Abstract
In the present work we investigated the dependence on temperature of the ionic conductance and gating of human muscle ClC-1 chloride channels, transiently expressed in human embryonic kidney (HEK 293) cells.
At normal pH, ClC-1 currents deactivated at negative potentials with a double-exponential time course. The time constants of the exponential components, corresponding to the relaxations of the fast and slow gates, were temperature dependent with Q10 values of ≈3 and ≈4, respectively. Current amplitude increased with increasing temperature with a Q10 of ≈1.6.
The voltage dependence of the two gating processes was shifted towards more positive potentials with increasing temperature. The half-saturation voltage (V1/2) of the steady-state open probability (Po) was shifted by ≈23 and ≈34 mV per 10 °C increase in temperature, for the fast and slow gate, respectively.
At low pH, the voltage dependence of ClC-1 was reversed and currents were activated by hyperpolarisation with a single-exponential time course. This type of gating in ClC-1 resembled the slow gating of the Torpedo ClC-0 homologue, but differed with respect to its kinetics and temperature dependence, with a Q10 of gating relaxations at negative potentials of ≈5. The Arrhenius plot of ClC-1 conductance at low pH had a clear break point at ≈25 °C, with higher Q10 values at lower temperatures.
The temperature sensitivity of relaxation and open probability of the slow gate, which in both ClC-0 and ClC-1 controls two pores simultaneously, implies that the slow gating of ClC-1 is mechanistically different from that of ClC-0.
Analysis of the temperature dependence of ion channel conductance and gating provides valuable information about the thermodynamics of the underlying processes (Frankenhaeuser & Moore, 1963; Bamberg & Läuger, 1974; Murrell-Lagnado & Aldrich, 1993; Nobile et al. 1997; Rodriguez et al. 1998). For most channels, the temperature dependence of conductance is not significantly different from that of a diffusion-limited process, with a Q10 of 1.2-1.7, while gating, in general, shows much greater sensitivity to temperature with Q10 values ranging between 1.5 and 40 (DeCoursey & Cherny, 1998). One of the most temperature-sensitive biological processes currently characterised is the so-called ‘slow gating’ of ClC-0, the Torpedo chloride channel (Pusch et al. 1997). ClC-0 is known for its ‘double-barrelled’ structure, in which each subunit of a homodimeric complex forms an identical ion-conducting pore (Ludewig et al. 1996; Middleton et al. 1996). Each of the twin pores is controlled independently on a millisecond time scale by its own (fast) gate, while a common (slow) gate opens and closes both pores simultaneously over a much longer time scale (Miller, 1982). The kinetics of the fast gate of ClC-0 are only moderately temperature dependent, with a Q10 of about 2.2. In contrast, the deactivation kinetics of the slow gate are highly temperature sensitive with a Q10 of about 40 (Pusch et al. 1997). Such a large temperature coefficient may reflect the involvement of inter-subunit interactions in slow gating (Pusch et al. 1997); however, the precise mechanism of slow gating in ClC-0 is currently unresolved.
The skeletal muscle chloride channel ClC-1 shares many structural and functional similarities with ClC-0 (Waldegger & Jentsch, 2000). As has been demonstrated for ClC-0, ClC-1 has a double-barrelled appearance (Saviane et al. 1999; Weinreich & Jentsch, 2001) and its open probability depends on the external Cl− concentration (Rychkov et al. 1996). There is a substantial difference between the two channels, however, with respect to the voltage dependence and kinetics of the slow gating process (Saviane et al. 1999). In ClC-0, slow gating is activated by hyperpolarisation, while in ClC-1 hyperpolarisation deactivates both fast and slow gates in parallel. In addition, the time constant of ClC-1 slow gating relaxation at negative potentials, unlike that in ClC-0, is just 3-5 times slower than that of the fast gate (Saviane et al. 1999). Interestingly, the voltage dependence of ClC-1 gating can be reversed by low pH, resembling the slow gating of ClC-0 (Rychkov et al. 1996).
The low single-channel conductance and multi-exponential deactivation kinetics of ClC-1 make it less amenable to biophysical characterisation than the ClC-0 Torpedo homologue. Consequently, studies of ClC-0 channel structure and activity are often used as a model for ClC-1, and other ClC-type chloride channels (Jentsch et al. 1999). Although there is no doubt that the fast gating mechanisms in ClC-0 and ClC-1 are very similar, and that they both depend on the permeating anion, little is known about the slow gating of either channel.
In the present work we have used temperature to probe the mechanisms underlying the ionic conductance and gating of ClC-1 under control conditions and at low pH. The Q10 of the fast gating transitions of ClC-1 was similar to that of ClC-0; however, the shift of the open probability (Po) of the fast gate towards positive potentials indicated that the closing rate constant of the ClC-1 fast gate, unlike that of ClC-0, is more temperature sensitive than the opening rate constant. Neither the slow gate at normal pH nor the hyperpolarisation-activated gate at low pH of ClC-1 had a temperature dependence comparable with that of the slow gate of ClC-0.
METHODS
Channel expression
Human ClCN1 (Steinmeyer et al. 1994; kindly provided by Professor T. J. Jentsch, Centre for Molecular Neurobiology, Hamburg, Germany), digested with Eco RV and Eco RI restriction enzymes (Promega Corp., Madison, WI, USA), was ligated into the Nhe I and Eco RI sites of the pCIneo mammalian expression vector (Promega Corp.) using a PCR-generated adapter with 5′Nhe I and 3′Eco RV sites. The PCR-derived fragment was later sequenced to exclude errors. Human embryonic kidney (HEK 293) cells (American Type Culture Collection, Rockville, MD, USA) were transfected with a mixture of the construct and pEGFP-N1 reporter plasmid (Clontech, Palo Alto, CA, USA) at a molar ratio of 3:1 using Lipofectamine Plus (Life Technologies Inc., NY, USA), according to the manufacturer's instructions. Transfected cells were later identified by reporter plasmid-driven expression of green fluorescent protein.
Electrophysiology
Patch-clamp experiments on HEK 293 cells were performed in the whole-cell configuration using a List EPC 7 (List, Darmstadt, Germany) patch-clamp amplifier and associated standard equipment. The standard bath solution contained (mm): NaCl, 144; MgCl2, 1; CaCl2, 2; Hepes, 5; adjusted to pH 7.4 with NaOH. Mes (5 mm) was used instead of Hepes in the external solution to buffer the pH to 5.5. The standard pipette solution contained (mm): CsCl, 40; caesium glutamate, 80; EGTA-K, 10; Hepes, 10; adjusted to pH 7.2 with KOH. For low pH internal solution, 10 mm Mes was substituted for Hepes and adjusted to pH 6.2 with KOH. The temperature of the bath was varied using a Peltier-based device with negative feedback, and measured (within ± 0.05 K) approximately 1-5 mm from the clamped cell using a thermistor in the bath solution.
Patch pipettes of 1-3 MΩ were pulled from borosilicate glass and coated with Sylgard (Dow Corning, Midland, MI, USA). Series resistance did not exceed 5 MΩ and was 80-85 % compensated. Currents obtained were filtered at 3 kHz, collected and analysed using pCLAMP software (Axon Instruments, Foster City, CA, USA). Potentials listed are pipette potentials expressed as intracellular potentials relative to outside zero.
Data analysis
The temperature dependence of the rate constants was analysed according to the Arrhenius equation:
where k is a rate constant at the absolute temperature T, R is the gas constant and A is a constant factor. The activation energy (Ea) was obtained from the slope of the Arrhenius plot (lnk versus 1/T), to allow calculation of the enthalpic component:
Values for Q10 were calculated from the Arrhenius plots according to:
Single-channel recording of ClC-1 recently performed by Saviane et al. (1999) was consistent with the presence of two independent gates in this channel: a fast gate that works on each single protopore, and a slow gate that operates on both protopores simultaneously. The time constants of the fast and slow gates obtained from single-channel recordings were very similar to the time constants of two exponential components that could be fitted to the macroscopic currents. If the time constants extracted from whole-cell macroscopic currents reflect relaxations of the fast and slow gates, it is possible to derive the open probabilities of the fast and slow gates from the relative amplitudes of the corresponding exponential components. During the voltage step from a membrane potential V1 to membrane potential V, the Po of each gate changes exponentially from one steady state to another. The dependence of Po of the fast gate (Po,f) on time can be described by the following equation:
where Po,fV1 and Po,fV are the steady-state Po of the fast gate at membrane potentials V1 and V, respectively, and τf is the time constant of the fast gate.
Similarly, for the slow gate:
where τs is the time constant of the slow gate.
The open probability of the channel overall is given by the equation:
If the initial voltage V1 is set positive to +60 mV, Po of the fast and slow gates is close to unity (Saviane et al. 1999). Consequently, the result of multiplication of open probabilities of the fast and slow gates will be as follows:
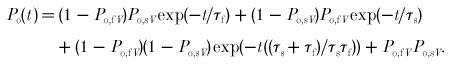 |
This equation contains three exponential terms. However, it can be simplified making the following assumption:
(in practice the latter is true if the slow time constant is at least 3 times longer than the fast time constant). As a result, the time dependence of the Po of the channel can be described by the following equation:
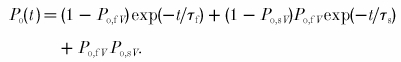 |
The time dependence of the current relaxation is given by the equation:
where Imax is the peak current at time zero.
On the other hand, the raw current data points can be fitted with an equation comprising two exponential components of the form:
where A1, A2 and C are the amplitudes of the fast, slow and steady-state components of the current, respectively. Combining eqns (7), (8) and (9) and dividing by Imax, it is possible to show that the solution of the final equation at each time point exists only if τf = τ1 and τs = τ2, and the coefficients in front of the corresponding exponentials are equal. Consequently:
and
where a1, a2 and c are A1/Imax, A2/Imax and C/Imax, respectively.
This analysis is based on the assumption that the fast and slow gating processes are independent of each other. Our recent results (Aromataris et al. 2001) show that some mutations and drugs can affect the Po of one type of gate without affecting the other, supporting the validity of the present approach. On the other hand, some other properties of ClC-1 indicate that the fast and slow gating in ClC-1 might be coupled (Accardi & Pusch, 2000; Aromataris et al. 2001). The method of separation of Po,f and Po,s presented by Accardi & Pusch (2000), similar to that in the present study, relies on mutual independence of the fast and slow gating. However, these authors showed that the assumption of independence is not crucial for the analysis, provided that the time constant of fast gating is at least 3 times smaller than that of slow gating. The same consideration is also valid for the present study.
Normalised peak tail currents for voltage steps to −100 mV after test pulses in the range -160 to +100 mV were used to produce apparent Po curves by fitting with a Boltzmann distribution with an offset, of the form:
 |
where Pmin is an offset, or a minimal open probability at very negative potentials, V is the membrane potential, V1/2 is the half-maximal activation potential, and k is the slope factor. The same equation was used to produce Po curves for the fast and slow gates with the data points calculated using eqns (10) and (11).
Results are presented as means ± s.e.m.
RESULTS
Temperature dependence of whole-cell currents
Raising the temperature increased the amplitude of ClC-1 currents and accelerated their deactivation at negative potentials (Fig. 1A and B). The characteristic inward rectification of instantaneous I-V plots was preserved at all temperatures (Fig. 1C). The Arrhenius plot for the normalised inward conductance measured as a chord conductance at −120 mV was essentially linear, and the straight line fitted to the data points pooled from several cells had a slope corresponding to a Q10 of 1.6 (Fig. 1D). The chord conductance at +60 mV also increased with temperature with a Q10 of 1.6 (not shown).
Figure 1. Temperature dependence of ClC-1 macroscopic currents.

A and B, current traces recorded from the same cell at 20 °C (A) and 30 °C (B) in response to the following activation voltage protocol: a 100 ms prepulse to +60 mV from a holding potential of −30 mV was followed by voltage steps ranging from -140 to +80 mV in 20 mV increments. C, I -V plots for peak current (Imax, current at t = 0, eqn (9)) at different temperatures. D, Arrhenius plot for inward conductance (Gin). Data pooled from 5 cells were normalised to the inward conductance at 25 °C for each cell. The mean chord conductance calculated from the peak current at −120 mV at 25 °C was 63 ± 23 nS (n = 5). The Q10 calculated from the fit of the Arrhenius equation was 1.6. E, apparent Po curves derived from normalised peak tail currents at −100 mV at different temperatures. The data are fitted with Boltzmann distributions as detailed in Methods.
The apparent Po obtained from normalised peak tail currents was shifted to more positive potentials at higher temperatures (Fig. 1E). Nevertheless, at a potential of +60 mV, which was routinely used as a prepulse potential to activate the channels, the apparent Po reached its maximal value at all temperatures examined.
Temperature dependence of fast gating
Current deactivation at negative potentials could consistently be described by the sum of two exponential components and an offset (eqn (9)) in the temperature range examined. The time constants of the two exponentials that can be extracted from the deactivating currents are believed to represent the time constants of relaxation of the fast and slow gating processes (Saviane et al. 1999). Therefore we refer to the time constants of exponential components τ1 and τ2 (eqn (9)) as the time constants of fast and slow gating, respectively. As expected, the time course of the fast gating relaxation became exponentially shorter with increasing temperature (Fig. 2). At temperatures higher than approximately 35 °C the inward current through ClC-1 could not be reliably dissected from the capacitive transient at the beginning of the voltage pulse. The dependence of the fast gating time constant on temperature could be well fitted by the Arrhenius equation (eqn (1)), yielding a mean activation enthalpy (ΔH‡) for the transition at −140 mV of 84 ± 3 kJ mol−1, with a corresponding Q10 of 3.1 ± 0.1 (n = 5). The apparent activation enthalpies and the corresponding Q10 values were voltage dependent, decreasing at less negative potentials (Fig. 2, Table 1).
Figure 2. Temperature dependence of the time constant of the fast deactivating exponential component of the inward current.

Arrhenius plots for τf from a representative cell at different voltages. The mean parameters of the fits from 5 cells are presented in Table 1.
Table 1.
Voltage dependence of activation enthalpy of fast gating
| ΔH‡ | Q10 | |
|---|---|---|
| (kJ mol-1) | ||
| –140 mV | 84.1 ± 2.5 | 3.1 ± 0.1 |
| –120 mV | 82.9 ± 2.8 | 3.1 ± 0.1 |
| –100 mV | 72.9 ± 3.0 | 2.7 ± 0.1 |
| –80 mV | 59.6 ± 2.5 | 2.2 ± 0.1 |
Mean values of apparent activation enthalpy (δH‡) and temperature coefficient (Q10) for the fast exponential component (τf) were determined from Arrhenius plots shown in Fig. 2.
The apparent Po of ClC-1, as shown in Fig. 1E, represents the combined Po of both fast and slow gates; therefore it is not possible to determine from these data how the Po of different gates was affected by temperature. Steady-state open probabilities of the fast and slow gates can be dissected by deriving them from the relative amplitudes of the exponential components of deactivating inward currents, as shown in Methods. The open probability of the fast gate was temperature dependent such that the half-saturation voltage (V1/2) was shifted towards more positive potentials by 23.4 ± 0.5 mV per 10 °C increase in temperature, while concurrently the minimal Po of the fast gate was reduced (Fig. 3).
Figure 3. Effect of temperature on steady-state Po of the fast gate.
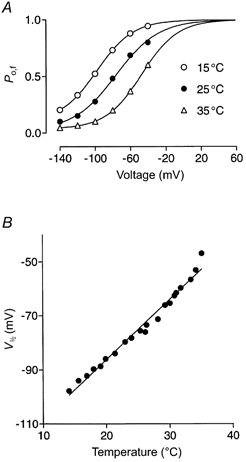
A, Po of the fast gate was derived from the relative amplitude of the fast exponential component as shown in Methods (eqn (10)). Curves represent Boltzmann fits to the calculated data; the maximal value of Po,f was constrained to 1. B, V1/2 determined from Boltzmann fits for the same cell shown in A plotted against temperature. The continuous line is a fit of the form V1/2 = mT + b, where T is temperature, b is the V1/2 at 0 °C, and m is the slope. The mean value of m calculated from 5 cells was 23.4 ± 0.5 mV per 10 °C increase in temperature (in the range 14-35 °C).
Temperature dependence of slow gating
Slow gating relaxations also became faster with increasing temperature. Arrhenius plots of the slow deactivation time constant yielded an apparent activation enthalpy of 103 ± 7 kJ mol−1 for this process, corresponding to a Q10 of 4.1 ± 0.4 (n = 5; Fig. 4). The activation enthalpy of slow gating relaxations was essentially voltage independent between -80 and −140 mV. As with the fast gate, Po of the slow gate was also dependent on temperature (Fig. 5). The V1/2 of slow gating was shifted to more depolarising potentials by 34 ± 2 mV per 10 °C increase in temperature between 14 and 35 °C. The minimal Po of the slow gate showed a tendency to decrease with rising temperature. At high temperatures, however, when the minimal Po of the fast gate was in the range 0.03, the calculation of the Po of the slow gate could be subject to a significant error as a leakage current of just 100 pA at −140 mV could significantly increase the relative amplitude of the steady-state current and increase the estimate of the minimal Po of the slow gate (see eqn (11)). Consequently, it is impossible to make a firm conclusion about the temperature dependence of this parameter of the slow gating process.
Figure 4. Temperature dependence of the time constant of the slow deactivating exponential component of the inward current.

Arrhenius plots for τs from a representative cell at different voltages.
Figure 5. Effect of temperature on steady-state Po of the slow gate.

A, Po of the slow gate was derived from the relative amplitude of the slow exponential component as shown in Methods (eqn (11)). Curves represent Boltzmann fits to the calculated data; the maximal value of Po,s was constrained to 1. B, V1/2 determined from Boltzmann fits plotted against temperature. The mean slope of the line calculated from 5 cells was 34 ± 2 mV per 10 °C increase in temperature (in the range 14-35 °C).
Hyperpolarisation-activated gating of ClC-1 at low pH
A major difference between ClC-1 and ClC-0 channels is in the voltage dependence and kinetics of their slow gating processes. The slow gating of ClC-1 is three orders of magnitude faster than that of ClC-0, and has opposite voltage dependence (Miller, 1982). However, it is possible to induce a type of gating in ClC-1 that resembles the slow gating of ClC-0 by specific point mutations or low pH (Fahlke et al. 1995; Rychkov et al. 1996; Zhang et al. 2000). In the present work, we used an internal pH of 6.2 and an external pH of 5.5 to reverse the voltage dependence of ClC-1 gating. To record hyperpolarisation-activated currents through ClC-1 the holding potential was set to +40 mV and voltage steps to negative potentials were applied at 20 s intervals (Fig. 6A and B). The time course of current activation, with the exception of a short initial phase (2-5 ms), could be fitted with a single exponential. At higher temperatures and potentials negative to −80 mV, inward currents showed some inactivation (Fig. 6B). For these currents, time constants of activation were determined by fitting current traces with a single exponential between 5 ms after the beginning of the pulse and the maximal negative amplitude of the current. The nature of the inactivation was not investigated further in the present study.
Figure 6. Temperature dependence of macroscopic ClC-1 currents at low pH.

Internal pH was 6.2 and external pH was 5.5. A and B, current traces recorded from the same cell at 19 °C (A) and 28 °C (B) in response to the following voltage protocol. For the course of the protocol the holding potential was clamped at +40 mV. The membrane potential was stepped from -120 to +40 mV in 20 mV increments, followed by a tail pulse to +60 mV. The membrane was returned to the holding potential for 20 s between each iteration of the protocol. C, apparent Po curves for hyperpolarisation-activated gating derived from normalised peak tail currents at +60 mV at different temperatures. The data are fitted with Boltzmann distributions as detailed in Methods. Curves fitted at 23 ± 1 °C yielded V1/2 of -30.6 ± 0.4 mV, and an apparent gating valence of 1.3 (n = 7). D and E, Arrhenius plots of peak inward (Gin; D) and outward (Gout; E) conductance. Data pooled from 7 cells were normalised to the inward conductance (D) and outward conductance (E) at 25 °C for each cell. The mean chord conductance calculated from the peak current at −120 mV at 25 °C was 77 ± 22 nS.
Deactivating tail currents recorded on membrane repolarisation required two exponentials for an adequate fit. Peak tail currents at +60 mV, as shown in Fig. 6A and B, were used to construct apparent Po curves of the hyperpolarisation-activated gate (Fig. 6C). A fit of the Boltzmann distribution to the experimental data points gave a V1/2 of -30.6 ± 0.4 mV and an apparent gating charge of approximately 1.3. The apparent Po of hyperpolarisation-activated gating was essentially independent of temperature in the range 15-35 °C.
The amplitude of the inward currents was highly temperature dependent in the range 15-25 °C. The Arrhenius plot for the inward conductance in this temperature range gave a Q10 of about 6.5. At higher temperatures (25-35 °C), however, the Q10 was only 1.2, characteristic of a simple diffusion-limited process (Fig. 6D). Similar temperature dependence was found for the peak tail currents at +60 mV (Fig. 6E). Changes of the current amplitude were fully reversible upon return to the starting temperature.
Arrhenius plots of the time course of current activation (Fig. 7A) yielded an activation enthalpy of 120 ± 3 kJ mol−1, corresponding to a Q10 of 5.4 ± 0.2 (n = 7). This value was independent of the voltage at which activation was measured.
Figure 7. Temperature dependence of current kinetics at low pH.
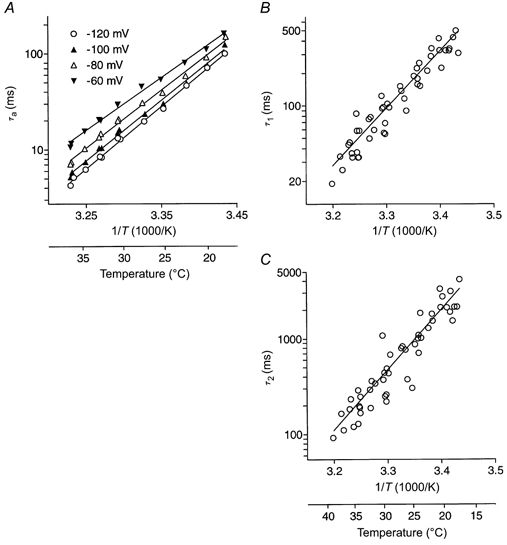
Internal pH was 6.2 and external pH was 5.5. A, Arrhenius plots of the activating time constant (τa) at various potentials for a representative cell. The mean activation enthalpy calculated from 7 cells was 120 ± 3 kJ mol−1, and Q10 was 5.4 ± 0.2. B, Arrhenius plot for the faster deactivating component of tail current at +60 mV measured following a prepulse to −100 mV. Data are pooled from 7 cells. C, Arrhenius plot of the slower deactivating component of tail current measured under the same conditions as in B.
Current deactivation at positive potentials followed a double-exponential time course in the temperature range examined, with time constants of typically less than 650 ms for the fast component (τ1) and 5 s for the slow component (τ2). At +60 mV, the relative amplitudes were approximately 0.6-0.7 and 0.3-0.4 for the fast and slow components, respectively. Pooled data from seven cells were fitted to the Arrhenius equation to yield an apparent activation enthalpy for the fast component of 102 ± 5 kJ mol−1, corresponding to a Q10 of 4.0 (Fig. 7B). The slow component was more steeply dependent on temperature, with an activation enthalpy of 120 ± 7 kJ mol−1, corresponding to a Q10 of 5.1 (Fig. 7C).
DISCUSSION
Temperature dependence of ClC-1 at normal pH
The mean Q10 for inward and outward conductance of ClC-1 under normal conditions was 1.6, a value at the upper end of the range characteristic of diffusion-limited processes (Q10, 1.2-1.7). The Arrhenius plots for individual cells were not quite linear (not shown), with higher Q10 values at lower temperatures. Within the pooled data the curvature in the plot was obscured by variability between cells.
The Q10 of fast gating relaxations varied between 3.1 at −140 mV and 2.2 at −80 mV (Table 1, Fig. 2). The voltage dependence of Q10 indicates that the opening and closing rates of the fast gate are not equally sensitive to temperature. Greater Q10 values for gating at more negative potentials are consistent with a steeper temperature dependence of the closing rate than the opening rate, since the time constant of gating relaxations at very negative potentials is dominated by the closing rate constant. This conclusion is supported by the rightward shift of the apparent Po of fast gating at higher temperatures (Fig. 3). The Po of a channel is related to its opening (α) and closing (β) rates by the expression: Po = α/(α+β). Consequently, if the dependence of β on temperature is steeper than that of α, then, with increasing temperature, the equilibrium will be shifted such that the Po of the channel at a given voltage will be reduced.
Our results differ from those reported by Pusch et al. (1997) for fast gating of ClC-0. The Q10 value obtained for fast gating transitions of ClC-1 was only moderately greater than that demonstrated for ClC-0 (Q10, 2.2). In contrast to ClC-1, however, the apparent activation enthalpies calculated for ClC-0 fast gating were insensitive to voltage, and similarly steady-state voltage dependence was independent of temperature (Pusch et al. 1997). These data indicate that for the fast gate in ClC-0 both forward and backward transitions to the closed state have approximately equivalent temperature dependence, and that neither open nor closed states are energetically favoured.
The slow gating process, which simultaneously regulates the activity of both ClC-1 protopores, has been identified at the single-channel level (Saviane et al. 1999). It differs from the corresponding process in ClC-0 with respect to its time course and voltage dependence and is very different in its dependence on temperature. ClC-0 slow gating relaxations have a remarkable temperature dependence, with an apparent activation enthalpy of 260 kJ mol−1 corresponding to a Q10 of ≈40, which suggests that the transitions between open and closed states of the slow gate in ClC-0 involve complex structural rearrangements (Pusch et al. 1997). Our measurements of the temperature sensitivity of this process in ClC-1 gave an activation enthalpy of 103 kJ mol−1, corresponding to a Q10 of about 4. Unlike the corresponding processes in ClC-0, both fast and slow gating of ClC-1 show similar sensitivity to temperature, with the activation enthalpy and Q10 of slow gating being only modestly greater than those of fast gating. This observation suggests that the structural changes that accompany transitions between open and closed states of the fast and slow gates are thermodynamically similar in their degree of complexity. The similarity between the two gates in ClC-1 extends to the parallel shift of Po with temperature (Fig. 3 and Fig. 5). By contrast, the Po of the slow gate of ClC-0 is not shifted along the voltage axis with temperature, but, rather, it changes with respect to its maximal and minimal values (Pusch et al. 1997). This unusual effect of temperature on the slow gate of ClC-0 was described by a model in which the open and closed states are connected by steps that depend solely on temperature (Pusch et al. 1997). In this respect, ClC-1 differs from ClC-0 in that the main effect of temperature appears to be on steps that are voltage dependent.
The temperature-dependent displacement of steady-state open probability of both the fast and slow gating processes of ClC-1 indicates that the free energy of the open to closed transition is reduced with increasing temperature. As enthalpy is essentially temperature independent, the variation of free energy with temperature is due predominantly to the product of temperature and entropy. Knowing V1/2 at temperatures T1 and T2, it is possible to calculate the entropic change (ΔS) as:
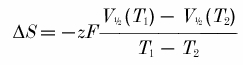 |
(Correa et al. 1992), where z is the gating charge and F is Faraday's constant. Calculations give negative entropic change during the opening of both fast and slow gates of -226 ± 4 and -332 ± 21 J mol−1 K−1, respectively.
Temperature dependence of ClC-1 at low pH
Protonation of ClC-1 at low pH reverses the voltage dependence of gating and alters the kinetics of inward currents (Fig. 6A and B; Rychkov et al. 1996; Rychkov et al. 1997). Specific point mutations introduced in different regions of the primary structure of ClC-1 have very similar effects on channel characteristics to those of low pH (Fahlke et al. 1995; Zhang et al. 2000). The titratable residues responsible for reversing the voltage dependence of ClC-1 remain unidentified at present. At low pH, the voltage dependence and kinetics of ClC-1 gating, although appreciably faster, resemble those of ClC-0 slow gating, and point mutations in ClC-0 have been described which speed up the kinetics of slow gating severalfold without altering other gating characteristics (Ludewig et al. 1997). Therefore, the differences in the kinetics of ClC-1 gating at low pH and slow gating in ClC-0 do not exclude the possibility of a common gating mechanism. We investigated the temperature dependence of the hyperpolarisation-activated gate to determine whether it shared the high activation barrier and unusual decrease of maximal Po with increasing temperature that characterise the slow gate of ClC-0.
The temperature dependence of the inward and outward conductance of ClC-1 at low pH was adequately described by two separate fits to the Arrhenius equation, such that a clear break point occurred at around 25 °C (Fig. 6D and E). Above this temperature, the Q10 for inward and outward conductance was 1.2 and 1.7, respectively, indicating that at higher temperatures the rate-limiting process involves low activation barriers, similar to those associated with diffusion. At temperatures below 25 °C, however, the temperature dependence was much steeper with Q10 values that ranged between 6.0 and 6.5. Break points in the temperature dependence of conductance (Lass & Fischbach, 1976; Chiu et al. 1979; Hagiwara & Yoshii, 1980; Quartararo & Barry, 1988) or gating kinetics (Schwarz, 1979; Chiu et al. 1979; Kirsch & Sykes, 1987) have previously been described in many other channels, and are generally interpreted as being indicative of phase transitions in the membrane lipids. If a phase transition in the membrane lipids of HEK 293 cells were responsible for the break point in the Arrhenius plot for conductance, then we would also expect to observe a similar effect on the temperature sensitivity of gating transitions. The Arrhenius plots for all gating parameters at normal and low pH were linear, and plots of conductance at normal pH, although slightly non-linear, showed no tangible intersection (Fig 1,Fig. 2,Fig. 4 and Fig. 7). Break points in Arrhenius plots of conductance have also been reported for voltage-dependent H+ currents in several different cell types, with higher activation enthalpy and Q10 values at lower temperatures (DeCoursey & Cherny, 1998). The macroscopic current amplitudes that we have used to determine ClC-1 conductance depend on the number of single channels in the membrane available for activation, their Po, and the single-channel current amplitude. The number of functional ClC-1 channels in the membrane appears to remain constant, as the changes in current amplitude are fully reversible. Therefore the temperature dependence of macroscopic current amplitude reflects the temperature dependence of single-channel conductance alone, or in combination with the temperature dependence of Po. The apparent Po curves for hyperpolarisation-activated gating were not shifted along the voltage axis with temperature; however, it was not possible to determine whether the maximal Po of the channel was changed. If maximal Po did vary with temperature, then transitions between conducting and non-conducting states of this gate depend uniquely on temperature. A four-state kinetic model, with uniquely temperature-dependent transitions between open and closed states, was proposed for ClC-0, to explain the dependence of the maximal and minimal Po on temperature (Pusch et al. 1997). In ClC-0, raising the temperature reduced the maximal Po, while in ClC-1, the current amplitude increased with a temperature coefficient that suggests that maximal Po increases. Alternatively, all of the temperature dependence of macroscopic currents may lie in the single-channel conductance. The biphasic temperature sensitivity of ClC-1 conductance suggests that around a critical temperature a different process becomes rate limiting, such that at higher temperatures permeation is diffusion limited, but at lower temperatures the height of energy barriers in the permeation pathway limits conductance. Clearly, further experiments are required to determine the mechanism underlying the high temperature sensitivity of ClC-1 conductance at low pH.
While gating of ClC-1 at low pH parallels the voltage dependence of ClC-0 slow gating, the time course is considerably shorter in ClC-1 than in ClC-0. The gating kinetics are also more complicated in ClC-1, requiring two exponential components to describe current deactivation. The fact that the deactivating kinetic components are described by different temperature coefficients implies that they are governed by different rate-limiting processes. We note that the temperature coefficients that we derived for activation of the current and the slower component of deactivation are approximately equal (Q10, ≈5), and are greater than the coefficients that we have described for other transitions. The faster component of deactivating current has a temperature coefficient (Q10, ≈4) similar to that of the slow gate at normal pH. The nature of the multiple deactivating components remains uncertain, and at this stage we are unable to speculate upon the underlying gating processes. It is apparent, however, that none of the kinetic components we have described are associated with temperature coefficients of the magnitude seen in deactivation of the slow gate of ClC-0.
In conclusion, the temperature dependence of the fast and slow gating of ClC-1 is consistent with ‘true’ conformational changes in the protein structure during open-closed transitions, which exclude the simple blocking mechanism suggested earlier (Rychkov et al. 1996). The difference between fast and slow gating complexity in ClC-1 is much less pronounced than in the Torpedo homologue, ClC-0; nevertheless, the processes underlying fast and slow gating in ClC-1 are not thermodynamically identical. Our results demonstrate that increasing temperature favours closure of both the fast and slow gates of ClC-1, which is consistent with a net decrease in entropy upon opening of each gate. These observations suggest that the open state in ClC-1 is more ordered than the closed state, or that the degrees of conformational freedom of the channel are restricted in the open state.
Acknowledgments
We are grateful to Professor T. J. Jentsch for providing the human ClC-1 clone. This work was supported by the Neuromuscular Research Foundation of the Muscular Dystrophy Association of South Australia and the Australian Research Council.
References
- Accardi A, Pusch M. Fast and slow relaxations in the muscle chloride channel ClC-1. Journal of General Physiology. 2000;116:433–444. doi: 10.1085/jgp.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromataris E C, Rychkov G Y, Bennetts B, Hughes B P, Bretag A H, Roberts M L. Fast and slow gating of ClC-1: differential effects of 2-(4-chlorophenoxy) propionic acid and dominant negative mutations. Molecular Pharmacology. 2001;60:200–208. doi: 10.1124/mol.60.1.200. [DOI] [PubMed] [Google Scholar]
- Bamberg L, Läuger P. Temperature-dependent properties of gramicidin A channels. Biochimica et Biophysica Acta. 1974;367:127–133. doi: 10.1016/0005-2736(74)90037-6. [DOI] [PubMed] [Google Scholar]
- Chiu S Y, Mrose H E, Ritchie J M. Anomalous temperature dependence of the sodium conductance in rabbit nerve compared with frog nerve. Nature. 1979;279:327–328. doi: 10.1038/279327a0. [DOI] [PubMed] [Google Scholar]
- Correa A M, Bezanilla F, Latorre R. Gating kinetics of batrachotoxin-modified Na+ channels in the squid giant axon. Voltage and temperature effects. Biophysical Journal. 1992;61:1332–1352. doi: 10.1016/S0006-3495(92)81941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T E, Cherny V V. Temperature dependence of voltage-gated H+ currents in human neutrophils, rat alveolar epithelial cells, and mammalian phagocytes. Journal of General Physiology. 1998;112:503–522. doi: 10.1085/jgp.112.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Rüdel R, Mitrovic N, Zhou M, George A L. An aspartic acid residue important for voltage dependent gating of human muscle chloride channels. Neuron. 1995;15:463–472. doi: 10.1016/0896-6273(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser B, Moore L E. The effect of temperature on the sodium and potassium permeability changes in myelinated nerve fibres of Xenopus laevis. Journal of Physiology. 1963;169:431–437. doi: 10.1113/jphysiol.1963.sp007269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Yoshii M. Effect of temperature on the anomalous rectification of the membrane of the egg of the starfish, Mediaster aequalis. Journal of Physiology. 1980;307:517–527. doi: 10.1113/jphysiol.1980.sp013451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T J, Friedrich T, Schriever A, Yamada H. The ClC chloride channel family. Pflügers Archiv. 1999;437:783–795. doi: 10.1007/s004240050847. [DOI] [PubMed] [Google Scholar]
- Kirsch G E, Sykes J S. Temperature dependence of Na+ currents in rabbit and frog muscle membranes. Journal of General Physiology. 1987;89:239–251. doi: 10.1085/jgp.89.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass Y, Fischbach G D. A discontinuous relationship between the acetylcholine-activated channel conductance and temperature. Nature. 1976;263:150–151. doi: 10.1038/263150a0. [DOI] [PubMed] [Google Scholar]
- Ludewig U, Jentsch T J, Pusch M. Analysis of a protein region involved in permeation and gating of the voltage-gated Torpedo chloride channel ClC-0. Journal of Physiology. 1997;498:691–702. doi: 10.1113/jphysiol.1997.sp021893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig U, Pusch M, Jentsch T J. Two physically distinct pores in the dimeric ClC-0 chloride channel. Nature. 1996;383:340–343. doi: 10.1038/383340a0. [DOI] [PubMed] [Google Scholar]
- Middleton R E, Pheasant D J, Miller C. Homodimeric architecture of a ClC-type chloride ion channel. Nature. 1996;383:337–340. doi: 10.1038/383337a0. [DOI] [PubMed] [Google Scholar]
- Miller C. Open-state subconductance of single chloride channels from Torpedo electroplax. Philosophical Transactions of the Royal Society. 1982;B 299:401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Murrell-Lagnado R D, Aldrich R W. Energetics of Shaker K channels block by inactivation peptides. Journal of General Physiology. 1993;102:977–1003. doi: 10.1085/jgp.102.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile M, Olcese R, Toro L, Stefani E. Fast inactivation of Shaker K+ channels is highly temperature dependent. Experimental Brain Research. 1997;114:1381–1442. doi: 10.1007/pl00005613. [DOI] [PubMed] [Google Scholar]
- Pusch M, Ludewig U, Jentsch T J. Temperature dependence of fast and slow gating relaxations of ClC-0 chloride channels. Journal of General Physiology. 1997;109:105–116. doi: 10.1085/jgp.109.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartararo N, Barry P H. Ion permeation through single ACh-activated channels in denervated adult toad sartorius skeletal muscle fibres: effect of temperature. Pflügers Archiv. 1988;411:101–112. doi: 10.1007/BF00581653. [DOI] [PubMed] [Google Scholar]
- Rodriguez B M, Sigg D, Bezanilla F. Voltage gating of Shaker K+ channels. The effect of temperature on ionic and gating currents. Journal of General Physiology. 1998;112:223–242. doi: 10.1085/jgp.112.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychkov G Y, Astill D StJ, Bennetts B, Hughes B P, Bretag A H, Roberts M L. pH-dependent interactions of Cd2+ and a carboxylate blocker with the rat ClC-1 chloride channel and its R304E mutant in the Sf-9 insect cell line. Journal of Physiology. 1997;501:355–362. doi: 10.1111/j.1469-7793.1997.355bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychkov G Y, Pusch M, Astill D StJ, Roberts M L, Jentsch T J, Bretag A H. Concentration and pH dependence of skeletal muscle chloride channel ClC-1. Journal of Physiology. 1996;497:423–435. doi: 10.1113/jphysiol.1996.sp021778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saviane C, Conti F, Pusch M. The muscular chloride channel ClC-1 has a double barreled appearance that is differentially affected in dominant and recessive myotonia. Journal of General Physiology. 1999;113:457–467. doi: 10.1085/jgp.113.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz W. Temperature experiments on nerve and muscle membranes of frogs: indications for a phase transition. Pflügers Archiv. 1979;382:27–34. doi: 10.1007/BF00585900. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K, Lorenz C, Pusch M, Koch M C, Jentsch T J. Multimeric structure of ClC-1 chloride channel revealed by mutations in dominant myotonia congenita (Thomsen) EMBO Journal. 1994;13:737–743. doi: 10.1002/j.1460-2075.1994.tb06315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldegger S, Jentsch T J. From tonus to tonicity: physiology of ClC chloride channels. Journal of the American Society of Nephrology. 2000;11:1331–1339. doi: 10.1681/ASN.V1171331. [DOI] [PubMed] [Google Scholar]
- Weinreich F, Jentsch T J. Pores formed by single subunits in mixed dimers of different ClC chloride channels. Journal of Biological Chemistry. 2001;276:2347–2353. doi: 10.1074/jbc.M005733200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Sanguinetti M C, Kwiecinski H, Ptacek L J. Mechanism of inverted activation of ClC-1 channels caused by a novel myotonia congenita mutation. Journal of Biological Chemistry. 2000;275:2999–3005. doi: 10.1074/jbc.275.4.2999. [DOI] [PubMed] [Google Scholar]


