Abstract
In this study we investigated the expression and function of the KVα1 subfamily of voltage-gated K+ channels in terminal arterioles from rabbit cerebral circulation.
K+ current was measured from smooth muscle cells within intact freshly isolated arteriolar fragments. Current activated on depolarisation positive of about –45 mV and a large fraction of this current was blocked by 3,4-diaminopyridine (3,4-DAP) or 4-aminopyridine (4-AP), inhibitors of KV channels. Expression of cRNA encoding KV1.6 in Xenopus oocytes also generated a 4-AP-sensitive K+ current with a threshold for activation near –45 mV.
Immunofluorescence labelling revealed KV1.2 to be specifically localised to endothelial cells, and KV1.5 and KV1.6 to plasma membranes of smooth muscle cells.
KV channel current in arteriolar fragments was blocked by correolide (which is specific for the KVα1 family of KV channels) but was resistant to recombinant agitoxin-2 (rAgTX2; which inhibits KV1.6 but not KV1.5). Heterologously expressed KV2.1 was resistant to correolide, and KV1.6 was blocked by rAgTX2.
Arterioles that were mildly preconstricted and depolarised by 0.1–0.3 nm endothelin-1 constricted further in response to 3,4-DAP, 4-AP or correolide, but not to rAgTX2.
We suggest that KVα1 channels are expressed in smooth muscle cells of terminal arterioles, underlie a major part of the voltage-dependent K+ current, and have a physiological function to oppose vasoconstriction. KVα1 complexes without KV1.5 appear to be uncommon.
That K+ channel activation has a vasodilator function is well established by the fact that there is vasodilatation in response to ATP-sensitive K+ (KATP) channel-opener drugs and vasoconstriction in response to block of large-conductance Ca2+-activated K+ (BKCa) channels by iberiotoxin or following knock-out of the BKCa channel β1-subunit (Brayden & Nelson, 1992; Mayhan & Faraci, 1993; Beech, 1997; Prior et al. 1998; Brenner et al. 2000). The focus of this study was on another family of K+ channels - the voltage-gated K+ channels (KV channels). We did this firstly because of the common observation that K+ current through KV channels in vascular smooth muscle cells is dominant in the physiological potential range -45 to 0 mV (Beech & Bolton, 1989; Volk et al. 1991; Robertson & Nelson, 1994; Ahn & Hume, 1997). Secondly, in smooth muscle cells of many blood vessels KATP channels are normally closed and BKCa channels activate only at more positive potentials (Faraci & Heistad, 1993; Wang & Mathers, 1993; Gebremedhin et al. 1994; Prior et al. 1998; Jackson & Blair, 1998). It is also notable that KV channels are inhibited by [Ca2+]i above the physiological level, whereas BKCa channels are activated (Gelband & Hume, 1995; Cox & Petrou, 1999), and in the spontaneously hypertensive rat, KV channel current is suppressed and BKCa channel expression and current are enhanced (Martens & Gelband, 1996; Liu et al. 1997, 1998). Thus, it appears reasonable to speculate that KV channels are physiologically important and that there is a switch over to dominance of BKCa channels only when [Ca2+]i is high in response to high concentrations of vasoconstrictor agonist or in disease.
It might be surprising that KV channels should be relevant in a cell type that often functions without firing action potentials. However, there is evidence from work on smooth muscle in major arteries that KV channel currents provide tonic negative feedback against sustained small depolarisations evoked by stretch (e.g. Knot & Nelson, 1995; Quan & Sobey, 2000). It is plausible, therefore, that KV channels have a major role in controlling physiological blood pressure. We sought evidence that KV channels serve this function in smooth muscle cells of terminal arterioles, which have a primary role in controlling blood pressure and local tissue perfusion. Having found evidence for such a role we aimed to reveal mammalian KV channel genes that are expressed and functionally important, focusing on the equivalents of the Shaker gene of Drosophila, i.e. the KCNA genes encoding KVα1 subunits (Coetzee et al. 1999).
METHODS
Male Dutch dwarf rabbits (1.0-1.5 kg) were killed by an intravenous overdose of 70 mg kg−1 sodium pentobarbitone, in accordance with the Code of Practice as set out by The UK Animals Scientific Procedures Act 1986. The brain was removed and placed in ice-cold Hanks’ solution bubbled with 100 % O2.
Pieces of pial membrane were removed from the cerebral cortex and incubated with 0.032 mg ml−1 protease and 0.2 mg ml−1 collagenase in Hanks’ solution at 37 °C for 10 min. The mixture was then kept at 4 °C for 15 min and subsequently agitated using a fire-polished Pasteur pipette before being washed with enzyme-free Hanks’ solution followed by centrifugation at 1000 r.p.m. for 1 min. Superficial supernatant was discarded and the remaining suspension of arterioles was stored on glass coverslips at 4 °C in Hanks’ solution and used within 10 h. Isolated arterioles used for experiments had an external diameter of about 30-40 μm and a thick wall of circularly arranged smooth muscle cells (Fig. 2A), in contrast to venules (Fig. 2B). This visual distinction was used to ensure that all recordings were from arterioles. Endothelial cells may appear to be present in these vessels (Guibert & Beech, 1999); in some cases they can be seen clearly under the light microscope (Fig. 2C) and can be detected by immunostaining (Fig. 4C). However, short arteriolar fragments can be visually identified as endothelium denuded (Fig. 2A). In confirmation that many vessel fragments are endothelium denuded, antibody specific for endothelial nitric oxide synthase detects endothelial cells in only about 25 % of fragments, and arterioles dilate very rarely in response to 1 μm acetylcholine (C. Guibert, R. Flemming and D. J. Beech, unpublished observations).
Figure 2. Conventional whole-cell patch-clamp recordings from smooth muscle cells within intact arterioles.
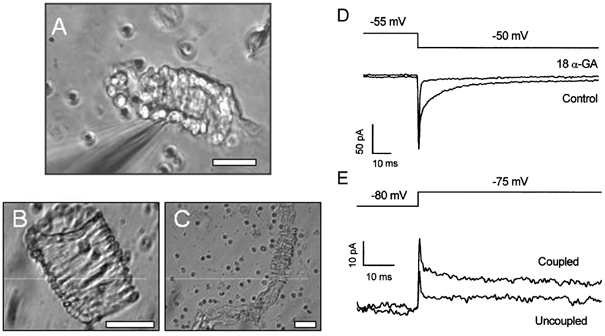
A, digital image of an arteriolar fragment with an attached patch pipette (out of focus object entering the image from the left corner). B, an isolated venule. C, a long isolated arteriole with exposed endothelial cells. Small circular cells are erythrocytes. The scale bars in A-C represent 30 μm. D and E, capacity currents recorded from arteriolar fragments in response to 5 mV steps in voltage. D, two currents from the same arteriole, one under control conditions, and the other in the presence of 50 μm 18α-glycyrrhetinic acid (18α-GA). E, two current traces from different arterioles, one showing electrical coupling between cells (Coupled) and the other in which the patched cell was spontaneously uncoupled from other cells in the arteriole (Uncoupled).
Figure 4. Immunofluorescence staining of native KV1.2, KV1.5 and KV1.6 in enzymatically isolated arterioles.
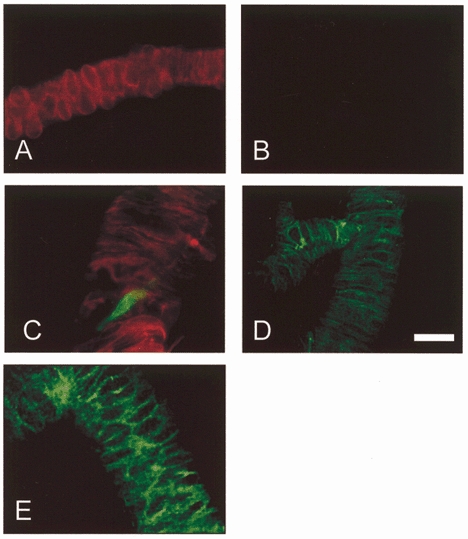
A and B, control experiments showing an arteriole stained with anti-α-SMA-Cy3 (A) and secondary antibody conjugated to FITC but without primary (anti-KV) antibody (B). C, double staining with anti-α-SMA-Cy3 (red) and anti-KV1.2RK(461-480) (green). A fracture in the arteriole is evident, through which an endothelial cell (green) is exposed. D, staining with anti-Kv1.5R(578-598). E, staining with anti-Kv1.6RK(509-526). Scale bar, 30 μm.
Arteriolar diameter measurements were made by placing arterioles in a modified culture dish on the stage of an inverted trinocular microscope (Nikon TMS, Japan), which had an attached video camera (Sony, Japan). The external diameter of arteriolar segments was measured using a video-dimension analyser (Living Systems Instrumentation, VT, USA) and calibrated using a stage micrometer. The signal was captured on-line via an A/D converter (Picolog software, Pico Technology, Cambridge, UK) and stored on a computer. All diameter measurements were made in artificial cerebrospinal fluid (aCSF) gassed with 5 % CO2-95 % O2 at 37 °C.
Current- and voltage-clamp experiments were performed using conventional whole-cell recording (Quinn & Beech, 1998). Gigaseals were formed directly on a smooth muscle cell embedded in an intact arteriolar segment (Fig. 2A). The patch-clamp amplifier used was an Axopatch-1D (Axon Instruments, USA), and command- and data-sampling protocols were controlled by Patch and Vclamp 6 software (Cambridge Electronic Design, UK). Except for the capacity current recordings in Fig. 2, current signals were filtered at 1 kHz and sampled at 2 kHz. Capacity currents were filtered at 10 kHz and sampled at 20 kHz. Patch pipettes were made from borosilicate glass capillary tubing with an outside diameter of 1 mm and inside diameter of 0.58 mm (Clark Electromedical Instruments, UK). Patch pipettes had resistances, after fire-polishing and when filled with solution, of 2-3 MΩ. Voltage clamp was applied to arteriolar segments < 150 μm in length. All patch-clamp experiments were performed at 23 °C. Substances were applied to arterioles by exchanging the solution in the recording chamber, which took < 30 s.
For immunofluorescence experiments, enzymatically isolated arterioles were allowed to adhere to polylysine-coated glass slides prior to fixation in 2 % paraformaldehyde for 30 min. This was followed by a 60 min incubation at room temperature in 1 % bovine serum albumin (BSA) and 0.1 % Triton X-100 in phosphate-buffered saline (PBS). Rabbit polyclonal antibodies were used to detect KVα1 subunits. Antibodies were targeted to rat (R) or mouse (M) sequences: anti-Kv1.2RK(461-480), anti-Kv1.5R(578-598) and anti-Kv1.6RK(509-526), kindly provided by Dr H. G. Knaus (Koch et al. 1997), and anti-Kv1.2RA(417-498) and anti-Kv1.5M(513-602), obtained from Alomone Labs (Israel). The locations of the peptide sequences in the channels are shown subscripted in parentheses. All antisera were used at 1:250 dilution. Goat anti-rabbit IgG conjugated to fluorescein isothiocyanate (FITC; Sigma) was used as the secondary antibody. Use of enzymatically cleaned arterioles minimised background staining associated with using anti-rabbit secondary antibody on rabbit tissue. For double labelling, anti-smooth muscle α-actin antibody pre-conjugated to indocarbocyanine 3 (α-SMA-Cy3; Sigma) was used at 1:200 dilution. The specificity of all antibodies was investigated by parallel experiments in the absence of primary antibody, and in the case of anti-Kv1.5R(578-598) the specificity was also confirmed by preadsorbing the antibody with 10 μm antigenic peptide. Stained cells were viewed using a fluorescence microscope with a × 40 1.3-NA Nplan objective (Nikon, Japan). Images were captured with a Hamamatsu 4880-82 cooled CCD camera at five focal planes separated by 0.5 μm, background subtracted, and haze removed by a deconvolution algorithm (Improvision, UK).
Xenopus laevis were killed by an overdose of tricaine anaesthetic followed by destruction of the brain and spinal cord. Oocytes were surgically removed from the abdomen and transferred into Ca2+- free Ringer solution. Follicular cells were dissociated from oocytes by incubation in 1 mg ml−1 collagenase (Type 1A) at 23 °C for 2 h. Oocytes were microinjected with 50 nl of water or cRNA (20-25 ng) encoding human KV1.6 (GenBank accession number NP002226) or rat KV2.1 (GenBank accession number P15387). Oocytes were then incubated in Barth's solution (mm: NaCl, 88; KCl, 1; NaHCO3, 2.4; Hepes, 10; MgSO4, 0.82; Ca(NO3)2, 0.33; CaCl2, 0.41; pH 7.4) at 19 °C. Recordings were made 2 days later using a GeneClamp 500 amplifier (Axon Instruments) for two-electrode voltage clamp. Recording electrodes were pulled from borosilicate tubing (Clark Electromedical Instruments) and had resistances of 0.5-5 MΩ when filled with 3 m KCl solution. Current and voltage signals were digitised by a 1401 A/D converter (Cambridge Electronic Design) and stored on a computer. The extracellular bathing solution during recordings was Ringer solution (115 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 10 mm Hepes, pH 7.4). During recordings, oocytes were placed in a 0.2 ml recording chamber through which solution flowed at about 2 ml min−1.
Results are expressed as means ±s.e.m. The n values indicate the number of arterioles in electrophysiological and diameter experiments or the number of staining protocols per animal in immunofluorescence experiments, which mostly involved observation of > 1 arteriole and > 1 microscope slide. Groups of data were compared using Student's unpaired t test and statistical significance was concluded if P < 0.05. Mathematical functions were fitted to data points and data are presented using Origin 6 software (Microcal Inc., USA).
Hanks’ solution contained (mm): NaCl, 137; KCl, 5.4; CaCl2, 0.01; NaH2PO4, 0.34; K2HPO4, 0.44; d-glucose, 8; Hepes, 5. aCSF contained 0.5 μm tetrodotoxin and (mm): NaCl, 125; KCl, 1.72; NaHCO3, 24; MgSO4, 1.74; KH2PO4, 1.17; d-glucose, 5.35; CaCl2, 2.47; EDTA, 0.023. Standard bath solution contained (mm): NaCl, 130; KCl, 5; d-glucose, 8; Hepes, 10; MgCl2, 1.2; CaCl2, 1.5. The pipette solution contained (mm): KCl, 130; EGTA, 0.2; MgCl2, 2; Hepes, 10; Na2ATP, 3; NaGTP, 0.5. The final pH of all solutions was titrated to 7.4 using NaOH. Solutions containing 3,4-diaminopyridine (3,4-DAP) or 4-aminopyridine (4-AP) were pH 7.4. BSA (0.01 %) was present in all solutions in experiments involving recombinant agitoxin-2.
EGTA, EDTA, Hepes, protease (Type E), collagenase (Type 1A), glibenclamide, apamin, penitrem A, barium chloride (BaCl2), fatty acid-free bovine serum albumin (BSA), 18α-glycyrrhetinic acid, dimethylsulphoxide (DMSO) and 4-AP were from Sigma. 3,4-DAP was from Fluka Chemie AG. Recombinant agitoxin-2 (rAgTX2) was from Alomone Labs. Endothelin-1 (ET-1) was from Calbiochem. General salts were from BDH or Sigma/Aldrich. 18α-Glycyrrhetinic acid was prepared as a 10 mm stock in 100 % DMSO and freshly added to solutions and vortexed prior to experiments.
RESULTS
Enzymatically isolated arterioles appear functionally normal. They constrict in response to 20-60 mm extracellular K+ (Quinn & Beech, 1998; Quinn et al. 2000) and 0.1-10 nm ET-1 (Guibert & Beech, 1999) or arginine vasopressin (data not shown). To study KV channel function, arterioles were first exposed to 0.1-0.3 nm ET-1, which induces a small degree of tone and depolarisation to about -40 mV (Guibert & Beech, 1999). Tetrodotoxin (0.5 μm) was included to block effects originating from nerve terminals not removed by the collagenase-protease treatment. In the continued presence of ET-1, arterioles constricted strongly (and lengthened and straightened) in response to 1 mm 3,4-DAP (Fig. 1) or 1 mm 4-AP (not shown, but see Fig. 7A), which are inhibitors of many KV channels (Robertson & Nelson, 1994; Coetzee et al. 1999). The reduction in external diameter in response to 3,4-DAP was 32.0 ± 4.0 % of the initial diameter (n = 4). The maximum possible reduction of external diameter, starting in the absence of ET-1, is about 40 % (Quinn & Beech, 1998; Guibert & Beech, 1999). Thus, a significant role for KV channels in controlling arteriolar diameter is indicated.
Figure 1. Contraction in an arteriole exposed to 3,4-diaminopyridine, a blocker of KV channels.
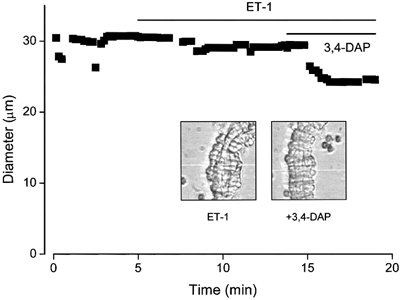
The filled squares in the plot indicate the external diameter of the arteriole. The bath solution was aCSF and a small level of preconstriction was induced with 0.3 nm endothelin-1 (ET-1) prior to application of 1 mm 3,4-diaminopyridine (3,4-DAP). Actual video images of the arteriole are shown inset for the two conditions. The white horizontal line in each image is the scan line used to detect the outer edge of the arteriole. It was moved slightly during the recording to ensure that edge-detection was maintained.
Figure 7. Effects of correolide and recombinant agitoxin-2 on arteriolar diameter.
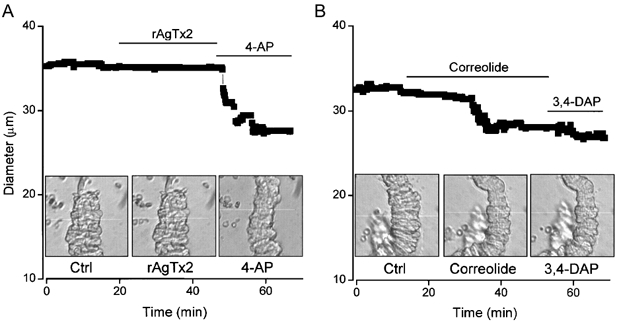
The filled squares in the plots indicate external diameter. The bath solution was aCSF and a small level of preconstriction was induced in both experiments with 0.3 nm ET-1. Actual video images of the arterioles are shown inset for the three conditions in each case. The white horizontal line in each image is the scan line used to detect the outer edge of the arteriole. It was moved slightly during recordings to maintain edge-detection. A, 1 nm rAgTX2 and 1 mm 4-AP. B, 1 μm correolide and 1 mm 3,4-DAP. Ctrl, control.
Smooth muscle cells within arterioles were subjected to patch-clamp recording (Fig. 2A) to determine the properties of the aminopyridine-sensitive current. Cells within these arteriolar fragments were often electrically coupled as shown by the presence of fast and slow components of capacity current elicited following a square step in voltage (Fig. 2D and E). The slow, but not the fast, capacity current was inhibited by 18α-glycyrrhetinic acid (Fig. 2D), which is a gap junction blocker (Davidson & Baumgarten, 1988; Taylor et al. 1998). Occasionally, the slow component was spontaneously absent even though the patched cell remained in the vessel (Fig. 2E).
Whether or not there was electrical coupling between cells, an outward current component was activated by depolarisation. Furthermore, replacement of extracellular Ca2+ by Mg2+ and application of penitrem A, glibenclamide and niflumic acid (inhibiting voltage-dependent Ca2+ entry and blocking BKCa, KATP and ClCa (Ca2+-activated chloride) channels, respectively; Quinn et al. 2000) had only a small inhibitory effect, whereas 1 mm 3,4-DAP inhibited a major component of the current (Fig. 3A). A concentration- response curve was constructed for 4-AP, rather than 3,4-DAP, to facilitate comparison with other published studies. About 40 % inhibition occurred with a low concentration (0.05 mm), there was little further effect at 0.1 mm, but then further inhibition occurred at concentrations up to 5 mm (Fig. 3B). Thus, there appeared to be two components of voltage-dependent K+ current, one very sensitive and one less sensitive to 4-AP.
Figure 3. KV channel currents in arterioles and through heterologously expressed KV1.6.
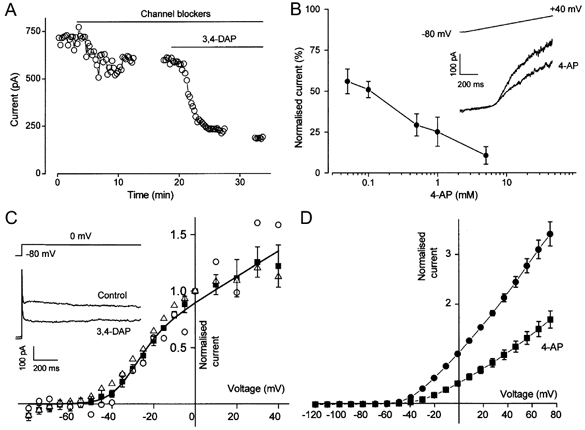
A, open circles show current amplitude at the end of 1 s ramp changes in voltage from -80 to +40 mV. ‘Channel blockers’ means replacement of extracellular Ca2+ by Mg2+, and application of 100 nm penitrem A, 1 μm glibenclamide and 50 μm niflumic acid. 3,4-DAP (1 mm) was applied as indicated. Gaps in the trace indicate the periods during which current-voltage relationships were constructed. B, plot of mean ±s.e.m. (n = 3-5) concentration-dependent block by 4-aminopyridine (4-AP) of outward current at 0 mV. Currents were normalised to the pre-4-AP amplitude. Inset, voltage protocol and example currents for control and in the presence of 50 μm 4-AP. C, voltage dependence of 1 mm 3,4-DAP-sensitive current measured at the end of 1 s square voltage steps and normalised to the amplitude at 0 mV. ▵, coupled cells under control conditions (s.e.m. bars not shown, n = 9); ▪, cells in the presence of 50 μm 18α-glycyrrhetinic acid (n = 4); ○, a spontaneously uncoupled cell. The curve is fitted to the 18α-glycyrrhetinic acid data and is the equation: gmax(V -Vrev)/{1 + exp((V - V1/2)/dV)}, in which V1/2 is the half-activation potential (-31.4 mV), Vrev is the reversal potential (-85 mV), dV is the slope (6.5 mV) and gmax is the maximum normalised conductance. Inset, example currents for one arteriole. The bath solution contained ‘channel blockers’. D, voltage dependence of current (means ±s.e.m.) through KV1.6 expressed as a homomultimer in Xenopus oocytes under control conditions (•, n = 7) and in 1 mm 4-AP (▪, n = 7). Currents were normalised to the amplitude at 0 mV.
The voltage dependence of 3,4-DAP-sensitive current was determined by measuring current amplitude at the end of long square steps in voltage, well after the settling time of the clamp (Fig. 3C). The current-voltage relationships were compared for arterioles with electrically coupled cells, arterioles in the presence of 18α-glycyrrhetinic acid and an arteriole in which the patched cell was spontaneously uncoupled. In each case the voltage dependence of current was similar. The apparent threshold of activation (when current was first detected) was near -45 mV, and the half-activation potential was not significantly different for the control (n = 9) and 18α-glycyrrhetinic acid (n = 4) data sets (-33.7 ± 2.5 mV compared with -30.4 ± 2.3 mV). For comparison, current-voltage relationships were constructed for KV1.6 expressed as a homomultimer (Fig. 3D). Again the activation threshold was near -45 mV and the current was sensitive to 4-AP. These data indicate that KVα1 might underlie part of the native voltage-dependent K+ current in arterioles.
Immunofluorescence staining with polyclonal antibodies was used to test for KV1.2, KV1.5 and KV1.6 expression in arterioles. Arterioles were routinely double-stained with anti-α-SMA-Cy3 to confirm that structures were arterioles. Fluorescence was not observed in parallel control experiments in the absence of primary antibody (e.g. Fig. 4A and B). Inclusion of primary antibody revealed KV1.2 to be localised to endothelial cells (Fig. 4C; n = 5 for anti-Kv1.2RK(461-480), and confirmed with anti-Kv1.2RA(417-498) (data not shown)). In contrast, KV1.5 (n = 5 for anti-Kv1.5R(578-598)) and KV1.6 (n = 6 for anti-Kv1.6RK(509-526)) were localised to the plasma membranes of arteriolar smooth muscle cells (Fig. 4D and E). Anti-Kv1.5M(513-602) also stained smooth muscle cells and staining with anti-KV1.5R(578-598) was not observed in the presence of antigenic peptide (data not shown). Thus, KVα1 subunits are expressed in arteriolar smooth muscle.
Correolide is a triterpene from Spachea correa that selectively binds and blocks members of the KVα1 family of KV channel proteins including KV1.5 and KV1.6 (Felix et al. 1999; Hanner et al. 1999; Koo et al. 1999; Kaczorowski & Garcia, 1999). Consistent with the involvement of KVα1 in the native arteriolar K+ current, 1 μm correolide had a marked inhibitory effect (Fig. 5A), reducing currents at 0 and +40 mV by 43.5 ± 7.6 and 43.9 ± 4.6 %, respectively (n = 9). Although correolide has no effect on an array of non-KVα1 proteins, it may have some effect on KV2.1 at high concentrations (Felix et al. 1999). To check the significance of this effect in our experimental protocol, we expressed KV2.1 cRNA in Xenopus oocytes. Correolide (1 μm) had no effect on KV2.1 (Fig. 5B).
Figure 5. Effect of correolide on K+ currents.
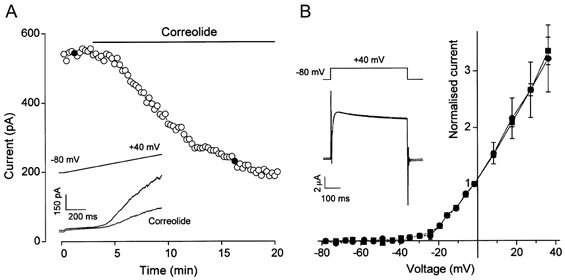
A, plot of current amplitude at the end of 1 s ramp changes in voltage from -80 to +40 mV. Inset, two example current traces taken at the points marked by filled circles in the time-series plot. Correolide (1 μm) was bath applied. B, current-voltage relationships for KV2.1 homomultimers expressed in Xenopus oocytes before (▪) and after (•) application of 1 μm correolide (means ±s.e.m., n = 3). Currents were normalised to the amplitude at 0 mV. Inset, example current traces for control and correolide.
Agitoxin-2 is a peptide originally derived from Leiurus quinquestriatus venom. It is a potent blocker of KVα1 channels except KV1.5, inhibiting KV1.6 with a Ki of 37 pm (Garcia et al. 1994; Kaczorowski & Garcia, 1999; Kotecha & Schlichter, 1999; Tytgat et al. 1999; M. Garcia, personal communication). rAgTX2 (1 nm) had no effect on voltage-dependent K+ current in arterioles at 0 or +40 mV (2.8 ± 2.8 % effect at 0 mV, n = 8) even though the current was shown subsequently to be sensitive to 3,4-DAP (Fig. 6A). In contrast, 1 nm rAgTX2 blocked heterologously expressed KV1.6 by 58.6 ± 8.1 %(n = 5) using a square step voltage protocol (Fig. 6B) and by 72.4 ± 4.8 %(n = 5) using the voltage ramp protocol described for Fig. 6A (data not shown).
Figure 6. Effect of recombinant agitoxin-2 on K+ currents.
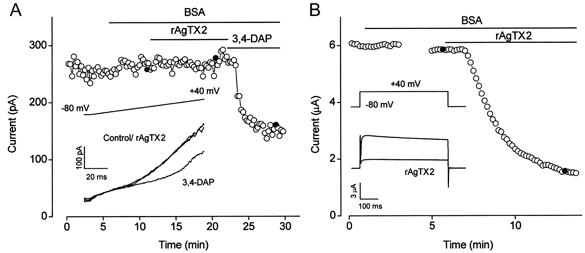
A, plot of arteriolar current amplitude at the end of 1 s ramp changes in voltage from -80 to +40 mV. Inset, three example current traces taken at the points marked by filled circles in the time-series plot. BSA (0.01 %), recombinant agitoxin-2 (rAgTX2; 1 nm) and 3,4-DAP (1 mm) were bath applied. B, plot of current amplitude at +40 mV for KV1.6 homomultimers expressed in a Xenopus oocyte. BSA (0.01 %) and rAgTX2 (1 nm) were bath applied. The gap in the trace indicates the period during which a current-voltage relationship was constructed. Inset, example current traces taken at the points marked by filled circles in the time-series plot.
Correolide (1 μm) was an effective vasoconstrictor of arterioles (Fig. 7B), reducing the diameter by 28.0 ± 3.5 %(n = 7) relative to the effect of 3,4-DAP or 4-AP. In contrast, rAgTX2 (1 nm) had no effect (0.96 ± 0.96 %, n = 5) on arterioles that were sensitive to 3,4-DAP or 4-AP (Fig. 7A).
DISCUSSION
We measured KV channel current in smooth muscle cells within functional cerebral precapillary arterioles, blood vessels that have a primary physiological function in the control of blood pressure and local blood flow in the brain. The data show that current through KV channels is a major current component and is active in the physiologically crucial voltage range of -45 to 0 mV. We also show expression of KV1.5 and KV1.6 proteins in the plasma membrane of arteriolar smooth muscle cells, and reveal that there are electrophysiological and pharmacological similarities between the native KV channel current and that carried by heterologously expressed KCNA6 cRNA. Importantly, non-specific KV channel blockers and a selective KVα1 blocker are powerful arteriolar constrictor agents, suggesting that KVα1 channels play a major role in smooth muscle cells of these small blood vessels.
Inferences about the functional importance of KVα1 were made from in vitro studies of arteriolar diameter under quasi-physiological conditions, which included the presence of a low concentration of an endogenous vasoconstrictor (ET-1). The presence of ET-1 is important because it depolarises the arterioles to about -40 mV and causes a small amount of basal tone (Guibert & Beech, 1999). This is significant because, in vivo, arterioles are exposed to a small lumenal flow and pressure (30 mmHg), which causes an effect, at least by inference from studies of large arteries, similar to that of ET-1 (Knot & Nelson, 1995). In vivo, arterioles are also exposed to a range of endogenous vasoconstrictors, including ET-1 (Thorin et al. 1998). The induction of depolarisation or basal tone is probably important for the vasoconstrictor action of KV channel inhibitors. The mean resting membrane potential of isolated cerebral arterioles in the absence of stimuli is -69 mV (negative of the threshold for activation of KV channel current) and these arterioles are often resistant to a cocktail of K+ channel inhibitors including 1 mm 3,4-DAP (Quinn et al. 2000).
The functional importance of KV channels in arterioles is dependent on the existence of sustained channel activity, which may arise because of window-current, or because there is a non-inactivating channel state at physiological membrane potentials. There are three reasons to believe that sustained KV channel activity exists. Firstly, the data in Fig. 3C show that 3,4-DAP-sensitive KV channel current is non-inactivating over a time period of 1 s at 0 mV. Thus, if there is inactivation it must be very slow, and it would be even slower at, for example, -35 mV. Secondly, correolide and aminopyridine compounds caused arteriolar constriction, effects that could not occur in the absence of sustained non-inactivating KV channel current. Thirdly, in rabbit basilar artery myocytes, there is a 3 pA sustained current component at -40 mV that is inhibited by 5 mm 4-AP (Robertson & Nelson, 1994).
We suggest that agitoxin-2 is ineffective on rabbit arterioles because Kv1.5 is expressed in the smooth muscle cells. Although KV1.6 is also expressed, we presume that this subunit exists only within a heteromultimer with KV1.5, which prevents access of agitoxin-2 to the binding site on KV1.6. Indeed, such an effect has been demonstrated for charybdotoxin, which also blocks KV1.6 but not KV1.5 (Russell et al. 1994; Coetzee et al. 1999). Strikingly, cerebral arterioles from mouse do not express KV1.5 in the smooth muscle layer and are sensitive to agitoxin-2 (Cheong et al. 2001).
All previous studies suggesting a role for KV channels in vascular smooth muscle have employed aminopyridine compounds. 4-AP causes contraction in pulmonary, coronary, basilar and middle cerebral arteries (Knot & Nelson, 1995; O'Rourke, 1996; Archer et al. 1998; Shimizu et al. 2000; Quan & Sobey, 2000). One problem with aminopyridines is their high pKa (-log of the acid dissociation constant). Particularly at concentrations > 1 mm, they tend to alkalinise extracellular and intracellular solutions. Anti-proliferative effects of 4-AP have been attributed to intracellular alkalinisation (Pappas et al. 1994). Although it was shown that intracellular loading with anti-KV1.5 antibody inhibited the effect of 4-AP in pulmonary artery, the antibody surprisingly did not cause contraction (Archer et al. 1998). Thus, evidence for the functional importance of KVα1 has been lacking. Following the development of correolide (Felix et al. 1999), we now show that KVα1 channels have a primary role in terminal cerebral arterioles. We also show that, as with large arteries, aminopyridine compounds are very effective vasoconstrictors, and demonstrate the existence of KV channel current in the physiological voltage range and in smooth muscle cells that are still within the functional vessel, rather than in isolation.
Archer et al. (1998) and Hogg et al. (1999) previously observed a KVα1 channel (KV1.5) in endothelial cells of pulmonary arteries. Although we did not detect KV1.5 in cerebral endothelial cells we did detect KV1.2. In endothelial cells, KV channels do not have a well-recognised function (Nilius et al. 1997), although a 4-AP-sensitive KV channel current exists and is regulated by stretch (Fan & Walsh, 1999). KV1.2 may serve to inhibit depolarisation if it spreads electrotonically from smooth muscle cells.
In cerebral arteriolar smooth muscle cells we show that current through KVα1 contributes a critical balance against sustained excitation, preventing depolarisation at the steep rising phase of the voltage-gated Ca2+ channel activation curve. Too much KVα1 channel current will lead to quiescence, and too little to excess contraction and even regenerative spiking. Regulation of KVα1 function or gene expression presumably has major implications for the control of physiological blood pressure and local tissue perfusion.
Acknowledgments
This work was supported by the British Heart Foundation and A. Dedman had a Nuffield Foundation Undergraduate Bursary. We are grateful to H. G. Knaus (Innsbruck, Austria) for gifts of polyclonal antibodies and advice, J. B. C. Findlay and G. Bentley (Leeds, UK) for the gifts of cDNA encoding KV1.6 and KV2.1, V. Lapostolle for injection of cRNA into Xenopus oocytes (Leeds, UK) and G. Kaczorowski (Merck, USA) for the gift of correolide.
References
- Ahn DS, Hume JR. pH regulation of voltage-dependent K+ channels in canine pulmonary arterial smooth muscle cells. Pflügers Archiv. 1997;433:758–765. doi: 10.1007/s004240050342. [DOI] [PubMed] [Google Scholar]
- Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, KV1. 5 and KV2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. Journal of Clinical Investigation. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ. Actions of neurotransmitters and other messengers on Ca2+ channels and K+ channels in smooth muscle cells. Pharmacology and Therapeutics. 1997;73:91–119. doi: 10.1016/s0163-7258(97)87271-3. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Bolton TB. Two components of potassium current activated by depolarisation of single smooth muscle cells from the rabbit portal vein. Journal of Physiology. 1989;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- Cheong A, Dedman AM, Xu SZ, Beech DJ. KVα1 channels in murine arterioles: differential cellular expression and regulation of diameter. American Journal of Physiology. 2001 doi: 10.1152/ajpheart.2001.281.3.H1057. (in the Press) [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Annals of the New York Academy of Sciences. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Cox RH, Petrou S. Ca2+ influx inhibits voltage-dependent and augments Ca2+-dependent K+ currents in arterial myocytes. American Journal of Physiology. 1999;277:C51–63. doi: 10.1152/ajpcell.1999.277.1.C51. [DOI] [PubMed] [Google Scholar]
- Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. Journal of Pharmacology and Experimental Therapeutics. 1988;246:1104–1107. [PubMed] [Google Scholar]
- Fan J, Walsh KB. Mechanical stimulation regulates voltage-gated potassium currents in cardiac microvascular endothelial cells. Circulation Research. 1999;84:451–457. doi: 10.1161/01.res.84.4.451. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Role of ATP-sensitive potassium channels in the basilar artery. American Journal of Physiology. 1993;264:H8–13. doi: 10.1152/ajpheart.1993.264.1.H8. [DOI] [PubMed] [Google Scholar]
- Felix JP, Bugianesi RM, Schmalhofer WA, Borris R, Goetz MA, Hensens OD, Bao JM, Kayser F, Parsons WH, Rupprecht K, Garcia ML, Kaczorowski GJ, Slaughter RS. Identification and biochemical characterization of a novel nortriterpene inhibitor of the human lymphocyte voltage-gated potassium channel, KV1. 3. Biochemistry. 1999;38:4922–4930. doi: 10.1021/bi982954w. [DOI] [PubMed] [Google Scholar]
- Garcia ML, Garcia-Calvo M, Hidalgo P, Lee A, MacKinnon R. Purification and characterization of three inhibitors of voltage-dependent K+ channels from Leiurus quinquestriatus var. hebraeus venom. Biochemistry. 1994;33:6834–6839. doi: 10.1021/bi00188a012. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Bonnet P, Greene AS, England SK, Rusch NJ, Lombard JH, Harder DR. Hypoxia increases the activity of Ca2+-sensitive K+ channels in cat cerebral arterial muscle cell membranes. Pflügers Archiv. 1994;428:621–630. doi: 10.1007/BF00374586. [DOI] [PubMed] [Google Scholar]
- Gelband CH, Hume JR. [Ca2+]i inhibition of K+ channels in canine renal artery. Novel mechanism for agonist-induced membrane depolarisation. Circulation Research. 1995;77:121–130. doi: 10.1161/01.res.77.1.121. [DOI] [PubMed] [Google Scholar]
- Guibert C, Beech DJ. Positive and negative coupling of the endothelin ETA receptor to Ca2+-permeable channels in rabbit cerebral cortex arterioles. Journal of Physiology. 1999;514:843–856. doi: 10.1111/j.1469-7793.1999.843ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Schmalhofer WA, Green B, Bordallo C, Liu J, Slaughter RS, Kaczorowski GJ, Garcia ML. Binding of correolide to KV1 family potassium channels. Mapping the domains of high affinity interaction. Journal of Biological Chemistry. 1999;274:25237–25244. doi: 10.1074/jbc.274.36.25237. [DOI] [PubMed] [Google Scholar]
- Hogg DS, Albarwani S, Davies AR, Kozlowski RZ. Endothelial cells freshly isolated from resistance-sized pulmonary arteries possess a unique K+ current profile. Biochemical and Biophysical Research Communications. 1999;263:405–409. doi: 10.1006/bbrc.1999.1338. [DOI] [PubMed] [Google Scholar]
- Jackson WF, Blair KL. Characterization and function of Ca2+-activated K+ channels in arteriolar muscle cells. American Journal of Physiology. 1998;274:H27–34. doi: 10.1152/ajpheart.1998.274.1.H27. [DOI] [PubMed] [Google Scholar]
- Kaczorowski GJ, Garcia ML. Pharmacology of voltage-gated and calcium-activated potassium channels. Current Opinion in Chemical Biology. 1999;3:448–458. doi: 10.1016/S1367-5931(99)80066-0. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. American Journal of Physiology. 1995;269:H348–355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- Koch RO, Wanner SG, Koschak A, Hanner M, Schwarzer C, Kaczorowski GJ, Slaughter RS, Garcia ML, Knaus HG. Complex subunit assembly of neuronal voltage-gated K+ channels. Basis for high-affinity toxin interactions and pharmacology. Journal of Biological Chemistry. 1997;272:27577–27581. doi: 10.1074/jbc.272.44.27577. [DOI] [PubMed] [Google Scholar]
- Koo GC, Blake JT, Shah K, Staruch MJ, Dumont F, Wunderler D, Sanchez M, McManus OB, Sirotina-Meisher A, Fischer P, Boltz RC, Goetz MA, Baker R, Bao J, Kayser F, Rupprecht KM, Parsons WH, Tong XC, Ita IE, Pivnichny J, Vincent S, Cunningham P, Hora D, Jr, Feeney W, Kaczorowski G. Correolide and derivatives are novel immunosuppressants blocking the lymphocyte KV1. 3 potassium channels. Cellular Immunology. 1999;197:99–107. doi: 10.1006/cimm.1999.1569. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Schlichter LC. A KV1. 5 to KV1.3 switch in endogenous hippocampal microglia and a role in proliferation. Journal of Neuroscience. 1999;19:10680–10693. doi: 10.1523/JNEUROSCI.19-24-10680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hudetz AG, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in the cerebral microcirculation of genetically hypertensive rats: evidence for their protection against cerebral vasospasm. Circulation Research. 1998;82:729–737. doi: 10.1161/01.res.82.6.729. [DOI] [PubMed] [Google Scholar]
- Liu Y, Pleyte K, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in aorta of hypertensive rats. Hypertension. 1997;30:1403–1409. doi: 10.1161/01.hyp.30.6.1403. [DOI] [PubMed] [Google Scholar]
- Martens JR, Gelband CH. Alterations in rat interlobar artery membrane potential and K+ channels in genetic and nongenetic hypertension. Circulation Research. 1996;79:295–301. doi: 10.1161/01.res.79.2.295. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Faraci FM. Responses of cerebral arterioles in diabetic rats to activation of ATP-sensitive potassium channels. American Journal of Physiology. 1993;265:H152–157. doi: 10.1152/ajpheart.1993.265.1.H152. [DOI] [PubMed] [Google Scholar]
- Nilius B, Viana F, Droogmans G. Ion channels in vascular endothelium. Annual Review of Physiology. 1997;59:145–170. doi: 10.1146/annurev.physiol.59.1.145. [DOI] [PubMed] [Google Scholar]
- O'Rourke ST. Effects of potassium channel blockers on resting tone in isolated coronary arteries. Journal of Cardiovascular Pharmacology. 1996;27:636–642. doi: 10.1097/00005344-199605000-00004. [DOI] [PubMed] [Google Scholar]
- Pappas CA, Ullrich N, Sontheimer H. Reduction of glial proliferation by K+ channel blockers is mediated by changes in pHi. NeuroReport. 1994;6:193–196. doi: 10.1097/00001756-199412300-00049. [DOI] [PubMed] [Google Scholar]
- Prior HM, Yates MS, Beech DJ. Functions of large conductance Ca2+-activated (BKCa), delayed rectifier (KV) and background K+ channels in the control of membrane potential in rabbit renal arcuate artery. Journal of Physiology. 1998;511:159–169. doi: 10.1111/j.1469-7793.1998.159bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan L, Sobey CG. Selective effects of subarachnoid haemorrhage on cerebral vascular responses to 4-aminopyridine in rats. Stroke. 2000;31:2460–2465. doi: 10.1161/01.str.31.10.2460. [DOI] [PubMed] [Google Scholar]
- Quinn K, Beech DJ. A method for direct patch-clamp recording from smooth muscle cells embedded in functional brain microvessels. Pflügers Archiv. 1998;435:564–569. doi: 10.1007/s004240050553. [DOI] [PubMed] [Google Scholar]
- Quinn K, Guibert C, Beech DJ. Sodium-potassium-ATPase electrogenicity in cerebral precapillary arterioles. American Journal of Physiology - Heart and Circulatory Physiology. 2000;279:H351–360. doi: 10.1152/ajpheart.2000.279.1.H351. [DOI] [PubMed] [Google Scholar]
- Robertson BE, Nelson MT. Aminopyridine inhibition and voltage dependence of K+ currents in smooth muscle cells from cerebral arteries. American Journal of Physiology. 1994;267:C1589–1597. doi: 10.1152/ajpcell.1994.267.6.C1589. [DOI] [PubMed] [Google Scholar]
- Russell SN, Overturf KE, Horowitz B. Heterotetramer formation and charybdotoxin sensitivity of two K+ channels cloned from smooth muscle. American Journal of Physiology. 1994;267:C1729–1733. doi: 10.1152/ajpcell.1994.267.6.C1729. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Yokoshiki H, Sperelakis N, Paul RJ. Role of voltage-dependent and Ca2+-activated K+ channels on the regulation of isometric force in porcine coronary artery. Journal of Vascular Research. 2000;37:16–25. doi: 10.1159/000025709. [DOI] [PubMed] [Google Scholar]
- Taylor HJ, Chaytor AT, Evans WH, Griffith TM. Inhibition of the gap junctional component of endothelium-dependent relaxations in rabbit iliac artery by 18-α glycyrrhetinic acid. British Journal of Pharmacology. 1998;125:1–3. doi: 10.1038/sj.bjp.0702078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorin E, Nguyen TD, Bouthillier A. Control of vascular tone by endogenous endothelin-1 in human pial arteries. Stroke. 1998;29:175–180. doi: 10.1161/01.str.29.1.175. [DOI] [PubMed] [Google Scholar]
- Tytgat J, Chandy KG, Garcia ML, Gutman GA, Martin-Eauclaire MF, van der Walt JJ, Possani LD. A unified nomenclature for short-chain peptides isolated from scorpion venoms: α-KTx molecular subfamilies. Trends in Pharmacological Sciences. 1999;20:444–447. doi: 10.1016/s0165-6147(99)01398-x. [DOI] [PubMed] [Google Scholar]
- Volk KA, Matsuda JJ, Shibata EF. A voltage-dependent potassium current in rabbit coronary artery smooth muscle cells. Journal of Physiology. 1991;439:751–768. doi: 10.1113/jphysiol.1991.sp018691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mathers DA. Ca2+-dependent K+ channels of high conductance in smooth muscle cells isolated from rat cerebral arteries. Journal of Physiology. 1993;462:529–545. doi: 10.1113/jphysiol.1993.sp019567. [DOI] [PMC free article] [PubMed] [Google Scholar]


