Abstract
A fast ATP-mediated synaptic current was identified in CA3 pyramidal cells in organotypic hippocampal slice cultures. In the presence of inhibitors for ionotropic glutamate and GABA receptors, extracellular stimulation in the pyramidal cell layer evoked fast synaptic currents that reversed near 0 mV, reflecting an increase in a non-selective cationic conductance. This response was mimicked by focal application of ATP. Antagonists of ionotropic P2X receptors reduced both synaptic and ATP-induced currents.
Using a pharmacological approach, the source of synaptically released ATP was determined. Synaptic ATP responses were insensitive to presynaptic blockade of GABAergic transmission between interneurons and CA3 pyramidal cells with the μ-opioid receptor agonist D-Ala2,MePhe4,Met(O)5-ol-enkephalin (FK33-824), but were blocked by adenosine, which inhibits glutamate release from synaptic terminals in the hippocampus. However, selective inhibition of mossy fibre glutamatergic transmission with the metabotropic glutamate receptor group II agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG IV) did not affect the response. This result points to the associational fibres as the source of the ATP-mediated synaptic response.
These results suggest that ATP, coreleased with glutamate, induces a synaptic response in CA3 pyramidal cells that is observed mainly under conditions of synchronous discharge from multiple presynaptic inputs.
There is much evidence to indicate that ATP mediates and/or modulates neurotransmission in many brain areas. Both classes of ATP receptors, the ionotropic P2X receptors and the metabotropic P2Y receptors, are widely distributed in the brain (Ralevic & Burnstock, 1998). Furthermore, ATP is present in synaptic vesicles derived from brain tissue and is released when nerve endings are depolarized (Sperlágh & Vizi, 1996; Sperlágh et al. 1998). Only a few studies, however, have provided functional data showing that action potential-dependent release of ATP induces postsynaptic responses. To date, synaptic responses in the brain mediated by ATP have only been described in the medial habenula (Edwards et al. 1992), locus ceruleus (Nieber et al. 1997), and CA1 pyramidal cells of the hippocampus (Pankratov et al. 1998). In these examples, fast synaptic ATP responses were recorded indicating an activation of P2X receptors, which form non-selective cation channel-receptor complexes. Seven subtypes (P2X1-7) have been cloned (North & Surprenant, 2000), of which P2X2, P2X4, and P2X6, are expressed in the brain, often with overlapping distribution (Collo et al. 1996; Lêet al. 1998b; Kanjhan et al. 1999; Rubio & Soto, 2001).
We have recently observed a fast synaptic current in CA3 pyramidal cells that was insensitive to antagonists for ionotropic glutamate and GABA receptors, and was tentatively attributed to an activation of ATP receptors (Heuss et al. 1999). A similar synaptic current was evoked in CA1 pyramidal cells by stimulation of the Schaffer collateral-comissural pathway (Pankratov et al. 1998). In that study, the receptors mediating the response were not characterized and the source of the released ATP remained unclear, with both a neuronal and a glial origin being considered. Nevertheless, the rapid kinetics of the ATP current in the hippocampus (Pankratov et al. 1998) and analysis of miniature ESPCs in the habenula (Edwards et al. 1992) suggest that these fast ATP responses are of vesicular origin.
Here we characterize a fast current mediated by P2X receptors in CA3 pyramidal cells and identify the terminals of the CA3 pyramidal cell associational fibres as the source of the released ATP.
METHODS
Preparation of slice cultures
Slice cultures were prepared from 6- to 7-day-old Wistar rat pups killed by decapitation following a protocol approved by the Veterinary Department of the Canton of Zurich, and maintained as previously described (Gähwiler, 1981; Gähwiler et al. 1998). In brief, 400 μm-thick hippocampal slices were attached to glass coverslips using clotted chicken plasma, placed in sealed test tubes with serum-containing medium, and maintained in a roller-drum in an incubator at 36 °C for at least 14 days.
Electrophysiological recordings
After 14-28 days in vitro, cultures were transferred to a recording chamber mounted onto the stage of an upright microscope (Axioskop FS; Zeiss, Jena, Germany) and superfused with an external solution (28 °C, pH 7.4) containing 148.8 mm Na+, 2.7 mm K+, 149.2 mm Cl−, 2.8 mm Ca2+, 2.0 mm Mg2+, 11.6 mm HCO3−, 0.4 mm H2PO4−, 5.6 mmd-glucose and 10 mg l−1 Phenol Red (pH 7.4). Synaptic currents were evoked with monopolar metal electrodes placed in the CA3 pyramidal cell layer using single pulses (100 μs, 0.5-30 μA, 0.03-0.05 Hz). Patch-clamp recordings were obtained from CA3 pyramidal cells (Axopatch 200B amplifier; Axon Instruments, Foster City, CA, USA) with patch pipettes (2-5 MΩ) filled with a solution containing: 130 mm caesium methanesulphonate; 10 mm Hepes; 10 mm EGTA; 5 mm 2-(triethylamino)-N-(2,6-dimethylphenyl)acetamide (QX-314), pH 7.25. Series resistance (typically between 5 and 15 MΩ) was regularly checked, and if a change of more than 10 % occurred cells were excluded. ATP was applied locally with a glass pipette positioned about 50 μm from the soma of the recorded pyramidal cell (NeuroPhore; Medical Systems, Greenvale, NY, USA).
Drugs and chemicals
1,2,3,4-Tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulphonamide (NBQX, 40 μm), 3-((R)-2-carboxypiperazin-4yl)-propyl-1-phosphonic acid (CPP, 40 μm) and picrotoxin (100 μm) were always present in the bathing fluid, except for the controls indicated in Fig. 1. NBQX, DCG IV, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), Reactive Blue 2, suramin and ivermectin were purchased from Tocris Cookson (Bristol, UK); EGTA was from Fluka (Buchs, Switzerland), ATP, α,β-methylene ATP, adenosine, apyrase, hexamethonium, picrotoxin and 3-tropanyl-indole-3-carboxylate hydrochloride (ICS 205-930) were from Sigma (Buchs, Switzerland); tetrodotoxin (TTX) was obtained from Latoxan (Rossans, France); CPP and FK 33-824 were gifts from Novartis AG (Basel, Switzerland). QX-314 was purchased from Alomone Labs (Jerusalem, Israel).
Figure 1. Fast synaptic currents evoked in CA3 pyramidal cells of the hippocampus.
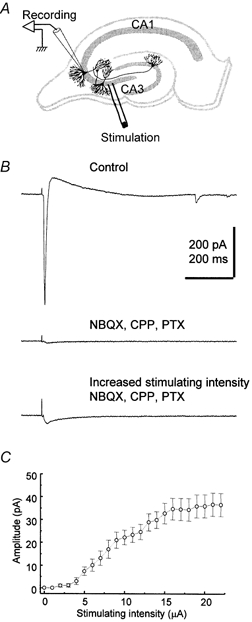
A, schematic diagram illustrating the position of the recording and stimulating electrodes in the hippocampal slice culture. B, electrically evoked fast synaptic currents at a holding potential of −80 mV before (upper trace), and after perfusion of the antagonists NBQX (40 μm), CPP (40 μm) and picrotoxin (PTX, 100 μm) (middle trace) with a stimulating intensity of 7 μA. In the presence of these antagonists, a non-glutamatergic non-GABAergic fast response becomes apparent (lower trace) when the stimulating intensity is increased to 15 μA. C, the relationship between peak amplitude of fast synaptic currents and stimulating intensity at a holding potential of −80 mV in the presence of NBQX (40 μm), CPP (40 μm) and picrotoxin (100 μm). Each point represents the mean of 8 cells. Error bars indicate s.e.m.
Data acquisition and analysis
Signals were filtered at 2 kHz, digitally recorded on computer using Clampex 7 software (Axon Instruments) and stored on tape for later analysis. Numerical data in the text are expressed as means ± s.e.m. Student's t test was used to compare values when appropriate. P < 0.05 was considered significant.
RESULTS
A non-glutamatergic, non-GABAergic synaptic response in CA3 pyramidal cells
Polysynaptic currents were evoked in CA3 pyramidal cells in response to brief (100 μs, single current pulse) extracellular stimulation in the CA3 pyramidal cell layer in the absence of neurotransmitter receptor inhibitors (Fig. 1A and B). The stimulation intensity was then increased until the fast inward current response began to plateau (usually with less than 10 μA). Under these conditions, addition of the ionotropic receptor antagonists NBQX (40 μm), CPP (40 μm) and picrotoxin (100 μm) to block, respectively, AMPA/kainate, NMDA, and GABAA receptor-mediated responses, resulted in a small residual inward current in most cases (Fig. 1B). Initial experiments were performed with the more conventional concentrations of 20 μm NBQX and CPP. Although increasing the concentrations of NBQX and CPP to 40 μm did not alter the residual current response, all subsequent experiments were performed with these higher concentrations to rule out possible polysynaptic effects. The response persisting after blockade of AMPA, NMDA, and GABAA receptors peaked when the stimulation intensity was increased up to 20 μA, reaching an average current of 37.6 ± 4.8 pA at a holding potential of −80 mV (n = 14; Fig. 1B and C). The synaptic latency was 7.1 ± 0.6 ms (n = 14) and the rise-time of the current was 12.3 ± 1.0 ms (10-90 %; n = 14), consistent with a fast synaptic response mediated by ionotropic receptors. The decay phase of the current was best fitted by two exponentials (τ1 = 32.4 ± 3.4 ms, 61.3 %; τ2 = 163.8 ± 19.9 ms, 38.7 %; n = 14; not shown). TTX (0.5 μm) or cadmium (50 μm) completely blocked the evoked current (data not shown; n = 5), further suggesting that this was a synaptic response.
A plot of the I-V relation of the non-glutamatergic, non-GABAergic fast synaptic current revealed a reversal potential near 0 mV (-9.3 ± 4.2 mV; n = 5), indicating an increase in a non-selective cationic conductance (Fig. 2A). The I-V relationship was usually linear, with some cells showing slight inward rectification when hyperpolarized below −70 mV. Other ionotropic receptors that may gate non-selective cation channels include the nicotinic cholinergic receptors, the 5-HT3 serotonergic receptor, and the P2X ATP receptors. Cholinergic and serotonergic fibres are not likely to survive in organotypic hippocampal slice cultures 3-4 weeks after being severed from their cell bodies. Moreover, neither the nicotinic acetylcholine receptor antagonist hexamethonium (100 μm; n = 4), nor the 5-HT3 receptor antagonist ICS 205-930 (10 μm; n = 4) affected this fast synaptic current (Fig. 2B).
Figure 2. Properties of the non-glutamatergic, non-GABAergic fast synaptic current evoked in CA3 pyramidal cells.

A, a typical example of the fast synaptic currents at different voltages (range, -80 to +20 mV in 20 mV increments) in the presence of NBQX (40 μm), CPP (40 μm) and picrotoxin (100 μm) (upper panel) and a plot of the current-voltage relationship for this cell (lower panel). B, perfusion with a nicotinic acetylcholine receptor antagonist, hexamethonium (100 μm) (upper trace), or a selective 5-HT3 receptor antagonist, ICS 205-930 (10 μm) (lower trace), has no effect on the non-glutamatergic, non-GABAergic fast synaptic currents. The holding potential was −80 mV. Traces recorded before and 5 min after drug perfusion are superimposed. Each trace and data point represent an average of four sweeps.
P2X receptors mediate the fast inward current in CA3 pyramidal cells
To determine whether the non-glutamatergic, non-GABAergic fast synaptic current is mediated by ATP, we examined the sensitivity of the response to a series of ATP receptor antagonists. The synaptic current was partially inhibited (see Fig. 3H) by bath application of the P2X receptor antagonists suramin (n = 8), PPADS (n = 8), and Reactive Blue 2 (n = 9), at relatively high concentrations (0.1 and 1 mm). Reactive Blue 2 and suramin were more potent than PPADS (Fig. 3A, B, C and H). The time course of inhibition following bath application of Reactive Blue 2 (1 mm) is illustrated in Fig. 3D. As previously reported (Khakh et al. 1995; Buell et al. 1996; Collo et al. 1996), washout of purinergic antagonists was slow and only partial recovery of responses was observed (51.8 % recovery in 2 cells after 60 min of wash). The fast synaptic current was reduced when ATP breakdown was enhanced by the diphosphohydrolase apyrase (5 U ml−1; n = 6; Fig. 3E and H), under conditions where presynaptic inhibitory effects of adenosine could be excluded because of the presence of the specific antagonist DPCPX (1 μm) (Thompson et al. 1992). In an attempt to characterize the subunit composition of the P2X receptor, we examined the action of ivermectin and zinc. However, the allosteric modulator ivermectin (10 μm; n = 8), which acts on P2X4 receptors (Khakh et al. 1999), had no effect (Fig. 3F and H), whereas zinc (10 μm; n = 6), which enhances responses mediated by homomeric P2X2 and P2X4 receptors (North & Suprenant, 2000), inhibited the non-glutamatergic, non-GABAergic fast synaptic current (Fig. 3G and H).
Figure 3. The effects of antagonists or modulators of P2X receptor-mediated responses on the non-glutamatergic, non-GABAergic fast synaptic current.
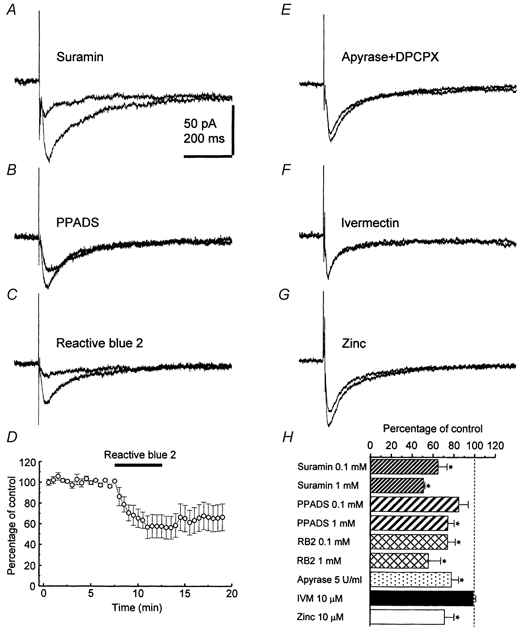
Averaged traces (n = 4) recorded from cells at a holding potential of −80 mV before and 5 min after drug perfusion are superimposed. Inhibition of responses with suramin (1 mm) (A), PPADS (1 mm) (B), Reactive Blue 2 (1 mm) (C), and the time course of the effect of Reactive Blue 2 (1 mm) (D). E, apyrase (5 U ml−1), a diphosphohydrolase, reduces the current. F, the allosteric P2X4 receptor modulator ivermectin (10 μm) has no effect. G, zinc (10 μm) reduces the current. H, summary bar graph. Each column shows the mean effect in 4-8 cells. Error bars indicate s.e.m. with asterisks indicating significant differences. RB2, Reactive Blue 2; IVM, ivermectin.
To confirm that P2X receptors are present on CA3 pyramidal cells, we investigated the effects of focal ATP application by pressure ejection to minimize receptor desensitization associated with prolonged exposure. Because ATP is rapidly degraded to adenosine by endogenous ectonucleotidase (Dunwiddie et al. 1997; Zimmermann, 1999), these experiments were performed in the presence of DPCPX (1 μm). Pulses of ATP applied for 0.4 s induced transient inward currents that reversed near 0 mV (-6.4 ± 4.6 mV; n = 5), similar to the value obtained for the pharmacologically isolated synaptic ATP response (Fig. 4A). Another P2X receptor agonist, α,β-methylene ATP, failed to induce a response (n = 5). In four cells it was possible to show that suramin (1 mm) reduced the current induced by exogenous ATP and the fast synaptic current to a similar extent (n = 4; Fig. 4B), indicating that both responses are mediated by ATP acting at P2X receptors.
Figure 4. Responses induced by local application of ATP in CA3 pyramidal cells.
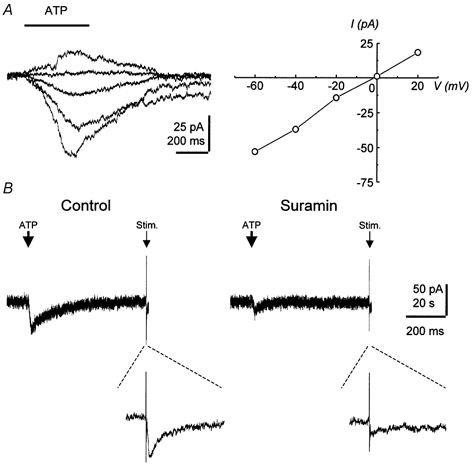
A, currents recorded from a typical cell in response to pressure application of ATP every 2 min from a glass pipette (ATP pipette concentration, 10 μm) shown superimposed at holding potentials ranging from -60 to +20 mV (left panel) and the current-voltage relationship (right panel). B, inhibition by suramin (1 mm, 5 min bath application) of both the current induced by ATP application and the synaptically evoked current. Pressure application of ATP and synaptic stimulation were alternated every 60 s. The holding potential was −80 mV. Responses to synaptic stimulation are shown as expanded traces below.
What is the source of the released ATP?
CA3 pyramidal cells in slice cultures receive inputs from mossy fibres originating from the dentate granule cells, from associational fibres that are axon collaterals of CA3 pyramidal cells, and from diverse inhibitory interneurons. To identify which of these synaptic pathways mediates the fast ATP current, experiments were performed in which each input was selectively inhibited. The μ-opioid receptor agonist FK 33-824 (1 μm), which inhibits transmitter release from inhibitory interneurons contacting CA3 pyramidal cells (Capogna et al. 1993), was without effect on the ATP-mediated fast synaptic current (n = 7; Fig. 5A and D). In contrast, adenosine (100 μm), which presynaptically inhibits glutamatergic synapses with no effect on GABAergic terminals in the hippocampus (Lambert & Teyler, 1991; Scanziani et al. 1992; Thompson et al. 1992), significantly inhibited the ATP response (n = 6; Fig. 5B and D). To determine which glutamatergic projection to the CA3 cells mediates the ATP response, mossy fibre transmission was selectively blocked by activating group II metabotropic glutamate receptors (Kamiya et al. 1996), which are not expressed on other glutamatergic terminals in the hippocampus (Shigemoto et al. 1997). The group II metabotropic glutamate receptor agonist DCG IV (5 μm) had no effect on the ATP-mediated fast synaptic current (n = 6; Fig. 5C and D). These results indicate that ATP is released from glutamatergic associational fibres originating from neighbouring CA3 pyramidal cells.
Figure 5. ATP is released from the associational fibres of CA3 pyramidal cells.

A, FK 33-824 (1 μm), which inhibits interneuron input to CA3 pyramidal cells, has no effect on the fast synaptic ATP current. B, adenosine (100 μm), which reduces excitatory neurotransmission, blocks the ATP response. C, DCG IV (5 μm), which selectively inhibits the mossy fibre glutamatergic input, is without effect. The holding potential was −80 mV. Averaged traces (n = 4) recorded before and 5 min after perfusion with drugs are superimposed. D, summary bar graph. Each column shows the mean effect in 6 or 7 cells. Error bars indicate s.e.m. with asterisks indicating significant differences.
DISCUSSION
In this study an excitatory synaptic current mediated by ionotropic ATP receptors is characterized in CA3 pyramidal cells and ATP is shown to be released synaptically from the associational fibres of neighbouring CA3 pyramidal cells.
The properties of P2X receptors expressed in CA3 pyramidal cells
The rapid time course of the fast synaptic current examined in this study is consistent with a response mediated by an ionotropic receptor. An activation of cation channels gated by nicotinic, serotoninergic or glutamatergic receptors (NMDA receptors and AMPA/ kainate receptors) can be ruled out by the complete lack of effect of the respective antagonists, hexamethonium (100 μm), ICS 205-930 (10 μm), CPP (40 μm) and NBQX (40 μm) (Fig. 1B and Fig. 2B). This leaves the family of ionotropic ATP receptors as a remaining candidate. Indeed, the fast synaptic current was significantly reduced in the presence of the established P2X receptor antagonists suramin, PPADS, and Reactive Blue 2 (Fig. 3A, B, C and H).
The ionotropic ATP receptors found in the hippocampus are composed of P2X2, P2X4 and P2X6 subunits, which are often coexpressed in the same cells (Collo et al. 1996; Lêet al. 1998b; Kanjhan et al. 1999; Rubio & Soto, 2001). Native receptors are thought to be heteromeric (Lewis et al. 1995), with subunits probably assembled as trimers (Nicke et al. 1998). This complexity of P2X receptor structure is thought to underlie the difficulties in reconciling the well-characterized pharmacological effects of antagonists on homomeric P2X receptors with those observed in studies with native receptors. Furthermore, of the subunits expressed in the hippocampus, only the P2X2 subunit shows strong sensitivity to available antagonists (North & Surprenant, 2000). Thus in our experiments, the incomplete blockade obtained with ATP antagonists is likely to reflect the heteromeric nature of the receptors involved. The allosteric modulator ivermectin, which enhances current through homomeric P2X4 receptors or heteromeric P2X4/P2X6 receptors in heterologous expression systems (Khakh et al. 1999), had no effect on ATP responses in CA1 pyramidal cells (Khakh et al. 1999), or in CA3 pyramidal cells in this study (Fig. 3F and H), ruling out these receptors as mediators of the fast synaptic current. Zinc is another modulator of P2X receptors, which potentiates currents conducted by homomeric P2X2 and P2X4 receptors (Brake et al. 1994; Séguéla et al. 1996; Soto et al. 1996; Wildman et al. 1998; Xiong et al. 1999), and by heteromeric P2X4/P2X6 and P2X2/P2X6 receptors (Lêet al. 1998a; King et al. 2000). In dorsal root ganglion neurons from bullfrog (Li et al. 1997), however, and in CA3 pyramidal cells in our study, zinc inhibited currents mediated by P2X receptors (Fig. 3G and H). Consistent with the absence of P2X1 and P2X3 receptors in the hippocampus (Collo et al. 1996), α,β-methylene ATP, a P2X receptor agonist, which acts on homomeric P2X1 and P2X3 receptors (North & Surprenant, 2000), failed to induce a current in CA3 pyramidal cells. On the basis of these results, the receptor mediating the fast synaptic ATP current is likely to be poor in P2X2 and P2X4 subunits, although additional subunit-specific antagonists must be awaited before the combination of subunits comprising the underlying receptor can be identified.
Although ATP receptor localization on presynaptic nerve terminals has been described, this appears not to be the case in the hippocampus (Rubio & Soto, 2001). However, even if, for example, suramin or Reactive Blue 2 were modulating synaptic transmission via a presynaptic receptor, this would be difficult to investigate, as these compounds also inhibit postsynaptic NMDA and AMPA receptors in hippocampus (Nakazawa et al. 1995).
ATP is released from CA3 associational fibres in the hippocampus
To evoke synaptic responses by extracellular stimulation, we applied current through an electrode positioned in the CA3 pyramidal layer (Fig. 1A). Under these conditions, it is likely that mossy fibres, the fibres emanating from inhibitory neurons, and CA3 pyramidal cells and their axons are activated indiscriminately, as reflected by the biphasic currents depicted in Fig. 1B. Therefore, one possible source of ATP release is from interneurons. However, we found that activation of μ-opioid receptors, which prevents GABA release from presynaptic terminals making synapses with hippocampal pyramidal cells (Lambert et al. 1991; Cohen et al. 1992; Capogna et al. 1993), did not modify the fast synaptic response (Fig. 5A and D), suggesting that interneurons are not the source of ATP. The ATP-mediated synaptic current was reduced by adenosine (Fig. 5B and D), which selectively inhibits the release of glutamate from excitatory terminals in the hippocampus (Lambert & Teyler, 1991; Scanziani et al. 1992; Thompson et al. 1992). The results with adenosine, however, do not allow us to distinguish between ATP release from mossy fibres or CA3 associational fibres. Therefore it was necessary to test the effect of DCG IV (Kamiya et al. 1996), which had no effect (Fig. 5C and D), indicating that ATP is not released from mossy fibre terminals. These findings are consistent with the release of ATP from glutamatergic associational fibres terminating in the CA3 area. There is no evidence for a specific purinergic pathway in the hippocampus, as has been described, for instance, in the habenula (Robertson & Edwards, 1998). Stimulation of the Schaffer collateral-comissural pathway, which also originates from CA3 pyramidal cells, evokes a similar ATP-mediated fast synaptic current in CA1 pyramidal cells (Pankratov et al. 1998). Together with this report, our data indicate that ATP is released from CA3 pyramidal cell terminals projecting to both CA1 and neighbouring CA3 pyramidal cells.
Are ATP and glutamate packaged in separate vesicles subject to different mechanisms of release or are these transmitters coreleased? Previous findings suggest that the latter is likely. Vesicles can contain and release more than one fast-acting neurotransmitter (Jonas et al. 1998), and a recent report provides evidence for the corelease of ATP and GABA from the same vesicles in spinal neurons (Jo & Schlichter, 1999).
Functional role of P2X receptors in the hippocampus
In CA1 pyramidal cells, the ATP component has been reported to contribute 25 % of the amplitude of EPSCs (Pankratov et al. 1998). This was not the case in CA3 pyramidal cells (Fig. 1B), where P2X receptors are not as readily activated by synaptically released neurotransmitter as are glutamatergic or GABAergic ionotropic receptors. A number of factors could account for the lower efficacy of ATP-mediated transmission as compared to synaptic responses mediated by other ionotropic receptors in hippocampal pyramidal cells. These include (i) low release probability for ATP, (ii) high ectonucleotidase activity in the synaptic cleft resulting in rapid degrading of ATP, (iii) low expression of postsynaptic ATP receptors, (iv) a predominantly extrasynaptic localization of ATP receptors, (v) mediation of synaptic currents by heteromeric P2X receptors with low affinity for ATP, and (vi) rapid desensitization of P2X receptors mediating the fast synaptic current. At present, it is difficult to evaluate the relative importance of these factors because of a lack of specific experimental tools. Our results show, however, that the pharmacological profile of the P2X receptors mediating the fast synaptic response does not correspond to those described for homomeric receptors, but rather that heteromeric P2X receptors are involved, whose properties are not yet well characterized. Although immunohistochemical studies have shown that P2X2, P2X4 and P2X6 subunits are located preferentially at extrasynaptic sites on CA1 hippocampal pyramidal cells (Rubio & Soto, 2001), the receptors mediating the fast synaptic current in CA3 cells may be heteromeric assemblies expressing other subunits. Thus, the response we have studied may be mediated by extrasynaptic heteromeric receptors that are usually located at a distance from the transmitter release site. The synaptic latency of 7.1 ms for the ATP response, as compared to a latency of 4.2 ms at the excitatory mossy fibre-CA3 pyramidal cell synapse (Jonas et al. 1993), may also reflect an extrasynaptic localization of these receptors.
In conclusion, our results establish that ATP is released from associational fibres of CA3 pyramidal cells thereby inducing a fast postsynaptic inward current. Comparison of the properties of the ATP response with the fast glutamatergic and GABAergic responses in CA3 pyramidal cells, and taking into consideration the stimulation parameters required to evoke this response, it is likely that activation of these P2X receptors requires ATP release from multiple synapses. Therefore, ATP may not mediate point to point information transfer at this synapse, but may modulate hippocampal network behaviours characterized by simultaneous oscillatory activity in neighbouring neurons.
Acknowledgments
We thank L. Heeb, L. Rietschin, S. Giger, H. Kasper, R. Schöb and Dr R. Dürr for excellent technical assistance and Drs K. Fischer, C. Gee and M. Scanziani for valuable discussions and critical reading of the manuscript. This work was supported by the Professor Dr Max Cloëtta Foundation (U.G.), the Swiss National Science Foundation (U.G.), and the Ministry of Education, Science, and Culture of Japan (M.M.).
References
- Brake A J, Wagenbach M J, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North R A, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO Journal. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Capogna M, Gähwiler B H, Thompson S M. Mechanism of μ-opioid receptor-mediated presynaptic inhibition in the rat hippocampus in vitro. Journal of Physiology. 1993;470:539–558. doi: 10.1113/jphysiol.1993.sp019874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G A, Doze V A, Madison D V. Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron. 1992;9:325–335. doi: 10.1016/0896-6273(92)90171-9. [DOI] [PubMed] [Google Scholar]
- Collo G, North R A, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T V, Diao L, Proctor W R. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. Journal of Neuroscience. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F A, Gibb A J, Colquhoun D. ATP receptor-mediated fast synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Gähwiler B H. Organotypic monolayer cultures of nervous tissue. Journal of Neuroscience Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gähwiler B H, Thompson S M, McKinney R A, Debanne D, Robertson R T. Organotypic slice cultures of neural tissue. In: Banker G, Goslin K, editors. Culturing Nerve Cells. 2. Cambridge, MA, USA: MIT Press; 1998. pp. 461–498. [Google Scholar]
- Heuss C, Scanziani M, Gähwiler B H, Gerber U. G-protein-independent signaling mediated by metabotropic glutamate receptors. Nature Neuroscience. 1999;2:1070–1077. doi: 10.1038/15996. [DOI] [PubMed] [Google Scholar]
- Jo Y H, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nature Neurosience. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Jonas P, Major G, Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. Journal of Physiology. 1993;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. Journal of Physiology. 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjhan R, Housley G D, Burton L D, Christie D L, Kippenberger A, Thorne P R, Luo L, Ryan A F. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. Journal of Comparative Neurology. 1999;407:11–32. [PubMed] [Google Scholar]
- Khakh B S, Humphrey P P, Surprenant A. Electrophysiological properties of P2X-purinoceptors in rat superior cervical, nodose and guinea-pig coeliac neurones. Journal of Physiology. 1995;484:385–395. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh B S, Proctor W R, Dunwiddie T V, Labarca C, Lester H A. Allosteric control of gating and kinetics at P2X4 receptor channels. Journal of Neuroscience. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B F, Townsend-Nicholson A, Wildman S S, Thomas T, Spyer K M, Burnstock G. Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. Journal of Neuroscience. 2000;20:4871–4877. doi: 10.1523/JNEUROSCI.20-13-04871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert N A, Harrison N L, Teyler T J. Evidence for μ opiate receptors on inhibitory terminals in area CA1 of rat hippocampus. Neuroscience Letters. 1991;124:101–104. doi: 10.1016/0304-3940(91)90831-d. [DOI] [PubMed] [Google Scholar]
- Lambert N A, Teyler T J. Adenosine depresses excitatory but not fast inhibitory synaptic transmission in area CA1 of the rat hippocampus. Neuroscience Letters. 1991;122:50–52. doi: 10.1016/0304-3940(91)90190-5. [DOI] [PubMed] [Google Scholar]
- Lê K T, Babinski K, Séguéla P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. Journal of Neuroscience. 1998a;18:7152–7159. doi: 10.1523/JNEUROSCI.18-18-07152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê K T, Villeneuve P, Ramjaun A R, McPherson P S, Beaudet A, Séguéla P. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptors. Neuroscience. 1998b;83:177–190. doi: 10.1016/s0306-4522(97)00365-5. [DOI] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North R A, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Li C, Peoples R W, Weight F F. Inhibition of ATP-activated current by zinc in dorsal root ganglion neurones of bullfrog. Journal of Physiology. 1997;505:641–653. doi: 10.1111/j.1469-7793.1997.641ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Inoue K, Ito K, Koizumi S, Inoue K. Inhibition by suramin and reactive blue 2 of GABA and glutamate receptor channels in rat hippocampal neurons. Naunyn-Schmiedeberg's Archives of Pharmacology. 1995;351:202–208. doi: 10.1007/BF00169334. [DOI] [PubMed] [Google Scholar]
- Nicke A, Bäumert H G, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO Journal. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieber K, Poelchen W, Illes P. Role of ATP in fast excitatory synaptic potentials in locus coeruleus neurones of the rat. British Journal of Pharmacology. 1997;122:423–430. doi: 10.1038/sj.bjp.0701386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R A, Surprenant A. Pharmacology of cloned P2X receptors. Annual Review of Pharmacology and Toxicology. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Castro E, Miras-Portugal M T, Krishtal O. A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. European Journal of Neuroscience. 1998;10:3898–3902. doi: 10.1046/j.1460-9568.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- Robertson S J, Edwards F A. ATP and glutamate are released from separate neurones in the rat medial habenula nucleus: frequency dependence and adneosine-mediated inhibition of release. Journal of Physiology. 1998;508:691–701. doi: 10.1111/j.1469-7793.1998.691bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio M E, Soto F. Distinct localization of P2X receptors at excitatory postsynaptic specialization. Journal of Neuroscience. 2001;21:641–653. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Capogna M, Gähwiler B H, Thompson S M. Presynaptic inhibition of miniature excitatory fast synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Haghighi A, Soghomonian J J, Cooper E. A novel neuronal P2x ATP receptor ion channel with widespread distribution in the brain. Journal of Neuroscience. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor P J, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. Journal of Neuroscience. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Gomez-Hernandez J M, Hollmann M, Karschin C, Stühmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proceedings of the National Academy of Sciences of the USA. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperlágh B, Maglóczky E S, Vizi E S, Freund T F. The triangular septal nucleus as the major source of ATP release in the rat habenula: a combined neurochemical and morphological study. Neuroscience. 1998;86:1195–1207. doi: 10.1016/s0306-4522(98)00026-8. [DOI] [PubMed] [Google Scholar]
- Sperlágh B, Vizi E S. Neuronal synthesis, storage and release of ATP. Seminars in the Neurosciences. 1996;8:175–186. [Google Scholar]
- Thompson S M, Haas H L, Gähwiler B H. Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. Journal of Physiology. 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman S S, King B F, Burnstock G. Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. British Journal of Pharmacology. 1998;123:1214–1220. doi: 10.1038/sj.bjp.0701717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Peoples R W, Montgomery J P, Chiang Y, Stewart R R, Weight F F, Li C. Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. Journal of Neurophysiology. 1999;81:2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Two novel families of ectonucleotidases: molecular structures, catalytic properties and a search for function. Trends in Pharmacological Sciences. 1999;20:231–236. doi: 10.1016/s0165-6147(99)01293-6. [DOI] [PubMed] [Google Scholar]


