Abstract
Non-image forming, irradiance-dependent responses mediated by the human eye include synchronisation of the circadian axis and suppression of pineal melatonin production. The retinal photopigment(s) transducing these light responses in humans have not been characterised.
Using the ability of light to suppress nocturnal melatonin production, we aimed to investigate its spectral sensitivity and produce an action spectrum. Melatonin suppression was quantified in 22 volunteers in 215 light exposure trials using monochromatic light (30 min pulse administered at circadian time (CT) 16-18) of different wavelengths (λmax 424, 456, 472, 496, 520 and 548 nm) and irradiances (0.7-65.0 μW cm−2).
At each wavelength, suppression of plasma melatonin increased with increasing irradiance. Irradiance-response curves (IRCs) were fitted and the generated half-maximal responses (IR50) were corrected for lens filtering and used to construct an action spectrum.
The resulting action spectrum showed unique short-wavelength sensitivity very different from the classical scotopic and photopic visual systems. The lack of fit (r2 < 0.1) of our action spectrum with the published rod and cone absorption spectra precluded these photoreceptors from having a major role. Cryptochromes 1 and 2 also had a poor fit to the data. Fitting a series of Dartnall nomograms generated for rhodopsin-based photopigments over the λmax range 420-480 nm showed that rhodopsin templates between λmax 457 and 462 nm fitted the data well (r2≥ 0.73). Of these, the best fit was to the rhodopsin template with λmax 459 nm (r2 = 0.74).
Our data strongly support a primary role for a novel short-wavelength photopigment in light-induced melatonin suppression and provide the first direct evidence of a non-rod, non-cone photoreceptive system in humans.
In addition to image generation, the mammalian eye is capable of detecting changes in environmental light irradiance resulting in non-image forming light responses. Non-image forming, irradiance-dependent responses in humans include synchronisation of the circadian clock (Arendt & Broadway, 1986; Czeisler et al. 1986; Boivin et al. 1996; Zeitzer et al. 2000), suppression of pineal melatonin production (Lewy et al. 1980; Bojkowski et al. 1987; McIntyre et al. 1989; Brainard et al. 1997; Zeitzer et al. 2000), elevation of core body temperature (Badia et al. 1991), pupil constriction, reduced slow eye movements and enhanced alertness (Badia et al. 1991; Cajochen et al. 2000). Current evidence points to these responses being mediated by the eyes. For example, melatonin suppression cannot be induced in blindfolded or bilaterally enucleated subjects (Czeisler et al. 1995) or by extraocular light exposure (Lockley et al. 1998). In addition, only non-24 h (free running) circadian rhythms were observed in bilaterally enucleated people (Lockley et al. 1997; Skene et al. 1999), suggesting that, in the absence of eyes, the light-dark cycle is unable to synchronise the human circadian clock to the 24-h day.
The neural pathways mediating some non-image forming responses are anatomically distinct from the classical visual pathways. Circadian (phase shifting) and acute (melatonin suppression) responses to ocular light are transmitted from a discrete subset of retinal ganglion cells via the retinohypothalamic tract (RHT) to the hypothalamic suprachiasmatic nuclei (SCN), the site of the human circadian pacemaker (Moore et al. 1995). Efferent signals from the SCN are transmitted through a multisynaptic pathway (hypothalamic subparaventricular nuclei, thoracic intermediolateral cell column, superior cervical ganglia) to the pineal gland and acute suppression of melatonin occurs (Klein & Moore, 1979; Larsen et al. 1998).
Although the retinal rod and cone cells are vital for image formation, their role in non-image forming light responses may not be as essential. Recent studies using rodless/coneless mice have shown that novel non-rod, non-cone photoreceptor(s) may mediate circadian photoentrainment (Freedman et al. 1999), acute light responses (melatonin suppression) (Lucas et al. 1999) and pupil constriction (Lucas et al. 2001). Several candidate opsin-based photopigments have been identified including vertebrate ancient opsin (Soni & Foster, 1997), melanopsin (Provencio et al. 2000) and OP479 (Lucas et al. 2001). A role for a retinal vitamin B2-based photopigment, cryptochrome, in mammalian circadian photoentrainment has also been proposed (Miyamoto & Sancar, 1998). A recent report, however, has shown the classical opsins and cryptochromes to be functionally redundant in mediating light masking of behaviour in mice (Selby et al. 2000). Whether these photoreceptors are functionally redundant in circadian photoentrainment and melatonin suppression remains to be determined.
The retinal photopigment(s) mediating non-image forming light responses in humans have not been characterised. Identification of a photopigment requires investigation of the spectral sensitivity of the light-dependent response and construction of an action spectrum. Here we report the first complete action spectrum conducted in humans. Suppression of night-time melatonin production was used as the light-dependent response. Although previous research has shown wavelength dependence of melatonin suppression (Brainard et al. 1985, 2001; Skene et al. 1999) and suggested that an intact trichromatic visual system was not essential (Ruberg et al. 1986), these studies have fallen short of producing an action spectrum to characterise the photopigment. Our action spectrum data describe a novel opsin-based, non-rod, non-cone photoreceptor system in the human retina.
METHODS
Subjects
Subjects (18 males, 4 females) aged 18-45 years (mean ± s.d. = 27 ± 7 years) completed between 1 and 16 study sessions, each session consisting of three consecutive nights in the Clinical Investigation Unit. The subjects were healthy and drug free. They had no colour vision deficiencies according to the Ishihara colour blindness plate test. All had regular sleep-wake cycles with no complaints of sleep disorders (Pittsburgh sleep quality index < 5). All subjects gave written informed consent. The study was approved by The University of Surrey Advisory Committee on Ethics and all procedures were carried out in accordance with the Declaration of Helsinki.
Pre-study measurements
For at least three nights prior to the study, subjects were required to keep a regular sleep-wake cycle (sleeping from 23.00 to 07.00 h). The subjects’ activity and light exposure levels were monitored during this time (Actillumes, Ambulatory Monitoring Inc., Ardsley, NY, USA) to ensure compliance to a stable sleep-wake pattern. For 24 h preceding each study session and for its duration, subjects refrained from caffeinated drinks, alcohol, excessive exercise and bright light in order to minimise factors capable of affecting the circadian clock and the melatonin rhythm.
Prior to the study subjects collected sequential 4-hourly (8 h overnight) urine samples for 48 h for measurement of 6-sulphatoxymelatonin, the major metabolite of melatonin. Antiserum and radiolabel were supplied by Stockgrand Ltd, University of Surrey, Guildford, UK. The time of light administration was individualised to occur on the rising phase of the subjects’ endogenous melatonin rhythm predicted from cosinor analysis of the 6-sulphatoxymelatonin data.
Protocol
Each study session consisted of three consecutive nights (19.00-07.00 h); night 1 was a baseline night (no light exposure) followed by two light exposure nights. The baseline night controls for short- (e.g. posture, pupil dilation) and long-term factors (stage of menstrual cycle, season, changes in circadian phase) which may affect the melatonin rhythm (Skene et al. 1999). Posture and environmental light exposure (21.00-23.00 h < 10 lux; 23.00-07.00 h complete darkness with subjects wearing eye-masks) were controlled throughout. On light exposure nights subjects received 30 min of monochromatic light at a set circadian time (CT 16-18) which occurred between 23.30 and 02.30 h. A single drop of pupil dilator, Mimins Tropicamide 0.5 % (Chauvin Pharmaceuticals, Romford, UK) was placed in each eye 90 min before light exposure. Blood samples were taken via an indwelling cannula at -90, -15, 0, 15, 30, 45, 60, 75, 90 and 120 min after lights on. Plasma melatonin was assayed by radioimmunoassay (Stockgrand Ltd). The limit of detection of the assay was 5 pg ml−1 and the interassay coefficients of variation (CV) were 12 % at 28 pg ml−1, 14 % at 78 pg ml−1 and 13 % at 161 pg ml−1.
Light exposure
Monochromatic light (half-bandwidth (λ1/2) ≤ 13 nm) of different wavelengths (λmax 424, 456, 472, 496, 520 and 548 nm) and irradiances (0.70-65 μW cm−2) was administered for 30 min via a sphere (45 cm diameter, Apollo Lighting, Leeds, UK) coated with white reflectance paint (Kodak, Hemel Hempstead, UK). The sphere was illuminated via a fibre optic cable connected to a metal halide arc lamp light source (Enlightened Technology Associates Inc., Fairfax, VA, USA). The light source did not emit ultraviolet, infrared or electromagnetic radiation outside the visual range. The input port of the sphere housed the monochromatic (Coherent Ealing, Watford, UK) and neutral density (Kodak, Hemel Hempstead, UK) filters used to alter wavelength and irradiance, respectively. This light sphere provided constant uniform illumination of the entire retina in the pupil-dilated subjects. Light irradiance (μW cm−2) was measured at the subjects’ eye level (optical powermeter, Macam Photometrics, Livingstone, UK). Each wavelength was tested at five to eight irradiances (0.70-65.0 μW cm−2) and for each irradiance between three and seven subjects were studied. During light exposure subjects were instructed to keep their eyes open (verified by research observers) and to fix their gaze on a target dot in the centre of the sphere. A chin rest and head band limited movement of the subjects’ head. The design of the light sphere altered the spectral quality of the monochromatic filters slightly from the manufacturer's specifications. Measurements with a spectroradiometer (Spectrascan 650 portable, Photoresearch, Chatsworth, CA, USA) confirmed the actual λmax wavelengths at eye level to be 424, 456, 472, 496, 520 and 548 nm (λ1/2 5-13 nm) instead of 430, 460, 480, 500, 530 and 560 nm (λ1/2 10 nm), respectively. The measured wavelengths were used for all subsequent photon density calculations.
Statistics
Melatonin suppression was calculated from the difference in melatonin concentrations between the baseline night and the light exposure night for samples collected at 30 and 45 min after lights on (time of maximum melatonin suppression). For each wavelength, irradiance response curves (IRCs) were constructed using the mean melatonin suppression at each irradiance and fitted with curves generated (SAS 6.13, SAS Institute, Cary, NC, USA) using a four-parameter logistic model of the form: y = ((a - c)/(1 + (x/b)d)) +c, where a = response when irradiance = 0, c = response when irradiance is maximum, b = half-maximal response, d = steepness of curve. The generated best fit curve where a = 0, c = 70 and d = 1.5 closely matched each IRC (r2≥ 0.99). The half-maximal responses (irradiance required to produce 50 % suppression, IR50) were calculated from the fitted IRCs. These values were corrected for pre-receptoral filtering by the lens (Stockman & Sharpe, 2000). Action spectra (original and corrected) were constructed by plotting log(relative sensitivity) against wavelength.
The published absorption spectra templates of the rod and cone visual pigments (Dartnall et al. 1983) and cryptochromes 1 and 2 (Hsu et al. 1996) were fitted to a third order polynomial (Prism 3.0, Graphpad Software, San Diego, CA, USA). Fits of r2 = 0.99 were achieved and the resultant polynomial curves were fitted to the melatonin suppression action spectrum and goodness of fit values (r2) obtained. Third order polynomial curves, generated from rhodopsin templates with λmax ranging between 420-480 nm, were also fitted to the action spectrum by the same method.
RESULTS
The spectral sensitivity of melatonin suppression was quantified in 22 subjects in 215 light exposure trials. At all wavelengths (λmax 424, 456, 472, 496, 520 and 548 nm) tested, nocturnal plasma melatonin concentrations were suppressed by monochromatic light in a dose-dependent manner. Maximum suppression of melatonin was observed 30-45 min after lights on. Although all irradiances tested exceeded the threshold for vision, no significant melatonin suppression was observed with the lowest irradiances of 520 nm light (0.70-3.3 μW cm−2). The minimum irradiances required for statistically significant suppression were 1.9, 2.0, 1.8, 3.0, 7.0 and 7.2 μW cm−2 for 424, 456, 472, 496, 520 and 548 nm, respectively. Figure 1A shows a typical example of light-induced suppression of melatonin in a subject using low irradiances of monochromatic light. The effectiveness of short-wavelength light in the suppression of melatonin is shown in Fig. 1B.
Figure 1. The effect of wavelength on the suppression of nocturnal plasma melatonin.
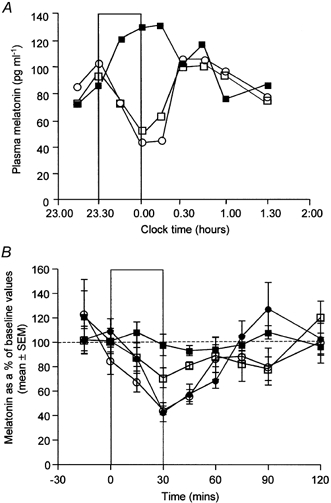
A, time course of plasma melatonin concentrations during a baseline night (▪) and during two consecutive light treatment nights (□, 424 nm, 11 μW cm−2; ○, 472 nm, 31 μW cm−2) in an 18-year-old man. The bar indicates the time of light exposure (30 min). Maximum suppression of melatonin was observed 30-45 min after lights on. B, the ability of light to suppress melatonin as a function of wavelength. Light of different wavelengths (λmax 424 nm (○), 456 nm (•), 520 nm (□) and 548 nm (▪)) was administered at approximately the same photon density (2.00 × 1013 photons cm−2 s−1 ± 10 %) for 30 min. Order of efficacy: λmax 424 ≈ 456 nm > 520 nm > 548 nm. Each point is the mean ± s.e.m. of 5-7 subjects. The bar indicates the period of light exposure.
At each wavelength, suppression of plasma melatonin increased with increasing irradiance (Fig. 2A). The data produced irradiance response curves (IRCs) which were fitted with curves generated using the four-parameter logistic equation. Each IRC had a significant fit (r2≥ 0.99) to the generated best fit curve where a = 0, c = 70, d = 1.5 demonstrating univariance (Fig. 2B). The half-maximal responses, calculated from the fitted IRCs and corrected for lens density changes, show 424 nm to be the most effective wavelength followed by 456 nm > 472 nm > 500 nm > 520 nm > 548 nm. The effect of pre-receptoral filtering by the lens on the shape of the action spectrum is shown in Fig. 2C. Correcting for lens density shifted the maximum sensitivity of the action spectrum to a shorter wavelength. The uncorrected action spectrum best fits a rhodopsin template with λmax 468 nm (r2 = 0.85). The action spectrum corrected for lens transmission best fits a rhodopsin template with λmax 459 nm (r2 = 0.74) (Fig. 2D).
Figure 2. Sensitivity of plasma melatonin to monochromatic light exposure.
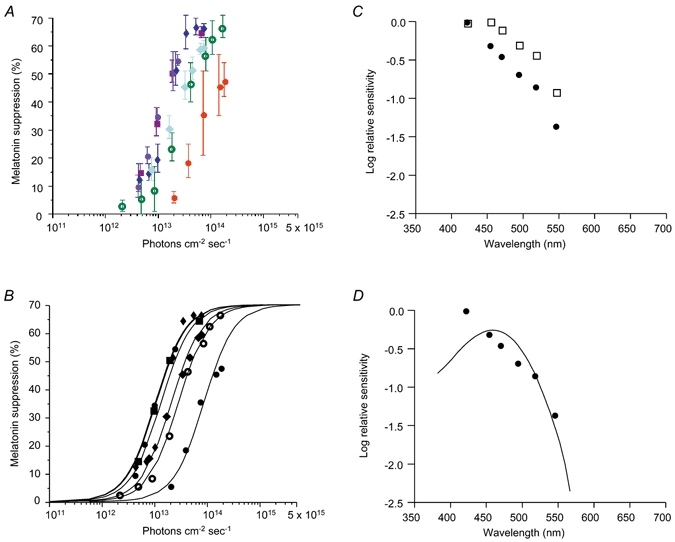
A, percentage melatonin suppression (mean ± s.e.m.) at varying irradiances for 424 (purple circles), 456 (maroon squares), 472 (dark blue diamonds), 496 (pale blue diamonds), 520 (hollow green circles) and 548 nm (red circles). B, irradiance response curves (IRCs) fitted to the data using the four-parameter logistic equation where a = 0, c = 70 and d = 1.5. For each IRC the goodness of fit was r2≥ 0.99. C, action spectrum for melatonin suppression physiologically derived (□) compared with the action spectrum corrected for lens filtering (•). D, the corrected action spectrum best fits a rhodopsin template with λmax 459 nm (r2 = 0.74).
The observed action spectrum for melatonin suppression shows short-wavelength sensitivity that is very different from the known spectral sensitivity of the scotopic and photopic response curves (being shifted approximately 50 and 100 nm to the left of the scotopic and photopic response, respectively) (Fig. 3).
Figure 3. Action spectrum for melatonin suppression physiologically derived (▪) compared to scotopic (λmax 505 nm, continuous line) and photopic (λmax 555 nm, dashed line) vision curves.
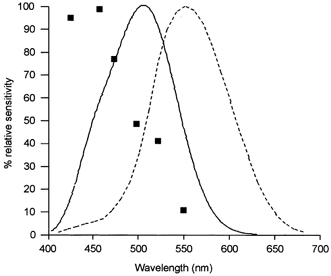
Scotopic and photopic luminosity functions after CIE 1951 and 1924, respectively.
The human retina contains one class of rod (λmax 496 nm) and three classes of cone photoreceptors (S-cones, λmax 419 nm; M-cones, λmax 531 nm; L-cones, λmax 558 nm). In order to assess the involvement of these in melatonin suppression, the observed action spectrum (corrected for lens filtering) was fitted with the published absorption spectra of the rod and cone photopigments (Dartnall et al. 1983) (Fig. 4). The lack of fit (r2 < 0.1) provides strong evidence that no single known retinal photoreceptor (rod or cone) could account solely for this response. In addition, fitting the action spectrum with the cryptochromes 1 and 2 absorption spectra (from 400 nm onwards to account for lens transmission) does not produce a good fit: r2 = 0.19 and 0.41 for cryptochromes 1 and 2, respectively.
Figure 4. Action spectrum for melatonin suppression compared to the rod and cone absorption spectra.
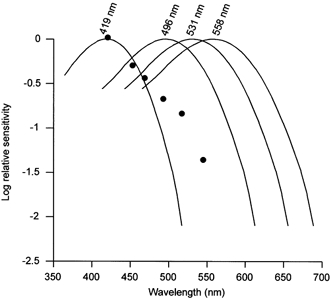
Action spectrum for melatonin suppression corrected for lens filtering (•) compared to the known rod and cone photoreceptor absorption spectra (from Dartnall et al. 1983). Short wavelength sensitive (S) cone absorption spectrum (λmax 419 nm); rod absorption spectrum (λmax 496 nm); medium wavelength sensitive (M) cone absorption spectrum (λmax 531 nm); and long wavelength sensitive (L) cone spectrum (λmax 558 nm).
The data show a spectral sensitivity curve that may derive from a novel non-rod, non-cone photopigment. In order to determine the best fit to the lens-corrected action spectrum, a series of Dartnall nomograms generated for rhodopsin-based photopigments over the λmax range of 420-480 nm were fitted to the data. Rhodopsin photopigments with λmax ranging from 457-462 nm fitted the data well (r2≥ 0.73). Of these, an opsin with a λmax 459 nm best fits the data (lowest sum of squares, r2 = 0.74) (Fig. 2D). For comparison Dartnall nomograms were also fitted to the original, uncorrected action spectrum. Rhodopsin photopigments with λmax ranging from 465 to 471 nm fitted the data well (r2 > 0.80) and of these, an opsin with a λmax 468 nm best fits the data (lowest sum of squares, r2 = 0.85).
DISCUSSION
Action spectroscopy methodology (construction of an action spectrum) has been used to characterise a non-image forming, circadian response in humans. The enormous number of trials (a number of wavelengths each tested over a range of irradiances), length of study, expense, need for controlled delivery of light and standardised protocol to minimise confounding factors have, until now, deterred its use.
Our action spectrum for melatonin suppression in humans has revealed unique short-wavelength sensitivity very different from the classical scotopic and photopic visual systems. These data provide strong evidence that the rod and cone cells are not the major photoreceptor systems involved in melatonin suppression in humans. In support of our findings, a recent study assessing the effect of λmax 505 and 555 nm light on melatonin suppression concluded that the cone photopic system was not the primary photoreceptor (Brainard et al. 2001).
In the present study the spectral response curve was physiologically derived and would include the effect of prereceptoral filtering by the lens and macula pigment. Prereceptoral filtering is known to shift the spectral sensitivities of the S-, M- and L-cones to longer wavelengths in vivo:λmax 440, 540 and 565 nm, respectively (Stockman & Sharpe, 2000). In order to determine the spectral characteristics of the underlying photopigment responsible for melatonin suppression, the filtering effect of the lens was removed (Stockman & Sharpe, 2000). This correction altered the shape of the action spectrum and shifted the maximum sensitivity to shorter wavelengths. Lens density shows inter-individual differences so, although the lens density data used were calculated from mean data (Stockman & Sharpe, 2000), the correction is at best an approximation. Lens opacity also changes with age. However, this was not corrected for owing to the relatively narrow age range of our study subjects.
Given the method of light exposure in the present study and the restricted distribution of the macula pigment (primarily in the fovea), macula pigment filtering is not likely to make a contribution to the observed response and thus this was not corrected for. In the unlikely event that the candidate photoreceptor is found to be confined to the macula then a further correction would be necessary.
Neither action spectrum (original or lens corrected) fitted the published absorption spectra of the human rod and S-, M- and L-cone photopigments (r2 < 0.1). Although the short wavelength data points of the corrected action spectrum (n = 3) lie on the S-cone absorption spectrum (λmax 419 nm), the longer wavelength data points do not, and thus a poor fit with the S-cone template results (r2 < 0.1). The lack of fit with the rod and S-, M- and L-cone photopigments allows us to conclude that none of the known visual photoreceptors in the human retina are solely responsible for light-induced melatonin suppression. Our data strongly support a primary role for a novel non-rod, non-cone photopigment in light-induced melatonin suppression. The IRCs show univariance indicating that a single opsin photopigment drives the observed response. A novel opsin with peak sensitivity around 459 nm (457-462 nm, r2≥ 0.73) best fits the data and is the most likely candidate. Although the data are best described by this single novel opsin template, the possibility that other photopigments may make a minor contribution cannot be ruled out. For example, the absence of a reduction in sensitivity at the shortest wavelength (424 nm) may indicate a contribution from another photopigment, perhaps the S-cone. A similar phenomenon was observed in the action spectrum for melatonin suppression in cultured Xenopus eyecups (Cahill et al. 1998).
In the present study suppression of nocturnal melatonin production has been used as the light-dependent response to characterise the underlying photopigment. Whether or not other non-image forming light responses such as circadian phase resetting show a similar spectral sensitivity and are regulated by the same novel photopigment cannot be determined from the current data. However, at present there is no firm evidence to contradict the assumption that the photoreceptor system is the same. On the contrary, anatomical studies (Klein & Moore, 1979; Moore et al. 1995; Larsen et al. 1998) support the existence of a common neural circuit (type III retinal ganglion cells-RHT-SCN) for circadian resetting and melatonin suppression. The recent demonstration in humans of similar illuminance-response curves for circadian phase shifting and melatonin suppression (Zeitzer et al. 2000) adds further support for a common photoreceptor-mediated pathway. If this proves to be the case, the short-wavelength sensitivity demonstrated in the present study will have a major impact in the design and use of lighting for the treatment of certain types of sleep disorders, seasonal affective disorder, adaptation to shift work, jetlag, improving alertness and performance and reducing sleepiness and accidents during night work with broad applications in, for example, factories, hospitals, old age homes.
In conclusion, we have demonstrated the existence of a novel non-rod, non-cone photoreceptor system in the human retina. This is the first direct evidence that non-image forming photoreception is distinct from image formation in humans. A novel short-wavelength opsin-based photopigment has been shown to be the primary transducer of light-induced melatonin suppression and it is likely to be responsible for other non-image forming light responses such as circadian phase resetting. The current findings will allow the spectral composition of light to be optimised to manipulate (or to avoid manipulating) the human circadian axis. This knowledge has broad applications in situations where light therapy is used to synchronise the circadian clock (such as treatment of circadian rhythm sleep disorders and adaptation to shift work, or transmeridian travel).
Acknowledgments
We thank Drs Simon Archer, Derk Jan Dijk, Judie English, Steven W. Lockley, Robert J. Lucas and Ms Selvamalar Ratnasingham for their contribution to this work. This work was partially supported by EUBIOMED2 (BMH4-CT97-2327).
References
- Arendt J, Broadway J. Phase response of human melatonin rhythms to bright light in Antarctica. Journal of Physiology. 1986;377:68P. [Google Scholar]
- Badia P, Myers B, Boecker M, Culpepper J, Harsh J R. Bright light effects on body temperature, alertness, EEG and behaviour. Physiology and Behaviour. 1991;50:583–588. doi: 10.1016/0031-9384(91)90549-4. [DOI] [PubMed] [Google Scholar]
- Boivin D B, Duffy J F, Kronauer R E, Czeisler C A. Dose-response relationships for the resetting of human circadian clock by light. Nature. 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- Bojkowski C J, Aldhous M E, English J, Franey C, Poulton A L, Skene D J, Arendt J. Suppression of nocturnal plasma melatonin and 6-sulphatoxymelatonin by bright and dim light in man. Hormone and Metabolic Research. 1987;19:437–440. doi: 10.1055/s-2007-1011846. [DOI] [PubMed] [Google Scholar]
- Brainard G C, Hanifin J P, Rollag M D, Greeson J, Byrne B, Glickman G, Gener E, Standford B. Human melatonin regulation is not mediated by the three cone photopic visual system. Journal of Clinical Endocrinology and Metabolism. 2001;86:433–436. doi: 10.1210/jcem.86.1.7277. [DOI] [PubMed] [Google Scholar]
- Brainard G C, Lewy A J, Menaker M, Friedrickson R H, Miller L S, Weleber R G, Cassone V, Hudson D. Effect of light wavelength on the suppression of nocturnal plasma melatonin in normal volunteers. Annals of the New York Academy of Sciences. 1985;453:376–378. [Google Scholar]
- Brainard G C, Rollag M D, Hanifin J P. Photic regulation of melatonin in humans: ocular and neural signal transduction. Journal of Biological Rhythms. 1997;12:537–546. doi: 10.1177/074873049701200608. [DOI] [PubMed] [Google Scholar]
- Cahill G M, Parsons S E, Besharse J C. Spectral sensitivity of melatonin synthesis suppression in Xenopus eyecups. Visual Neuroscience. 1998;15:499–502. doi: 10.1017/s0952523898153099. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Zeitzer J M, Czeisler C A, Dijk D-J. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behavioural Brain Research. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Czeisler C A, Allan J S, Strogatz S H, Ronda J M, Sanchez R, Rios C D, Freitag W O, Richardson G S, Kronauer R E. Bright light resets the human circadian pacemaker independent of timing of sleep-wake cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- Czeisler C A, Shanahan T L, Klerman E B, Martens H, Brotman D J, Emens J S, Klein T, Rizzo J F., III Suppression of melatonin secretion in some blind patients by exposure to bright light. New England Journal of Medicine. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- Dartnall H J A, Bowmaker J K, Mollon J D. Human visual pigments: microspectrophotometric results from the eyes of seven persons. Proceedings of the Royal Society. 1983;B 220:115–130. doi: 10.1098/rspb.1983.0091. [DOI] [PubMed] [Google Scholar]
- Freedman M S, Lucas R J, Soni B, Von Schantz M, Muñoz M, David-Gray Z, Foster R G. Regulation of mammalian circadian behaviour by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- Hsu D S, Zhao X, Zhao S, Kazantsev A, Wang R P, Todo T, Wei Y F, Sancar A. Putative human blue-light photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry. 1996;35:13871–13877. doi: 10.1021/bi962209o. [DOI] [PubMed] [Google Scholar]
- Klein D C, Moore R Y. Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Research. 1979;174:245–262. doi: 10.1016/0006-8993(79)90848-5. [DOI] [PubMed] [Google Scholar]
- Larsen P J, Enquist L W, Card J P. Characterization of the multisynaptic neuronal control of the rat pineal gland using viral transneuronal tracing. European Journal of Neuroscience. 1998;10:128–145. doi: 10.1046/j.1460-9568.1998.00003.x. [DOI] [PubMed] [Google Scholar]
- Lewy A J, Wehr T A, Goodwin F K, Newsome D A, Markey S P. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- Lockley S W, Skene D J, Arendt J, Tabandeh H, Bird A C, Defrance R. Relationship between melatonin rhythms and visual loss in the blind. Journal of Clinical Endocrinology and Metabolism. 1997;82:3763–3770. doi: 10.1210/jcem.82.11.4355. [DOI] [PubMed] [Google Scholar]
- Lockley S W, Skene D J, Thapan K, English J, Ribeiro D, Haimov I, Hampton S, Middleton B, von Schantz M, Arendt J. Extraocular light exposure does not suppress plasma melatonin in humans. Journal of Clinical Endocrinology and Metabolism. 1998;83:3763–3770. doi: 10.1210/jcem.83.9.5244. [DOI] [PubMed] [Google Scholar]
- Lucas R J, Douglas R H, Foster R G. Characterisation of an ocular photopigment capable of driving pupillary constriction in mice. Nature Neuroscience. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Lucas R J, Freedman M S, Muñoz M, Garcia-Fernández J M, Foster R G. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- McIntyre I M, Norman T R, Burrows G D, Armstrong S. Human melatonin suppression is intensity dependent. Journal of Pineal Research. 1989;6:149–156. doi: 10.1111/j.1600-079x.1989.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Sancar A. Vitamin B2-based blue light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proceedings of the National Academy of Sciences of the USA. 1998;95:6079–6102. doi: 10.1073/pnas.95.11.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R Y, Speh J C, Card J P. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. Journal of Comparative Neurology. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rodriguez I R, Jiang G, Hayes W P, Moreira E F, Rollag M D. A novel human opsin in the inner retina. Journal of Neuroscience. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberg F L, Skene D J, Hanifin J P, Rollag M D, English J, Arendt J, Brainard G C. Melatonin regulation in humans with color vision deficiencies. Journal of Clinical Endocrinology and Metabolism. 1986;81:2980–2985. doi: 10.1210/jcem.81.8.8768862. [DOI] [PubMed] [Google Scholar]
- Selby C P, Thompson C, Schmitz T M, Van Gelder R N, Sancar A. Functional redundancy of cryptochromes and classical photoreceptors for nonvisual ocular photoreception in mice. Proceedings of the National Academy of Sciences of the USA. 2000;97:14697–14702. doi: 10.1073/pnas.260498597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene D J, Lockley S W, Thapan K, Arendt J. Effects of light on human circadian rhythms. Reproduction Nutrition and Development. 1999;39:295–304. doi: 10.1051/rnd:19990302. [DOI] [PubMed] [Google Scholar]
- Soni B G, Foster R G. A novel and ancient vertebrate opsin. FEBS Letters. 1997;406:279–283. doi: 10.1016/s0014-5793(97)00287-1. [DOI] [PubMed] [Google Scholar]
- Stockman A, Sharpe L T. Spectral sensitivities of the middle- and long-wavelength-sensitive cones derived from measurements in observers of known genotype. Vision Research. 2000;40:1711–1737. doi: 10.1016/s0042-6989(00)00021-3. [DOI] [PubMed] [Google Scholar]
- Zeitzer J M, Dijk D-J, Kronauer R E, Brown E N, Czeisler C A. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. Journal of Physiology. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


