Abstract
The action potential clamp technique was exploited to evaluate the rate dependency of delayed rectifier currents (IKr and IKs) during physiological electrical activity. IKr and IKs were measured in guinea-pig ventricular myocytes at pacing cycle lengths (CL) of 1000 and 250 ms.
A shorter CL, with the attendant changes in action potential shape, was associated with earlier activation and increased magnitude of both IKr and IKs. Nonetheless, the relative contributions of IKr and IKs to total transmembrane current were independent of CL.
Shortening of diastolic interval only (constant action potential shape) enhanced IKs, but not IKr.
IKr was increased by a change in the action potential shape only (constant diastolic interval).
In ramp clamp experiments, IKr amplitude was directly proportional to repolarization rate at values within the low physiological range (< 1.0 V s−1); at higher repolarization rates proportionality became shallower and finally reversed.
When action potential duration (APD) was modulated by constant current injection (I-clamp), repolarization rates > 1.0 V s−1 were associated with a reduced effect of IKr block on APD. The effect of changes in repolarization rate was independent of CL and occurred in the presence of IKs blockade.
In spite of its complexity, the behaviour of IKr was accurately predicted by a numerical model based entirely on known kinetic properties of the current.
Both IKr and IKs may be increased at fast heart rates, but this may occur through completely different mechanisms. The mechanisms identified are such as to contribute to abnormal rate dependency of repolarization in prolonged repolarization syndromes.
Among the conductances supporting ventricular repolarization, the delayed rectifier-type currents IKr and IKs are of particular pharmacological and pathophysiological interest. While the former is the target of numerous substances active on repolarization (Moss, 1999), genetic abnormalities of both IKr and IKs are involved in the pathogenesis of congenital long-QT syndrome (Curran et al. 1995; Sanguinetti et al. 1995; Wang et al. 1996). Rate-dependent changes in the expression of delayed rectifier currents during the cardiac cycle are thought to play a pivotal role in the adaptation of repolarization to heart rate. Accordingly, the abnormalities in the rate dependency of repolarization that characterize some forms of QT prolongation (Schwartz et al. 1995) are likely to reflect the underlying imbalance between IKr and IKs. Albeit the biophysical properties and receptor modulation of IKr and IKs have been extensively characterized, the rate dependency of these currents has never been investigated under conditions mimicking physiological repetitive cardiac activity.
This led us to use an updated version (Zaza et al. 1998) of the ‘action-potential clamp’ technique (Doerr et al. 1990) to analyse the functional contributions of IKr and IKs to ventricular repolarization, with a focus on rate-dependent phenomena. This technique allows the measurement of ionic currents elicited by membrane potential changes which reproduce the physiological electrical activity of the cell. Currents are dissected by suitable blockers and, if the agent used is sufficiently selective, the ‘blocker-sensitive current’ may actually provide a good estimate of the current supported by an individual channel type.
Several goals of theoretical and practical relevance have been achieved in this study: (1) to define the rate dependency of IKr and IKs and relate it to changes in total membrane current flowing during repolarization; (2) to expose the different mechanisms underlying the rate dependencies of IKr and IKs; and (3) to disclose a novel form of mutual interdependency between IKr and repolarization rate that may provide clues to the interpretation of the response of repolarization to heart rate.
METHODS
Cell isolation
Guinea-pig ventricular myocytes were isolated by using a retrograde coronary perfusion method previously published except for minor modifications (Zaza et al. 1998). All the experiments were carried out according to the guidelines issued by the Animal Care Commitee of the University of Milan. In brief, guinea-pigs weighing 200-300 g were anaesthetised with a xylazine (1.5 mg (kg body wt)−1 and ketamine (20 mg (kg body wt)−1 mix, killed by cervical dislocation and exanguinated. Hearts were quickly removed, and the ascending aorta was connected to the outlet of a Langendorff column and perfused with a modified Tyrode solution (37 °C) containing (mm): NaCl, 130; KCl, 4.5; CaCl2, 0.75; MgCl2, 5; Hepes, 23; d-glucose, 21; NaH2PO4, 1; taurine, 20; creatine, 5; pyruvate, 5; adjusted to pH 7.3, and equilibrated with 100 % O2. Tyrode perfusion was maintained until vigorous mechanical activity resumed and blood was completely removed. The heart was then perfused for 5 min with a nominally Ca2+-free Tyrode solution containing 3.3 μm EGTA and adjusted to pH 7.0, followed by the same solution to which 140 U ml−1 collagenase (Worthington Type 1), 0.17 U ml−1 protease (Sigma Type XIV), 0.05 mm CaCl2 and 1 mg ml−1 bovine serum albumin were added. When the effluent became slightly viscous (after about 4 min), the atria were dissected and the ventricles were chopped to 1 mm fragments in the same solution without enzymes, adjusted to pH 7.3. The fragments were exposed to gentle oxygen bubbling at 37 °C. Samples of the suspension were collected every 5 min, filtered through a nylon mesh and centrifuged at 2.5 g for 3 min. To separate myocytes and non-myocyte cells, the pellets were resuspended (50 %, v/v) in a continuous Percoll gradient, created by combining Percoll solution (Sigma) with 9 % NaCl (final [NaCl] 0.9 %), and centrifuged at 2.5 g for 15 min. Finally, isolated myocytes were resuspended in the modified Tyrode solution (final concentration 1 mm Ca2+) including gentamycine (10 μg ml−1) and stored at room temperature until use.
Rod shaped, Ca2+-tolerant myocytes, obtained with this procedure, were used within 12 h from dissociation. Measurements were performed only in quiescent myocytes with clear-cut striations. The investigation conforms to the Guide of the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996).
Solutions and recording apparatus
The myocyte suspension was placed in a 30 mm Petri dish, with a plastic ring to reduce total volume to ≈1 ml and mounted on the stage of an inverted microscope. The dish was perfused at 2 ml min−1 with standard external Tyrode solution, containing (mm): NaCl, 154; KCl, 4; CaCl2, 2; MgCl2, 1; Hepes, 5 (titrated with NaOH); d-glucose, 5.5; adjusted to pH 7.35. The cell under study was held within 300 μm from the tip (1 mm) of a thermostated multi-line pipette, connected to solution reservoirs through electrically driven valves. This system allowed us to expose the cell to different solutions, with changes completed in about 1 s. The solution temperature was monitored at the pipette tip with a fast-response digital thermometer (BAT-12, Physitemp) and kept at 35 ± 0.5 °C.
Membrane potential and current were measured in the whole cell configuration (Axopatch 200-A, Axon Instruments) by borosilicate glass pipettes with a tip resistance between 3 and 5 MΩ. The pipette solution contained (mm): potassium aspartate, 110; KCl, 23; CaCl2, 0.4; (calculated free Ca2+, 10−7m), MgCl2 3, Hepes, 5 (titrated with KOH); EGTA, 1 (titrated with KOH); GTP-Na salt, 0.4; ATP-Na salt, 5; creatine phosphate, 5; pH 7.3. Series resistances (< 5 MΩ) and membrane capacitance were measured in every cell, but were not compensated; since the currents measured never exceeded 200 pA, the voltage error generated by uncompensated series resistance was ≤ 1 mV. A mean junction potential of about 5 mV, measured upon moving the electrode tip from Tyrode to ‘intracellular’ (potassium aspartate) solution, was also left uncompensated.
The command potentials for the V-clamp amplifier were supplied through a 12-bit D/A converter (Digidata 1200A) by an IBM-compatible PC (Pentium II, 300 MHz). Potential and current signals were filtered at 2 kHz and were also tape recorded, together with trigger signals, with an adapted VCR system. Recorded signals were later acquired through a 12-bit A/D (sampling at 5 kHz) for analysis.
Nifedipine (Sigma) and chromanol 293B stock solutions were prepared by dissolving the substances in ethanol and dimethyl-sulfoxide (DMSO), respectively; E-4031 and chromanol 293B were generous gifts from Sanofi Recherche (Montpellier) and Roche Pharmaceuticals (Milan), respectively.
Protocols
The rapid and the slow components of the delayed rectifier were isolated by using the channel blockers E-4031 (specific for IKr; Sanguinetti & Jurkiewicz, 1990) and chromanol 293B (specific for IKs; Bosch et al. 1998) at high concentrations (5 μm E-4031 and 10 μm chromanol 293B). The protocol of ‘action-potential clamp’ (AP-clamp) experiments required full reversibility of current block, which is peculiar to 293B, but not to E-4031. Thus, in these experiments IKr was identified by a highly specific ERG channels toxin, isolated from the venom of the Mexican scorpion Centruroides noxius (Gurrola et al. 1999), characterized by fast and complete reversibility of effects. For this work we have used the toxin subtype ErgTx-1 (ErgTx), from which the primary structure and disulfide bridges were recently published (Bottiglieri et al. 2000). Preliminary experiments showed that, when measured within the same myocyte, the current sensitive to 1 μg ml−1 ErgTx (IC50 = 76 ± 2 ng ml−1) (Gurrola et al. 1999) was superimposable on that sensitive to 5 μm E-4031.
Membrane currents were studied by standard voltage-clamp (V-clamp) and the AP-clamp technique as previously described (Zaza et al. 1998).
Voltage-clamp protocols
Voltage-clamp experiments were carried out by using pCLAMP 7.0 software by Axon Instruments (Foster-City, CA, USA). Depolarizing steps to +30 mV (200 ms) were immediately followed by repolarizing ramps of variable steepness. The steps were separated by intervals of 8 s at a holding potential of -80 mV; the interpulse interval was largely sufficient for the gating variables of IKr to achieve their steady-state values. The protocol was repeated in control (Tyrode superfusion including 10 μm chromanol 293B and 5 μm nifedipine) and during challenge with 5 μm E-4031.
Action potential clamp protocols
Action potential clamp experiments were carried out using dedicated software developed in the laboratory.
The prototype experimental protocol (protocol A), which had to be completed within each single myocyte, included: (1) acquisition of one action potential waveform during steady-state stimulation (2 ms pulses) under control conditions (Tyrode superfusion) at the first cycle length (e.g. 1000 ms); (2) V-clamp of the cell with the acquired waveform, applied at the same cycle length under control conditions, until membrane current settled to a value close to zero (except for the stimulation artifact); (3) steady-state exposure to the specific channel blocker and acquisition of the resulting ‘compensation’ current, followed by complete wash out; and (4) repetition of steps 1, 2 and 3 at the second cycle length (e.g. 250 ms).
To address specific questions (Results) protocol A was modified as follows.
Protocol B: the action potential waveform was acquired at a single CL (step 1), but applied at different CLs by adjusting the diastolic interval (steps 2-4).
Protocol C: action potential waveforms were acquired at different CLs (step 1), but applied with the same diastolic interval (steps 2-4).
Recordings were considered acceptable only if: (1) the current recorded in control conditions was negligible, indicating that reproduction of the spontaneous activity was satisfactory (of course, this condition did not apply to the phases of protocols B and C in which the CLs at which an individual action potential waveform was recorded and applied were different); and (2) wash out of the blocker, performed after each exposure, caused return of the current to its control value.
Definitions
Throughout text and figures, data from AP-clamp experiments are presented in terms of ‘blocker-sensitive current’ (IE-4031, E-4031-sensitive current; IErgTx, ErgTx-sensitive current; I293B, current sensitive to chromanol 293B) obtained by digital subtraction of compensation current traces from control ones (3-5 cycles at steady state in each condition were averaged before subtraction). While IE-4031 and IErgTx are representative of IKr, I293B is representative of IKs. Accurate identification of a specific channel current through blocker-sensitive currents (e.g. IErgTx =IKr) requires the assumption that blockade is selective, unaffected by the time course of membrane potential and rate independent. While all the agents used in this study are highly selective, dependency of block from channel gating might cause the time course of drug-sensitive currents to diverge from the one of IKr and IKs, respectively; the implications of this potential artifact are addressed in the discussion.
Currents measured during the activation cycle were identified, according to the cycle phase in: (1) ‘peak current amplitude’, i.e. the maximum amplitude achieved by the specific current (e.g IErgTx) during repolarization; (2) ‘mean current amplitude’, obtained as the time integral of the current trace during repolarization divided by the integration interval; and (3) ‘instantaneous current’, obtained as the current point value measured during the action potential upstroke (at the end of the stimulation artifact). Conductance was obtained by dividing the specific current amplitude at time t by the difference between membrane potential at time t and the estimated K+ equilibrium potential.
Current density was estimated by dividing the current value by membrane capacitance (Cm).
Total membrane current during repolarization (Itot) was estimated by the product of Cm times the derivative (dV/dt) of the membrane potential signal (Itot = -CmdV/dt). In all figures, membrane potential and current traces are aligned.
Statistical analysis
Means were compared by Student's t test for paired or unpaired observations as appropriate. A probability level (P) < 0.05 was used to define significance throughout the study (n.s., not significant). In both text and figures, values are presented as means ± standard error of the mean.
RESULTS
Rate dependency of ErgTx-sensitive current (IKr)
The rate dependency of IKr flowing during the action potential was tested by using AP-clamp protocol A (Methods), i.e. each action potential waveform was acquired and applied at the same CL. If two CLs could not be tested within the same myocyte, the results were discarded.
The results obtained from seven cells are shown in Fig. 1. At both CL IErgTx was characterized by a gradual onset during phase 2 and a peak, corresponding to the final portion of phase 3 repolarization (Fig. 1A and B). Shortening of CL from 1000 to 250 ms was associated with an increase of mean IErgTx density (0.37 ± 0.03 vs. 0.24 ± 0.03 pA pF−1, P < 0.05; Fig. 1C), shortening of the time between fast depolarization and peak IErgTx (120 ± 7 ms vs. 224 ± 14 ms, P < 0.05; Fig. 1D), but no change in peak current density (1.03 ± 0.09 vs. 0.86 ± 0.12 pA pF−1, n.s.; Fig. 1E). The membrane potential at which peak IErgTx was recorded was remarkably consistent among cells and became slightly more negative at shorter CL (-43.6 ± 1.9 vs. -38.6 ± 1.4 mV, P < 0.05). The proportion of total membrane current (Itot) accounted for by IErgTx was estimated in each myocyte by the ratio between the mean values of IErgTx and Itot. This proportion was not affected by CL changes (0.39 ± 0.04 vs. 0.45 ± 0.06, n.s.; Fig. 1F). In summary, a shorter cycle was associated with a larger ErgTx-sensitive charge movement per unit time and an earlier activation of IErgTx. Nonetheless, the proportion of total current during repolarization contributed by IErgTx was independent of cycle length.
Figure 1. Rate dependency of ErgTx-sensitive current (IKr).
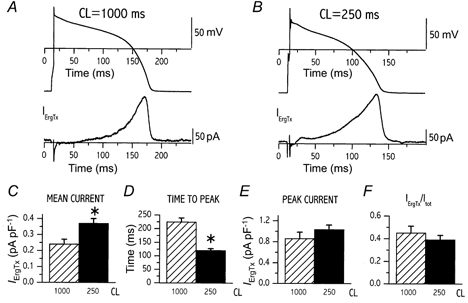
A and B, examples of action potential and IErgTx recorded at a cycle length (CL) of 1000 (A) and 250 ms (B) according to protocol A (Methods); the two CLs were tested within the same cell. C-F, comparison between cycle lengths of 1000 and 250 ms; mean repolarization current density (C), time-to-peak current (D), peak current density (E) and the ratio between IErgTx and total repolarizing current (Itot, see text for definitions, F). C-F, mean data from 7 cells; *P < 0.05.
Rate dependency of 293B-sensitive current (IKs)
Except for the use of chromanol 293B (instead of ErgTx), the protocol of this set of experiments was identical to the one described in the previous paragraph.
The results of this experiment, performed on 13 cells, are shown in Fig. 2. At both CLs I293B reached a significant amplitude very early in phase 2, without displaying a clear-cut current peak. Thus, in the case of I293B neither peak amplitude nor the potential at which peak occurred could be safely measured. Shortening of CL from 1000 to 250 ms caused an increase of mean I293B density (0.25 ± 0.05 vs. 0.18 ± 0.03 pA pF−1, P < 0.05); Fig. 2A-C) associated with a faster increase in current conductance (g293B). As shown in Fig. 2D, the latter was accounted for by an increase in both the instantaneous conductance (0.72 ± 0.18 vs. 0.32 ± 0.13 pS pF−1; P < 0.05) and the onset rate of the non-instantaneous component. The proportion of total membrane current (Itot) accounted for by I293B was estimated in each myocyte by the ratio between the mean values of I293B and Itot (Fig. 2E). This proportion was 0.35 ± 0.05 at CL 1000 and was unchanged at CL 250 ms (0.32 ± 0.06, n.s.). In summary, similarly to IErgTx (see above), mean I293B was increased when the cell was paced at the shorter cycle length. Also in the case of I293B, the proportion of total repolarizing current accounted for by the specific conductance was independent of cycle length.
Figure 2. Rate dependency of 293B-sensitive current (IKs).
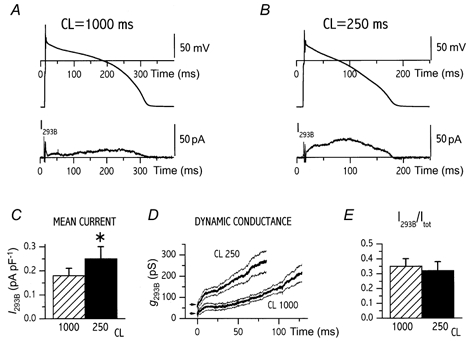
A and B, examples of action potential and I293B recorded at a cycle length (CL) of 1000 (A) and 250 ms (B) according to protocol A; the two CLs were tested within the same cell. C-E, comparison between cycle lengths of 1000 and 250 ms for mean repolarization current density (C), time course of I293B conductance (g293B) during the action potential (dynamic conductance, D) and the ratio between I293B and total repolarizing current (Itot, E). In D, mean traces (thick lines) and their confidence limits (thin lines) are shown; the arrows mark instantaneous conductance. C-E, mean data from 13 cells; *P < 0.05.
Mechanism for the rate dependency of IErgTx and I293B
This set of experiments was aimed at clarifying the mechanism of the rate-dependent increase in IErgTx and I293B.
Firstly we considered the hypothesis that current deactivation during diastole could be incomplete, thus leading to rate-dependent ‘accumulation’ of the channel activated state (Jurkiewicz & Sanguinetti, 1993). According to this hypothesis the relevant parameter would be the duration of the diastolic interval. Thus, a single action potential waveform was recorded at CL 1000 ms and then applied (V-clamp mode) with two different diastolic intervals (protocol B): the first one corresponded to CL 1000 ms and the second was made equal to 14 % of the first. Indeed, this was the mean change in diastolic interval that occurred at the steady state between CLs 1000 and 250 ms (data from a previous set of experiments). Measurements of IErgTx and I293B were obtained as previously. In seven cells (Fig. 3) mean IErgTx density (0.26 ± 0.03 vs. 0.27 ± 0.03 pA pF−1, n.s.; Fig. 3B) and peak current density (0.80 ± 0.05 vs. 0.84 ± 0.06 pA pF−1, n.s.; Fig. 3C) were not affected by shortening of the diastolic interval. In contrast, shortening of the diastolic interval sharply increased the mean I293B density (0.24 ± 0.01 vs. 0.14 ± 0.02 pA pF−1n = 4, P < 0.05; Fig. 4A and B).
Figure 3. Dependency of ErgTx-sensitive current (IKr) on diastolic interval only.
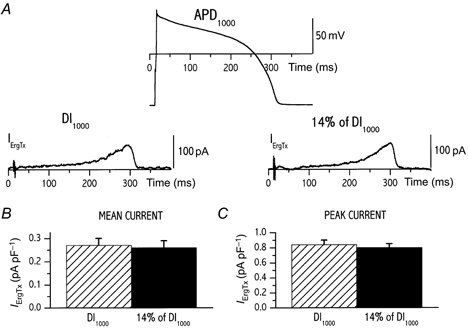
A, the same action potential waveform (recorded at CL of 1000 ms) was applied to measure IErgTx at different diastolic intervals (DI) as in protocol B (Methods); two DIs were tested within the same cell. Values of mean repolarization current density (B) and peak current density (C) measured at the two DIs are compared (n = 7).
Figure 4. Dependency of 293B-sensitive current (IKs) on diastolic interval only.
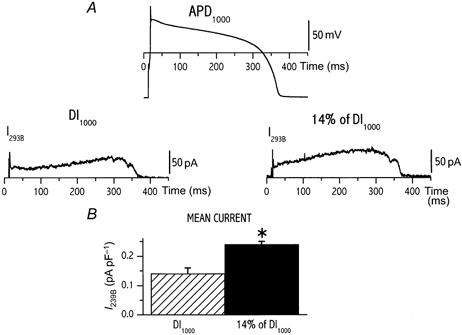
A, the same action potential waveform (recorded at CL of 1000 ms) was applied to measure I293B at different diastolic intervals (DI) as in protocol B (Methods); two DIs were tested within the same cell. Values of mean repolarization current density measured at the two CLs are compared in B (n = 4). *P < 0.05.
To summarize: when the diastolic interval alone was shortened (by keeping the action potential waveform constant), the response of the two currents differed: IErgTx remained unchanged but I293B was further increased. Then, the alternative hypothesis was considered that the CL-dependent increase in IErgTx, observed in the first set of experiments, might be due to the attendant change in the action potential waveform. This was tested in four cells by recording action potential waveforms at CL 1000 and 250 ms and applying them with a constant diastolic interval (the one occurring at CL 1000 ms). In all cases during the action potential corresponding to CL 250 ms IErgTx activated earlier and its mean density was larger (an example is shown in Fig. 5A and B). Thus, a change in the action potential contour, as the one occurring between cycle lengths of 1000 and 250 ms was, on its own, sufficient to increase IErgTx.
Figure 5. Dependency of ErgTx-sensitive current (IKr) on action potential contour only.
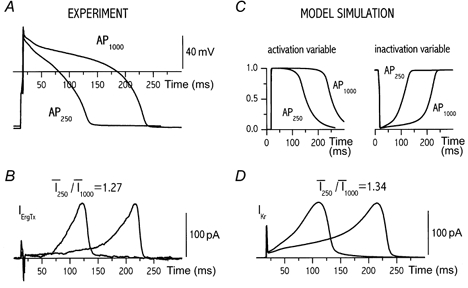
Action potential waveforms were recorded at two CLs (A) and applied with the same diastolic interval (B) to record IErgTx as in protocol C (Methods); two action potential waveforms were tested within the same cell. C, time course of IKr gating variables; D, IKr amplitude predicted by applying the action potential waveforms (from A) to the numerical model. The ratio between mean currents at the two CLs is specified for measured IErgTx and for simulated IKr above the respective panels.
The next question was whether the dependency of IKr on the action potential contour could be accounted for by the known kinetic properties of the current. To address this point, a numerical model of IKr was developed as described in Appendix. The same action potential waveforms as shown in Fig. 5A and B were used to drive the model, thus obtaining the simulated IKr traces shown in Fig. 5D. As well as reasonably reproducing the IErgTx time course, the model predicted that IKr mean amplitude should increase by approximately 30 % when switching from CL 1000 to 250 ms, a change compatible with the one observed for IErgTx. The model also predicts that, at both CLs, action potential duration may be adequate to fully activate IKr (Fig. 5C), irrespective of diastolic interval. This is consistent with the observation that IErgTx amplitude was independent of diastolic interval.
Dependency of E-4031-sensitive current (IKr) on repolarization rate
The results presented above suggest that the amplitude of IKr during repolarization may be a function of the rate of change of membrane potential during the repolarization phase (dV/dt). This hypothesis was tested in ventricular myocytes by applying the ramp protocol, shown in the inset of Fig. 6A. In each cell the protocol was repeated during perfusion with E-4031 (5 μm) and IE-4031 was identified by subtraction (Fig. 6A). Mean data from a total of nine cells show that while IE-4031 amplitude steeply increased with dV/dt values in the range between 0 and 1 V s−1, an opposite and shallower dependence would occur at dV/dt > 1.5 V s−1 (Fig. 6C, circles). To test whether such a dependency would be consistent with the known properties of IKr, the ramp protocol was also applied to the numerical model. Simulations reproduced the profile of IE-4031 during the ramp (Fig. 6B) and accurately predicted the dependency of IE-4031 on the rate of repolarization (Fig. 6C, continuous line). Since published parameters of inactivation had to be scaled to be used in the model (see Appendix), we tested how inaccuracy in the scaling might affect model predictions. To this end, simulations were repeated after changing the time constant of IKr inactivation to ±30 % of its initial value (Fig. 6D). While the downsloping portion of the relation was sensitive to such changes, the direct dependency observed at dV/dt < 1 V s−1 was robust with respect to potential inaccuracy of inactivation parameters.
Figure 6. Dependency of E-4031-sensitive current (IKr) on the rate of repolarization.
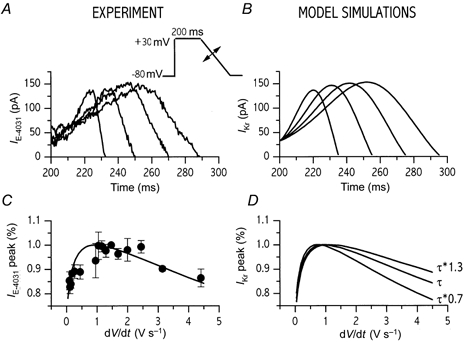
A, IE-4031 recorded, by applying the voltage protocol shown in the inset, during ramps of variable steepness (dV/dt). B, model simulation of the experiment of A. C, peak IE-4031 magnitude recorded from 9 cells as a function of ramp dV/dt (•); in order to eliminate differences between cells, IE-4031 magnitude is expressed as the percentage of the value observed at dV/dt = 1.5 V s−1, which was measured in all cells. The continuous line represents the relationship independently predicted by the model. D, effect of changes in the time constant of inactivation (τ) on the predicted relation between IKr amplitude and dV/dt (data expressed as C.
Overall, these data confirm that the amount of IKr available for repolarization may be directly proportional to dV/dt of repolarization up to a ‘threshold’ value of about 1 V s−1; a shallower or reversed proportionality may be present at higher repolarization rates. The range of repolarization rates relevant to physiology may be estimated by considering dV/dt values measured from action potentials recorded at CLs of 1000 and 250 ms (Table 1). During both phase 2 and phase 3 repolarization dV/dt was below 1.5 V s−1, a range in which direct proportionality is expected. Thus the increase in repolarization rate occurring at shorter CLs may be responsible of the concomitant enhancement of IKr.
Table 1.
Action potential repolarization rates
| Cycle length (ms) | Phase 2 (V s−1) | Phase 3 (V s−1) |
|---|---|---|
| 1000 | 0.27 ± 0.02 | 1.04 ± 0.1 |
| 250 | 0.64 ± 0.05 | 1.34 ± 0.05 |
Dependency of E-4031 modulation of APD on repolarization rate
The shape of IKr dependency on repolarization rate (Fig. 6C) suggested that a primary deficit in IKr may have a different impact on action potential duration (APD) depending on the initial repolarization rate (see Implications). This series of experiments tests this hypothesis by measuring the effect of IKr blockade on action potentials of variable duration (i.e. variable repolarization rate), but in the presence of a constant CL. To be able to interpret the changes in terms of IKr behaviour only, the experiments were carried out during IKs blockade (chromanol 293B, 10 μm). Action potentials were recorded under I-clamp conditions at a pacing CL of 2000 ms (long enough to make the duration of diastolic interval non-influential). Action potential duration was shortened by the injection of a suitable amount of constant current during the repolarization phase; this allowed us to obtain action potentials with a wide spectrum of repolarization rates. All cells were recorded during superfusion in Tyrode solution (control) and during steady-state exposure to 3 μm E-4031 (Fig. 7). According to the cutoff value identified in the previous set of experiments, cells were divided into two groups having mean repolarization rates ≤ 1.0 V s−1 (Group 1, n = 7, mean dV/dt = -0.87 ± 0.07 V s−1) and > 1.0 V s−1 (Group 2, n = 6, mean dV/dt = -2.36 ± 0.31 V s−1). While in Group 1 E-4031 prolonged APD from 143 ± 12 to 160 ± 13 ms (P < 0.05), the change did not achieve significance in Group 2 (55 ± 6 vs. 58 ± 6 ms; n.s.). The difference in percentage APD prolongation between the two groups was significant (+12.3 ± 2.0 %vs.+4.8 ± 1.9 %; P < 0.05).
Figure 7. Dependency of E-4031 modulation of APD on repolarization rate.
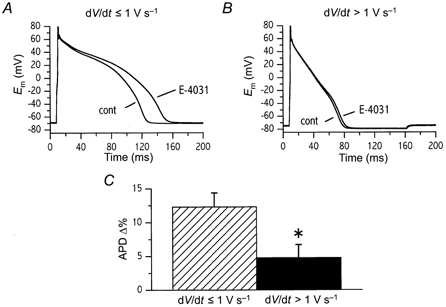
Effect of IKr inhibition (3 μm E-4031) on action potentials with repolarization rates (dV/dt)≤ 1 V s−1 (A) and > 1 V s−1 (B). C, E-4031-induced APD prolongation in cells with dV/dt≤ 1 V s−1 and > 1 V s−1. Action potential duration was modulated by constant current injection during the repolarization phase. Cells were paced at constant cycle length (2 s); IKs was blocked (10 μm chromanol 293B) throughout the experiment.
These experiments demonstrate that during shorter action potentials, in spite of IKr being larger (Fig. 5), the impact of its reduction on APD is minimized. On the contrary, slowing of initial repolarization rate below 1 V s−1 amplifies the effect of IKr inhibition. This occurs independently of CL and it does not depend on concomitant changes in IKs.
DISCUSSION
The results described above suggest the following: (1) both IKr and IKs increase when CL is shortened; (2) the relative contribution of IKr and IKs to repolarization does not depend on CL; (3) the mechanisms by which IKr and IKs increase at shorter CLs are profoundly different - while IKs amplitude depends on the ratio between systolic and diastolic times, IKr amplitude is solely a function of the action potential contour; and (4) the effects on APD of IKr inhibition are amplified during longer action potentials, i.e those having an mean repolarization rate below approximately 1 V s−1.
The results of this study confirm that in guinea-pig myocytes under conditions mimicking physiological electrical activity, IKs increases at shorter CLs. If this were due to incomplete diastolic deactivation, CL shortening should be associated with the appearance or enhancement of an ‘instantaneous’ component of IKs during the action potential upstroke, carried by the residual active channels exposed to an abrupt increase in the driving force. Consistent with this interpretation, instantaneous I293B conductance was increased at shorter CLs; nonetheless, the rate of onset of the non-instantaneous I293B component was also enhanced (Fig. 2D). While more positive potentials would be required to accelerate IKs activation, shorter CL was associated with slightly more negative plateau potentials. Thus, the change in the action potential contour is an unlikely explanation for the faster onset of non-instantaneous I293B conductance. An alternative explanation might be provided by recalling that: (1) IK channel opening from the resting state may require transition through multiple non-conductive states (Balser et al. 1990) and (2) deactivation and inactivation kinetics consistent with the presence of at least two sequential open states have been recently described in expressed KvLQT1 channels (Tristani-Firouzi & Sanguinetti, 1998). At the end of the diastolic interval, part of IKs channels might persist in an ‘intermediate’ state, other than the resting one, from which transition to the final open state would be faster, but still not instantaneous. Since, deactivation rate was found to be inversely proportional to the duration of the activating step (Tristani-Firouzi & Sanguinetti, 1998) the probability of such an ‘intermediate’ state would probably be larger when the ratio between action potential duration and diastolic interval is increased, as occurs at shorter CL.
It is fair to stress that the dependency of IKs on the duration of diastolic interval (i.e. ‘accumulation’ of the activated state), although able to account for the present observations, may not apply to other species, in which IKs deactivation kinetics are considerably faster than in the guinea-pig. However, it should be recognized that in vivo, additional factors are likely to contribute to enhancement of IKs at faster rates. These may include IKs sensitivity to increased intracellular Ca2+ concentration (Tohse et al. 1987; Nitta et al. 1994) and β-adrenergic activation (Yazawa & Kameyama, 1990).
A direct rate dependency of IKr magnitude was unexpected in view of its fast activation and/or deactivation and it might seem at odds with the reverse rate dependency of the effect of IKr block (Bosch et al. 1998). These apparent contradictions can be explained by the observation that IKr magnitude may be determined by action potential shape, rather than by diastolic interval. The observed proportionality between IE-4031 and repolarization rate was complex, being steeper when repolarization was slow (< 1 V s−1) and even reversed when repolarization became very fast (> 1.5 V s−1). Although its physiological role was previously unappreciated, the dependency of IKr on action potential shape can be justified by its known kinetic properties. Indeed, reconstruction of the current by model simulations, based entirely on published kinetic parameters, reasonably reproduced a IE-4031 dependency on action potential contour and repolarization rate. Such a dependency may result from a subtle interplay between activation and inactivation gating, which appears to be exquisitely sensitive to the action potential profile. As previously recognized, fast inactivation (rectification) is pivotal in limiting IKr magnitude during the action potential plateau (Hancox et al. 1998a,b), thus contributing to maintenance of a high input resistance during this phase. At variance with our results, in canine ventricular myocytes peak amplitude of IKr was found to be independent of repolarization rate (Gintant, 2000). This might result from IKr deactivation being considerably slower in dog; indeed, if the time required for deactivation were much larger than that required for repolarization, changes in the latter might become irrelevant. Nonetheless, it should be noted that, among dog myocytes, the voltage at which IKr peaked during the ramp was highly variable, thus implying the coexistence of cells with markedly different IKr kinetics (Gintant, 2000). This, in turn, would lead to a large variability in the profile of IKr dependency on repolarization rate which, when considering mean data, might conceal the dependency itself.
Limitations
Blockade of heterologously expressed KvLQT1+IsK proteins (representative of IKs) by chromanol 293B was reported to develop after channel opening with relatively slow kinetics and to dissipate with faster kinetics (Loussouarn et al. 1997). Although in ventricular myocytes potential contamination by other time-dependent components makes this measurement less reliable, roughly similar kinetics of block may be observed under the experimental conditions of this study (A. Zaza, unpublished observations). Thus, during the early phase of repolarization I293B might underestimate IKs. Nonetheless, the observation that CL shortening was associated with a sharp increase of the instantaneous I293B component indicates that IKs channels which failed to deactivate during the diastole remained associated to the blocker (Loussouarn et al. 1997). Being both accounted for by such non-deactivated/drug-bound channel population, rate-dependent enhancement of IKs and I293B are likely to be reasonably similar. On the other hand, direct use dependency of block might theoretically lead I293B to overstimate IKs rate dependency; however, in view of the high rate of block dissipation at diastolic potentials, direct use dependency is unlikely to occur under the conditions of this study.
Block of neuronal HERG current by ErgTx was inhibited by conditioning steps at positive potentials and recovered at resting potentials over several seconds (Gurrola et al. 1999). Such kinetics might theoretically result in enhancement of blockade at long diastolic intervals. However, in AP clamp on ventricular myocytes IErgTx amplitude was insensitive to changes in diastolic interval (see Results), thus suggesting lack of rate dependency of block. Moreover, the observations that IE-4031 may closely reflect IKr (Hancox et al. 1998a) and that the contours of IErgTx and IE-4031 during the action potential were superimposable (authors’ preliminary observations) suggest that IErgTx is suitable for representing IKr.
Implications
All the conditions leading to an increase in systolic/ diastolic time ratio (shorter CL with adapting APD; shorter CL with constant APD) were associated with an increase in IKs. Overall, IKs behaviour suggests that a sort of negative feedback might occur between primary and secondary modifications of APD induced by IKs blockade. Indeed, APD prolongation, the primary effect of IKs blockade, would increase the systolic/diastolic time ratio, thus leading to a secondary increase in the proportion of the residual IKs channels being in the activated state. Such a negative feedback would be enhanced at faster heart rates, when APD prolongation induced by IKs blockade would have a stronger impact on the already short diastolic interval. This might explain the apparently paradoxical finding that at faster rates, although IKs is enhanced, the effect of its block on APD may be unchanged (Bosch et al. 1998).
The dependency of IKr amplitude on the action potential shape has implications for relevant and poorly understood aspects of repolarization control. Firstly, a decrease in repolarization rate below approximately 1 V s−1 (due either to channel blockade or to channel malfunction) may decrease IKr, which might in turn produce a further slowing of repolarization. Thus, in contrast to IKs, a positive feedback may exist and cause the effects of IKr inhibition to be autoregenerative. Such a positive feedback may take place only when the repolarization rate falls below a threshold value, which may explain the apparently paradoxical observation that the effect of IKr blockade on APD was amplified in the presence of initial repolarization rates below 1 V s−1 (Fig. 7). It is fair to stress that, since IKr dependency on action potential shape is determined by its kinetic properties, its relevance to species with IKr kinetics widely different from those peculiar to the guinea-pig, possibly including man (Wang et al. 1994;Li et al. 1996), is hard to predict and would need to be tested directly.
Positive feedback control of IKr might play a role in the abnormal rate dependency often associated with delayed repolarization. While, exercise-induced QT shortening is enhanced in patients with prolonged repolarization due to primary Na+ channel abnormality (LQT3 syndrome), it is reduced in those with a primary IKr defect (LQT2 syndrome) (Schwartz et al. 1995). If based on causes other than IKr defects, as in LQT3, slowed repolarization may activate IKr feedback suppression, thus leading to amplification of rate-dependent APD changes. On the other hand, due to primary IKr deficiency, such an amplification mechanism would be absent in LQT2, thus explaining the genotype-related differences in the response of QT to exercise. Nonetheless, interpreting exercise-induced QT shortening simply as rate dependency of repolarization may be simplistic. Indeed, in LQTS patients, QTc prolongation induced by exercise or isoproterenol administration was not reproduced by fast atrial pacing (Shimizu et al. 1991). Based on current knowledge, catecholamine-induced QTc prolongation may be typical of primary IKs defects (LQT1) (Priori et al. 1999), in which subnormal IKs enhancement may leave the concomitant increase in calcium unopposed.
In conclusion, we suggest that dependency of IKr on repolarization rate may provide clues to the understanding of the enhanced rate dependency of repolarization observed in primary and secondary forms of QT prolongation. Our experiments also show that the relation between APD changes resulting from blockade of a specific current and the role of the same current in the repolarization process may be extremely complex.
Acknowledgments
The authors are grateful to Professer Enzo Wanke for providing insightful comments, to Mr Gaspare Mostacciuolo and Dr Roberto Cristina Reggiani for expert technical assistance. This work has been supported by a grant from the Ministry of University, Research and Technology (MURST 1997).
APPENDIX
Numerical model of IKr
The purpose of this modelling was to test whether the dependency of IKr amplitude on the repolarization rate (see above) could be predicted by the known kinetic properties of the current.
Repolarizing voltage ramps of different steepness were started from +30 mV (the initial IKr amplitude was the steady-state one predicted for this potential). IKr amplitude was computed from:
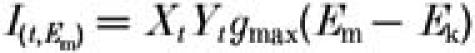 |
where Xt and Yt are the voltage-dependent activation and inactivation gating variables at time t during the ramp; Em and Ek are membrane and K+ equilibrium potentials, respectively; and gmax is fully activated IKr conductance.
Voltage dependency of the gating variables was reconstructed through the voltage dependence of the rate constants (α and β) of the opening and inactivation transitions. For activation:
For inactivation:
The values of the constants were estimated from IKr activation/deactivation kinetics of guinea-pig myocytes (Sanguinetti & Jurkiewicz, 1990) and for fast inactivation of expressed HERG channels (Spector et al. 1996). Inactivation parameters, which were originally measured at 20 °C, were modified so as to scale the resulting inactivation time constants by a factor of 3.5 (corresponding to Q10 = 2.3). Steady-state values of X and Y were computed at each potential as:
time constants (τ) were computed as 1/(α+β).
The value of the gating variables at time t during the Em changes was computed as (integration step = 1 ms):
Current deactivation and recovery from inactivation were considered as the reversal of activation and inactivation processes, respectively (e.g. the same equations were used to describe activation and deactivation).
The overall voltage dependency of IKr kinetics described by the model is shown in Fig. 8. Validation of the model is provided in Figs 5, 6 and 8.
Figure 8. Voltage dependency of IKr and of its gating variables as predicted by the numerical model.
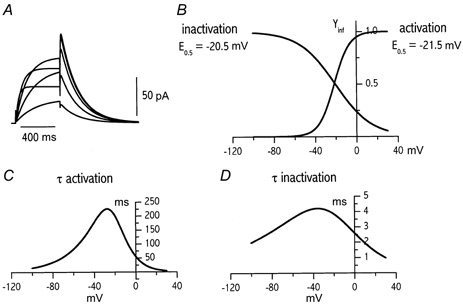
A, IKr activated during depolarizing steps at -30 to +10 mV from a holding of -40 mV. B, voltage dependency of gating variables at steady state (E0.5 = mid potential). C and D, voltage dependency of the time constants (τ) of activation and inactivation, respectively.
References
- Balser JR, Bennett PB, Roden DM. Time-dependent outward current in guinea pig ventricular myocytes. Gating kinetics of the delayed rectifier. Journal of General Physiology. 1990;96:835–863. doi: 10.1085/jgp.96.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch RF, Gaspo R, Busch AE, Lang HJ, Li GR, Nattel S. Effects of the chromanol 293B, a selective blocker of the slow component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovascular Research. 1998;38:441–450. doi: 10.1016/s0008-6363(98)00021-2. [DOI] [PubMed] [Google Scholar]
- Bottiglieri C, Ferrara L, Corona M, Gurrola GB, Batista C, Wanke E, Possani LD. Disulfide bridges of Ergtoxin, a member of a new sub-family of peptide blockers of the ether-a-go-go-related K+-channel. FEBS Letters. 2000;479:156–157. doi: 10.1016/s0014-5793(00)01891-3. [DOI] [PubMed] [Google Scholar]
- Curran ME, Splawski I, Timothy KW, Vincent MG, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- Doerr T, Denger R, Doerr A, Trautwein W. Ionic currents contributing to the action potential in single ventricular myocytes of the guinea pig studied with action potential clamp. Pflügers Archiv. 1990;416:230–237. doi: 10.1007/BF00392058. [DOI] [PubMed] [Google Scholar]
- Gintant GA. Characterization and functional consequences of delayed rectifier current transient in ventricular repolarization. American Journal of Physiology - Heart and Circulatory Physiology. 2000;278:H806–817. doi: 10.1152/ajpheart.2000.278.3.H806. [DOI] [PubMed] [Google Scholar]
- Gurrola GB, Rosati B, Rocchetti M, Pimienta G, Zaza A, Arcangeli A, Olivotto M, Possani LD, Wanke E. A toxin to nervous, cardiac, and endocrine ERG K+ channels isolated from Centuroides noxius scorpion venom. FASEB Journal. 1999;13:953–962. [PubMed] [Google Scholar]
- Hancox JC, Levi AJ, Witchel HJ. Time course and voltage dependence of expressed HERG current compared with native ‘rapid’ delayed rectifier K current during the cardiac ventricular action potential. Pflügers Archiv. 1998a;436:843–853. doi: 10.1007/s004240050713. [DOI] [PubMed] [Google Scholar]
- Hancox JC, Witchel HJ, Varghese A. Alteration of HERG current profile during the cardiac ventricular action potential, following a pore mutation. Biochemical and Biophysical Research Communications. 1998b;253:719–724. doi: 10.1006/bbrc.1998.9837. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz NK, Sanguinetti MC. Rate-dependent prolongation of cardiac action potentials by a methane sulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier current by dofetilide. Circulation Research. 1993;72:75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- Li G-R, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circulation Research. 1996;78:689–696. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- Loussouarn G, Charpentier F, Mohammad-Panah R, Kunzelmann K, Baro I, Escande D. KvLQT1 potassium channel but not IsK is the molecular target for trans-6-cyano-4-(N-ethylsulfonyl-N-methylamino)-3-hydroxy-2,2-dimethyl-chromane. Molecular Pharmacology. 1997;52:1131–1136. doi: 10.1124/mol.52.6.1131. [DOI] [PubMed] [Google Scholar]
- Moss AJ. The QT interval and torsade de pointes. Drug Safety. 1999;21(suppl. 1):5–10. doi: 10.2165/00002018-199921001-00002. [DOI] [PubMed] [Google Scholar]
- Nitta J-I, Furukawa T, Marumo F, Sawanobori T, Hiraoka M. Subcellular mechanism for Ca2+-dependent enhancement of delayed rectifier K+ current in isolated membrane patches of guinea pig ventricular myocytes. Circulation Research. 1994;74:96–104. doi: 10.1161/01.res.74.1.96. [DOI] [PubMed] [Google Scholar]
- Priori SG, Barhanin J, Hauer RW, Haverkamp W, Jongsma HJ, Kleber AG, Mckenna WJ, Roden DM, Rudy Y, Schwartz K, Schwartz PJ, Towbin JA, Wilde A. Genetic and molecular basis of cardiac arrhythmias - Impact on clinical management. European Heart Journal. 1999;20:174–195. doi: 10.1053/euhj.1998.1220. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. Journal of General Physiology. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantu F, Towbin JA, Keating MT, Hammoude H, Brown AM, Chen LS. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–3386. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- Shimizu A, Ohe T, Kurita T, Shimomura K. Differential response of QTU interval to exercise, isoproterenol, and atrial pacing in patients with congenital long QT syndrome. Pacing and Clinical Electrophysiology. 1991;14:1966–1970. doi: 10.1111/j.1540-8159.1991.tb02799.x. [DOI] [PubMed] [Google Scholar]
- Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. Journal of General Physiology. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohse N, Kameyama M, Irisawa H. Intracellular Ca2+ and protein kinase C modulate K+ current in guinea pig heart cells. American Journal of Physiology. 1987;253:H1321–1324. doi: 10.1152/ajpheart.1987.253.5.H1321. [DOI] [PubMed] [Google Scholar]
- Tristani-Firouzi M, Sanguinetti MC. Voltage-dependent inactivation of the human K+ channel KvLQT1 is eliminated by association with minimal K+ channel (MinK) subunits. Journal of Physiology. 1998;510:37–45. doi: 10.1111/j.1469-7793.1998.037bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, da Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nature Genetics. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fermini B, Nattel S. Rapid and slow components of delayed rectifier current in human atrial myocytes. Cardiovascular Research. 1994;28:1540–1546. doi: 10.1093/cvr/28.10.1540. [DOI] [PubMed] [Google Scholar]
- Yazawa K, Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. Journal of Physiology. 1990;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaza A, Rocchetti M, Brioschi A, Cantadori A, Ferroni A. Dynamic Ca2+-induced inward rectification of K+ current during the ventricular action potential. Circulation Research. 1998;82:947–956. doi: 10.1161/01.res.82.9.947. [DOI] [PubMed] [Google Scholar]


