Abstract
The primary aim of the present study was to explore whether in healthy subjects the muscle contractions required for unrestrained voluntary wrist dorsiflexions are adjusted in strength to thixotropy-dependent variations in the short-range stiffness encountered in measurements of passive torque resistance to imposed wrist dorsiflexions.
After a period of rest, only the first movement in a series of passive wrist dorsiflexions of moderate amplitude exhibited clear signs of short-range stiffness in the torque response. During analogous types of voluntary movements, the extensor EMG during the first movement after rest showed a steep initial rise of activity, which apparently served to compensate for the short-range stiffness.
The passive torque resistance to minute repetitive wrist dorsiflexions (within the range of short-range stiffness) was markedly reduced after various types of mechanical agitation. During analogous low-amplitude voluntary wrist dorsiflexions the extensor EMG signals were weaker after than before agitation.
Mechanical agitation also led to enhancement of passive dorsiflexion movements induced by weak constant torque pulses. In an analogous way, the movement-generating capacity of weak voluntary extensor activations (as determined by EMG recordings) was greatly enhanced by mechanical agitation.
The signals from a force transducer probe pressed against the wrist flexor tendons - during passive wrist dorsiflexions - revealed short-range stiffness responses which highly resembled those observed in the torque measurements, suggesting that the latter to a large extent emanated from the stretched, relaxed flexor muscles. During repetitive stereotyped voluntary wrist dorsiflexions, a close correspondence was observed between the degree of short-range stiffness as sensed by the wrist flexor tension transducer and the strength of the initial extensor activation required for movement generation.
The results provide evidence that the central nervous system in its control of voluntary movements takes account of and compensates for the history-dependent degree of inherent short-range stiffness of the muscles antagonistic to the prime movers.
Since the middle of the last century evidence has accumulated from animal as well as human studies that both extrafusal and intrafusal skeletal muscle fibres possess so-called thixotropic properties (Buchtal & Kaiser, 1951; Lakie et al. 1984; Proske et al. 1993). The term ‘thixotropy’ implies that the resting tension and stiffness of the fibres at any given moment are largely determined by the immediately preceding history of movements and contractions. A limited number of studies have been carried out on human subjects in an attempt to elucidate the motor control consequences of this inherent, plastic muscle behaviour. Most of these studies have dealt with the question of how intrafusal thixotropy can affect spindle afferent activity (Proske et al. 1993) and thereby contribute not only to history-dependent changes in the strength of stretch and vibration reflexes (Morgan et al. 1984; Hagbarth et al. 1985; Jahnke et al. 1989; Gregory et al. 1990; Nordin & Hagbarth, 1996) but also to the appearance of non-volitional, so called ‘postural after- contractions’ (Hagbarth & Nordin, 1998). Examples of human motor phenomena explicable in terms of extrafusal thixotropy have been observed (Hagbarth et al. 1995), but no systematic studies of such phenomena have as yet been made.
A relaxed human limb, kept at rest, is known to possess an inherent mechanical property which provides a disproportionately high resistance to the initial stages of imposed passive movements, a property often referred to as ‘short-range stiffness’. This comparatively high resistance to minute movements has been shown to exhibit thixotropic behaviour, i.e. it can be temporarily loosened up by mechanical agitation such as high amplitude joint movements (Lakie et al. 1984; Hufschmidt & Schwaller, 1987; Wiegner, 1987; Walsh & Wright, 1988). In their study of human ankle joint torque responses to imposed movements, Hufschmidt & Schwaller (1987) presented evidence that the observed short-range stiffness is an in vivo manifestation of what Hill (1968) in his in vitro study of amphibian skeletal muscles observed as a disproportionately high passive resistance to the initial stages of imposed stretch movements. Hill designated this phenomenon as the ‘short-range elastic component’ (SREC) where the initial steep linear (elastic) tension response was only seen up to the ‘elastic limit’ (of about 0.2 % of the total muscle length) from where the tension then levelled off during the remainder of the stretch. Subsequent to Hill's original study, the SREC has been studied from different aspects mainly in amphibian skeletal muscles or isolated muscle fibres (Lännergren, 1971; Haugen & Sten-Knudsen, 1981; Campbell & Lakie, 1998). Hill also proposed that a small part of the resting tension of the skeletal muscle is due to ‘stable’ cross-bridges between actin and myosin filaments - the filamentary resting tension (FRT) - and it was demonstrated that the FRT and SREC were both increased by hypertonicity of the bathing solution suggesting a common origin. The work by Campbell & Lakie (1998) supports Hill's proposal and a theoretical model - compatible with their experimental observations - was developed in which the FRT and the SREC were interpreted as manifestations of slowly cycling cross-bridges between actin and myosin filaments. Even though the SREC has commonly been attributed to the spontaneous formation of cross-bridges between actin and myosin filaments in resting muscle fibres - bridges that are easily detached by stretch movements exceeding the elastic limit - alternative explanations for the underlying mechanisms have also been discussed (Proske & Morgan, 1999). The terms ‘SREC’ and ‘short-range stiffness’ are sometimes used synonymously but we have preferred to use the less specific term short-range stiffness (cf. Rack & Westbury, 1974) in our human-based study where mechanical damping forces are naturally provided not only by the muscles but also other tissues such as the tendons, ligaments, skin, etc.
The present report is primarily concerned with the question whether the neural commands to the muscles during voluntary slow wrist movements are adjusted to compensate for the short-range stiffness. A few examples of motor control adjustments to thixotropy-dependent changes in muscle resting tension are given but will be further dealt with in a study in progress. In the present study integrated surface EMG (IEMG) recordings from extensor/flexor muscles served the dual function of (1) ensuring muscle relaxation during passive tests demonstrating the effect of ‘limbering-up’ manoeuvres on the short-range stiffness and (2) elucidating the effect of such manoeuvres also on the strength of extensor muscle contractions required for voluntary execution of various types of wrist dorsiflexion movements.
METHODS
Subjects
Experiments were carried out on 12 healthy volunteers, including the authors (age 19-73 years, mean 31 years; 7 males and 5 females). They gave informed written consent. The project was approved by the Ethics Committee of the Faculty of Medicine at Uppsala University (Dnr 99114) and was in accordance with the Declaration of Helsinki.
Experimental set-up
At a room temperature of about 20 °C, subjects were comfortably seated in a chair with the left elbow extended to approximately 100 deg and the forearm resting on a table. The forearm was fixed at the elbow and was distally supported by - and fitted into - a U-shaped wooden block holding the wrist joint axis in the vertical plane, thereby minimising the effect of gravitational forces on wrist joint movements. The wrist joint was aligned to the axis of a torque motor (Aeroflex, TQ 34W-33) that had a crank arm which via a force transducer - placed 0.09 m from the rotation axis - was connected to a wooden plate firmly strapped against the volar side of the hand. Angular displacements of the wrist were measured and expressed in degrees using a potentiometer connected to the axis of the torque motor. In the tests where the torque motor was used (Fig. 4A), it was driven by a power amplifier (BBC Axodyn 05LV09) which was fed by a function generator (HP3311A) producing weak rhythmical one-directional torque pulses at a rate of 0.4 Hz, pushing the hand towards dorsiflexion. In these tests the passive stiffness was evaluated by measuring the amplitude of the wrist dorsiflexions induced by the torque pulses.
Figure 4. Aftereffects of stirring manoeuvres on the amplitude of passive wrist dorsiflexions induced by weak repetitive torque pulses (A) and on the amplitude of dorsiflexions actively generated by repetitive weak extensor contractions (B).
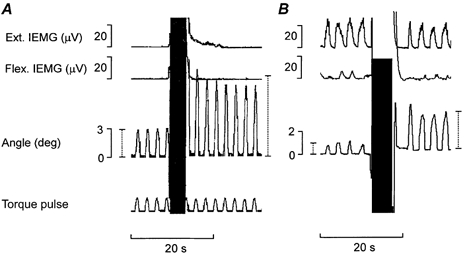
A, example of the Flap. manoeuvre causing a marked enhancement on the hand excursions induced by repetitive torque pulse applications. The two vertical dotted bars in the goniometer trace indicate the mean amplitude of three excursions induced by the torque pulses immediately before and after the Flap. maneouvre. B, example of a Flap. manoeuvre causing an equally marked enhancement of the movement-generating capacity of weak wrist extensor contractions. The two dotted bars in the goniometer trace indicate the mean amplitude of the excursions as in A. (In both A and B the goniometer amplifier was overloaded during the high amplitude Flap. manoeuvres.)
In another series of tests (Fig. 1A and Fig. 3A) stiffness was instead evaluated by measuring the signals from the above-mentioned force transducer when the operator via the crank arm moved the hand towards dorsiflexion. In complementary experiments another force transducer - fixed to a rigid external support - was via a small wooden plate firmly pressed against the flexor tendons in the distal part of the forearm (primarily the flexor carpi radialis tendon). It served to measure tension responses of the flexor musculotendinous structures during passive as well as active wrist dorsiflexions (Fig. 5 and Fig. 6).
Figure 1. Torque responses to passive repetitive wrist dorsiflexions exceeding the limit for the short-range stiffness.
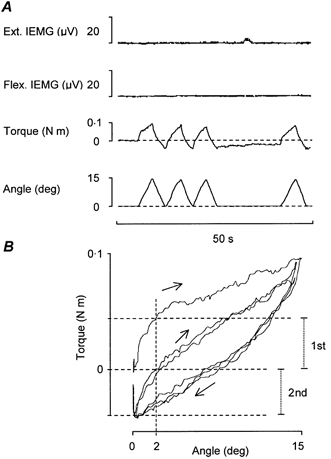
A, with the subject relaxed (as confirmed by IEMG records) the torque responses to manually imposed repetitive wrist dorsiflexions are displayed as up-going deflexions from the zero torque baseline (dashed line). In the goniometer trace wrist dorsiflexions are displayed as up-going deflexions from a baseline (dashed line) assigned as the zero level and indicating the wrist resting position after the standard ‘pre-test’ conditioning manoeuvre described in Methods. B, X-Y plot of torque against wrist displacement for the three consecutive movements shown in A. The upper horizontal dashed line indicates the torque response to the first dorsiflexion at 2 deg and the horizontal middle dashed line indicates the corresponding response to the second movement (which in this particular recording also represents the zero torque level). The lower horizontal dashed line indicates the torque level at the onset of the second movement. The two vertical dotted bars (to the right) indicate the increase in torque resistance for the two movements over the first 2 deg, i.e. measurements used for quantification. Note that the torque responses to the second and the third movements are almost indistinguishable.
Figure 3. Passive compared to active repetitive wrist dorsiflexions of low enough amplitude to not exceed the limit for the short-range stiffness: effects of stirring manoeuvres.
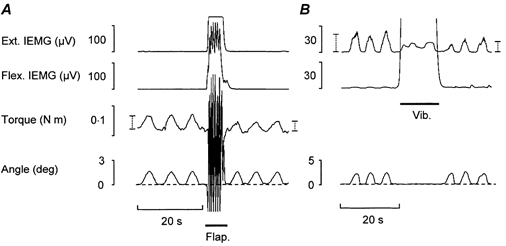
A, torque responses to passive repetitive low amplitude dorsiflexions induced before and after high-amplitude flapping hand movements (Flap.). The two vertical dotted bars in the torque trace indicate the mean torque response to three movements before and after the Flap. manoeuvre. B, strength of extensor contractions required for active repetitive dorsiflexions of similar low amplitude as the passive movements shown in A. The two vertical dotted bars in the extensor IEMG trace indicate the mean IEMG amplitude for three movements before and after a stirring manoeuvre consisting of mechanical vibration applied over the wrist flexor muscles (Vib.).
Figure 5. Wrist flexor tension responses as sensed by force transducer firmly pressed against the flexor tendons during passive wrist movements.
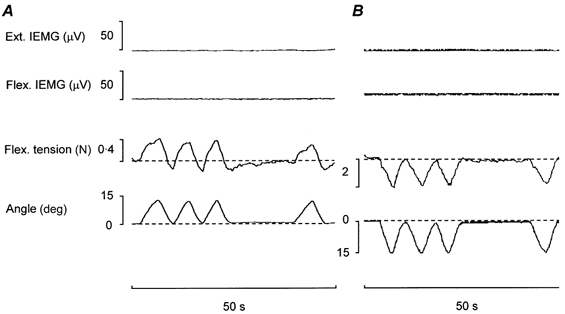
A, flexor tension responses to passive repetitive wrist dorsiflexions of similar speed and amplitude to those illustrated in Fig. 1A. Note the extent to which the signals from the flexor force transducer resemble the torque responses in Fig. 1A. Dashed lines in tension traces show the tension base line (arbitrarily assigned to be zero force) without account being taken of the steady-state pressure exerted by the strain gauge probe against the flexor tendons. B, example of wrist flexor tension responses to passive repetitive wrist volarflexion movements. Note that in response to the first (and fourth) passive shortening movements (after periods of rest) the relaxed flexors exhibit a steep initial fall in tension followed by a less prominent decline, i.e. a response which is the reverse of that induced by passive repetitive dorsiflexions (cf. responses to first and fourth movement in A).
Figure 6. Simultaneous recordings of wrist extensor IEMG activity and wrist flexor tension responses during active repetitive wrist dorsiflexions.
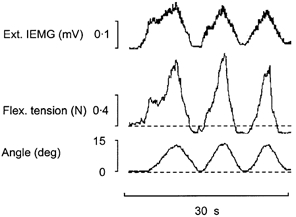
Dashed lines show tension base line (defined as in Fig. 5A) and resting wrist position (defined as in Fig. 1). Note the close correspondence between the strength of the short-range stiffness as exhibited by the flexor tension responses and the strength of the initial extensor activation required for movement generation.
Integrated surface EMG was recorded using two pairs of disposable surface EMG electrodes (blue sensor, type N-10-F, Medicotest, Denmark) placed over the bellies of the flexor carpi radialis and the extensor carpi muscles. The electrodes were placed 5-6 cm apart in a proximal-distal direction for bipolar recordings. The skin was rinsed with ethanol to lower the impedance. The surface EMG signals were amplified and integrated with a time constant of 200 ms. During the experiments, the mean voltage EMG signals were like the goniometer, strain gauge and function generator signals displayed on a four-channel digital oscilloscope (Lecroy 9314M). The signals were either directly printed out by the oscilloscope screen dump unit or stored on disk for further analysis.
Specifications of various terms and expressions used in the description of the test procedures
In view of the reported history dependency of the short-range stiffness, all tests in the present study were preceded by the following type of pre-test conditioning procedure (which is not shown in the figures): the subject performed high-amplitude rapid flapping movements of the hand for a few seconds and then relaxed for at least 20 s. The hand position obtained after this procedure was denoted as the ‘resting position’. It showed some inter-individual variations within the range of 5-15 deg of volarflexion.
Specific instructions were always given to the subjects before the performance of ‘voluntary wrist dorsiflexions’. They were told to execute the movements with a minimum of effort and to avoid co-contraction in the wrist flexor muscles. All movements (passive as well as active) were relatively slow (0.5-7 deg s−1) in order to reduce the influence of viscosity and to avoid stretch reflexes. With respect to their amplitude, the movements were divided into two categories: those that were meant to exceed and those meant to stay below the ‘limit for short-range stiffness’, i.e. the movement amplitude at which the initial steep resistance response levelled off (cf. Hufschmidt & Schwaller, 1987; Campbell & Lakie, 1998).
The terms ‘mechanical agitation’ and ‘stirring manoeuvres’ are used to describe such procedures as (1) large amplitude rapid flapping of the hand (Flap.), (2) mechanical high-frequency vibration (75 Hz, amplitude 2 mm) applied for 10-15 s over the bellies of the wrist flexors (Vib.), (3) 10-15 s of manual massage of the flexors and (4) a few seconds of forceful isometric voluntary contractions of the wrist flexor and extensor muscles (Cocontr.). The latter three manoeuvres were carried out with the hand fixed in the resting position and consequently did not involve any wrist movements.
Statistics
For all types of tests, pooled values of position, torque or IEMG from a given number (n) of subjects were reported as median together with the interquartile range (25th to the 75th percentile) unless n≤ 10 when the whole range was given. If not otherwise declared, changes in the above parameters were evaluated using one-tailed Wilcoxon paired-sample testing with P < 0.05 as the chosen level of significance.
RESULTS
Passive compared to active repetitive wrist dorsiflexions with an amplitude exceeding the limit for the short-range stiffness
Figure 1A shows an example of wrist torque responses to passive repetitive wrist dorsiflexions of equivalent slow speed and with an amplitude of about 15 deg. EMG recordings verified that the wrist muscles were relaxed and that no stretch reflexes were elicited. There was an initial steep rise in the torque response to the first dorsiflexion, i.e. a sign of short-range stiffness which was less pronounced during the immediately succeeding two movements. In Fig. 1B the torque responses to the three successive movements are plotted not against time but as an X-Y plot of torque against angular displacement. This plot highlights the fact that the first movement - characterised by its marked short-range stiffness response - acts as a stirring event which has a loosening-up effect on the short-range stiffness of the succeeding movements.
The limit for the short-range stiffness was commonly seen at 3-4 deg of dorsiflexion and for quantification of the pooled results, measurements were made of the increase in passive torque resistance over the first 2 deg of dorsiflexion (i.e. within the range of the short-range stiffness). Such measurements showed a significant reduction in torque resistance between the first and the second movement (median 39 × 10−3 N m, range (31-57) × 10−3 N m vs. median 32 × 10−3 N m, range (27-52) × 10−3 N m, P = 0.01, n = 11). The fourth movement in Fig. 1A demonstrates that the relatively high initial torque resistance reappears after a rest period of about 15 s. Quantification showed no significant difference in torque resistance over the first 2 deg between the first and the fourth movement (median 39 × 10−3 N m, range (31-57) × 10−3 N m vs. median 42 × 10−3 N m, range (28-62) × 10−3 N m, P = 0.73 (two-tailed), n = 11). Figure 1A also shows that after each imposed dorsiflexion, a pulling force (i.e. a force of opposite direction to that needed for dorsiflexion) is required to bring the wrist fully back to its initial resting position. During the 15 s rest period after the third movement this pulling force gradually subsides (i.e. it gradually approaches the zero torque level indicated by the dashed line in Fig. 1A). The findings are compatible both with those reported by Hufschmidt & Schwaller (1987) in their study of forces required for slow passive movements of the human ankle joint and with those reported by Campbell & Lakie (1998) who measured tension responses of frog skeletal muscle fibres to imposed length changes.
After having been exposed to the test procedure illustrated in Fig. 1, the subjects were instructed to make repetitive voluntary wrist dorsiflexions which, like the passive movements, fulfilled the requirements of having an equivalent triangular shape and an amplitude of about 15 deg (i.e. an amplitude which exceeded the limit for short-range stiffness). To help the subjects in their attempts to make stereotyped dorsiflexion movements, with care taken to return the wrist to its original resting position after each movement, they were during the trials allowed to watch the goniometer trace on the oscilloscope screen. Trials which did not fulfil the requirements mentioned above were dismissed. Figure 2A shows a representative example of voluntary movements, which with respect to amplitude, speed and repetition rate are similar to the passive movements shown in Fig. 1A. As illustrated, the first voluntary movement in the series (induced after a standard pre-test conditioning procedure) was accompanied by a steeper initial rise of extensor EMG activity than the succeeding two movements. This difference is most clearly seen in the X-Y display where the extensor IEMG signals are plotted against wrist displacements (Fig. 2B). By analogy with the passive tests, quantification of the extensor IEMG amplitude at 2 deg of dorsiflexion showed a significant reduction of IEMG between the first and the second movement (median 22 μV, range 17-31 μV vs. median 17 μV, range 13-17 μV, P = 0.01, n = 11). It was observed that, as a rule, the delay between the onset of the extensor IEMG and the movement initiation was longer for the first movement compared to the second but this difference was not further evaluated.
Figure 2. Muscle activation patterns during active repetitive wrist dorsiflexions of similar triangular shape and amplitude as the passive movements shown in Fig. 1.
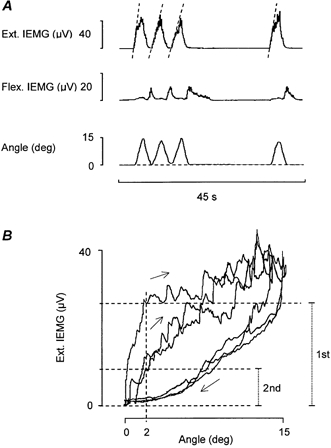
A, dashed lines in wrist extensor IEMG trace drawn to highlight that the speed of the initial rise of EMG activity depends on whether the dorsiflexion movement is imposed after a period of rest or immediately after a preceding similar dorsiflexion movement. B, X-Y plot of extensor IEMG activity against wrist displacement for the three consecutive movements shown in A. The upper and the middle horizontal dashed line indicates the IEMG level at 2 deg of dorsiflexion for the first and the second movement, respectively. The two vertical dotted bars (to the right) indicate the IEMG amplitudes used for quantification.
Figure 2A also shows that during the return movements towards the resting position the extensor IEMG activity ceased before the return movements were completed. Brief wrist flexor contractions are needed to bring the wrist fully back after the first two dorsiflexions and a longer lasting flexor contraction is needed to maintain the resting position after the final third dorsiflexion. It was also observed that in a similar way as the short-range stiffness reappeared after a rest period of about 15 s so did the initial increase of extensor EMG activity reappear after approximately the same rest interval and no significant difference in the amount of extensor IEMG at 2 deg of dorsiflexion was seen between the first and the fourth movement (median 22 μV, range 17-31 μV vs. median 23 μV, range 16-28 μV, P = 0.94 (two-tailed), n = 11). It was of interest to note that the subjects were not consciously aware of the fact that when, after a period of rest, they started a series of repetitive wrist dorsiflexions, the first movement required a stronger initial extensor activation than the succeeding ones. Nor were they aware of the fact that after completion of the rhythmical movements they could not maintain the initial resting position without wrist flexor activation.
Passive compared to active wrist dorsiflexions not exceeding the limit for the short-range stiffness: aftereffects of stirring manoeuvres
In tests like those illustrated in Fig. 3A, the torque responses to passive movements not exceeding 3 deg were measured before and after different types of stirring manoeuvres. In the case illustrated, the stirring manoeuvre consisted of a short period of high-amplitude voluntary flapping movements of the hand (Flap.). This manoeuvre caused a reduction of the torque responses to the imposed minute dorsiflexions. To obtain a quantitative evaluation of this reduction, calculations were made of the extent to which the mean amplitude of the torque responses to the three post-manoeuvre movements differed as compared to the corresponding value for the three pre-manoeuvre movements.
For the subjects exposed to this type of test, the Flap. manoeuvre caused a significant reduction of the mean torque resistance (median 44 × 10−3 N m, range (17-82) × 10−3 N m vs. median 34 × 10−3 N m, range (16-73) × 10−3 N m, P = 0.01, n = 8). In relative terms this reduction was 19 % on average for the group. Other types of stirring manoeuvres were found to cause similar loosening-up effects: 20 % after Cocontr. (median 42 × 10−3 N m, range (29-120) × 10−3 N m vs. median 40 × 10−3 N m, range (22-81) × 10−3 N m, P = 0.02, n = 5) and 19 % after Vib. (median 55 × 10−3 N m, range (21-138) × 10−3 N m vs. median 49 × 10−3 N m, range (15-120) × 10−3 N m, P = 0.01, n = 7).
Figure 3B illustrates the effect of a stirring manoeuvre on the strength of wrist extensor contractions required to generate voluntary rhythmical wrist dorsiflexions of similar low amplitude to the passive movements. In the example illustrated, the stirring manoeuvre consisted of a short period of mechanical vibration applied over the wrist flexor muscles (Vib.). The vibration period was probably not sufficient to induce any tonic vibration reflex but this could not be definitely verified since large vibration artefacts obscured the EMG recordings. The main message conveyed by the figure is that, as judged by the extensor IEMG record, the low amplitude dorsiflexion movements are generated by much weaker extensor contractions after than before the stirring manoeuvre.
When using the technique described above for quantitative evaluations the results showed that the Flap. manoeuvres caused an average reduction of 30 % in the strength of the movement-generating contractions (median 27 μV, range 10-51 μV vs. median 20 μV, range 7-40 μV, P = 0.01, n = 6). The other manoeuvres had similar effects: 30 % after Cocontr. (median 23 μV, range 8-59 μV vs. median 14 μV, range 4-38 μV, P = 0.02, n = 5) and 33 % after Vib. (median 24 μV, range 6-35 μV vs. median 16 μV, range 4-23 μV, P = 0.04, n = 6). It should be pointed out that many recordings of the type illustrated in Fig. 3B were dismissed because the subjects failed to keep the first dorsiflexion after stirring within the desired low range. This movement was greatly exaggerated as compared to the movements before stirring even though it was not generated by a stronger extensor contraction. When this happened, the subjects often made spontaneous comments on a loosening-up sensation and when being aware of the phenomenon they had in successive trials usually no problem in keeping the first post-manoeuvre movement within the desired range.
The effect of stirring manoeuvres on the amplitude of wrist dorsiflexions induced either by repetitive weak torque pulses or by repetitive weak voluntary extensor contractions
Tests of the type illustrated in Fig. 4 provided clear evidence that the movement-generating capacity of voluntary muscle contractions varies with the prevailing degree of inherent short-range stiffness of the moving parts. In these tests the technique introduced by Lakie et al. (1984) was used to demonstrate that in relaxed subjects the wrist movements induced by repetitive weak torque pulses are markedly enhanced by different types of stirring manoeuvres. It was observed that - as previously pointed out by Lakie et al. (1984) - the strength of the torque pulses was critical in the sense that movements generated by the torque motor near or exceeding the limit for the short-range stiffness sometimes caused a gradual loosening-up effect before the actual stirring manoeuvre was carried out. Such trials were dismissed and the torque strength was adjusted to produce movements within the desired small range. An example of the loosening-up effect caused by a Flap. manoeuvre is illustrated in Fig. 4A.
When quantitative comparisons were made between the mean amplitude of the movements induced by the three pre- and post-manoeuvre torque pulses, the latter movements were found to be on average 120 % larger after the Flap. manoeuvre (median 1.5 deg, range 0.5-3.0 deg vs. median 2.9 deg, range 0.9-8.6 deg, P = 0.01, n = 6), 110 % larger after Cocontr. (median 1.1 deg, range 0.6-3.4 deg vs. median 2.1 deg, range 1.8-5.3 deg, P = 0.01, n = 7) and 60 % larger after Vib. (median 2.4 deg, range 0.6-4.1 deg vs. median 3.1 deg, range 0.2-5.9 deg, P = 0.02, n = 8).
After having been exposed to the types of test illustrated in Fig. 4A the subjects were instructed to make repetitive low-amplitude wrist dorsiflexions while they watched the wrist extensor EMG trace on the oscilloscope screen and tried to keep the movement-generating extensor contractions equally strong after and before the stirring manoeuvres. An example of such a trial is illustrated in Fig. 4B. Even though in this case the mean strength of the extensor contractions (as judged by the amplitude of the IEMG) was somewhat lower after than before the Flap. manoeuvre, the movements generated by the post-manoeuvre contractions were markedly increased. Quantitative evaluations of the results showed that, on average, the post-manoeuvre increase amounted to 140 % after Flap. (median 0.7 deg, range 0.1-2.7 deg vs. median 1.7 deg, range 0.7-6.4 deg, P = 0.01, n = 10), 170 % after Cocontr. (median 1.6 deg, range 0.3-3.8 deg vs. median 3.7 deg, range 1.6-5.5 deg, P = 0.01, n = 6) and 45 % after Vib. (median 0.9 deg, range 0.5-5.3 deg vs. median 1.8 deg, range 0.9-5.2 deg, P = 0.04, n = 5).
Besides the stirring procedures mentioned above a few subjects were also exposed to manual massage of the flexor muscles while the subject's hand remained in its resting position. Five to ten seconds of such massage was sufficient to give a loosening-up effect similar to the effects illustrated in Fig. 3 and Fig. 4. Although we could not find any obvious differences in the ‘loosening-up’ efficiency of the various manoeuvres (Flap, Cocontr., Vib. and massage) employed in the above-presented tests, the material must be considered too small to make any reliable comparisons of that type.
Wrist flexor tension responses to passive and active wrist movements
In a series of experiments the strain gauge measuring the torque resistance to imposed wrist dorsiflexions was replaced by a strain gauge firmly pressed against the flexor tendons in the distal part of the forearm. Our primary aim was to determine whether the torque responses to imposed wrist dorsiflexions mainly originated from stretch-resisting forces generated by the relaxed wrist flexor muscles - forces that could be sensed as tension variations by the force transducer pressed against the tendons. It was found that during repetitive passive wrist dorsiflexions with an amplitude exceeding the limit for short-range stiffness, the flexor tension responses exhibited a marked similarity to the previously described torque responses (cf. Fig. 1A and Fig. 5). Like the torque responses illustrated in Fig. 1A, the flexor tension responses to individual dorsiflexions illustrated in Fig. 5A varied in shape depending on whether the movement in question was or was not preceded by a period of rest. Only the ‘post-rest’ responses had a distinct biphasic shape, indicative of short-range stiffness. Furthermore, the flexor tension responses exhibited another similarity to the torque responses: there was a decrease in resting tension (compared to the base level) when the hand was returned to the resting position after a preceding dorsiflexion, and when the consecutive movements ended, the resting tension gradually recovered.
Passive ‘post-rest’ wrist volarflexions evoked flexor tension responses of opposite polarity to those obtained during ‘post-rest’ wrist dorsiflexions. As illustrated in Fig. 5B, the tension response to the first passive shortening after a period of rest was biphasic with a steep initial fall in tension succeeded by a slower decline, i.e. a response reverse to the short-range stiffness responses shown in Fig. 5A. The initial steep fall in tension was commonly seen over the first 4 deg (median 4.2 deg, range 2.8-5.2 deg, n = 7).
The advantage of the technique used to measure wrist flexor tension responses was that it permitted direct insights into how these responses during repetitive voluntary wrist dorsiflexions vary in comparison with the strength of the wrist extensor contractions required to generate the movements. Figure 6 shows a representative example of how closely the shape of the wrist extensor IEMG deflections during repetitive voluntary wrist dorsiflexions vary in parallel with the shape of the flexor tension responses. The stronger the flexor short-range stiffness components are, the more prominent are the initial steep up-going deflections in the extensor IEMG traces. The pooled values for the increase in tension during the first 2 deg of dorsiflexion and for the concurrent increase in extensor IEMG were both significantly higher for the first as compared to the second movement (tension: median 0.34 N, range 0.25-0.48 N vs. median 0.19 N, range 0.13-0.38 N, P = 0.01, n = 11; IEMG: median 28 μV, range 22-44 μV vs. median 22 μV, range 17-27 μV, P = 0.01, n = 11). In relative terms, the reductions in tension and IEMG for the whole group were on average 34 % and 31 %, respectively.
DISCUSSION
The results presented provide clear evidence that the nervous system in its control of voluntary movements takes account of and compensates for thixotropy-dependent alterations in the degree of inherent muscular short-range stiffness. The results imply that when exploring the muscle activation patterns required for a specific type of movement it must be realised that these patterns vary with the immediately preceding history of movements and muscle contractions. As mentioned in Methods, all experiments described in the present study were preceded by a rest period of about 20 s, during which the wrist was left undisturbed and the wrist muscles remained relaxed. This rest period was in turn preceded by a short period of active, high-amplitude flapping wrist movements made with the intention of disrupting any pre-existing molecular bonds in thixotropic structures (Lakie et al. 1984; Proske et al. 1993).
It was not the prime aim of the present study to identify the peripheral structures responsible for the thixotropic changes in short-range stiffness and resting tension sensed by the torque meter during passive wrist movements. Still, observations were made which agree with earlier deductions that the structures mainly responsible are the extrafusal muscle fibres in the muscles being stretched by the movements and that consequently the short-range stiffness, as can be demonstrated in human in vivo studies, is equivalent to the short-range elastic component observed by Hill (1968) in resting frog muscles (cf. Hufschmidt & Schwaller, 1987; Walsh, 1992). It was for instance observed that the torque responses to repetitive wrist dorsiflexions illustrated in Fig. 1A exhibited a marked resemblance not only to the responses of frog skeletal muscle fibres to imposed length changes (Campbell & Lakie, 1998) but also to the wrist flexor tension responses illustrated in Fig. 5A. Furthermore, it was noted that the short-range stiffness could be effectively loosened-up by stirring manoeuvres which affected the wrist flexor muscles (Vib., Cocontr., flexor massage) but which did not involve any wrist movements which possibly could have loosened-up any joint or tendon ‘stickiness’.
The resistance to stretch inherent in the muscles themselves apparently has a damping, load compensating function which helps to maintain postural stability. As compared to the stretch reflex which serves a similar function, the inherent muscle stiffness has the advantage that it acts with less time delay (Grillner, 1972).
The question may be raised whether inactive components of the wrist extensors (which were shortened by the movements) might contribute to the short-range stiffness encountered in the torque measurements. Theoretically, this could be the case providing the extensor components generate a compressive force when the hand is dorsiflexed. Even though such compression forces might occur in response to wrist movements they are not likely to contribute to the observed short-range stiffness. As judged by the findings illustrated in Fig. 5B, there is a steep initial fall in resting tension of those muscles which are shortened by a movement, i.e. a response the reverse of that of the stretched antagonists.
The present findings give ample support to the predictive remark of Campbell & Lakie (1998) that the neuromuscular control system must allow for the inherent mechanical properties of inactive muscle fibres if it is to control movements with precision (cf. also Campbell & Moss, 2000). A relevant but as yet unanswered question is in which way the motor commands are appropriately adjusted to the prevailing degree of muscular short-range stiffness. The observed motor adjustments were ‘automatic’ in the sense that they occurred without the subjects being consciously aware of them and there were no indications that the subjects during the experiments learned to make appropriate motor compensations. Possibly, the adjustments were of reflex origin and dependent on the process of load compensation by spindles acting in alpha-gamma linkage (Granit, 1970; Hagbarth, 1993). They might also be dependent on learning processes occurring in connection with training of motor skills during early childhood.
It should be noted that all voluntary dorsiflexions in the present study were performed with visual feedback and with instructions to return the hand to its initial resting position after each movement. Without visual feedback the subjects were commonly unable to follow the instructions: the hand did not fully return to its resting position before the next dorsiflexion was initiated. This type of proprioceptive misjudgement was particularly evident after high-amplitude dorsiflexions, which markedly exceeded the limit for short-range stiffness. The observations are concordant with those previously made by Gregory et al. (1988) who interpreted this type of temporary error in position sense as a sign of intrafusal thixotropy leading to a change of muscle spindle resting discharge (cf. also Hagbarth et al. 1985).
In the present study, 15 s of rest following a stirring manoeuvre was usually sufficient to restore most of the initial short-range stiffness. However, earlier studies on human subjects (Lakie & Robson, 1988) as well as on isolated frog muscle fibres (Buchtal & Kaiser, 1951; Lännergren, 1971) have shown that under certain conditions it may take several minutes for full recovery. From a clinical point of view, long-term effects are of particular interest. There are studies showing that repetitive muscle stretches or maintained muscle stretch, particularly when combined with muscle contraction, can lead not only to muscle damage and soreness but also to a subsequent remodelling of the muscle (Macpherson et al. 1997; Goldspink, 1999). It is also of interest to note that in patients with long-lasting spasticity the increased resistance to passive stretch to a large extent may depend not on enhanced stretch reflexes but on enhanced inherent stiffness of the muscles concerned (Dietz & Berger, 1983; Hufschmidt & Mauritz, 1985). Further studies are needed to explore the extent to which long-lasting immobilisation, excessive muscular exercise or muscular diseases can affect the thixotropic behaviour of the muscles and thereby also the motor patterns required for control of voluntary movements. Still, before such pathophysiological studies are undertaken more must be known about the motor control consequences of muscle thixotropy under normal conditions.
The present study has not only demonstrated the motor control adjustments to thixotropic changes in muscular short-range stiffness but has also given examples of motor adjustments to thixotropy-dependent changes in resting tension of the muscles. In a study in progress further examples will be presented of such types of motor control adjustments.
References
- Buchthal F, Kaiser E. The rheology of the cross-striated muscle fibre with special reference to isotonic conditions. Det Kongelige Danske Videnskabernes Selskab. Biologiske Meddelerser. 1951;21:1–307. [Google Scholar]
- Campbell K S, Lakie M. A cross-bridge mechanism can explain the thixotropic short-range elastic component of relaxed frog skeletal muscle. Journal of Physiology. 1998;510:941–962. doi: 10.1111/j.1469-7793.1998.941bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K S, Moss R L. A thixotropic effect in contracting rabbit psoas muscle: prior movement reduces the initial tension response to stretch. Journal of Physiology. 2000;525:531–548. doi: 10.1111/j.1469-7793.2000.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Berger W. Normal and impaired regulation of muscle stiffness in gait: a new hypothesis about muscle hypertonia. Experimental Neurology. 1983;79:680–687. doi: 10.1016/0014-4886(83)90032-8. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. Journal of Anatomy. 1999;194:323–334. doi: 10.1046/j.1469-7580.1999.19430323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R. The Basis of Motor Control. London and New York: Academic Press; 1970. [Google Scholar]
- Gregory J E, Mark R F, Morgan D L, Patak A, Polus B, Proske U. Effects of muscle history on the stretch reflex in cat and man. Journal of Physiology. 1990;424:93–107. doi: 10.1113/jphysiol.1990.sp018057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J E, Morgan D L, Proske U. After-effects in the responses of cat muscle spindles and errors of limb position sense in man. Journal of Neurophysiology. 1988;59:1220–1230. doi: 10.1152/jn.1988.59.4.1220. [DOI] [PubMed] [Google Scholar]
- Grillner S. The role of muscle stiffness in meeting the changing postural and locomotor requirements for force development by the ankle extensors. Acta Physiologica Scandinavica. 1972;86:92–108. doi: 10.1111/j.1748-1716.1972.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Hagbarth K-E. Microneurography and applications to issues of motor control: Fifth Annual Stuart Reiner Memorial Lecture. Muscle and Nerve. 1993;16:693–705. doi: 10.1002/mus.880160702. review. [DOI] [PubMed] [Google Scholar]
- Hagbarth K-E, Hägglund J V, Nordin M, Wallin E U. Thixotropic behaviour of human finger flexor muscles with accompanying changes in spindle and reflex responses to stretch. Journal of Physiology. 1985;368:323–342. doi: 10.1113/jphysiol.1985.sp015860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K-E, Nordin M. Postural after-contractions in man attributed to muscle spindle thixotropy. Journal of Physiology. 1998;506:875–883. doi: 10.1111/j.1469-7793.1998.875bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K-E, Nordin M, Bongiovanni L G. After-effects on stiffness and stretch reflexes of human finger flexor muscles attributed to muscle thixotropy. Journal of Physiology. 1995;482:215–223. doi: 10.1113/jphysiol.1995.sp020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen P, Sten-Knudsen O. The dependence of the short-range elasticity on sarcomere length in resting isolated frog muscle fibres. Acta Physiologica Scandinavica. 1981;112:113–120. doi: 10.1111/j.1748-1716.1981.tb06793.x. [DOI] [PubMed] [Google Scholar]
- Hill D K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. Journal of Physiology. 1968;199:637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufschmidt A, Mauritz K H. Chronic transformation of muscle in spasticity: a peripheral contribution to increased tone. Journal of Neurology, Neurosurgery and Psychiatry. 1985;48:676–685. doi: 10.1136/jnnp.48.7.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufschmidt A, Schwaller I. Short-range elasticity and resting tension of relaxed human lower leg muscles. Journal of Physiology. 1987;391:451–465. doi: 10.1113/jphysiol.1987.sp016749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnke M T, Proske U, Struppler A. Measurements of muscle stiffness, the electromyogram and activity in single muscle spindles of human flexor muscles following conditioning by passive stretch or contraction. Brain Research. 1989;493:103–112. doi: 10.1016/0006-8993(89)91004-4. [DOI] [PubMed] [Google Scholar]
- Lakie M, Robson L G. Thixotropic changes in human muscle stiffness and the effects of fatigue. Quarterly Journal of Experimental Physiology. 1988;73:487–500. doi: 10.1113/expphysiol.1988.sp003169. [DOI] [PubMed] [Google Scholar]
- Lakie M, Walsh E G, Wright G W. Resonance at the wrist demonstrated by the use of a torque motor: an instrumental analysis of muscle tone in man. Journal of Physiology. 1984;353:265–285. doi: 10.1113/jphysiol.1984.sp015335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J. The effect of low-level activation on the mechanical properties of isolated frog muscle fibers. Journal of General Physiology. 1971;58:145–162. doi: 10.1085/jgp.58.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson P C, Dennis R G, Faulkner J A. Sarcomere dynamics and contraction-induced injury to maximally activated single muscle fibres from soleus muscles of rats. Journal of Physiology. 1997;500:523–533. doi: 10.1113/jphysiol.1997.sp022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D L, Prochazka A, Proske U. The after-effects of stretch and fusimotor stimulation on the responses of primary endings of cat muscle spindles. Journal of Physiology. 1984;356:465–477. doi: 10.1113/jphysiol.1984.sp015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin M, Hagbarth K-E. Effects of preceding movements and contractions on the tonic vibration reflex of human finger extensor muscles. Acta Physiologica Scandinavica. 1996;156:435–440. doi: 10.1046/j.1365-201X.1996.465180000.x. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan D L. Do cross-bridges contribute to the tension during stretch of passive muscle. Journal of Muscle Research and Cell Motility. 1999;20:433–442. doi: 10.1023/a:1005573625675. [DOI] [PubMed] [Google Scholar]
- Rack P M, Westbury D R. The short range stiffness of active mammalian muscle and its effect on mechanical properties. Journal of Physiology. 1974;240:331–350. doi: 10.1113/jphysiol.1974.sp010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E G. Muscles, Masses and Motion. The Physiology of Normality, Hypotonicity, Spasticity and Rigidity. Clinics in Developmental Medicine. Mac Keith Press/Cambridge University Press; 1992. p. 83. see no. 125. [Google Scholar]
- Walsh E G, Wright G W. Postural thixotropy at the human hip. Quarterly Journal of Experimental Physiology. 1988;73:369–377. doi: 10.1113/expphysiol.1988.sp003153. [DOI] [PubMed] [Google Scholar]
- Wiegner A W. Mechanism of thixotropic behavior at relaxed joints in the rat. Journal of Applied Physiology. 1987;62:1615–1621. doi: 10.1152/jappl.1987.62.4.1615. [DOI] [PubMed] [Google Scholar]


