Abstract
Postnatal development of synaptic efficacy was studied at a single glutamatergic synapse formed by the calyx of Held in the rat brainstem. Throughout postnatal development from day 7 (P7) to day 14 (P14), both the excitatory postsynaptic currents (EPSCs) evoked at a basal frequency (0.05 Hz) and spontaneous miniature EPSCs (mEPSCs) remained similar in their mean amplitudes, but became faster in their decay times.
During repetitive stimulation at 1–100 Hz, EPSCs underwent a depression. The magnitude of the depression significantly decreased from P7 to P14, whereas the time course of recovery from depression (after 10 Hz stimulation) remained similar throughout development.
The size of the readily releasable pool (RRP) of synaptic vesicles (N) and the release probability (p) were estimated from the cumulative amplitude histogram of EPSCs during high frequency stimulation. From P7 to P14, N increased 2-fold, whereas p decreased to a similar extent.
The open channel blocker MK-801 caused an activity-dependent attenuation of NMDA receptor-mediated EPSCs. The blocking rate became slower from P7 to P14, further supporting the developmental decrease in p.
Given that the mean amplitudes of mEPSCs (q) and evoked EPSCs (Npq) remain constant throughout the developmental period, these results suggest that a developmental increase in N compensates for a concomitant decrease in p. We conclude that the developmental decrease in the release probability will establish a stable synapse at which only a small fraction of releasable synaptic vesicles is depleted during high frequency transmission.
Soon after axon terminals establish contacts with postsynaptic target cells, synapses undergo morphological transformations (Takahashi et al. 1987; Kandler & Friauf, 1993) and molecular replacements. Postsynaptically, for example, immature-type subunits of various receptor ion channels switch to mature-types, thereby speeding the kinetics of postsynaptic currents (Mishina et al. 1986; Takahashi et al. 1992, 1996a;Okada et al. 2000). Presynaptically, Ca2+ channel subtypes that trigger transmitter release switch from a mixture of P/Q-, N- and R-types to predominantly P/Q-type at various central synapses (Iwasaki & Takahashi, 1998; Iwasaki et al. 2000).
Regarding a developmental change in presynaptic function, it has been reported that the amplitudes of postsynaptic currents decrease during postnatal development in association with a decrease in the mean quantal content (m), i.e. the mean number of transmitter quanta released by a presynaptic action potential, at hippocampal excitatory (Bolshakov & Siegelbaum, 1995) and cerebellar inhibitory (Pouzat & Hestrin, 1997) synapses. According to the quantum hypothesis (del Castillo & Katz, 1954), m is defined as Np, where N and p denote the pool size of releasable quanta and the probability of quantal release, respectively. At various central synapses, it has been suggested that p decreases with development because the paired-pulse depression becomes facilitation (Bolshakov & Siegelbaum, 1995; Choi & Lovinger, 1997; Pouzat & Hestrin, 1997) and failures in response to presynaptic action potentials increase (Bolshakov & Siegelbaum, 1995). In contrast, at a brainstem auditory synapse, the calyx of Held, it has been reported that p increases with development (Chuhma & Ohmori, 1998). Thus it is not known whether the developmental decrease in p is general among central synapses. Also, it remains open whether N changes with development.
In this study, we examined developmental changes in N and p at the calyx of Held synapse during the second postnatal week, when rats begin to hear sound (by P13; Jewett & Romano, 1972). During this critical period, the calyceal synapse undergoes morphological (Kandler & Friauf, 1993) and various molecular (Iwasaki & Takahashi, 1998; Futai et al. 2001) changes. Our results indicated a developmental increase in N and decrease in p. Being consistent with the decrease in p, the magnitude of depression during high frequency stimulation underwent a developmental decrease, whilst the recovery rate from depression did not change. It is suggested that this auditory synapse acquires stability for high frequency transmission through postnatal development. A preliminary report of this work has been published in abstract form (Iwasaki & Takahashi, 2000).
METHODS
All experiments were performed in accordance with the guidelines of the Physiological Society of Japan. Transverse slices of the superior olivary complex (200 μm in thickness) were prepared from 7- to 16-day-old Wistar rats killed by decapitation under halothane anaesthesia, as described previously (Forsythe & Barnes-Davies, 1993; Iwasaki & Takahashi, 1998). Each slice was placed in a recording chamber and superfused with an artificial cerebrospinal fluid (ACSF) containing (mm): 120 NaCl, 2.5 KCl, 26 NaHCO3, 10 glucose, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 3 myo-inositol, 2 sodium pyruvate and 0.5 ascorbic acid (pH 7.4 when bubbled with 5 % CO2 and 95 % O2). The principal neurons in the medial nucleus of the trapezoid body (MNTB) were visually identified using a × 40 water immersion objective (Zeiss) attached to an upright microscope (Axioskop, Zeiss). The perfusate routinely contained bicuculline methiodide (10 μm; Sigma) and strychnine hydrochloride (0.5 μm; Sigma) to block inhibitory synaptic responses. The following drugs were added to the ACSF: d(-)-2-amino-5-phosphopentanoic acid (d-AP5; 50 μm; Tocris) for recording non-NMDA EPSCs, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μm; Tocris) for recording NMDA EPSCs, and tetrodotoxin (TTX, 1 μm) for recording mEPSCs. The pipette solution contained (mm): 110 CsF, 30 CsCl, 10 Hepes, 5 EGTA and 1 MgCl2 (pH 7.4, adjusted with CsOH). N-(2,6-diethylphenylcarbamoylmethyl)-triethyl-ammonium bromide (QX-314; 5 mm) was routinely included in the internal solution to suppress action potential generation. The electrode resistance was 2-4 MΩ. Series resistance was 5-10 MΩ and compensated by > 70 %. Cells were voltage clamped at a holding potential of -70 mV unless otherwise noted. The liquid junction potential between the pipette and ACSF was not corrected for. Recordings were made at room temperature (24-28 °C).
EPSCs were evoked in MNTB principal neurons using a bipolar platinum electrode positioned half-way between the midline and the MNTB (Forsythe & Barnes-Davies, 1993). EPSCs derived from the calyx of Held were identified as those evoked in an all-or-none manner for graded stimulus intensity and having amplitudes larger than 1 nA at -70 mV. The latter criterion was set to exclude those mediated by collaterals of neurons in other nuclei (usually < 150 pA at -70 mV; Forsythe & Barnes-Davies, 1993). In fact, EPSCs satisfying these criteria were recorded most often from MNTB neurons surrounded by a visually identified calyceal structure. At earlier postnatal times than P7, just after the synaptic formation (P4-6), EPSCs were too small to determine whether they derived from calyces or collaterals. Although spontaneous mEPSCs may include those arising from collaterals, these were indistinguishable in amplitude from mEPSCs induced by direct depolarization of the calyx in TTX (Sahara & Takahashi, 2001). Records were low-pass filtered at 5 kHz and digitized at 10 kHz by a LM-12 interface (Dagan Corporation). The mean time constant of the MK-801 blocking rate (τm) was calculated from the blocking rate constant of the fast (τf) and slow components (τs) and their relative amplitude (af and as) as τm =τfaf+τsas. Values in the text and figures are given as means ±s.e.m., and the significance of differences was evaluated by Mann-Whitney U test, unless otherwise noted, with 0.05 taken as the level of significance.
RESULTS
Constant quantal content and quantal size throughout development
EPSCs arising from the calyx of Held were recorded from MNTB neurons using the whole-cell recording technique in rat brainstem slices. These EPSCs were evoked with a clear threshold and had amplitudes > 1 nA (Forsythe & Barnes-Davies, 1993). At stimulus frequencies below 0.05 Hz, EPSCs of stable amplitude were evoked without a depression or failure. Throughout development from P7 to P14, the mean amplitude of these EPSCs remained similar (Fig. 1A and B). During this period, the decay time of EPSCs became significantly faster (e-fold decay time, 2.6 ± 0.10 ms at P7, 1.7 ± 0.11 ms at P10 and 0.74 ± 0.05 ms at P14, n = 15-18, P < 0.001, ANOVA) as reported in rats (Taschenberger & Gersdorff, 2000; but see Chuhma & Ohmori, 1998) and mice (Futai et al. 2001). When [Ca2+]o was reduced from 2 to 1 mm, EPSCs diminished to a similar extent among P7-14 rats (Fig. 1C, P > 0.1, ANOVA). When [Ca2+]o was increased, however, EPSC amplitudes increased differentially with postnatal ages. Between 2 and 10 mm[Ca2+]o, EPSC amplitude increased linearly in P14 rats, whereas it reached a plateau at 4 mm in P10 and at 2 mm in P7 animals. In 10 mm[Ca2+]o, the EPSC amplitude was larger in older rats. Spontaneous mEPSCs recorded after blocking action potentials with tetrodotoxin (TTX, 1 μm) also had a similar mean amplitude throughout development (Fig. 1D, P > 0.9, ANOVA) as previously reported (Chuhma & Ohmori, 1998), and their cumulative amplitude profiles in P7 and P14 rats were indistinguishable (Fig. 1E, Kolmogorov-Smirnov test). Thus, both the mean quantal content (m = Np) deduced from the mean amplitude of evoked EPSCs (Npq) and the mean quantal size (q), i.e. mean amplitude of mEPSCs, stayed constant throughout development during the second postnatal week at this synapse. In parallel with evoked EPSCs, the decay time of mEPSCs became significantly shorter with development (Fig. 1F, P < 0.01, ANOVA). The quantal content deduced from the current amplitudes might be underestimated if there was an asynchronous quantal release (Diamond & Jahr, 1995; Isaacson & Walmsley, 1995). Thus, we further estimated the mean quantal content from the synaptic current charges of evoked and miniature EPSCs (Fig. 1G). The results were essentially the same, indicating no significant change in the quantal content throughout development during the second week.
Figure 1. Evoked and miniature EPSCs in developing rats.
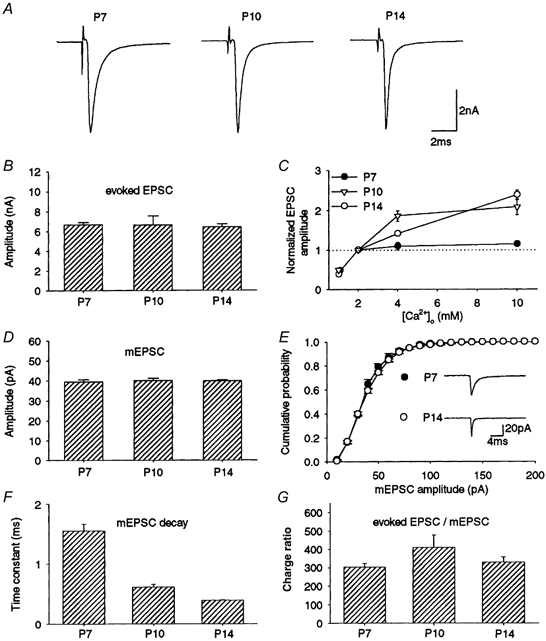
A, representative EPSCs evoked at 0.05 Hz from P7, P10 and P14 rats (averaged from 20 consecutive events in each neuron). B, the mean amplitude of EPSCs from P7 (n = 68 cells), P10 (n = 15) and P14 (n = 64) rats. Error bars in this and the following figures indicate s.e.m. C, the mean amplitude of EPSCs relative to those at 2 mm[Ca2+]o in P7 (•), P10 (▿) and P14 (○) rats (n = 5-8). D, the mean amplitude of spontaneous mEPSCs recorded in the presence of TTX (1 μm) in P7, P10 and P14 rats (4 cells each, derived from 2-3 rats). E, cumulative amplitude histogram of mEPSCs in P7 and P14 rats (each derived from 4 cells). No significant difference with Kolmogorov-Smirnov test. Sample records are mEPSCs each averaged from 120 events. F, the mean decay time constant of mEPSCs at P7, P10 and P14 (n = 3 cells at each age). The decay time of mEPSCs could be fitted by a single exponential function. The mean time constants were 1.6 ± 0.12 ms at P7, 0.61 ± 0.04 ms at P10 and 0.39 ± 0.01 ms at P14 (n = 3 at each age, significant difference between these values, P < 0.01, ANOVA). G, the mean quantal content assessed from charges of evoked EPSCs and mEPSCs at P7, P10 and P14 (n = 15-45 cells, no significant difference). Evoked EPSCs and mEPSCs were derived from different cells.
Decrease in synaptic depression with postnatal development
At this synapse in normal [Ca2+]o, paired-pulse stimulation caused a depression of the second response (Fig. 2A). The magnitude of the depression varied among interpulse intervals (5 ms to 10 s) and their relationship was multi-phasic, suggesting that multiple mechanisms might underlie the paired-pulse depression. At all intervals between 5 ms and 10 s, the magnitude of depression was greater at P7 than at P14 (Fig. 2B, P < 0.01). The results at this synapse are similar to those at other central synapses which suggest that the release probability decreases with development (Bolshakov & Siegelbaum, 1995; Choi & Lovinger, 1997; Pouzat & Hestrin, 1997).
Figure 2. Developmental decrease in paired-pulse depression.
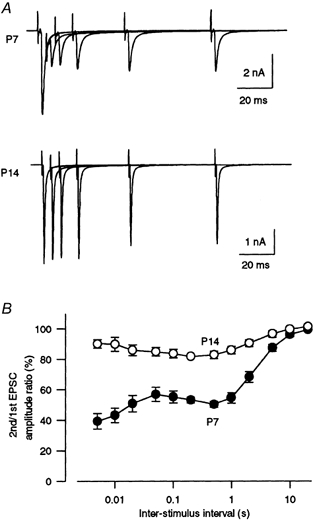
A, pairs of EPSCs evoked at interstimulus intervals of 5, 10, 20, 50 and 100 ms in P7 and P14 rats. B, the paired-pulse ratio of EPSCs in P7 (•) and P14 (○) rats expressed as the second EPSC amplitude relative to the first (n = 8-14). At an interstimulus interval of 10 s, this value was 96.1 ± 1.4 % in P7 and 99.6 ± 1.3 % in P14 rats. For all interstimulus intervals examined between 5 ms and 10 s, the magnitude of paired-pulse depression was significantly greater in P7 than in P14 rats (P < 0.01).
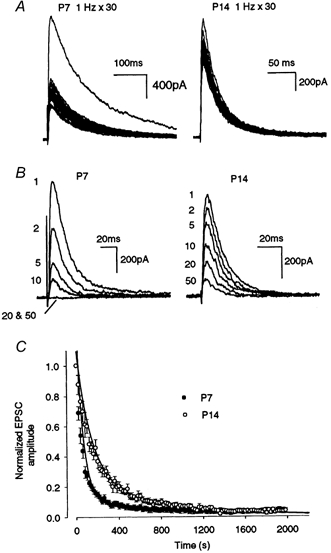
As expected from the paired-pulse depression, repetitive stimulation (> 0.1 Hz) depressed EPSCs in a frequency-dependent manner (Fig. 3D). During stimulation at 1 Hz, EPSCs declined and reached a low steady-state level (Iss, Fig. 3A and B). At 1 Hz stimulation, this level relative to the first EPSC amplitude (I1st) was 38 ± 1 %(n = 8) in P7 rats, whereas it was 75 ± 1 %(n = 6) in P14 rats. Thus, the magnitude of synaptic depression (Iss/I1st) at P14 was significantly less than that at P7 (P < 0.00001). The developmental decrease in the mean magnitude of depression was most prominent between P9 and P12 (Fig. 3C). At higher stimulation frequencies, the synaptic depression was greater, and the magnitude of depression in P14 rats was smaller than that in P7 rats for all frequencies examined (1-100 Hz, P < 0.05, Fig. 3D). Thus the amplitude of synaptic currents during high frequency stimulation increases with postnatal development, whereas that at basal frequency remains constant throughout development (Fig. 1).
Figure 3. Developmental decrease in synaptic depression.
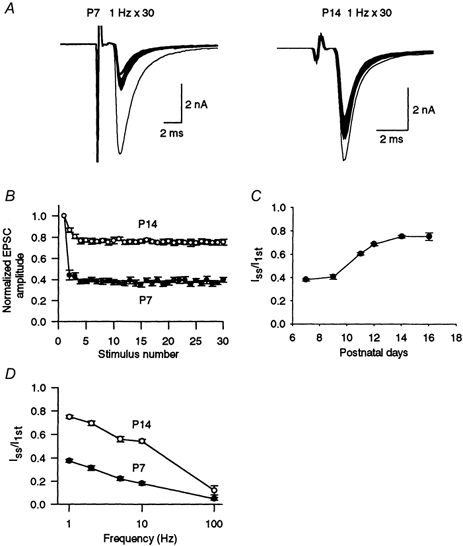
A, EPSCs evoked by a train of 30 stimuli at 1 Hz (superimposed) in P7 and P14 rats. B, EPSC amplitudes during a train of 30 stimuli at 1 Hz relative to the first EPSC in P7 and P14 rats (n = 5 each). C, magnitude of synaptic depression during the 1 Hz train expressed as the steady-state amplitude (Iss; mean of the 26th to 30th events) relative to the first EPSC amplitude (I1st, n = 5-6). D, frequency dependence of synaptic depression (n = 5-6). Iss/I1st was significantly greater at P14 than at P7 (P < 0.05).
Rate of recovery from synaptic depression
Synaptic depression during repetitive stimulation is primarily caused by the depletion of synaptic vesicles in the RRP, and the time course of recovery depends upon the speed of replenishment of the RRP (von Gersdorff et al. 1997; Dittman & Regehr, 1998; Stevens & Wasseling, 1998), although a decrease in p (Wu & Borst, 1999) secondary to adaptation of the release machinery (Hsu et al. 1996) may additionally be involved. During repetitive stimulation at a frequency of 10 Hz, postsynaptic receptors can recover each time from desensitization (Trussell et al. 1993; Otis et al. 1996) and presynaptic Ca2+ currents directly recorded from the calyx nerve terminal show little change (Takahashi et al. 2000). To examine whether the time course of recovery changes with development, a train of 30 stimuli at 10 Hz was followed by a test pulse at various intervals (0.5-16 s; Fig. 4A). The time course of recovery after the 10 Hz train could be fitted by a single exponential function with a mean time constant of 4.0 ± 0.6 s in both P7 (n = 5) and P14 (n = 6) rats. Thus the repriming process of synaptic vesicles after synaptic transmission at 10 Hz seems to stay constant throughout the developmental period.
Figure 4. Recovery from synaptic depression.
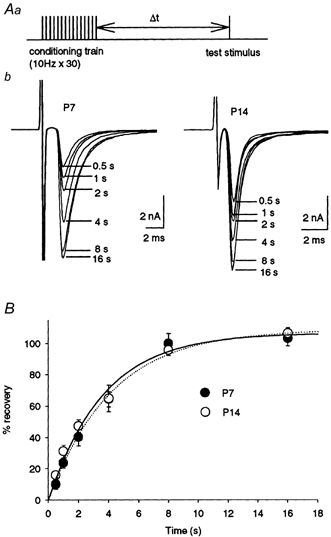
Aa, experimental protocol. A conditioning train (30 stimuli at 10 Hz) was followed by a test stimulus at different intervals (Δt = 0.5-16 s). This protocol was repeated every 60 s. b, EPSCs evoked by test stimuli after different intervals are superimposed for P7 and P14 rats. B, time courses of recovery from depression in P7 (•; n = 5) and P14 (○; n = 6) rats expressed as percentage recovery =(Itest - Iss)/(I1st - Iss) × 100. Data from both ages were fitted by a single exponential function with a time constant of 4.0 s in P7 rats (dotted line) and 4.1 s in P14 rats (continuous line).
Estimation of RRP size and release probability
The total amount of transmitter released during a train of high frequency stimuli can represent the RRP size provided that the pool is completely depleted and is not replenished. At the calyx of Held, a similar RRP size has been estimated from a cumulative amplitude histogram of EPSCs during a train of 100 Hz stimulation in high [Ca2+]o (4 mm), from the maximal EPSC amplitude estimated by extrapolating the amplitude-[Ca2+]o relationship, and from the maximal synaptic current amplitude obtained by photorelease of Ca2+ from a caged compound (Schneggenburger et al. 1999). During stimulation at 100 Hz in 4 mm[Ca2+]o, EPSCs underwent a marked depression and reached a steady low level (Fig. 5A). Assuming that the depression is primarily caused by the RRP depletion and also that a constant fraction remains by an immediate replenishment, the RRP size can be estimated from the zero time intercept of a line fitted to a cumulative amplitude plot of EPSCs (Fig. 5B; Schneggenburger et al. 1999). The RRP size (Nq) estimated in this way was 9.2 ± 0.9 nA in P7 (n = 7), 14 ± 1.0 nA (n = 6) in P10 (cf. Schneggenburger et al. 1999) and 18 ± 1.5 nA in P14 rats (n = 6, P < 0.05 ANOVA), indicating that Nq increases with development (Fig. 5C). By dividing the EPSC amplitude in normal [Ca2+]o (Npq, Fig. 5E) by the RRP size in high [Ca2+]o, p was then estimated (Schneggenburger et al. 1999). The p value was 0.75 ± 0.07 at P7 and decreased with development to 0.38 ± 0.07 at P10 and 0.29 ± 0.01 at P14 (Fig. 5D, P < 0.05 ANOVA).
Figure 5. Estimations of RRP size and release probability in developing rats.
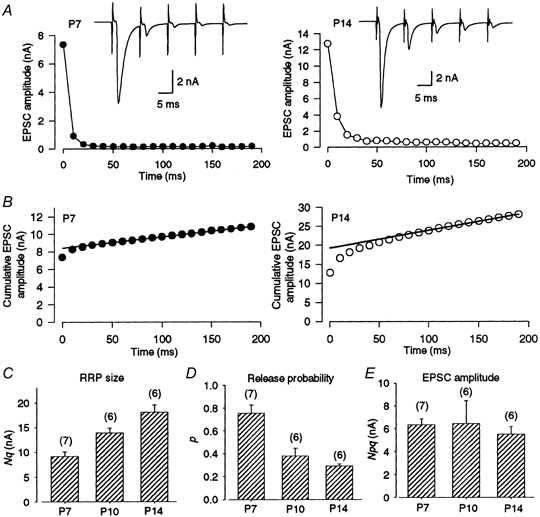
A, depression of EPSCs during a train of 20 stimuli at 100 Hz in P7 (left) and P14 (right) rats, both in 4 mm[Ca2+]o solution. Sample records are the 1st to 5th EPSCs during the train. The Iss/I1st values were 0.027 ± 0.006 at P7 (n = 7) and 0.070 ± 0.02 at P14 (n = 6). B, cumulative EPSC amplitudes during a 100 Hz train. Data points in the range 100-200 ms were fitted by linear regression, and extrapolated to time zero to estimate the RRP size (N). The N values estimated in these cells were 8.4 nA at P7 and 19 nA at P14. C, mean RRP sizes (Nq) estimated in P7 (n = 7), P10 (n = 6) and P14 rats (n = 6). These values were not significantly different from those estimated by drawing regression lines through data between 150 and 200 ms. D, mean release probabilities (p) estimated from the ratio of the EPSCs evoked at 0.05 Hz in 2 mm[Ca2+]o relative to the RRP size (Nq) estimated in 4 mm[Ca2+]o in P7 (n = 7), P10 (n = 6) and P14 rats (n = 6). E, the first EPSC amplitude (Npq) in 2 mm[Ca2+]o in P7, P10 and P14 rats (no significant difference, ANOVA).
Another way of assessing the release probability is to evaluate the speed of block of NMDA receptors by the irreversible open-channel blocker MK-801. If the probability of transmitter release is higher, a larger proportion of postsynaptic NMDA receptors is blocked at any one time, resulting in a faster decline of NMDA EPSCs (Rosenmund et al. 1993; Hessler et al. 1993). The NMDA EPSCs were recorded from MNTB neurons at a holding potential of +40 mV in the presence of CNQX (10 μm; Fig. 6). During 1 Hz stimulation, NMDA EPSCs were depressed to 42 ± 2 %(n = 5) in P7 and to 72 ± 2 %(n = 5) in P14 animals (Fig. 6A), values similar in magnitude to those for the non-NMDA EPSCs (P > 0.1). At a basal stimulus frequency (0.05 Hz), NMDA EPSCs were evoked constantly with no sign of decline or failure. After confirming a stable baseline, stimulation was stopped, MK-801 (40 μm) was applied, and stimulation was resumed 7 min later. When stimulated at 0.05 Hz in the presence of MK-801, NMDA EPSCs progressively declined in amplitude (Fig. 6B). The time course of this decline could be fitted with a double exponential function at both ages. The fast and slow blocking time constants (τf and τs) were 62 and 1280 s, respectively, for P7 animals (fast component 92 %, n = 5), and 142 and 860 s, respectively, for P14 animals (fast component 75 %, n = 6). The weighted mean time constant (τm) was 128 ± 20 s at P7, whereas it was 300 ± 20 s at P14 (P < 0.05). A longer incubation time with MK-801 prior to stimulation (15 min) did not change the mean blocking time constant (320 ± 20 s, n = 4 in P14 rats, P > 0.5), excluding the possibility that the difference resulted from a slower diffusion time of MK-801 in older animals. These results further support the proposal that p decreases with development at this synapse.
Figure 6. Developmental changes in the release probability assessed from the time course of block of NMDA EPSCs by MK-801.
A, NMDA EPSCs evoked at 1 Hz (30 stimuli) in P7 and P14 rats (superimposed) in the presence of CNQX (10 μm) at a holding potential of +40 mV. Values of Iss/I1st for NMDA EPSCs (0.42 ± 0.02 at P7 and 0.72 ± 0.02 at P14; n = 5 each) were not significantly different from those of non-NMDA EPSCs at each age (P > 0.1). B, sample records of NMDA EPSCs evoked at 0.05 Hz in the presence of MK-801 (40 μm) in P7 (left) and P14 (right) rats. The stimulus sequence numbers (1-50) are indicated. C, the time course of block of NMDA EPSCs by MK-801 in P7 (•; n = 5) and P14 (○; n = 5) rats. Ordinate indicates the amplitude of NMDA EPSCs normalized to the amplitude of the first EPSC. Abscissa indicates time after stimulation. Data were fitted by a double exponential function.
Contribution of metabotropic glutamate autoreceptors to synaptic depression
At the calyx of Held synapse in P8-18 rats, ligands for the group III metabotropic glutamate receptors (mGluRs) attenuate synaptic transmission through inhibition of presynaptic Ca2+ channels (Takahashi et al. 1996b). As reported for P8-11 rats (von Gersdorff et al. 1997), the group III mGluR antagonist (RS)-a-cyclopropyl-4-phosphonophenylglycine (CPPG, 300 μm) significantly reduced the magnitude of depression during 10 Hz stimulation in P7 animals (Fig. 7, P < 0.05, Student's paired t test), indicating the autoreceptor function of presynaptic mGluRs during this early postnatal period. However, at P14, CPPG no longer affected the magnitude of depression (P > 0.3). As reported previously (Takahashi et al. 1996b), the mGluR agonist l-1-2-amino-4-phosphonobutyrate (l-AP4, 50 μm) attenuated EPSCs at P7 (by 69.5 ± 3.6 %, n = 5) and P14 (by 64.5 ± 3.1 %, n = 6) to a similar extent (P > 0.3, data not shown). CPPG (300 μm) completely abolished the l-AP4-induced presynaptic inhibition both at P7 (by 102 ± 1.7 %, n = 5) and at P14 (by 99.2 ± 3.1 %, n = 6). These results suggest that mGluRs are expressed at the calyceal nerve terminal to a similar extent throughout development, but their autoreceptor function decreases with development.
Figure 7. Effect of a metabotropic glutamate receptor antagonist on synaptic depression during repetitive stimulation.
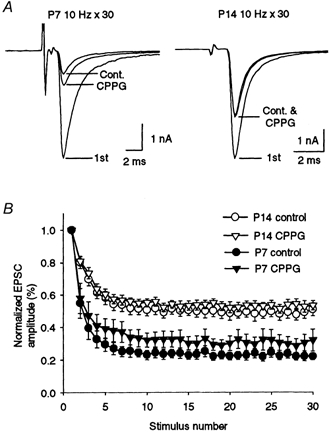
A, CPPG reduced synaptic depression at P7 but not at P14. CPPG had no effect on the amplitude of the first EPSCs at P7 and P14. The first EPSCs (1st) and the averages of the 26th to 30th EPSCs evoked at 10 Hz are superimposed for the control (Cont.) and in the presence of CPPG (300 μm) in P7 and P14 rats. B, time course of synaptic depression during 10 Hz stimulation in the presence (triangles) or absence (circles) of CPPG in P7 (filled symbols) and P14 (open symbols) rats (n = 5 for each). The Iss/I1st values with CPPG were significantly greater than control (P < 0.05) at P7, whereas the values were not different from control at P14 (P > 0.3, Student's paired t test).
DISCUSSION
Developmental regulation of synaptic efficacy
Our results indicate the constant mean amplitude of EPSCs (Npq) evoked at a basal frequency throughout the second postnatal week. Our estimations of Nq and p from cumulative histograms of EPSCs during high frequency stimulation indicate that Nq becomes larger, whereas p becomes smaller with development. Given that the mean quantal size (q) was nearly constant throughout development, these results suggest that the decrease in p was fully compensated for by the increase in N. Similar to our results, the mean amplitude of evoked EPSCs at this synapse is reported to stay constant from P5-7 to P12-14 (Taschenberger & von Gersdorff, 2000). However, calyceal EPSCs were too small to be distinguished from collateral EPSCs before P7 (see Methods), suggesting that the EPSC amplitude increases during the first postnatal week (Chuhma & Ohmori, 1998). At the calyx of Held synapse in mice, the mean amplitude of evoked EPSCs increases during the second postnatal week (Futai et al. 2001). Also, at the endbulb of Held synapse in the rat auditory brainstem, the mean amplitudes of both evoked and spontaneous EPSCs grow larger from P4-11 to P12-18 (Bellingham et al. 1998). It remains to be seen whether N and p change reciprocally during development at these synapses.
During high frequency transmission, EPSCs underwent a depression, and the magnitude of this depression decreased with development for all frequencies examined (1-100 Hz). In agreement with our results, the magnitude of depression is reported to be smaller in P12-14 rats compared with P5-7 rats for the frequencies of 10, 100 and 300 Hz (Taschenberger & von Gersdorff, 2000). Our results further indicate that the recovery rate after depression by 10 Hz stimulation does not change throughout the second postnatal week, suggesting a constant rate of synaptic vesicle replenishment. Thus the developmental decrease in depression may arise from a decrease in p reducing the rate of synaptic vesicle depletion. This would contribute to the maintenance of high synaptic efficacy during high frequency synaptic transmission.
Developmental decrease in release probability
At this synapse, the p value estimated as a ratio of the size of basal EPSCs relative to the RRP size decreased with development. A developmental decrease in p was further supported by the slower blocking rate of NMDA EPSCs by MK-801 in more mature animals. It is also consistent with the developmental decrease in both the paired-pulse depression ratio and the magnitude of depression during repetitive stimulation. However, a previous report estimated p assuming simple binomial statistics for EPSCs and suggested its developmental increase at this synapse (Chuhma & Ohmori, 1998). This discrepancy might arise from the simple binomial assumption, which might not be applicable to EPSCs in normal [Ca2+]o at this synapse (Sahara & Takahashi, 2001). The developmental decrease in p has also been reported at other synapses (Bolshakov & Siegelbaum, 1995; Choi & Lovinger, 1997; Pouzat & Hestrin, 1997), suggesting that it may be a common phenomenon among many central synapses.
What mechanisms underlie the developmental decrease of p? At the immature calyx of Held (P8-10), intracellular Ca2+ concentrations are determined by a relatively weak buffering capacity and high Ca2+ extrusion rate (Helmchen et al. 1997). If this balance shifts towards higher rates of Ca2+ sequestration with development, for example by a developmental increase in the expression of Ca2+-binding proteins (Lohman & Friauf, 1996), p might decrease. Developmental shortening of presynaptic action potentials (Taschenberger & von Gersdorff, 2000) and the ensuing reduction in Ca2+ influx may also contribute to the reduction in p. After synaptic formation, presynaptic Ca2+ channels cluster and form discrete active zones during development (Takahashi et al. 1987). It remains to be seen how the developmental clustering of presynaptic Ca2+ channels affects the release probability.
Developmental increase in the RRP size
Our results suggested a 2-fold increase in the RRP size during the second postnatal week. This is indicated by a larger cumulative EPSC amplitude during high frequency stimulation in 4 mm[Ca2+]o and is consistent with larger mean EPSC amplitudes in 10 mm[Ca2+]o in older animals. A similar result using the cumulative amplitude method has been reported recently (2.1-2.5-fold increase from P5-7 to P12-14; Taschenberger & von Gersdorff, 2000). The RRP size has been estimated to be about 700 by different methods at this synapse (Schneggenburger et al. 1999). However, this RRP size may be an underestimate because of postsynaptic receptor desensitization (Schneggenburger & Neher, 2000) and the possible saturation of postsynaptic receptors. Thus our present results may provide only a relative change in the RRP size determined using a given method. A recent morphological study at the calyx of Held synapse in adult cats indicates that 6200 synaptic vesicles are docked at the active zone (Rowland et al. 2000). It remains to be seen whether this number undergo developmental changes during postnatal development.
Transient role of autoreceptor function
The group III mGluR antagonist CPPG attenuated synaptic depression during 10 Hz stimulation in P7 rats but not in P14 rats. Glutamate released from nerve terminals may activate presynaptic mGluR autoreceptors, thereby reducing transmitter release through inhibition of presynaptic Ca2+ currents (Takahashi et al. 1996b). This autoreceptor function may contribute to saving transmitter in the early postnatal period when the release probability is high. Although the effect of CPPG on synaptic depression decreased with development, the mGluR agonist l-AP4 applied from the outside attenuated EPSCs to a similar extent throughout P7-14, and these effects were completely blocked by CPPG. Therefore, the developmental decline in the mGluR autoreceptor function observed here cannot be attributed to the developmental decrease in the expression of mGluRs as reported for mGluR4 at this synapse (Elezgarai et al. 1999). Presumably, glutamate released from the terminal may no longer reach a sufficient concentration for activating the autoreceptors at P14, for example, due to an increased diffusion distance between the release sites and autoreceptors, or increased strength of the glutamate uptake mechanism (Oliet et al. 2001).
Functional significance of development at the auditory synapse
The calyx of Held synapse forms an auditory pathway that processes sound source localization in auditory space by detecting interaural timing and intensity differences of sound detected at each cochlea. The ability to detect sound sources is dependent on highly reliable (high-fidelity) synaptic transmission and preservation of the timing of information in the auditory signal (Oertel, 1997; Trussel, 1997). The giant nerve terminal calyx of Held has many release sites (Rowland et al. 2000) and generates huge EPSCs that greatly exceed the action potential threshold, thereby providing a high safety factor for high-fidelity transmission (Trussel, 1997). With respect to the basal synaptic efficacy, the auditory transmission seems to be already established at P7 just after the formation of the synapse (P4-6; Kandler & Friauf, 1993). However, the establishment of high-fidelity transmission at high frequency awaits further development in the second postnatal week, during which time the RRP grows in size and the release probability declines, thereby establishing a stable and high-efficacy transmission. The fidelity of transmission at the calyx of Held synapse increases during the second postnatal week in mice (Futai et al. 2001), and in adult mice, pre- and postsynaptic action potentials can be phase-locked to 600 Hz at physiological temperature (Wu & Kelly, 1993). Thus the developmental changes in the properties of transmitter release observed at this synapse will contribute to the establishment of stable and high-fidelity synaptic transmission for timing recognition.
Acknowledgments
We thank Katsunori Kobayashi, David Saffen and Tetsuhiro Tsujimoto for discussion and comments on our manuscript. This work was supported by the ‘Research for the Future’ programme of The Japan Society for the Promotion of Sciences and also by Grants-in-Aid for Scientific Research on Priority Area C from the Ministry of Education, Culture, Science and Technology, Japan.
References
- Bellingham MC, Lim R, Walmsley B. Developmental changes in EPSC quantal size and quantal content at a central glutamatergic synapse in rat. Journal of Physiology. 1998;511:861–869. doi: 10.1111/j.1469-7793.1998.861bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov VY, Siegelbaum SA. Regulation of hippocampal transmitter release during development and long-term potentiation. Science. 1995;269:1730–1734. doi: 10.1126/science.7569903. [DOI] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proceedings of the National Academy of Sciences of the USA. 1997;94:2665–2670. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Ohmori H. Postnatal development of phase-locked high-fidelity synaptic transmission in the medial nucleus of the trapezoid body of the rat. Journal of Neuroscience. 1998;18:512–520. doi: 10.1523/JNEUROSCI.18-01-00512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Castillo J, Katz B. Quantal components of the end-plate potential. Journal of Physiology. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15:1097–1107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. Journal of Neuroscience. 1998;18:6147–6162. doi: 10.1523/JNEUROSCI.18-16-06147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elezgarai I, Benitez R, Mateos JM, Lazaro E, Osorio A, Azkue JJ, Bilbao A, Lingenhoehl K, van der Putten H, Hampson DR, Kuhn R, Knopfel T, Grandes P. Developmental expression of the group III metabotropic glutamate receptor mGluR4a in the medial nucleus of the trapezoid body of the rat. Journal of Comparative Neurology. 1999;411:431–440. doi: 10.1002/(sici)1096-9861(19990830)411:3<431::aid-cne6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Barnes-Davies M. The binaural auditory pathway: excitatory amino acid receptors mediate dual time course excitatory postsynaptic currents in the rat medial nucleus of the trapezoid body. Proceedings of the Royal Society B. 1993;251:151–157. doi: 10.1098/rspb.1993.0022. [DOI] [PubMed] [Google Scholar]
- Futai K, Okada M, Matsuyama K, Takahashi T. High-fidelity transmission acquired by a developmental decrease in NMDA receptor expression at an auditory synapse. Journal of Neuroscience. 2001;21:3342–3349. doi: 10.1523/JNEUROSCI.21-10-03342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F, Borst JGG, Sakmann B. Calcium dynamics associated with a single action potential in a CNS presynaptic terminal. Biophysical Journal. 1997;72:1458–1471. doi: 10.1016/S0006-3495(97)78792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA, Shirke AM, Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Hsu S-F, Augustine GJ, Jackson MB. Adaptation of Ca2+-triggered exocytosis in presynaptic terminals. Neuron. 1996;17:501–512. doi: 10.1016/s0896-6273(00)80182-8. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Counting quanta: Direct measurements of transmitter release at a central synapse. Neuron. 1995;15:875–884. doi: 10.1016/0896-6273(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Momiyama A, Uchitel OD, Takahashi T. Developmental changes in calcium channel types mediating central synaptic transmission. Journal of Neuroscience. 2000;20:59–65. doi: 10.1523/JNEUROSCI.20-01-00059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental changes in calcium channel types mediating synaptic transmission in rat auditory brainstem. Journal of Physiology. 1998;509:419–423. doi: 10.1111/j.1469-7793.1998.419bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental changes in synaptic efficacy at the medial nucleus of trapezoid body in rats. Japanese Journal of Physiology Supplement. 2000;50:S135. [Google Scholar]
- Jewett DL, Romano MN. Neonatal development of auditory system potentials averaged from the scalp of rat and cat. Brain Research. 1972;36:101–115. doi: 10.1016/0006-8993(72)90769-x. [DOI] [PubMed] [Google Scholar]
- Kandler K, Friauf E. Pre- and postnatal development of efferent connections of the cochlear nucleus in the rat. Journal of Comparative Neurology. 1993;328:161–184. doi: 10.1002/cne.903280202. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Friauf E. Distribution of the calcium-binding proteins parvalbumin and calretinin in the auditory brainstem of adult and developing rats. Journal of Comparative Neurology. 1996;367:90–109. doi: 10.1002/(SICI)1096-9861(19960325)367:1<90::AID-CNE7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986;321:406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Oertel D. Encoding of timing in the brain stem auditory nuclei of vertebrates. Neuron. 1997;19:959–962. doi: 10.1016/s0896-6273(00)80388-8. [DOI] [PubMed] [Google Scholar]
- Okada M, Onodera K, Van Renterghem C, Sieghart W, Takahashi T. Functional correlation of GABAA receptor α subunits expression with the properties of IPSCs in the developing thalamus. Journal of Neuroscience. 2000;20:2202–2208. doi: 10.1523/JNEUROSCI.20-06-02202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SHR, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Otis T, Zhang S, Trussell LO. Direct measurement of AMPA receptor desensitization induced by glutamatergic synaptic transmission. Journal of Neuroscience. 1996;16:7496–7504. doi: 10.1523/JNEUROSCI.16-23-07496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouzat C, Hestrin S. Developmental regulation of basket/stellate cell-Purkinje cell synapses in cerebellum. Journal of Neuroscience. 1997;17:9104–9112. doi: 10.1523/JNEUROSCI.17-23-09104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- Rowland KC, Irby NK, Spirou GA. Specialized synapse-associated structures within the calyx of Held. Journal of Neuroscience. 2000;20:9135–9144. doi: 10.1523/JNEUROSCI.20-24-09135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara Y, Takahashi T. Quantal components of the excitatory postsynaptic currents at a rat central auditory synapse. Journal of Physiology. 2001 doi: 10.1111/j.1469-7793.2001.00189.x. (in the Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Meyer AC. Neher E, editor. Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron. 1999;23:399–409. doi: 10.1016/s0896-6273(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wasseling JF. Activity-dependent modulation of the rate at which synaptic vesicles become available to undergo exocytosis. Neuron. 1998;21:415–424. doi: 10.1016/s0896-6273(00)80550-4. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Feldmeyer D, Suzuki N, Onodera K, Cull-Candy SG, Sakimura K, Mishina M. Functional correlation of NMDA receptor ε subunits expression with the properties of single-channel and synaptic currents in the developing cerebellum. Journal of Neuroscience. 1996a;16:4376–4382. doi: 10.1523/JNEUROSCI.16-14-04376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996b;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Hori T, Kajikawa Y, Tsujimoto T. The role of GTP-binding protein activity in fast central synaptic transmission. Science. 2000;289:460–463. doi: 10.1126/science.289.5478.460. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A, Hirai K, Hishinuma F, Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992;9:1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nakajima Y, Hirosawa K, Nakajima S, Onodera K. Structure and physiology of developing neuromuscular synapses in culture. Journal of Neuroscience. 1987;7:473–481. doi: 10.1523/JNEUROSCI.07-02-00473.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine tuning an auditory synapse for speed and fidelity: Developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. Journal of Neuroscience. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO. Cellular mechanisms for preservation of timing in central auditory pathways. Current Opinion in Neurobiology. 1997;7:487–492. doi: 10.1016/s0959-4388(97)80027-x. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- von Gersdorff HV, Schneggenburger R, Weis S, Neher E. Presynaptic depression at a calyx synapse: the small contribution of metabotropic glutamate receptors. Journal of Neuroscience. 1997;17:8137–8146. doi: 10.1523/JNEUROSCI.17-21-08137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L-G, Borst JGG. The reduced release probability of releasable vesicles during recovery from short-term synaptic depression. Neuron. 1999;23:821–832. doi: 10.1016/s0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- Wu SH, Kelly JB. Response of neurons in the lateral superior olive and medial nucleus of the trapezoid body to repetitive stimulation: intracellular and extracellular recordings from mouse brain slice. Hearing Research. 1993;68:189–201. doi: 10.1016/0378-5955(93)90123-i. [DOI] [PubMed] [Google Scholar]


