Abstract
Self-referencing ion-selective (SERIS) electrodes were used to measure the temperature and pressure dependence of Cl− efflux, during myogenic contraction of pressurized rat cerebral resistance arteries.
At room temperature (18–21 °C), a small, pressure-independent Cl− efflux was measured. On warming to 37 °C, arteries developed pressure-dependent myogenic tone, and this was associated with a pressure-dependent increase in Cl− efflux (n = 5).
Both myogenic tone and the pressure- and temperature-dependent Cl− efflux were abolished on application of 10 μm tamoxifen, a Cl− channel blocker (IC50 3.75 ± 0.2 μm). Tamoxifen (10 μm) also prevented contraction to 60 mm K+, suggesting non-specific effects of tamoxifen (n = 5).
Myogenic tone was abolished by 2 μm nimodipine, but Cl− efflux was unaffected. In the presence of nimodipine, 10 μm tamoxifen still abolished pressure- and temperature-dependant Cl− efflux (n = 3).
In summary, a Cl− efflux can be measured from rat cerebral arteries, with a temperature dependence that is closely correlated with myogenic contraction. We conclude that Cl− efflux through Cl− channels contributes to the depolarization associated with myogenic contraction.
Membrane depolarization from approximately -65 mV to as positive as -35 mV is associated with the myogenic response of renal and cerebral arteries of the rat (Harder, 1984; Harder et al. 1987). This depolarization is graded with transmural pressure, and is sufficient to increase significantly the open probability (Popen) of dihydropyridine (DHP)-sensitive Ca2+ channels, thus raising [Ca2+]i and augmenting calcium-dependent contraction (Knot & Nelson, 1998). However the ionic mechanism underlying this pressure-dependent depolarization is poorly understood. Evidence that Cl− channels are present in vascular smooth muscle (Pacaud et al. 1991; Large & Wang, 1996; Yamazaki et al. 1998), combined with an estimated reversal potential for chloride of -20 to -30 mV (Aickin, 1990) makes a Cl− conductance an obvious candidate for mediating the pressure-induced depolarization. Indeed, Cl− channel blockers have been demonstrated to inhibit depolarization and contraction in pressurized cerebral arteries (Nelson et al. 1997). Cl− channel blockers are poorly selective, as they also block Ca2+ channels and non-selective cation channels, and/or depress Ca2+-dependent force generation (Doughty et al. 1998; Kato et al. 1999), which could account for their effects on arterial tone. The poor selectivity of Cl− channel blockers poses problems for the interpretation of experiments that rely on measurements of contraction and, to a lesser extent, membrane potential.
To further study the potential role of Cl− channels in the myogenic response we have developed a non-invasive method of directly measuring Cl− flux from pressurized cerebral arteries, using a self-referencing ion-selective (SERIS) electrode (Kuhtreiber & Jaffe, 1990; Smith et al. 1994; Smith, 1995). Using this technique we have found evidence that increased Cl− efflux from rat cerebral arteries is correlated with the myogenic response.
METHODS
Wistar rats (250-300 g) were killed by an intraperitoneal injection of sodium pentobarbitone (500 mg kg−1). The brain was removed into an ice-cold Hepes-buffered saline solution (HBSS), with a low concentration of Cl− (mm: NaCl 20; sodium gluconate 120; KCl 4.7; NaHCO3 4.2; KH2PO4 1.18; CaCl2 1.8; MgSO4 1.2; glucose 10; EDTA 0.027; Hepes 10, pH 7.4). Extracellular [Cl−] was reduced in order to increase the resolution of the SERIS electrode (Doughty & Langton, 1998b). Posterior cerebral arteries were dissected from the surface of the brain as previously described (Doughty & Langton, 1998a).
Halpern pressure myography
Leak-free segments of artery, of at least 1 mm in length, were mounted between two glass cannulae in an arteriograph (Living Systems Instrumentation, Burlington, VT, USA) (Halpern et al. 1984) at room temperature (18-21°C) and pressurized to 80 mmHg, under conditions of no lumenal flow. Both the lumen of the artery, and the arteriograph contained the low-Cl− HBSS. A pre-determined level of pressure was maintained via a pressure-servo control system (PS200, Living Systems Instrumentation). Arteries were viewed through a Leica DM IRB inverted microscope and a measurement of the internal diameter was made from a video image using a video dimension analyser (V91, Living Systems Instrumentation). The HBSS was warmed to 37 °C via a glass heat exchanger within the arteriograph chamber, so that constant superfusion of the artery was not required. If a sustained reduction in artery diameter was seen on warming to 37 °C, then a myogenic response was considered to be present and the artery was used for further experiments. Arteries were then cooled to room temperature, and allowed to equilibrate for at least 30 min before electrophysiological measurements were made.
SERIS electrode techniques
Self-referencing electrode technology was originally developed to detect steady extracellular currents by measuring the electrical field density (Jaffe & Nuccitelli, 1974; Jaffe, 1985). Kuhtreiber & Jaffe (1990) described a derivative of the original voltage-sensing ‘vibrating probe’ that was sensitive to specific ion species (SERIS electrodes). SERIS electrodes are based on standard neutral carrier ion-selective microelectrode technology (Ammann, 1986), enabling changes in the activity of an ion to be measured potentiometrically. In practice, a SERIS electrode is repeatedly stepped between two positions, to make a self-referencing measurement of the weak changes in ionic activity that result from and reflect the presence of a steady-state ion flux from a cell or a piece of tissue. This is advantageous over standard microelectrode techniques because it is non-invasive, allowing flux measurements to be made from an artery that is actively contracting. Because the measurement made is self-referencing, it has the potential, under optimal conditions, to resolve nanovolt differences; far below the noise level of conventional microelectrodes. Measurements resolving fluxes in the order of pmol cm−2 s−1 have been reported using the SERIS electrode technique (see Smith et al. 1994).
Preparation of Cl−-selective electrodes
Electrodes with tip diameters of 3 μm were pulled from thin walled borosilicate glass (Clark Electromedical: GC150T), and oven-dried under a beaker at 180 °C for 24 h. Once the electrodes were dry, 0.05 ml of a silanizing compound, N,N-dimethyltrimethylsilylamine (Fluka, Dorset, UK), was applied under the lip of the beaker, still at 180 °C. After 30 min, the beaker was removed and the silanizing compound allowed to evaporate for 1 h at 180 °C. Electrodes were now ready for use, and could be stored in a dessicator at room temperature for several days.
Electrodes were back-filled with 10 mm NaCl, and then front-filled with a 100 μm column of a Cl−-selective ionophore (Fluka Cl− ionophore I - cocktail A).
Electrophysiological set-up
The electrode was attached to the amplifier headstage via a Ag-AgCl wire. The bath electrode was a Ag-AgCl pellet attached to a glass capillary filled with 3 m sodium acetate in 3 % agar. The electrode was positioned using a motor-driven manipulator (Newport, UK), controlled by ‘Ionview’ software (R. Sanger, Marine Biology Laboratories, Woods Hole, MA, USA). The electrode could be positioned with micrometre accuracy using a calibrated video monitor.
Electrode calibration
The Nernstian properties of each electrode were measured by placing the electrode in a series of standard NaCl solutions (1, 10 and 100 mm) balanced to a physiological osmolarity (300 mosmol l−1) with sodium gluconate. By plotting the voltage output of the probe against log Cl− concentration, a linear regression yielded a Nernstian slope of -60.5 ± 1.25 mV (n = 15; mean ±s.e.m.). An example is shown in Fig. 1A. This linear relationship enables the voltage output of the probe to be correlated to a Cl− concentration.
Figure 1. Calibration of Cl−-selective SERIS electrodes.
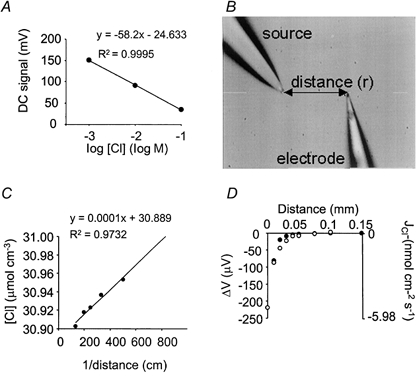
A, the Nernstian properties of each electrode were tested by measuring the voltage output (DC signal) of the probe in 1, 10 and 100 mm NaCl (balanced to 300 mosmol l−1 with sodium gluconate). A regression of the voltage ouput (mV) against the log of the Cl− concentration yielded a line with a mean Nernstian slope of -60.5 ± 1.25 mV (n = 15; mean ±s.e.m.). A single example is shown, with a Nernstian slope of -58.2 mV. R2 = regression coefficient. B, chloride-selective probes were verified against an artificial point source - a glass pipette filled with 0.5 % agar containing 120 mm NaCl and 30 mm sodium gluconate. The electrode was positioned at known distances from the source. Two measurements were made at any one distance: (1) a static measurement (DC signal), (2) A self-referencing measurement (AC signal) over a 10 μm excursion at 0.3 Hz. C, the DC signal measurements were converted to concentrations using the Nernstian relationship in A. The characteristics of the Cl− gradient were defined by a plot of Cl− concentration (C) against 1/distance, fitted with a linear regression in the format: C = CB+K/r, where CB = background Cl− concentration, K = empirical constant, r = distance from source (cm). D, from the characteristics of the Cl− gradient, a prediction of the differential voltage (AC signal) (mV; •), self-referenced over a 10 μm excursion, can be made from the equation: ΔV = S[(-KΔr)/(CBr2+Kr)]/2.3, where S = Nernstian slope (see panel A), CB = background Cl− concentration, K = empirical constant, r = distance from source (cm), and Δr = excursion distance (cm). This follows closely the measured AC signal (○).
Verification of the SERIS probe technique using an artificial Cl− source
A steady-state Cl− gradient was established using an artificial point source that was transduced by the SERIS electrode into a voltage gradient. The Cl− source was a glass electrode with a 3-5 μm tip, filled with 0.5 % agar containing 120 mm NaCl and 30 mm sodium gluconate. This was placed in a bath containing a reciprocal ionic composition of 30 mm NaCl and 120 mm sodium gluconate, and allowed to reach a steady state for 1 h.
The SERIS electrode was positioned at known distances from the source, and static voltage measurements were made (i.e. without self-referencing movements) to obtain the ‘DC signal’ (Fig. 1B). These static DC signal measurements were converted to Cl− concentrations (μmol cm−3) using the Nernstian calibration (Fig. 1A). By regressing 1/distance against Cl− concentration (C), as previously described (Kuhtreiber & Jaffe, 1990; Smith et al. 1994), the characteristics of the Cl− gradient was defined as:
where CB = background Cl− concentration (μmol cm−3), K = the calculated empirical constant (μmol cm−2), which defines the diffusion properties of the source, and r = distance from source (cm) (Fig. 1C).
A prediction for the differential (self-referencing) voltage change (AC signal) (ΔV) as a function of distance from the source can be made using the equation:
where S = Nernstian slope (mV) and Δr = excursion distance (cm). An example is shown in Fig. 1D.
Measured differential AC signals were compared with the prediction. At known distances from the source, the motor-driven manipulator was used to move the SERIS electrode over a 10 μm excursion at a rate of 0.3 Hz and the difference in the signal at the two extremes of the excursion was measured. The measured differential voltage (AC signal) was shown to follow closely the prediction (Fig. 1D).
Measuring Cl− flux from a pressurized artery
Measurements were made from the artery, either at room temperature (no myogenic tone), or at 37 °C. The SERIS electrode has very poor temporal resolution (see section below: ‘Data collection and analysis’) and is not suitable for measuring rapid changes in flux, so all measurements were made in the steady state at least 15 min after the development of a myogenic contraction.
At room temperature, the electrode was positioned so that the tip just touched the artery wall. This was set as distance zero. The electrode was then moved 1 μm away from the artery, and self-referencing measurements made over a 10 μm excursion, at 0.3 Hz for a range of pressures (20, 40, 60 and 80 mmHg), applied in random order. At each pressure, the electrode was reset to the zero position, to account for the change in artery diameter (see Fig. 2A). Between each set of measurements ‘at tissue’, a set of ‘background’ measurements were made 500 μm away from the artery, far beyond the limits of any gradient in ion activity.
Figure 2. Measurement of pressure-dependent Cl− fluxes.
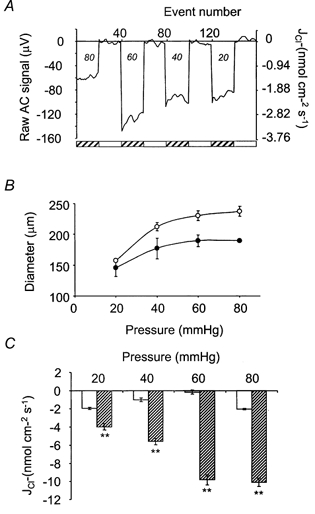
The electrode was positioned so that the tip just touched the artery wall. This was set as distance zero. The electrode was then moved 1 μm away from the artery, and self-referencing measurements made over a 10 μm excursion, at 0.3 Hz. Between each set of measurements ‘at tissue’, a set of ‘background’ measurements were made 500 μm away from the artery. A, a representative data trace. Typically, at background, the noise of the probe was less than ± 5 μV, which equates to a flux of approximately 0.1 nmol cm−2 s−1. These measurements were made at room temperature. Measurements made close to the artery (at tissue) (hatched bars), over a range of pressures (mmHg; indicated in italics), are clearly resolvable from background measurements (open bars). B, at 37 °C (•), the diameter of the artery reduced, compared with 18-21 °C (○), because of myogenic contraction (n = 5). C, myogenic contraction was accompanied by an increase in efflux of Cl−. This increase in efflux at 37 °C ( ) was significant, when compared with measurements at the equivalent flux at 18-21 °C (□) at each pressure. **P < 0.01(n = 5).
) was significant, when compared with measurements at the equivalent flux at 18-21 °C (□) at each pressure. **P < 0.01(n = 5).
On warming to 37 °C, at 80 mmHg, myogenic tone developed. The SERIS electrode remained in position during warming, and there was no significant change in the DC signal, indicating that the electrode remained stable. The electrode was then repositioned as previously described, by just touching the artery wall and then moving 1 μm away for the ‘at tissue’ measurements, and the self-referencing measurements were repeated over a range of pressures.
Data collection and analysis
The control of movement of the SERIS electrode and acquisition of data were accomplished using the Ionview software application (R. Sanger, Marine Biological Laboratories). An analog-digital converter provided AC and DC measurements at 1 ms intervals.
At 0.3 Hz, the electrode was static at each extreme of the 10 μm excursion, for 1.66 s. Data collected during the static period at both extremes of the excursion were divided into 10 bins, the first three of which were discarded to avoid mixing artefacts. The data in each of remaining seven bins were averaged, and seven points added to a separate data buffer for each position. Each buffer reported a running average of 40 data points. This impacts directly on the temporal resolution of SERIS electrode measurements. In practice, SERIS electrode measurements are best suited to measurement of fluxes in the steady state. Both the direct voltage output of the electrode (DC signal), and differential voltage measurement (AC signal) were observed for at least 20 excursions (to purge the data buffers) and then recorded for 20 excursions. Data are expressed as mean values ±s.e.m. If the DC signal changed by more than 2 mV h−1 at background, data were not used for further analysis.
Voltage measurements were converted to fluxes. For small voltage changes (Smith et al. 1994), the change in Cl− concentration (ΔC) can be calculated using:
where ΔC = change in concentration (μmol cm−3) and ΔV = differential voltage (mV).
Substituting ΔC into Fick's Law:
where JCl = Cl− flux (μmol cm−2 s−1) and D = diffusion constant (cm2 s−1) for Cl− (2.01 × 10−5 at 21 °C and 2.11 × 10−5 at 37 °C) (Vanysek, 2000).
The flux at room temperature was used as the control condition for these experiments. Significant differences from control were tested using one-way analysis of variance (ANOVA). P < 0.05 was regarded as indicating a significant difference, and P < 0.01 as indicating a highly significant difference.
Drugs
All drugs were made up as 1000 × stock in Milli-Q, unless otherwise stated. 5-Nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), indanyloxyacetic acid 94 (IAA-94) and 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS) were dissolved in dimethyl sulphoxide. IAA-94 and NPPB were supplied by Research Biochemicals International (distributed by Semat, St Albans, UK). Other drugs were supplied by Sigma UK. The Nernstian properties of the electrodes were measured in each drug, before use, to confirm that electrodes remained stable. Drugs were applied from the stock solution directly to the bath, and mixed with a Pasteur pipette to achieve the required final concentration.
RESULTS
Resolution of the Cl−-selective electrode
Typically, at background, the noise of the probe was less than ±5 μV, which would equate to a flux resolution of the electrode of approximately 0.1 nmol cm−2 s−1. A raw data recording is shown in Fig. 2A. These measurements were made at room temperature. Measurements made close to the artery (at tissue), over a range of pressures, are clearly resolvable from background measurements.
Temperature- and pressure-dependent Cl− efflux
On warming from room temperature (18-21 °C) to 37 °C, at 80 mmHg, arteries constricted from 237 ± 3.5 μm to 189 ± 2.0 μm (n = 5), demonstrating a temperature-dependent myogenic contraction (Fig. 2B). The temperature dependence of the myogenic response has been described previously (Doughty & Langton, 1998a; Doughty et al. 1998). Myogenic contraction was accompanied by a pressure- and temperature-dependent increase in Cl− efflux from these arteries. At 80 mmHg, efflux (a negative flux) increased from -2.03 ± 0.071 nmol cm−2 s−1 at room temperature to -10.1 ± 0.54 nmol cm−2 s−1 at 37 °C. (n = 5) (Fig. 2C), which is consistent with an increase in Cl− conductance.
Cl− channel blockers
Cl− channel blockers have been shown previously to block myogenic tone in cerebral resistance arteries. IAA-94 and DIDS (Nelson et al. 1997) and NPPB (Doughty et al. 1998) were all tested against Cl−-selective electrodes for use in these experiments; 100 μm IAA-94 (n = 3), 100 μm NPPB (n = 3) and 500 μm DIDS (n = 3) disrupted the electrical properties of the SERIS electrodes such that they no longer displayed Nernstian properties and therefore these drugs could not be used for further experiments (Fig. 3A). An alternative Cl− channel blocker, tamoxifen, has been shown to be a selective inhibitor of ClC-3 type chloride channels in vascular smooth muscle (Yamazaki et al. 1998), and had no effect on SERIS electrode properties (Fig. 3B). Therefore tamoxifen was used as the drug of choice for further experiments.
Figure 3. Testing Cl− channel blockers on SERIS electrodes.
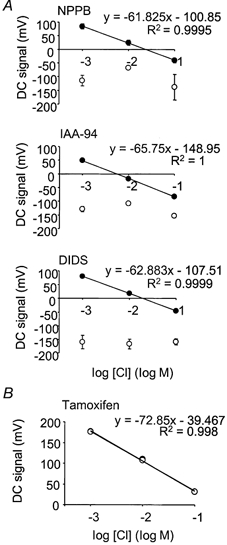
A, 100 μm NPPB (n = 3), 100 μm IAA-94 (n = 3) and 500 μm DIDS (n = 3) disrupted the Nernstian properties of the electrodes. Control, •; drug, ○. B, 10 μm tamoxifen did not affect the Nernstian properties of the Cl−-selective electrodes (n = 3), and was used for further investigations. Control, •; tamoxifen, ○.
The effect of tamoxifen on myogenic tone and Cl− efflux
In pressurized rat cerebral arteries, tamoxifen concentration-dependently inhibited myogenic tone, and was fitted with a Hill equation with an IC50 of 3.75 ± 0.2 μm, and a slope of -1.1 ± 0.1 (n = 5) (Fig. 4A). This effect was poorly reversible on washout. At 80 mmHg, mean artery diameter was reduced from 237 ± 8.0 μm at room temperature to 189 ± 2.0 μm at 37 °C. This reduction in diameter was abolished by 10 μm tamoxifen (235 ± 12.5 μm) (n = 4) (Fig. 4B). Cl− efflux increased significantly from -1.83 ± 0.075 nmol cm−2 s−1 at room temperature to -9.21 ± 0.55 nmol cm−2 s−1 at 37°C (P < 0.01). This increase in efflux was abolished by 10 μm tamoxifen (-1.53 ± 0.19 nmol cm−2 s−1) (n = 4) (Fig. 4C).
Figure 4. The effects of tamoxifen on myogenic tone and Cl− fluxes.
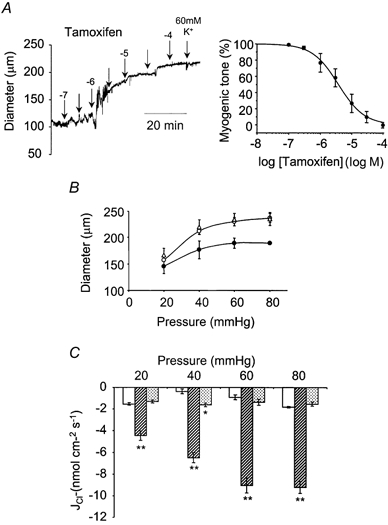
A, tamoxifen concentration-dependently depressed myogenic contraction. An example data trace is shown. Inhibition of myogenic tone by tamoxifen was fitted to a Hill Equation, with an IC50 of 3.75 ± 0.2 μm and a slope of -1.1 ± 0.1 (n = 5). B, 10 μm tamoxifen abolished myogenic tone at all pressures tested. (18-21 °C, ○; 37 °C, •; 10 μm tamoxifen, 37 °C, ▵) (n = 4). C, 10 μm tamoxifen also abolished pressure- and temperature-dependent Cl− efflux at all pressures tested (18-21 °C, □; 37 °C,  ; 10 μm tamoxifen, 37 °C,
; 10 μm tamoxifen, 37 °C,  ) (n = 4). **P < 0.01, *P < 0.05, compared to control at 18-21 °C.
) (n = 4). **P < 0.01, *P < 0.05, compared to control at 18-21 °C.
Non-specific effects of tamoxifen
Tamoxifen has also been shown to block Ca2+ channels in vascular smooth muscle (Song et al. 1996). At 80 mmHg, in the presence of 10 μm tamoxifen, depolarization with 60 mm KCl did not produce contraction (an example is shown in Fig. 4A), which is consistent with an effect of tamoxifen on Ca2+channels in this tissue.
The effects of a Ca2+ channel blocker, nimodipine, were compared with the effects of tamoxifen (Fig. 5). At 80 mmHg, artery diameter was reduced from 241 ± 10 to 190 ± 2.4 μm on warming to 37 °C. Nimodipine (2 μm) abolished this reduction in diameter (240 ± 14 μm) (n = 3). There was no further effect of tamoxifen (10 μm) on artery diameter in the presence of nimodipine (242 ± 15 μm) (Fig. 5A). In paired measurements from the same arteries Cl− efflux increased from -1.76 ± 0.071 nmol cm−2 s−1 at room temperature to -7.74 ± 0.69 nmol cm−2 s−1 at 37 °C. Nimodipine (2 μm) did not affect this flux increase (-8.46 ± 0.80 nmol cm−2 s). In the presence of nimodipine, 10 μm tamoxifen abolished the flux increase observed on warming (-1.28 nmol cm−2 s−1) (n = 3) (Fig. 5B).
Figure 5. The effects of nimodipine on myogenic tone and Cl− fluxes.
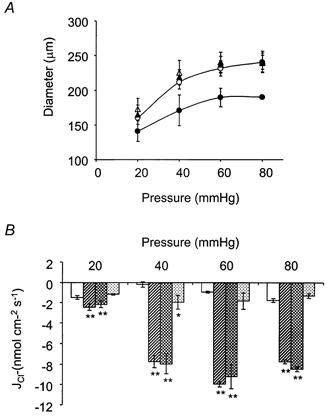
A, 2 μm nimodipine abolished myogenic tone at all pressures tested. (18-21 °C, ○; 37 °C, •; 2 μm nimodipine, 37 °C, ▴). Tamoxifen (10 μm) showed no further effect on tone in the presence of nimodipine (▵) (n = 3). B, 2 μm nimodipine did not affect pressure- and temperature-dependent Cl− fluxes at all pressures tested. Tamoxifen (10 μm), in the presence of 2 μm nimodipine, abolished pressure- and temperature-dependent Cl− efflux at all pressures tested (18-21 °C, □; 37 °C,  ; 2 μm nimodipine, 37 °C,
; 2 μm nimodipine, 37 °C,  ; 2 μm nimodipine + 10 μm tamoxifen, 37 °C,
; 2 μm nimodipine + 10 μm tamoxifen, 37 °C,  ) (n = 4). **P < 0.01, *P < 0.05, compared to control at 18-21 °C.
) (n = 4). **P < 0.01, *P < 0.05, compared to control at 18-21 °C.
DISCUSSION
This study is the first application of a Cl−-selective SERIS electrode technique to an intact artery. The aim of the study was to establish that the technique can be applied to small blood vessels and whether the myogenic response is associated with a change in Cl− flux. SERIS electrode technology has been developed to enable a non-invasive measurement of ion flux from isolated tissue and/or cells. Several reviews have been written, the most recent being that of Smith & Trimarchi (2001). In addition, several studies enable direct comparison of fluxes measured using SERIS electrodes and other techniques, such as whole-cell patch clamp (Knox et al. 1996; Shirihai et al. 1998) and micro-Ussing chambers (Wangemann et al. 1995). In each case there was broad agreement between the techniques. In practice, the ratio of signal to noise is a limiting factor. According to Smith et al. (1994), the intrinsic electrode noise is influenced by the neutral carrier being used, and the length of the column, which together determine the electrode resistance. Under optimal conditions, the intrinsic noise can be reduced to about ±0.5 μV (Smith et al. 1994). In our hands, the background noise was closer to ±5 μV and under our conditions this equates to a flux of less than 0.1 nmol cm−2 s−1. Cl− fluxes from rat cerebral resistance arteries were clearly resolvable. Fluxes measured from an artery ranged between 1 and 10 nmol cm−2 s−1, so the signal: noise ratio was always better than 10:1. This technique therefore provides a powerful tool for the study of membrane flux in small arteries.
Data from the present study demonstrate multiple correlations between a tamoxifen-sensitive Cl− efflux and the myogenic response of rat cerebral arteries. The Cl− efflux was graded with the applied transmural pressure, mirroring the graded depolarization that has been previously reported for this preparation (Knot & Nelson, 1998), and blocked by tamoxifen. Both myogenicity and Cl− flux were reversibly depressed by cooling to room temperature. The Cl− efflux was unaffected by nimodipine, a selective Ca2+ antagonist that inhibits myogenic tone but which does not hyperpolarize myogenic rat cerebral arteries (Knot & Nelson, 1998). In the continued presence of nimodipine, the Cl− efflux was inhibited by tamoxifen.
Non-selectivity of Cl− channel blockers
Cl− channel blockers have been shown to depress myogenic contraction of pressurized rat cerebral arteries (Nelson et al. 1997; Doughty et al. 1998). Interpretation of contraction data is difficult as many Cl− channel blockers have also been shown to be effective blockers of Ca2+ channels, non-specific cation channels and Ca2+-dependent force generation in rat cerebral and pulmonary arteries (Doughty et al. 1998; Kato et al. 1999; Welsh et al. 2000).
The study of Nelson et al. (1997) combined measurements of membrane potential and vessel diameter to show that the Cl− channel blockers IAA-94 and DIDS hyperpolarized membrane potential in pressurized arteries. In the same study it was shown that a reduction in extracellular [Cl−] enhanced the myogenic response, consistent with depolarization, but in this instance measurements of membrane potential were not made. The interpretation placed on the data in the Nelson et al. (1997) study was that an increase in Cl− conductance underlies the myogenic depolarization. Block of Ca2+ channels is not likely to explain these data as selective Ca2+ channel blockers, which are effective at relaxing myogenic tone in cerebral arteries, do not hyperpolarize rat cerebral arteries (Knot & Nelson, 1995). However, the report of Welsh et al. (2000), in which IAA-94, DIDS and tamoxifen (chemically disparate blockers of Cl− channels) are shown to inhibit a volume-sensitive, non-selective cation conductance in rat cerebral artery myocytes, does weaken the findings of Nelson et al. (1997).
What channels underlie myogenic depolarization?
Pressure-induced depolarization appears to be mediated by more than one mechanism. In renal afferent arterioles, for example, myogenic tone is unaffected by Cl− channel blockers or changes in [Cl−] but is sensitive to Gd3+, at concentrations that block non-selective cation channels but not voltage-gated Ca2+ channels (Takenaka et al. 1998). Interestingly, Welsh et al. (2000) have recently suggested that a volume-regulated, non-specific cation current may underlie both pressure-induced myogenic depolarization, and hyposmotic stress-induced depolarization, in rat cerebral arteries. This current is not only inhibited by Gd3+ ions, but also by a range of Cl− channel blockers, including tamoxifen. However, Gd3+ has been identified as a blocker of native volume-regulated, Ca2+-independent Cl− channels in Xenopus oocytes (Ackerman et al. 1994). Thus, agents that collectively have actions on Cl− and non-specific cation channels currently define the ionic changes that underlie myogenic depolarization. Pharmacological tools alone appear to discriminate poorly between Cl− channels and non-specific cation channels in pressurized arteries, which has consequences for interpretation of data based on measurements of force or diameter.
In our experiments, the relaxation of myogenic tone by tamoxifen could be partly attributable to block of a non-specific cation current. However, our experiments provide direct evidence of a tamoxifen-sensitive pressure-induced Cl− efflux that is closely associated with myogenicity in cerebral arteries. Augmentation of Cl− efflux equates to a depolarizing inward current, which would produce a smooth muscle contraction. Although the pharmacology of Cl− channels is poorly defined, volume-regulated Cl− channels are known to exist in vascular smooth muscle. The tamoxifen sensitivity of Cl− efflux measured in these experiments suggests a volume-regulated Cl− channel is present. This is consistent with the expression of ClC-3 subunits, either as homomeric channels, or in combination with another ClC subunit, as heterodimers (Duan et al. 1997).
Alternatively, depolarization could be achieved by inhibition of an outward current. At moderately negative potentials, the principal conductance mediating hyperpolarizing outward current is K+. Although there are many studies demonstrating that arterial tone is sensitive to changes in K+ conductance (Brayden et al. 1991; Brayden & Nelson, 1992; Knot et al. 1997), only the report of McPherson & Keily (1995), which shows depression of an inwardly rectifying potassium current, has suggested that myogenic depolarization is the direct result of a reduction in potassium conductance. There is evidence, however, that large-conductance calcium-activated K+ channels, activated by Ca2+ sparks, serve to limit myogenic depolarization (Jaggar et al. 1998).
Why use a SERIS electrode?
We have developed a Cl−-selective SERIS electrode, so that a direct, non-invasive measurement of Cl− flux can be made from actively contracting, myogenic cerebral arteries. Assessing the effects of Cl− channel blockers simultaneously on both Cl− efflux and arterial tone overcomes some of the problems of interpretation, due to the possible effects of Cl− channel blockers on Ca2+ and non-specific cation channels. We have shown that a blocker of ClC-3, tamoxifen (Duan et al. 1997;Yamazaki et al. 1998), abolishes both myogenic tone and pressure-dependent Cl− eflux. Tamoxifen is known to block Ca2+ channels (Song et al. 1996), which could account directly for the abolition of tone. Block of Ca2+ current and/or reduction in [Ca2+]i appears not to explain the inhibition of Cl− efflux, as nimodipine, a selective Ca2+ channel antagonist, inhibited myogenic tone but was without effect on Cl− flux.
Interpretation of net fluxes measured with SERIS electrodes
It must be remembered that the SERIS electrode technique measures net movement of ions and may therefore detect the activity of pumps and exchangers as well as ion channels. In smooth muscle, intracellular Cl− is substantially higher than would be predicted by a passive Nernstian distribution, and there is general agreement that Cl− is actively accumulated (Aickin, 1990). Three mechanisms of chloride accumulation have been described: (1) Na+-K+-2Cl− co-transport, (2) Cl−-HCO3− exchange and (3) a Na+-independent ATPase, designated pump III (Chipperfield et al. 1997; Davis et al. 1997). In our experiments Cl− accumulation is likely to be attenuated by lowering the concentration of extracellular Cl− to approximately 30 mm. Extracellular [Cl−] was reduced to improve the resolution of the Cl−-selective electrode (Doughty & Langton, 1998b). It also displaces the Cl− equilibrium potential, ECl, to more positive potentials, increasing the driving force on Cl− and thus increasing Cl− efflux (Nelson et al. 1997).
Although accumulation mechanisms are electroneutral, and do not therefore account directly for the myogenic depolarization, they cause intracellular Cl− accumulation, thus increasing the driving force on a depolarizing Cl− channel current (Davis et al. 1997). Cl− accumulation is stimulated by noradrenaline in rat femoral artery and saphenous vein, an effect correlated to depolarisation and constriction (Davis et al. 1997). Furthermore, in the rat model of deoxycorticosterone/salt-induced hypertension, Cl− accumulation is enhanced (Brown et al. 2000). To date, augmentation of Cl− accumulation in response to intramural pressure has not been demonstrated, although inhibition of Cl− accumulation, using the loop diuretic, bumetanide (Na+-K+-2Cl− co-transport; Haas, 1994), low extracellular HCO3− (depresses Cl−-HCO3− exchange; Davis, 1992) or acetazolamide (pump III; Chipperfield et al. 1993), has no significant effect on the myogenic contraction of cerebral arteries (Doughty & Langton, 1998b). An increase in Cl− accumulation would not of itself explain the large increase in Cl− efflux associated with myogenic contraction. It is more likely that increased Cl− flux is a result of a direct channel effect, for example, and increased probability of opening or numbers of channels.
In summary, the Cl−-selective SERIS electrode technique provides electrophysiological evidence to support the hypothesis that increases in Cl− conductance underlie the pressure-induced depolarization of myogenic cerebral arteries in the rat. The poor selectivity of Cl− channel blockers has limited their application in studies of intact arteries. By making dual measurements of the effect of these blockers on both Cl− flux and arterial tone, these problems are attenuated.
Acknowledgments
The authors wish to thank Dr Peter Smith, Mr Richard Sanger and Mrs Kasia Hammar (Marine Biology Laboratories, Woods Hole, MA, USA) for advice on the SERIS probe. This work was supported by the British Heart Foundation, grant no. PG97/182.
References
- Ackerman MJ, Wickman KD, Clapham DE. Hypotonicity activates a native chloride current in Xenopus oocytes. Journal of General Physiology. 1994;103:153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin CC. Chloride transport across the sarcolemma of vertebrate smooth and skeletal muscle. In: Alvarez-Leefmans FJ, Russel JM, editors. Chloride Channels and Carriers in Nerve, Muscle and Glial Cells. New York: Plenum Press; 1990. pp. 209–249. [Google Scholar]
- Ammann D. In: Ion-selective Microelectrodes: Principles, Design and Application. Ammann D, editor. New York: Springer-Verlag; 1986. [Google Scholar]
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Brayden JE, Quayle JM, Standen NB, Nelson MT. Role of potassium channels in the vascular response to endogenous and pharmacological vasodilators. Blood Vessels. 1991;28:147–153. doi: 10.1159/000158854. [DOI] [PubMed] [Google Scholar]
- Brown RA, Chipperfield AR, Davis JPL, Harper AA. Increased (Na+K+Cl−) cotransport in rat arterial smooth muscle in deoxycorticosterone (DOCA)/salt-induced hypertension. Journal of Vascular Research. 2000;36:492–501. doi: 10.1159/000025692. [DOI] [PubMed] [Google Scholar]
- Chipperfield AR, Davis JPL, Harper AA. An acetazolamide-sensitive inward chloride pump in vascular smooth muscle. Biochemical and Biophysical Research Communications. 1993;194:407–412. doi: 10.1006/bbrc.1993.1834. [DOI] [PubMed] [Google Scholar]
- Chipperfield AR, Davis JPL, Harper AA. Activation of two inward chloride pumps in rat arterial smooth muscle by noradrenaline in vitro. Journal of Physiology. 1997;501.P:116P. [Google Scholar]
- Davis JPL. The effects of Na+-K+-Cl− co-transport and Cl−-HCO3− exchange blockade on the membrane potential and intracellular chloride levels of rat arterial smooth muscle in vitro. Experimental Physiology. 1992;77:857–862. doi: 10.1113/expphysiol.1992.sp003652. [DOI] [PubMed] [Google Scholar]
- Davis JPL, Harper AA, Chipperfield AR. Stimulation of intracellular chloride accumulation by noradrenaline and hence potentiation of its depolarization of rat arterial smooth muscle in vitro. British Journal of Pharmacology. 1997;122:639–642. doi: 10.1038/sj.bjp.0701431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty JM, Langton PD. A transient dilatation of pressurised rat cerebral arteries during rapid pressure increases is mediated by nitric oxide. Pflügers Archiv. 1998a;436:220–226. doi: 10.1007/s004240050625. [DOI] [PubMed] [Google Scholar]
- Doughty JM, Langton PD. Chloride accumulation and the myogenic response. Journal of Physiology. 1998b;509.P:133P. doi: 10.1111/j.1469-7793.2001.t01-1-00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty JM, Miller AL, Langton PD. Non-specificity of chloride channel blockers in rat cerebral arteries: block of the L-type calcium channel. Journal of Physiology. 1998;507:433–439. doi: 10.1111/j.1469-7793.1998.433bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- Haas M. The Na-K-Cl cotransporters. American Journal of Physiology. 1994;267:C869–885. doi: 10.1152/ajpcell.1994.267.4.C869. [DOI] [PubMed] [Google Scholar]
- Halpern W, Osol G, Coy GS. Mechanical behavior of pressurized in vitro prearteriolar vessels determined with a video system. Annals of Biomedical Engineering. 1984;12:463–479. doi: 10.1007/BF02363917. [DOI] [PubMed] [Google Scholar]
- Harder DR. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circulation Research. 1984;55:197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- Harder DR, Gilbert R, Lombard JH. Vascular muscle cell depolarization and activation in renal arteries on elevation of transmural pressure. American Journal of Physiology. 1987;253:F778–781. doi: 10.1152/ajprenal.1987.253.4.F778. [DOI] [PubMed] [Google Scholar]
- Jaffe LF. Extracellular current measurements with a vibrating probe. Trends in Neurosciences. 1985;8:517–521. [Google Scholar]
- Jaffe LF, Nuccitelli R. An ultrasensitive vibrating probe for measuring steady electrical currents. Journal of Cell Biology. 1974;63:614–628. doi: 10.1083/jcb.63.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH, Stevenson AS, Nelson MT. Voltage dependence of Ca2+ sparks in intact cerebral arteries. American Journal of Physiology. 1998;274:C1755–1761. doi: 10.1152/ajpcell.1998.274.6.C1755. [DOI] [PubMed] [Google Scholar]
- Kato K, Evans AM, Kozlowski RZ. Relaxation of endothelin-1-induced pulmonary arterial constriction by niflumic acid and NPPB: mechanism(s) independent of chloride channel block. Journal of Pharmacology and Experimental Therapeutics. 1999;288:1242–1250. [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. American Journal of Physiology. 1995;269:H348–355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. Journal of Physiology. 1998;508:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ, Zimmermann PA, Nelson MT. Extracellular K+-induced hyperpolarisation and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. Journal of Physiology. 1997;492:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox RJ, Kao LS, Jonas E, Smith PJS, Connor JA, Kaczmarek LK. Ca2+ influx and activation of a cation current are coupled to an intracellular Ca2+ mobilization of peptidergic neurons. Journal of Physiology. 1996;494:627–639. doi: 10.1113/jphysiol.1996.sp021520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhtreiber WM, Jaffe LF. Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. Journal of Cell Biology. 1990;110:1565–1573. doi: 10.1083/jcb.110.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. American Journal of Physiology. 1996;271:C435–454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- McPherson GA, Keily SG. Electrophysiological properties of the rat middle cerebral artery at different levels of passive wall tension. Clinical and Experimental Pharmacology and Physiology. 1995;22:724–731. doi: 10.1111/j.1440-1681.1995.tb01926.x. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Conway MA, Knot HJ, Brayden JE. Chloride channel blockers inhibit myogenic tone in rat cerebral arteries. Journal of Physiology. 1997;502:259–264. doi: 10.1111/j.1469-7793.1997.259bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P, Loirand G, Baron A, Mironneau C, Mironneau J. Ca2+ channel activation and membrane depolarization mediated by Cl− channels in response to noradrenaline in vascular myocytes. British Journal of Pharmacology. 1991;104:1000–1006. doi: 10.1111/j.1476-5381.1991.tb12540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirihai O, Smith PJS, Hammar K, Dagan D. H+ and K+ gradient generated by microglea H/K-ATPase. Glia. 1998;23:339–348. [PubMed] [Google Scholar]
- Smith PJS. Noninvasive ion probes - tools for measuring transmembrane ion flux. Nature. 1995;378:645–646. doi: 10.1038/378645a0. [DOI] [PubMed] [Google Scholar]
- Smith PJS, Hammar K, Porterfield DM, Sanger RH, Trimarchi JR. A self-referencing, non-invasive, ion-selective electrode for single cell detection of trans-plasma membrane calcium flux. Microscopy Research Techniques. 1999;46:398–417. doi: 10.1002/(SICI)1097-0029(19990915)46:6<398::AID-JEMT8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Smith PJS, Sanger RH, Jaffe LF. The vibrating Ca2+ electrode: A new technique for detecting plasma membrane regions of Ca2+ influx and efflux. In: Nuccitelli R, editor. A Practical Guide to the Study of Calcium in Living Cells. San Diego, New York, Boston, London: Academic Press; 1994. pp. 115–133. [DOI] [PubMed] [Google Scholar]
- Smith PJS, Trimarchi JR. Noninvasive measurement of hydrogen and potassium ion flux from single cells and epithelial structures. American Journal of Physiology - Cell Physiology. 2001;280:C1–11. doi: 10.1152/ajpcell.2001.280.1.C1. [DOI] [PubMed] [Google Scholar]
- Song J, Standley PR, Zhang F, Joshi D, Gappy S, Sowers JR, Ram JL. Tamoxifen (estrogen antagonist) inhibits voltage-gated calcium current and contractility in vascular smooth muscle from rats. Journal of Pharmacology and Experimental Therapeutics. 1996;277:1444–1453. [PubMed] [Google Scholar]
- Takenaka T, Suzuki H, Okada H, Hayashi K, Kanno Y, Saruta T. Mechanosensitive cation channels mediate afferent arteriolar myogenic constriction in the isolated rat kidney. Journal of Physiology. 1998;511:245–253. doi: 10.1111/j.1469-7793.1998.245bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanysek P. Ionic conductivity and diffusion at infinite dilution. In: Lide DR, editor. Handbook of Chemistry and Physics. Boca Raton, FL, USA: CRC Press; 2000. [Google Scholar]
- Wangemann P, Liu J, Marcus DC. Ion transport mechanisms responsible for K+ secretion and the transepithelial voltage across marginal cells of stria vacularis in vitro. Hearing Research. 1995;84:19–29. doi: 10.1016/0378-5955(95)00009-s. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Nelson MT, Eckman DM, Brayden JE. Swelling-actvated cation channels mediate depolarization of rat cerebrovascular smooth muscle by hyposmolarity and intravascular pressure. Journal of Physiology. 2000;527:139–148. doi: 10.1111/j.1469-7793.2000.t01-1-00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki J, Duan D, Janiak R, Kuenzli K, Horowitz B, Hume JR. Functional and molecular expression of volume-regulated chloride channels in canine vascular smooth muscle cells. Journal of Physiology. 1998;507:729–736. doi: 10.1111/j.1469-7793.1998.729bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


