motor units from 6.7 ± 2.4 to 3.4 ± 1.3 % MVC, a value that was not significantly different (P > 0.05) from the prefatigue value (3.5 ± 1.3 % MVC).
DISCUSSION
In the present experiments, the mechanical properties, the recruitment and derecruitment thresholds, and the discharge frequencies were recorded concurrently in single motor units during intermittent fatigue tests performed at 50 % MVC. A large number of motor units, including those with low and high recruitment thresholds, were studied in the FDI of human subjects. The behaviour of each motor unit was recorded throughout the fatigue test. The main finding in this study was that although the central drive intensified during the fatiguing contraction, as indicated by the recruitment of motor units and an increase in the surface EMG, motor unit behaviour differed as a function of recruitment threshold. These findings suggest that motor unit activity during a fatiguing contraction could not be individually controlled by the central drive, but must also be influenced by motoneurone adaptation and afferent feedback from the active muscle.
The FDI was selected for this study because motor unit recruitment is nearly complete at 50 % MVC (Milner-Brown et al. 1973; De Luca et al. 1996). This permitted the study of the entire motor unit population. Furthermore, the intermittent fatigue task was used to allow recovery of the electrical changes in the membrane of the muscle fibres (Duchateau & Hainaut, 1985). This enabled reliable recognition of the motor unit action potential and the recording of time-related changes in threshold (Enoka et al. 1989).
Changes in motor units contractile properties and thresholds forces
The motor units that were activated throughout the fatigue tests showed different changes that were related to their recruitment thresholds. Because the rate of force development did not change during the fatiguing contraction, changes in recruitment threshold should be related to a neural adaptation strategy rather than to a force-rate related change in threshold (Desmedt & Godaux, 1978). Whereas the low-threshold motor units showed either no change or an increase in recruitment threshold, the high-threshold ones were activated at lower forces by the end of the fatigue test. The observation of a non-systematic change in threshold force for the low-threshold motor units during intermittent contractions confirms previous works in the same muscle (Enoka et al. 1989) and extends this finding to higher threshold motor units. All these changes are transient, and recovered within 15-30 min following the test. As observed in the study of Enoka et al. (1989), the derecruitment threshold increased in most of the motor units with fatigue. However, it should be noted that the initial rate of muscle force decrease could not be maintained by the subjects at the end of the fatigue test, and that these data should be interpreted with caution.
Changes in the mechanical motor unit properties, recorded by the spike-triggered averaging method (Milner-Brown et al. 1973), also differed for units with low- and high-recruitment thresholds. The motor units activated below 25 % MVC increased their contractile force, an observation which is consistent with other studies (Stephens & Usherwood, 1977; Nordstrom & Miles, 1990; Thomas et al. 1991). In contrast, motor units recruited above 25 % MVC experienced a decrease in force. These results agree with the observation that after fatigue the motor units with an enhanced contractile force also showed an increase in recruitment threshold, whereas motor units with a decreased force went in the other direction. Since motor unit recruitment order was not modified during fatigue (see Fig. 1), the relationship between the changes in recruitment threshold and in recorded force (Fig. 6) suggests that the central nervous system adapts the recruitment threshold of motor units as a function of the actual level of their force.
The difference in motor unit force before and after fatigue cannot be due to a change in the recording conditions because the discharge rate during the spike-triggered averaging was similar. Instead, change must be a consequence of the fatiguing contraction. The increase in motor unit force during fatigue could be explained either by force potentiation (Olson & Swett, 1971) or by enhanced muscle stiffness (Suzuki et al. 1990). This has been suggested as being related to residual cross-bridges in the intra- and extrafusal muscle fibres after a previous contraction (Hagbarth et al. 1985). Consistent with the latter proposition, we found that quickly stretching the muscle at the end of the test immediately reset the force and recruitment threshold of low-threshold motor units, but did not influence the high-threshold ones, which experienced a decrease in force and recruitment threshold during fatigue. In contrast, such a manoeuvre has no effect on the twitch potentiation during staircase phenomenon (J. Duchateau & K. Hainaut, unpublished observation). The effect of muscle stretch also indicated that the results were not contaminated by motor unit synchronization during spike-triggered averaging, because stretch would not alter synchronization.
Changes in neural drive and in motor units discharge frequencies
As indicated by the increase in the surface EMG, the central drive was intensified during the fatigue task (Bigland-Ritchie et al. 1986a; Garland et al. 1994; Löscher et al. 1996). In agreement with other studies, this observation is consistent with the recruitment of additional higher threshold motor units during the fatigue tests (Bigland-Ritchie et al. 1986a; Enoka et al. 1989; Maton & Gamet, 1989; Dorfman et al. 1990). These motor units showed first an increase in discharge frequency during the holding phase of the contraction and then a progressive decrease toward the end of the fatigue test. However, as also reported by other studies (Enoka et al. 1989; Christova & Kossev, 1998), motor units activated from the beginning of the fatigue test only showed a progressive decrease in discharge rate during these intermittent contractions. This difference in discharge pattern is further evidence of a different behaviour of motor units with low- and high-recruitment thresholds during fatigue. The motor units activated at the onset of the test showed a decrease in discharge frequency, which was greater for low-threshold compared with high-threshold units. Such findings cannot be explained by intrinsic motoneurone adaptation only, because it was reported to be greater in high-threshold than in small-threshold units (Kernell & Monster, 1982b). Interestingly, some high-threshold motor units were abruptly deactivated during the contractions (cf. also Peters & Fuglevand, 1999) although the central drive had not reached its maximum and the units displayed only a minimum reduction in discharge frequency. However, this deactivation appeared when the recruitment threshold was reduced substantially. This was a true deactivation because these units recovered their activity after the end of the test. Altogether, these observations of different behaviours of low- and high-threshold motor units, although the central drive to the muscle was increased during the fatigue test, suggests the specific influence of motoneurone adaptation and feedback mechanisms.
Three main mechanisms are usually proposed to explain the reduction in motoneurone discharge rate during fatigue (for review see Gandevia, 1998): (1) motoneurone adaptation to a constant excitatory input (Kernell & Monster, 1982a,b); (2) reflex disfacilitation by a decline in the group Ia excitatory input from muscle spindle afferents (Macefield et al. 1991); (3) reflex inhibition from group III and IV muscle afferents (Bigland-Ritchie et al. 1986b; Woods et al. 1987; Garland & McComas, 1990; Duchateau & Hainaut, 1993). The decline in the discharge rate due to reflex inhibition has been related to the accumulation of metabolites that seems to activate metaboreceptors and induce an inhibition of homonymous motoneurones via the small-diameter muscle afferents (Kaufman et al. 1983; Garland & McComas, 1990; Sinoway et al. 1993).
The muscle wisdom hypothesis proposed that a reduction in motor unit discharge rate matches the slowing of motor unit contractile speed during fatigue, optimizes force, and prevents energy loss (Marsden et al. 1983; Bigland-Ritchie et al. 1983). Our results on low-threshold motor units are more or less consistent with this hypothesis because the slowing of their mechanical responses is associated with a decline in discharge rate. However, such an adaptation did not occur in high-threshold units that displayed a speeding of their mechanical time course with only a modest decline in discharge rate. In the absence of a reduction in the mechanical contribution of low-threshold motor units during the fatigue task, it is suggested that the failure point may be primarily related to changes in the contractile function of the high-threshold motor units and to the derecruitment of some of these units. Furthermore, it appears clearly that in the FDI, low-threshold units (< 25 % MVC) are fatigue resistant whereas high-threshold units (> 25 % MVC) are not.
Concluding remarks
It has been claimed that the difficulty of having a clear view of motor unit behaviour during fatigue is related to a lack of data on different types of units (Thomas, 1995; Fuglevand et al. 1999). Our data were recorded from a wide range of identified motor unit recruitment thresholds, and the same units were followed throughout the tests. These data confirm previous reports (Bigland-Ritchie et al. 1986a; Maton & Gamet, 1989; Enoka et al. 1989; Garland et al. 1994; Löscher et al. 1996; Christova & Kossev, 1998) that the central drive to the motoneurones is intensified during fatiguing submaximal contractions. Moreover, they clearly indicate a different pattern of behaviour during fatigue for motor units based on differences in force thresholds. Such differences in discharge behaviour between high- and low-threshold units with fatigue could be explained in terms of intrinsic adaptations in motoneurones and of peripheral feedback. Because it was reported that motoneurone adaptation was greater in high-threshold units compared to low-threshold ones (Kernell & Monster, 1982b), our finding of a greater discharge rate decrease in the latter units underscores the significance of peripheral afferents on the motoneurone pool. It is concluded that during fatigue some specific influence of motoneurone adaptation and afferent feedback modulate the central control of individual motor unit behaviour.
Acknowledgments
The authors gratefully acknowledge Drs R. Enoka, L. de Montigny and E. Godaux for critical reading of the paper and Mrs A. Deisser for assistance in the preparation of the manuscript. This work was supported by NATO CRG no. 930261, the Fonds National de la Recherche Scientifique of Belgium and the Conseil de la Recherche of the Université Libre de Bruxelles.
References
- Bigland-Ritchie B, Cafarelli E, Vollestad NK. Fatigue of submaximal static contractions. Acta Physiologica Scandinavica. 1986a;(suppl. 128):137–148. [PubMed] [Google Scholar]
- Bigland-Ritchie B, Dawson NJ, Johansson RS, Lippold OCJ. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. Journal of Physiology. 1986b;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson RS, Lippold OCJ, Smith S, Woods JJ. Changes in motoneurone firing rates during sustained maximal voluntary contractions. Journal of Physiology. 1983;340:335–346. doi: 10.1113/jphysiol.1983.sp014765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christova P, Kossev A. Motor unit activity during long-lasting intermittent muscle contractions in humans. European Journal of Applied Physiology. 1998;77:379–387. doi: 10.1007/s004210050348. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Foley PJ, Erim Z. Motor units control properties in constant-force isometric contractions. Journal of Neurophysiology. 1996;76:1503–1516. doi: 10.1152/jn.1996.76.3.1503. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Godaux E. Ballistic skilled movements: load compensation and patterning of motor commands. In: Desmedt JE, editor. Progress in Clinical Neurophysiology. Vol. 4. Basel: Karger; 1978. pp. 21–55. [Google Scholar]
- Dorfman LJ, Howard JE, McGill KC. Triphasic behavioural response of motor units to submaximal fatiguing exercise. Muscle and Nerve. 1990;13:621–628. doi: 10.1002/mus.880130711. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Electrical and mechanical failures during sustained and intermittent contractions in humans. Journal of Applied Physiology. 1985;58:942–947. doi: 10.1152/jappl.1985.58.3.942. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Effects of immobilization on contractile properties, recruitment and firing rates of human motor units. Journal of Physiology. 1990;422:55–65. doi: 10.1113/jphysiol.1990.sp017972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Behaviour of short and long latency reflexes in fatigued human muscles. Journal of Physiology. 1993;471:787–799. doi: 10.1113/jphysiol.1993.sp019928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Fuglevand AJ. Motor unit physiology: some unresolved issues. Muscle and Nerve. 2001;24:4–17. doi: 10.1002/1097-4598(200101)24:1<4::aid-mus13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Robinson GA, Kossev AR. Task and fatigue effects on low-threshold motor units in human hand muscle. Journal of Neurophysiology. 1989;62:1344–1359. doi: 10.1152/jn.1989.62.6.1344. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Macefield VG, Bigland-Ritchie B. Force-frequency and fatigue properties of motor units in muscles that control digits of the human hand. Journal of Neurophysiology. 1999;81:1718–1729. doi: 10.1152/jn.1999.81.4.1718. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Neural control in human muscle fatigue: changes in muscle afferents, motoneurones and motor cortical drive. Acta Physiologica Scandinavica. 1998;162:275–283. doi: 10.1046/j.1365-201X.1998.0299f.x. [DOI] [PubMed] [Google Scholar]
- Gantchev GN, Gatev P, Ivanova T, Tankov N. Motor unit activity during fatigue. Biomedica et Biochemica Acta. 1986;45:69–75. [PubMed] [Google Scholar]
- Garland SJ, Enoka RM, Serrano LP, Robinson GA. Behaviour of motor units in human biceps brachii during a submaximal fatiguing contraction. Journal of Applied Physiology. 1994;76:2411–2419. doi: 10.1152/jappl.1994.76.6.2411. [DOI] [PubMed] [Google Scholar]
- Garland SJ, McComas AJ. Reflex inhibition of human soleus muscle during fatigue. Journal of Physiology. 1990;429:17–27. doi: 10.1113/jphysiol.1990.sp018241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett RAF, O'Donovan MJ, Stephens JA, Taylor A. Motor unit organization of human medial gastrocnemius. Journal of Physiology. 1979;287:33–43. doi: 10.1113/jphysiol.1979.sp012643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatev P, Ivanova T, Gantchev GN. Changes in the firing pattern of high-threshold motor units due to fatigue. Electromyography and Clinical Neurophysiology. 1986;26:83–93. [PubMed] [Google Scholar]
- Hagbarth KE, Hagglund JV, Nordin M, Wallin EU. Thixotropic behaviour of human finger flexor muscles with accompanying changes in spindle and reflex responses to stretch. Journal of Physiology. 1985;368:323–342. doi: 10.1113/jphysiol.1985.sp015860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effect of static muscular contraction on impulse activity of groups III and IV afferents in cats. Journal of Applied Physiology. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Time course and properties of late adaptation in spinal motoneurones of the cat. Experimental Brain Research. 1982a;46:191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Motoneurone properties and motor fatigue. Experimental Brain Research. 1982b;46:197–204. doi: 10.1007/BF00237177. [DOI] [PubMed] [Google Scholar]
- Löscher WN, Cresswell AG, Thorstensson A. Excitatory drive to the α-motoneuron pool during a fatiguing submaximal contraction in man. Journal of Physiology. 1996;491:271–280. doi: 10.1113/jphysiol.1996.sp021214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield G, Hagbarth KE, Gorman R, Gandevia SC, Burke D. Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. Journal of Physiology. 1991;440:497–512. doi: 10.1113/jphysiol.1991.sp018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Meadows JC, Merton PA. ‘Muscular wisdom’ that minimizes fatigue during prolonged effort in man: peak rates of motoneuron discharge and slowing of discharge during fatigue. In: Desmedt JE, editor. Motor Control Mechanisms in Health and Disease. New York: Raven Press; 1983. pp. 169–211. [PubMed] [Google Scholar]
- Maton B, Gamet D. The fatigability of two agonistic muscles in human isometric voluntary submaximal contraction: an EMG study. European Journal of Applied Physiology. 1989;58:369–374. doi: 10.1007/BF00643511. [DOI] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. The contractile properties of human motor units during voluntary isometric contractions. Journal of Physiology. 1973;228:285–306. doi: 10.1113/jphysiol.1973.sp010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom MA, Miles TS. Fatigue of single motor units in human masseter. Journal of Applied Physiology. 1990;68:26–34. doi: 10.1152/jappl.1990.68.1.26. [DOI] [PubMed] [Google Scholar]
- Olson CB, Swett CPA. Effect of prior activity on properties of different type of motor units. Journal of Neurophysiology. 1971;34:1–16. doi: 10.1152/jn.1971.34.1.1. [DOI] [PubMed] [Google Scholar]
- Person RS, Kudina LP. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalography and Clinical Neurophysiology. 1972;32:471–483. doi: 10.1016/0013-4694(72)90058-2. [DOI] [PubMed] [Google Scholar]
- Peters EJ, Fuglevand AJ. Cessation of human motor unit discharge during sustained maximal voluntary contraction. Neuroscience Letters. 1999;15:66–70. doi: 10.1016/s0304-3940(99)00666-7. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Hill JM, Pickar JG, Kaufman PM. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. Journal of Neurophysiology. 1993;69:1053–1059. doi: 10.1152/jn.1993.69.4.1053. [DOI] [PubMed] [Google Scholar]
- Stephens JA, Usherwood TP. The mechanical properties of human motor units with special reference to their fatigability and recruitment threshold. Brain Research. 1977;125:91–97. doi: 10.1016/0006-8993(77)90361-4. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Hayami M, Suzuki M, Watanabe S, Hutton RS. Reductions in recruitment force thresholds in human single motor units by successive voluntary contractions. Experimental Brain Research. 1990;82:227–230. doi: 10.1007/BF00230858. [DOI] [PubMed] [Google Scholar]
- Thomas CK. Human motor units studied by spike-triggered averaging and intraneural motor axon stimulation. In: Gandevia SC, Enoka RM, McComas AJ, Stuart DB, Thomas CK, editors. Fatigue: Neural and Muscular Mechanisms. New York: Plenum; 1995. pp. 17–160. [Google Scholar]
- Thomas CK, Johansson RS, Bigland-Ritchie B. Attempts to physiologically classify human thenar motor units. Journal of Neurophysiology. 1991;65:1501–1508. doi: 10.1152/jn.1991.65.6.1501. [DOI] [PubMed] [Google Scholar]
- Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. Journal of Neurophysiology. 1987;58:125–137. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]


