Abstract
This study examined the cellular actions of cannabinoids on neurons in the substantia gelatinosa of the spinal trigeminal nucleus pars caudalis, using whole-cell and perforated patch recording in brain slices.
The cannabinoid agonist WIN55,212-2 (3 μm) decreased the amplitude of both GABAergic and glycinergic electrically evoked inhibitory postsynaptic currents (IPSCs) by 35 and 41%, respectively. This inhibition was completely reversed by the CB1 receptor-selective antagonist N-piperidino-5-(4-chlorophenyl)-l-(2,4-dichlorophenyl)-4-methyl-3-pyrazole-carboxamide) (SR141716A, 3 μm). WIN55,212-2 also produced relative facilitation of the second evoked IPSC to paired stimuli.
WIN55,212-2 decreased the rate of both GABAergic and glycinergic miniature IPSCs by 44 and 34%, respectively, without changing their amplitude distributions or kinetics.
WIN55,212-2 did not affect the amplitude of electrically evoked non-NMDA glutamatergic excitatory postsynaptic currents (EPSCs).
WIN55,212-2 produced no postsynaptic membrane current and had no significant effect on membrane conductance over a range of membrane potentials (–60 to –130 mV).
These results suggest that, within the superficial medullary dorsal horn, cannabinoids presynaptically inhibit GABAergic and glycinergic neurotransmission. At the cellular level, the analgesic action of cannabinoids on these medullary dorsal horn neurons therefore differs from that of μ-opioids, which have both pre- and postsynaptic actions.
Cannabinoids mediate antinociception via actions in a number of regions throughout the central nervous system. Systemic and intracerebroventricular administration of cannabinoid agonists both produce analgesia (Lichtman & Martin, 1991; Hohmann et al. 1999b). Microinjection of cannabinoids into several brain regions, including the rostral ventromedial medulla (RVM) and periaqueductal grey (PAG), also produces antinociception (Lichtman et al. 1996; Meng et al. 1998; Martin et al. 1999). Furthermore, cannabinoid CB1 receptors are present in the areas of the brain involved in modulation of nociception (Herkenham et al. 1991; Tsou et al. 1998; Farquhar-Smith et al. 2000).
Intrathecal administration of cannabinoid agonists also produces analgesia (Lichtman & Martin, 1991; Smith & Martin, 1992) and CB1 receptors are reported to be expressed in the superficial dorsal horn (Herkenham et al. 1991; Tsou et al. 1998; Ong & Mackie, 1999; Farquhar-Smith et al. 2000). Studies in vivo demonstrate that cannabinoid agonists diminish excitation in deep convergent dorsal horn neurons following noxious heat stimulation (Hohmann et al. 1998, 1999b), and suppress wind-up elicited by transcutaneous stimulation (Strangman & Walker, 1999). Furthermore, the CB1-specific antagonist SR141716A has been shown to facilitate nociceptive responses of deep dorsal horn neurons (Chapman, 1999), suggesting that the spinal actions of cannabinoids are mediated through the CB1 receptor.
The cellular actions of cannabinoids on supraspinal descending antinociception pathways have been studied (Vaughan et al. 1999, 2000), but the cellular actions of cannabinoids in the superficial dorsal horn are unknown. Thus, the aim of the present study was to examine the cellular and synaptic actions of the cannabinoid agonist WIN55,212-2 in brain slices containing the superficial medullary dorsal horn, which is reported to be a major site of termination of small-diameter, nociceptive, primary afferent fibres (Ambalavanar & Morris, 1992).
METHODS
Sprague-Dawley rats (12-21 days old) were anaesthetized with halothane and decapitated, and horizontal brain slices (250 μm) containing the trigeminal nucleus caudalis (Vc) were cut (Grudt & Williams, 1994). All procedures reported conformed with the University of Sydney Ethics Committee guidelines. Briefly, a block of brainstem containing the medulla caudal to the obex was placed in a vibratome bath containing ice-cold (< 4 °C) artificial cerebrospinal fluid (ACSF). Two to three slices were taken from near the dorsal surface of the medulla and hemisected before being transferred to a submerged chamber containing ACSF equilibrated with 95 % O2 and 5 % CO2 and maintained at 34 °C. The slices were transferred to a superfusing chamber (32 °C) for recording (Vaughan et al. 2000). The ACSF contained (mm): NaCl, 126; KCl, 2.5; NaH2PO4, 1.4; MgCl2, 1.2; CaCl2, 2.4; glucose, 11; and NaHCO3, 25. For those experiments examining excitatory postsynaptic currents (EPSCs), the concentration of MgCl2 was increased to 1.8 mm to reduce polysynaptic recurrent EPSCs (R. Bardoni, personal communication).
Neurons in the substantia gelatinosa (SG) of the Vc were clearly visible as a translucent band just medial to the spinal trigeminal tract, which enters the slice 5-6 mm rostral to the Vc and travels along the lateral edge of the slice (Grudt & Williams, 1994). Neurons were visualized using infrared Nomarski optics, and whole-cell patch clamp recordings, under voltage clamp, were made of inhibitory postsynaptic currents (IPSCs; holding potential, -74 mV), using a CsCl-based internal solution containing (mm): CsCl, 140; EGTA, 10; Hepes, 5; CaCl2, 2; and MgATP, 2. Whole-cell patch clamp recordings were also made of EPSCs (holding potential, -70 mV), using a caesium methane sulphonate-based internal solution containing (mm): CsMeSO3, 135; EGTA, 10; NaCl, 15; MgCl2, 1.14; Hepes, 10; NaGTP, 0.25; and MgATP, 2. Perforated patch clamp recordings of postsynaptic currents (holding potential, -60 mV) were performed using a potassium acetate-based pipette solution of composition (mm): potassium acetate, 120; Hepes, 40; EGTA, 10; and MgCl2, 5; and containing 0.25 mg ml−1 pluronic F-127 and 0.12 mg ml−1 amphotericin B. Recordings were made with patch electrodes (3-7 MΩ) filled with one of the above internal solutions (all solutions had a pH of 7.3; osmolarity, 275-285 mosmol l−1). Series resistance (< 15 MΩ) was compensated by 80 % and continuously monitored during experiments, except in the perforated patch experiments. Liquid junction potentials of -13 mV for CsMeSO3-, -12 mV for potassium acetate- and -4 mV for CsCl-based internal solutions were corrected. Junction potentials were calculated using the program JPCalc, written by P. Barry (Barry, 1994).
Electrically evoked IPSCs (eIPSCs) were elicited via NaCl-filled glass stimulating electrodes (≈5 μm tip) placed 100-400 μm either rostral or caudal to the recording electrode in the SG (rate, 0.07-0.1 Hz; stimuli, 5-70 V, 60-400 μs) in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 5 μm), strychnine (5 μm) and (±)-2-amino-5-phosphopentanoic acid (APV, 20 μm). For paired-pulse experiments, two stimuli of identical strength were applied with an inter-stimulus interval of 70 ms. Evoked IPSCs and miniature IPSCs (mIPSCs) were filtered (1 kHz low-pass filter) and sampled at 5 kHz for on-line and later off-line analysis (Axograph 4.2, Axon Instruments). The amplitudes of eIPSCs were calculated to construct time plots of eIPSC amplitude. For analysis of the paired-pulse experiments, the amplitudes of the first eIPSC were normalized to measure the relative amplitudes of the second eIPSC (these reflected a ratio of the second eIPSC to the first eIPSC (eIPSC2/eIPSC1)). Miniature IPSCs above a preset threshold (4.5-5.5 standard deviations above baseline noise) were automatically detected by a sliding template algorithm, then manually checked off-line. Miniature IPSCs were then counted in 12 s epochs every 15 s to construct rate-time plots. The mIPSC rate and amplitude recorded during a 4 min period in the absence (control) and presence of WIN55,212-2 were compared.
Electrically evoked EPSCs (eEPSCs) were elicited via bipolar tungsten stimulating electrodes placed in the spinal trigeminal tract about 1 mm rostral to the site of recording. Stimuli were delivered at the same rates and intensities described above, in the presence of picrotoxin (100 μm), APV (20 μm) and strychnine (5 μm). Evoked EPSCs were filtered (2 kHz low-pass filter), and sampled at 10 kHz for analysis.
Stock solutions of all drugs were diluted to working concentrations using ACSF immediately before use and applied by superfusion. Stock solutions of cannabinoids were prepared in dimethylsulphoxide and diluted using ACSF to a final concentration of 0.01-0.1 % dimethylsulphoxide. The superfusion system was dismantled and rinsed with ethanol after each recording involving superfusion of a cannabinoid. Stock solutions of all other drugs were made in distilled water, or added directly to the ACSF. Methionine-enkephalin (met-enkephalin), baclofen, bicuculline methiodide, picrotoxin and APV were obtained from Sigma (Sydney, Australia); CNQX was from Tocris Cookson (Bristol, UK); tetrodotoxin (TTX) was from Alomone Labs Ltd (Jerusalem, Israel); naloxone hydrochloride and WIN55,212-2 mesylate were from Research Biochemicals Inc. (Natick, MA, USA); N-piperidino-5-(4-chlorophenyl)-l-(2,4-di chlorophenyl)-4-methyl-3-pyrazole-carboxamide (SR141716A) was donated by Sanofi Recherche. All pooled data are expressed as means ±s.e.m. (n, number of neurons), and statistical comparisons were made using Student's paired t test.
RESULTS
Cannabinoids inhibit both evoked GABAergic and glycinergic synaptic currents
GABAergic IPSCs were electrically evoked (eIPSCs) by focal stimulation in the superficial dorsal horn of the Vc in the presence of the non-N-methyl-D-aspartate (non-NMDA), NMDA and glycinergic antagonists, CNQX (5 μm), APV (20 μm) and strychnine (5 μm), respectively. Evoked IPSCs had a mean amplitude of 190 ± 27 pA (n = 14), and were abolished by the GABAA antagonist bicuculline (30 μm). Superfusion of the non-selective cannabinoid agonist WIN55,212-2 (3 μm) reduced the amplitude of the eIPSC by 35 ± 3 % (range, 18-55 %; n = 14; Fig. 1A and B) in all cells tested. This inhibition was reversed by the CB1 receptor-specific antagonist SR141716A (3 μm; 99 ± 7 % of control; n = 14; Fig. 1A and B). The decay time of eIPSCs was similar in the absence (τ = 15 ± 4 ms) and presence of WIN55,212-2 (3 μm; τ = 14 ± 4 ms; n = 5). The opioid agonist met-enkephalin (10 μm) caused a 25 ± 5 % decrease in GABAA eIPSC amplitude in all cells tested, and this was reversed by naloxone (1 μm; n = 4; data not shown).
Figure 1. Cannabinoids inhibit GABAAergic eIPSCs in medullary dorsal horn neurons.
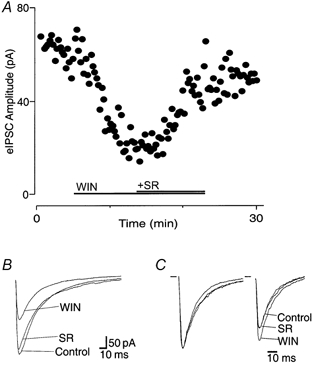
A, time course of eIPSC amplitude during application of WIN55,212-2 (WIN, 3 μm), and after addition of SR141716A (SR, 3 μm). B, averaged eIPSCs before (Control) and during application of WIN55,212-2 (3 μm), then after application of SR141716A (3 μm). C, normalized average responses to identical paired stimuli (inter-stimulus interval, 70 ms), with the eIPSC1 normalized (left) to demonstrate the relative facilitation of eIPSC2 during application of WIN55,212-2, and its reversal by SR141716A. Neurons were voltage clamped at -74 mV, and IPSCs were evoked at 15 s intervals in the presence of CNQX (5 μm), APV (20 μm) and strychnine (5 μm).
Under control conditions, the mean ratio of the amplitudes of the paired GABAergic eIPSCs (inter-stimulus interval, 70 ms) was 0.9 ± 0.1 (eIPSC2/eIPSC1; range, 0.64-1.3; n = 5; Fig. 1C). Superfusion of WIN55,212-2 (3 μm) produced a significant increase in the mean ratio of eIPSC2/eIPSC1 (127 ± 10 % of control; P < 0.05), which was reversed by the addition of SR141716A (3 μm; 99 ± 7 % of control; P = 0.97; n = 5; Fig. 1C).
Glycinergic eIPSCs, following focal stimulation in the superficial Vc, were recorded in the presence of CNQX (5 μm), APV (20 μm) and bicuculline (30 μm). The mean amplitude of these eIPSCs was 221 ± 34 pA and they were abolished by the application of strychnine (5 μm; Fig. 2A). Superfusion of WIN55,212-2 (3 μm) caused a decrease in the eIPSC amplitude of 41 ± 5 % (range, 21-68 %; n = 10) in all cells tested. This inhibition was reversed by SR141716A (3 μm; 113 ± 8 % of control; n = 10; Fig. 2A and B). The decay time of eIPSCs was similar in the absence (τ = 8 ± 1 ms) and presence of WIN55,212-2 (3 μm; τ = 9 ± 1 ms; n = 8).
Figure 2. Cannabinoids inhibit glycinergic eIPSCs in medullary dorsal horn neurons.
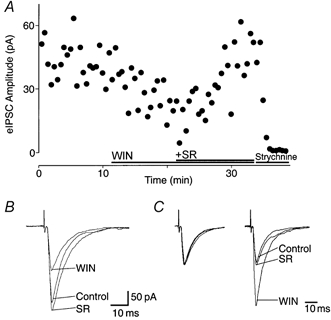
A, time course of eIPSC amplitude, before and during application of WIN55,212-2 (WIN, 3 μm), and after addition of SR141716A (SR, 3 μm) and strychnine (5 μm). B, averaged eIPSCs from the cell in A, showing amplitude size before (Control) and during application of WIN55,212-2 (3 μm), and after application of SR141716A (3 μm). C, normalized average responses to identical paired stimuli (inter-stimulus interval, 70 ms), with eIPSC1 normalized (left) to demonstrate the facilitation of eIPSC2 relative to eIPSC1 during application of WIN55,212-2, and its recovery after application of SR141716A. Neurons were voltage clamped at -74 mV, and IPSCs evoked at 15 s intervals in the presence of APV (20 μm), bicuculline (30 μm) and CNQX (5 μm).
The mean ratio of the amplitude of paired glycinergic eIPSCs (inter-stimulus interval, 70 ms) under control conditions was 1.0 ± 0.1 (eIPSC2/eIPSC1; range, 0.43-1.58; n = 10; Fig. 2C). Superfusion of WIN55,212-2 (3 μm) produced a significant increase in the mean eIPSC2/eIPSC1 ratio (207 ± 34 % of control; P < 0.01; Fig. 2C), which was reversed by the addition of SR141716A (3 μm; 113 ± 8 % of control).
It was noted that, during the course of recording glycinergic eIPSCs, there was a negative shift in the holding current, which was largely reversed by strychnine. This phenomenon has been reported previously (Grudt & Henderson, 1998), and was not analysed further in these experiments.
Cannabinoids inhibit the rate of miniature IPSCs without affecting their amplitude
mIPSCs were recorded from superficial Vc neurons in the presence of CNQX (5 μm), TTX (0.3 μm) and either strychnine (5 μm) or bicuculline (30 μm). GABAA-mediated mIPSCs had a mean rate of 0.47 ± 0.13 Hz (n = 6) and a mean amplitude of 15 ± 1.7 pA (range, 10-21 pA), and were abolished by bicuculline (30 μm; n = 2; Fig. 3A). Superfusion of WIN55,212-2 (3 μm) reduced the rate of mIPSCs by 44 ± 10 %(n = 6; P < 0.05; Fig. 3A), but had no effect on their amplitude distribution or kinetics. The mean amplitude of mIPSCs was similar in the absence (15 ± 1.7 pA) or presence of WIN55,212-2 (3 μm; 15 ± 1.4 pA; n = 6; P = 0.9; Fig. 3B and C). The mean decay time of GABAergic mIPSCs was similar in the absence (9.1 ± 1.0 ms) or presence of WIN55,212-2 (3 μm; 8.8 ± 0.7 ms; n = 6; P = 0.8). In all cases, the decay could be fitted with a single exponential.
Figure 3. Cannabinoids decrease the rate but not the amplitude of GABAAergic mIPSCs.
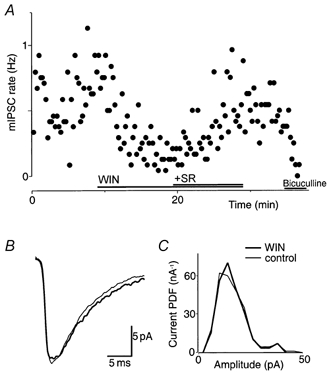
A, time plot of the mIPSC rate showing the decrease after application of WIN55,212-2 (WIN, 3 μm), and the reversal of this effect with the addition of SR141716A (SR, 3 μm). Superfusion of bicuculline (30 μm) abolished the mIPSC rate. B, averaged mIPSCs before (control; thin line) and during application of WIN55,212-2 (3 μm; thick line). C, probability density distributions of mIPSC amplitude. In B and C, the number of events was 125 and 53 for 16 epochs (4 min) of control and WIN55,212-2, respectively. Data in A-C are taken from one neuron that was voltage clamped at -74 mV in the presence of TTX (0.3 μm), CNQX (5 μm) and strychnine (5 μm).
Glycinergic mIPSCs had a mean rate of 0.9 ± 0.1 Hz (n = 5), and a mean amplitude of 36 ± 8 pA (range, 16-61 pA). Glycinergic mIPSCs were abolished by the addition of strychnine (5 μm; n = 3). Superfusion of WIN55,212-2 (3 μm) also caused a significant inhibition of the rate (34 ± 6 %; n = 5; P < 0.05; Fig. 4A), without affecting the amplitude (97 ± 12 %; P = 0.8; Fig. 4B and C). The addition of the CB1 antagonist SR141716A (3 μm) reversed the inhibition (116 ± 15 %; n = 4). The mean decay time of the glycinergic mIPSCs was 3.3 ± 0.4 ms, and this was not significantly changed following superfusion of WIN55,212-2 (3.4 ± 0.2 ms; n = 5; P = 0.8). In both cases, the decay could be fitted with a single exponential.
Figure 4. Cannabinoids decrease the rate but not the amplitude of glycinergic mIPSCs.
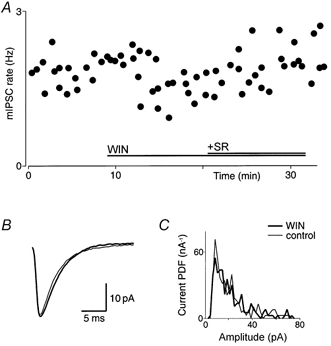
A, time plot showing that the rate of mIPSCs decreased in the presence of WIN55,212-2 (WIN, 3 μm), and returned to baseline with the addition of SR141716A (SR, 3 μm). B, averaged traces of mIPSCs before (control; thin line) and during application of WIN55,212-2 (3 μm; thick line). C, probability density distributions of mIPSC amplitude for this cell. In B and C, the number of events was 252 and 226 for 16 epochs (4 min) for control and WIN55,212-2, respectively. Data in A-C are taken from one neuron that was voltage clamped at -74 mV in the presence of TTX (0.3 μm), CNQX (5 μm) and bicuculline (30 μm).
Cannabinoids do not affect the amplitude of evoked glutamatergic non-NMDA-mediated EPSCs
Electrical stimulation of the trigeminal tract evoked non-NMDA glutamatergic EPSCs (eEPSCs) in neurons in the superficial Vc. These eEPSCs were recorded in the presence of picrotoxin (100 μm), strychnine (5 μm) and APV (20 μm). They had a mean amplitude of 440 ± 34 pA (n = 14), and were abolished by CNQX (5 μm; n = 4). Superfusion of WIN55,212-2 (3 μm; n = 14) had little or no effect on the eEPSC amplitude, with a mean reduction in the amplitude of 1 ± 2 % (range, -14 to 14 %; Fig. 5A and B). Similarly, addition of SR141716A (3 μm; n = 13; Fig. 5A and B) had no effect on the eEPSC amplitude. In five of these neurons, met-enkephalin (10 μm) inhibited the eEPSC amplitude (41 ± 9 %; Fig. 5A and B), and this was reversed by naloxone (1 μm). In another six of these neurons, met-enkephalin had no significant effect on the eEPSC amplitude (3 ± 2 % inhibition; data not shown).
Figure 5. Cannabinoids do not affect the amplitude of non-NMDA-mediated eEPSCs.
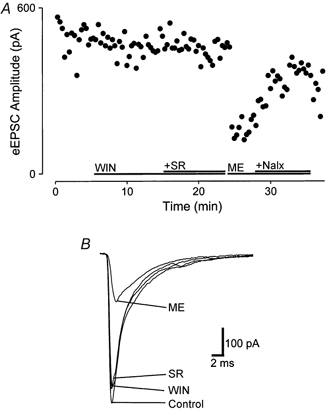
A, time course of eEPSC amplitude before and during application of WIN55,212-2 (WIN, 3 μm), and after addition of SR141716A (SR, 3 μm), and, for comparison, during application of met-enkephalin (ME, 10 μm), and after the addition of naloxone (Nalx, 1 μm). B, averaged eEPSCs from the cell in A, showing amplitude size before (Control) and during application of WIN55,212-2 (3 μm), after additional superfusion of SR141716A (3 μm) and during application of met-enkephalin (10 μm). Neurons were voltage clamped at -70 mV in the presence of picrotoxin (100 μm), APV (20 μm) and strychnine (5 μm).
Cannabinoids do not affect postsynaptic conductances
The effect of cannabinoids on postsynaptic currents in SG neurons was examined using perforated patch recordings. Superfusion of WIN55,212-2 (3 μm) produced no significant membrane current (1 ± 0.4 pA; n = 12; Fig. 6A) in neurons voltage clamped at -60 mV. Addition of SR141716A (3 μm) also produced no significant membrane current (n = 12; Fig. 6A). Superfusion of met-enkephalin (10 μm) produced a reversible outward current of 19 ± 4 pA in eight of these neurons (Fig. 6A). In one cell, met-enkephalin produced no change in holding current. Superfusion of the GABAB agonist baclofen (10 μm) produced a reversible outward current of 33 ± 5 pA in all neurons tested (n = 11; Fig. 6A).
Figure 6. Postsynaptic effects of cannabinoids on membrane conductance.
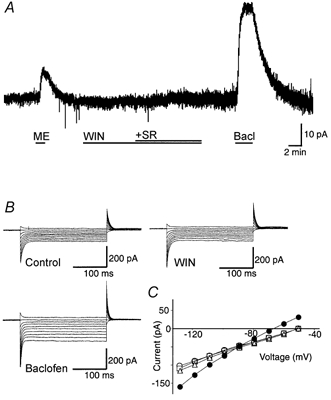
A, membrane trace of a superficial medullary dorsal horn neuron during superfusion of met-enkephalin (ME, 10 μm), WIN55,212-2 (WIN, 3 μm), SR141716A (SR, 3 μm) and baclofen (Bacl, 10 μm). B, amplitudes of currents evoked by voltage command steps in 10 mV increments from a holding potential of -50 mV to -130 mV (250 ms duration) for control, WIN55,212-2 (3 μm) and baclofen. C, current-voltage analysis plotted, from B, for control (○), WIN55,212-2 (□), WIN55,212-2 + SR141716A (▵) and baclofen (•). The neuron was voltage clamped at -60 mV.
The resting membrane conductances of trigeminal SG neurons were 1.3 ± 0.1 and 1.9 ± 0.3 nS when measured at -60 to -90 mV and at -110 to -130 mV, respectively (n = 6; Fig. 6B and C). Superfusion of WIN55,212-2 (3 μm) had no significant effect on the conductances when measured over the same potentials (1.3 ± 0.1 and 2.0 ± 0.2 nS; P = 0.4 and 0.2, respectively; n = 8). Addition of SR141716A (3 μm) also had no significant effect on the conductances when measured over the same potentials (1.4 ± 0.2 and 1.9 ± 0.2 nS; P = 0.07 and 0.9, respectively; n = 8). Baclofen (10 μm) increased the conductances to 2.2 ± 0.2 and 3.6 ± 0.3 nS when measured over the same potentials (P < 0.002 and 0.005; n = 5). The baclofen-induced current reversed polarity at -92 ± 4 mV (n = 5; Fig. 6C).
DISCUSSION
This study demonstrated that cannabinoids acting via CB1 receptors presynaptically inhibited both GABAAergic and glycinergic synaptic transmission from presumptive interneurons, without altering non-NMDA receptor-mediated glutamatergic transmission from primary afferents in the medullary dorsal horn. In addition, cannabinoids had no postsynaptic effects on medullary SG neurons.
Cannabinoid inhibition of GABAergic synaptic transmission has been reported in a number of brain regions, including the basal ganglia (Chan et al. 1998; Szabo et al. 1998), hippocampus (Katona et al. 1999), PAG (Vaughan et al. 2000) and RVM (Vaughan et al. 1999). The lack of cannabinoid inhibition of glutamatergic transmission in the medullary dorsal horn differs from some brain regions where effects have been reported (Shen et al. 1996; Levenes et al. 1998; Szabo et al. 2000; Vaughan et al. 2000). There have been no previous reports of cannabinoid actions on glycine-mediated neurotransmission.
It is likely that the cannabinoid inhibition of GABAergic and glycinergic synaptic transmission observed in the present study was mediated by a presynaptic mechanism. The inhibition of GABAA and glycine receptor-mediated eIPSCs by WIN55,212-2 was associated with a relative facilitation of the second IPSC to paired stimuli. This paired-pulse facilitation is a presynaptic process, arising from an increase in the probability of transmitter release (del Castillo & Katz, 1954). Confirmation of the presynaptic action is provided by the experiments showing that WIN55,212-2 significantly decreased the rate of both GABAA and glycine receptor-mediated miniature IPSCs, but had no effect on their amplitudes or decay times. The amplitudes of both the GABAA and glycine receptor-mediated mIPSCs were smaller than those previously reported (Grudt & Henderson, 1998).
Further evidence for the lack of postsynaptic effect of cannabinoids in the medullary dorsal horn is illustrated by the absence of cannabinoid effect on membrane conductance measured between -130 and -60 mV. This was unlikely to be due to cell damage, because we used perforated patch recordings and all of the neurons responded to the GABAB receptor agonist baclofen, which has previously been shown to hyperpolarize dorsal horn neurons in the spinal cord (Kangrga et al. 1991). This lack of direct postsynaptic action in the medullary dorsal horn is similar to that reported in the PAG and RVM (Vaughan et al. 1999, 2000), but differs from the hippocampus and substantia nigra, where cannabinoids have both pre- and postsynaptic actions (Twitchell et al. 1997; Chan et al. 1998).
The cannabinoid CB1 receptor has been detected in both the spinal and medullary superficial dorsal horn (Hohmann & Herkenham, 1998; Tsou et al. 1998; Hohmann et al. 1999a; Farquhar-Smith et al. 2000). Autoradiographic studies suggest that the majority (60 %) of μ-opioid receptors are located presynaptically on capsaicin-sensitive C afferent fibres. While 50 % of cannabinoid receptors are located presynaptically on primary afferent fibres, only 16 % are located on C fibre afferents (Hohmann & Herkenham, 1998; Hohmann et al. 1999a). Immunoreactivity for CB1 receptors is predominantly found in interneurons of the spinal cord dorsal horn, particularly in lamina I, IIi-III transition and X (Farquhar-Smith et al. 2000). Moreover, the CB1 receptor is located primarily on the axons of intrinsic interneurons in the superficial dorsal horn, rather than on the somata (Tsou et al. 1998; Farquhar-Smith et al. 2000), consistent with the presynaptic locus of action demonstrated in the present study. A study in the primate, however, reported CB1 receptor expression throughout the dorsal horn, with both soma and neuropil labelled (Ong & Mackie, 1999), suggesting either a species difference or different specificities in the antibodies used. GABAergic neurons are found throughout the superficial dorsal horn (Todd & Spike, 1993), so the eIPSCs recorded in this study may have originated in the superficial dorsal horn. The majority of neurons in lamina II are thought to arborize locally, having a role in the modulation of nociceptive information before it is passed to deeper laminae or to higher centres (Light, 1992). It is possible, therefore, that cannabinoid modulation of nociception, at the spinal cord level, is through a disinhibitory action on lamina II neurons. A recent study reported that, in the trigeminal ganglia, CB1 receptor mRNA is located in medium and large cells that do not express the nociceptor marker VR1 (Parghi et al. 2000). Since the majority of the fibres projecting to the Vc SG are small-diameter unmyelinated fibres (Light, 1992), it is unlikely that primary afferents terminating in this nucleus have presynaptic CB1 receptors on their central terminals, thus explaining the lack of cannabinoid effect on glutamatergic primary afferent transmission.
While cannabinoids and opioids both produce analgesia within the dorsal horn, their mechanisms of action differ. Unlike cannabinoids, μ-opioids inhibit release of glutamate from primary afferent terminals at the level of the spinal (Hori et al. 1992; Kohno et al. 1999) and medullary (Grudt & Williams, 1994) dorsal horn. In addition, opioids have direct postsynaptic effects in the dorsal horn (Grudt & Williams, 1994; Kohno et al. 1999). Like cannabinoids, μ-opioids presynaptically inhibit both glycinergic and GABAergic synaptic transmission in the medullary dorsal horn (Grudt & Henderson, 1998), but not in the spinal cord (Kohno et al. 1999). Differences in the cellular mechanisms of opioids and cannabinoids also occur at the supraspinal level, where they act to disinhibit antinociceptive descending projection neurons. While cannabinoids and μ-opioids presynaptically inhibit GABAergic synaptic transmission, only μ-opioids directly inhibit putative GABAergic neurons in the PAG and RVM (Chieng & Christie, 1994; Vaughan et al. 1999, 2000).
One straightforward interpretation of the present results is that cannabinoids are hyperalgesic at the level of the medullary dorsal horn because of their selective inhibition of GABAergic and glycinergic transmission. Indeed, the analgesic efficacy of cannabinoid agonists is greatly reduced following spinal cord transection (Lichtman & Martin, 1991; Hohmann et al. 1999b). In two extracellular electrophysiological studies in vivo, reporting the analgesic effects of cannabinoid agonists on wind-up in the dorsal horn, the drugs were delivered systemically (Hohmann et al. 1999b; Strangman & Walker, 1999). It is therefore possible that cannabinoid agonists inhibited wind-up in these studies via supraspinal actions. Furthermore, the CB1 receptor is confined to the superficial dorsal horn (Herkenham et al. 1991; Tsou et al. 1998; Farquhar-Smith et al. 2000), suggesting that the reported effects of cannabinoids on wind-up (Hohmann et al. 1999b; Strangman & Walker, 1999) are unlikely to have resulted from direct actions on deep convergent neurons. The presence of CB1 receptors in the dorsolateral funiculus (Farquhar-Smith et al. 2000) further suggests the importance of descending controls in cannabinoid-mediated analgesia. However, we were unable to test the role of descending inhibitory controls in this slice preparation.
In conclusion, the cannabinoid agonist WIN55,212-2 had no effect on either primary afferent-evoked excitatory glutamatergic transmission or postsynaptic K+ conductance in lamina II neurons in the medullary dorsal horn. Rather, cannabinoids presynaptically inhibited both GABAAergic and glycinergic neurotransmission in these neurons. It is likely, therefore, that cannabinoid analgesia, at the level of the superficial dorsal horn, is mediated through different pathways to those characterized for μ-opioid analgesia. This difference might be exploited in the treatment of intractable pain states, many of which are resistant to conventional opioid analgesics. Indeed, it has been reported that cannabinoid agonists retain their efficacy, whilst morphine does not, in an animal model of neuropathic pain (Mao et al. 2000).
Acknowledgments
E.A.J. is supported by the Wellcome Trust, C.W.V. is an NH & MRC RD Wright Research Fellow and M.J.C. is supported by the Medical Foundation, University of Sydney. Donation of SR141716A from Dr Madeline Mossé, Sanofi Recherche, Montpellier, France is gratefully acknowledged.
References
- Ambalavanar R, Morris R. The distribution of binding by isolectin I-B4 from Griffonia simplicifolia in the trigeminal ganglion and brainstem trigeminal nuclei in the rat. Neuroscience. 1992;47:421–429. doi: 10.1016/0306-4522(92)90256-2. [DOI] [PubMed] [Google Scholar]
- Barry PH. Jpcalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. Journal of Neuroscience Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Chan PK, Chan SC, Yung WH. Presynaptic inhibition of GABAergic inputs to rat substantia nigra pars reticulata neurones by a cannabinoid agonist. NeuroReport. 1998;9:671–675. doi: 10.1097/00001756-199803090-00020. [DOI] [PubMed] [Google Scholar]
- Chapman V. The cannabinoid CB1 receptor antagonist, SR141716A, selectively facilitates nociceptive responses of dorsal horn neurones in the rat. British Journal of Pharmacology. 1999;127:1765–1767. doi: 10.1038/sj.bjp.0702758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Hyperpolarization by opioids acting on μ-receptors of a sub-population of rat periaqueductal gray neurones in vitro. British Journal of Pharmacology. 1994;113:121–128. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Statistical factors involved in neuromuscular facilitation and depression. Journal of Physiology. 1954;124:574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Egertová M, Bradbury EJ, McMahon SB, Rice ASC, Elphick MR. Cannabinoid CB1 receptor expression in rat spinal cord. Molecular and Cellular Neuroscience. 2000;15:510–521. doi: 10.1006/mcne.2000.0844. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Henderson G. Glycine and GABAA receptor-mediated synaptic transmission in rat substantia gelatinosa: inhibition by μ-opioid and GABAB agonists. Journal of Physiology. 1998;507:473–483. doi: 10.1111/j.1469-7793.1998.473bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudt TJ, Williams JT. μ-Opioid agonists inhibit spinal trigeminal substantia gelatinosa neurons in guinea pig and rat. Journal of Neuroscience. 1994;14:1646–1654. doi: 10.1523/JNEUROSCI.14-03-01646.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. Journal of Neuroscience. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Briley EM, Herkenham M. Pre- and postsynaptic distribution of cannabinoid and μ opioid receptors in rat spinal cord. Brain Research. 1999a;822:17–25. doi: 10.1016/s0006-8993(98)01321-3. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Regulation of cannabinoid and μ opioid receptors in rat lumbar spinal cord following neonatal capsaicin treatment. Neuroscience Letters. 1998;252:13–16. doi: 10.1016/s0304-3940(98)00534-5. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Tsou K, Walker JM. Cannabinoid modulation of wide dynamic range neurons in the lumbar dorsal horn of the rat by spinally administered WIN55, 212–2. Neuroscience Letters. 1998;257:119–122. doi: 10.1016/s0304-3940(98)00802-7. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Tsou K, Walker JM. Cannabinoid suppression of noxious heat-evoked activity in wide dynamic range neurons in the lumbar dorsal horn of the rat. Journal of Neurophysiology. 1999b;81:575–583. doi: 10.1152/jn.1999.81.2.575. [DOI] [PubMed] [Google Scholar]
- Hori Y, Endo K, Takahashi T. Presynaptic inhibitory action of enkephalin on excitatory transmission in superficial dorsal horn of rat spinal cord. Journal of Physiology. 1992;450:673–685. doi: 10.1113/jphysiol.1992.sp019149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangrga I, Jiang MC, Randic M. Actions of (-)-baclofen on rat dorsal horn neurons. Brain Research. 1991;562:265–275. doi: 10.1016/0006-8993(91)90630-e. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. Journal of Neuroscience. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Kumamoto E, Higashi H, Shimoji K, Yoshimura M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. Journal of Physiology. 1999;518:803–813. doi: 10.1111/j.1469-7793.1999.0803p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenes C, Daniel H, Soubrie P, Crepel F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. Journal of Physiology. 1998;510:867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. Journal of Pharmacology and Experimental Therapeutics. 1996;276:585–593. [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Spinal and supraspinal components of cannabinoid-induced antinociception. Journal of Pharmacology and Experimental Therapeutics. 1991;258:517–523. [PubMed] [Google Scholar]
- Light AR. The organization of nociceptive neurons in the spinal grey matter. In: Gildenberg PL, editor. The Initial Processing of Pain and its Descending Control: Spinal and Trigeminal Systems. Basel: Karger; 1992. pp. 109–177. [Google Scholar]
- Mao J, Price DD, Lu J, Keniston L, Mayer DJ. Two distinctive antinociceptive systems in rats with pathological pain. Neuroscience Letters. 2000;280:13–16. doi: 10.1016/s0304-3940(99)00998-2. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Coffin PO, Attias E, Balinsky M, Tsou K, Walker JM. Anatomical basis for cannabinoid-induced antinociception as revealed by intracerebral microinjections. Brain Research. 1999;822:237–242. doi: 10.1016/s0006-8993(98)01368-7. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in the primate spinal cord. Journal of Neurocytology. 1999;28:39–45. doi: 10.1023/a:1007011700677. [DOI] [PubMed] [Google Scholar]
- Parghi D, Hargreaves KM, Flores CM. Distribution and phenotype of cannabinoid type one receptor-expressing neurons in the trigeminal ganglion of the rat. Society for Neuroscience Abstracts. 2000;26:815.4. [Google Scholar]
- Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. Journal of Neuroscience. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PB, Martin BR. Spinal mechanisms of Δ9-tetrahydrocannabinol-induced analgesia. Brain Research. 1992;578:8–12. doi: 10.1016/0006-8993(92)90222-u. [DOI] [PubMed] [Google Scholar]
- Strangman NM, Walker JM. Cannabinoid WIN 55, 212–2 inhibits the activity-dependent facilitation of spinal nociceptive responses. Journal of Neurophysiology. 1999;82:472–477. doi: 10.1152/jn.1999.82.1.472. [DOI] [PubMed] [Google Scholar]
- Szabo B, Dorner L, Pfreundtner C, Norenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403. doi: 10.1016/s0306-4522(97)00597-6. [DOI] [PubMed] [Google Scholar]
- Szabo B, Wallmichrath I, Mathonia P, Pfreundtner C. Cannabinoids inhibit excitatory neurotransmission in the substantia nigra pars reticulata. Neuroscience. 2000;97:89–97. doi: 10.1016/s0306-4522(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I-III of the mammalian spinal dorsal horn. Progress in Neurobiology. 1993;41:609–645. doi: 10.1016/0301-0082(93)90045-t. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. Journal of Neurophysiology. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Connor M, Bagley EE, Christie MJ. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Molecular Pharmacology. 2000;57:288–295. [PubMed] [Google Scholar]
- Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. British Journal of Pharmacology. 1999;127:935–940. doi: 10.1038/sj.bjp.0702636. [DOI] [PMC free article] [PubMed] [Google Scholar]


