Abstract
Catfish (Ictalurus punctatus) retinal cone horizontal cells contain an L-type calcium current that has been proposed to be involved in visual processing. Here we report on the modulation of this current by activation of glutamate receptors and calcium-induced calcium release (CICR) from intracellular calcium stores.
Fluorescence data obtained from isolated horizontal cells loaded with indo-1 provided evidence of calcium release from an intracellular calcium store sensitive to caffeine, calcium and ryanodine. In the presence of caffeine, ryanodine-sensitive stores released calcium in a transient manner. Release of calcium was blocked when cells were preincubated in BAPTA, in the presence of ruthenium red, or in low concentrations of ryanodine.
The release of calcium from ryanodine-sensitive stores directly corresponded with a decrease of the voltage-gated L-type calcium current amplitude. Caffeine-induced modulation of the calcium current was reduced in the presence of ruthenium red.
Activation of ionotropic kainate receptors on catfish cone horizontal cells triggered CICR from ryanodine-sensitive stores and mimicked inhibition of the voltage-gated calcium current. Kainate-induced inhibition of the calcium current was diminished when intracellular calcium stores were inhibited with ruthenium red or depleted with ryanodine, or when calmodulin antagonists or CaM kinase II inhibitors were present.
These results provide evidence that activation of an ionotropic glutamate receptor on catfish cone horizontal cells is linked to calcium release from ryanodine-sensitive intracellular calcium stores and modulation of the L-type calcium current activity. Inhibition of this calcium current directly or indirectly involves calmodulin and CaM kinase II and represents a possible mechanism used by horizontal cells to affect response properties of these cells.
Understanding the cellular mechanisms regulating changes in the concentrations of intracellular calcium ([Ca2+]i) is essential for understanding the role of calcium in cell function. In the nervous system, changes of [Ca2+]i have been linked to diverse events that modulate membrane excitability and cellular function (Ghosh & Greenberg, 1995; Berridge, 1997). These events can trigger calcium-activated membrane conductances or can modulate calcium-dependent regulation of enzymes. Large increases of [Ca2+]i can be caused by calcium flux through agonist-gated channels or voltage-gated calcium channels, or by release of calcium from intracellular stores.
The ability of intracellular stores to increase [Ca2+]i has been studied in many tissue types (Berridge, 1997). Two major non-mitochondrial intracellular calcium stores have been identified in neurons. One is sensitive to IP3, formed by the hydrolysis of phosphatidylinositol 4,5-bisphosphate following receptor activation in the plasma membrane (the IP3-sensitive intracellular store). Once formed, IP3 binds to an intracellular IP3 receptor located on the surface of the intracellular calcium store. This intracellular calcium store is functionally distinct from another store that is sensitive to calcium and agents such as caffeine and ryanodine (the ryanodine-sensitive intracellular store). Caffeine-evoked release of calcium has been proposed to be amplified by calcium-induced calcium release (CICR) where raised intracellular calcium levels trigger the release of calcium from intracellular stores. CICR can be inhibited with specific concentrations of ryanodine or ruthenium red. In neurons, these stores have been previously described in sensory (Neering & McBurney, 1984) and sympathetic cells (Lipscombe et al. 1988).
CICR has also been demonstrated in isolated cone horizontal cells in the catfish retina (Linn & Christensen, 1992). Permeation of calcium through a calcium-permeable AMPA receptor in these cells (Linn & Christensen, 1992; Eliasof & Jahr, 1997) triggers an increase in [Ca2+]i due to CICR from intracellular calcium stores. However, the effect of this increased [Ca2+]i is unknown. In previous studies, intracellular calcium ions have been shown to modulate characteristics of voltage-gated ion channels, either directly or through enzyme-activated phosphorylation (Levitan, 1988).
In this study, we have looked at the modulatory effect of releasing calcium from the ryanodine-sensitive intracellular calcium stores on the calcium current found in catfish cone horizontal cells and the physiological trigger that activates calcium release from intracellular stores. This high voltage-activated (HVA), sustained calcium current has been well characterized both pharmacologically and electrophysiologically (Shingai & Christensen, 1986) and closely resembles the L-type sustained calcium current described elsewhere (Nowycky et al. 1985), which is enhanced by the dihydropyridine agonist Bay K8644, and inhibited by dihydropyridine antagonists such as nitrendipine, nimodipine and nifendipine. In the vertebrate retina, voltage-gated calcium currents have been shown to be important in neuronal function and may play a role in physiological processing of sensory information through the retina (Winslow, 1989; Sullivan & Lasater, 1992; Schmitz & Witkovsky, 1997). Because of the importance of this channel, understanding the mechanisms of its modulation is essential for understanding neuronal processing in general, as well as gaining a better understanding of retinal processing in the outer retina.
METHODS
Cell isolation
All experiments were carried out in accordance with guidelines of the local Institutional Animal Care and Use Committee. The procedure for catfish cone horizontal cell isolation was modified from O'Dell & Christensen (1989). Briefly, after dark adapting the fish for 1 h, channel catfish (Ictalurus punctatus) were anaesthetized by placing tricaine methanosulfonate (100 mg ml−1) in the bath until the animal no longer reacted to tactile stimulation. The spinal column was then rapidly transected surgically, followed by immediate pithing. Both eyes were subsequently excised under dim red light and the cornea and lens were removed. The remaining eyecups were placed in a low calcium catfish saline (126 mm NaCl, 4 mm KCl, 0.3 mm CaCl2, 1 mm MgCl2, 15 mm dextrose, 2 mm Hepes, pH adjusted to 7.4) containing hyaluronidase (0.1 mg ml−1; 5270 U mg−1), which digested the vitreous fluid covering the retina. After 4 min, the eyecups were placed in a papain-activated solution (27 U mg−1) containing cysteine (0.7 mg ml−1 papain), which had been prepared in low calcium catfish saline 30 min before use.
Once enzymatically treated, the retina was manually peeled away from the underlying pigment epithelium. The retina was then placed in fresh papain-activated saline for 4 min more, rinsed and subsequently cut into eight pieces. Retinal pieces were stored in normal catfish saline (126 mm NaCl, 4 mm KCl, 3 mm CaCl2, 1 mm MgCl2, 15 mm dextrose, 2 mm Hepes, pH adjusted to 7.4) with bovine serum albumin (1 mg ml−1) at 4 °C until used. Consistent recordings were obtained from these cells for 48 h after preparation (O'Dell & Christensen, 1989).
Before voltage clamping cells, a piece of retina was manually dissociated to yield isolated catfish cone horizontal cells. This process involved triturating a piece of retina through a series of progressively smaller tipped glass Pasteur pipettes. Once the retinal tissue was dissociated completely, cells were loaded with the calcium indicator dye indo-1 (see below).
Measurement of cytosolic free [Ca2+]i
Intracellular calcium concentration ([Ca2+]i) in voltage-clamped catfish cone horizontal cells was measured using the fluorescence calcium indicator indo-1. Isolated cells were loaded for 20 min at room temperature in a solution containing 2 μm indo-1 AM in normal catfish saline. After 20 min of loading cells with the membrane-permeant form of indo-1, the dye was washed away and cone horizontal cells were voltage clamped on the stage of an inverted microscope (Nikon Diaphot) equipped for indo-1 measurement. Cells were continuously perfused after they adhered to the bottom of the chamber.
To prevent a gradual decrease of the fluorescence intensity of the intracellular dye, the recording pipette solution also contained 1 μm of the membrane-impermeant form of indo-1. We found that this application of indo-1 at room temperature eliminated any decrease of the signal over time. Due to compartmentalization problems that frequently accompany use of the free indo-1 indicator (Gray-Keller & Detwiler, 1994), several experiments were conducted using indo-dextran instead of free indo-1 in catfish cone horizontal cells. We found that the fluorescence ratio results obtained using free indo-1 or indo-dextran were indistinguishable.
Loaded cells were imaged using the Noran Instruments Intervision Odyssey system with dual fluorescence channels. The emitted fluorescence was measured at 405 and 490 nm. Recordings of images and ratio data were collected using the systems software driven by a SGI Indy R4000SC workstation. Raw fluorescence signals were background subtracted before being converted to calcium concentration as first described by Grynkiewicz et al. (1985):
where Kd is the equilibrium dissociation constant for indo-1 (288 nm), Fo/Fs is the fluorescence ratio obtained at low and saturating levels with 490 nm emission and R is the fluorescence ratio using 405 and 490 nm excitation. At the end of each experiment, the minimal and maximal fluorescence ratios (Rmin and Rmax) were recorded from each cell. These values were obtained by superfusion of a buffered 10 nm calcium catfish saline with 10 μm ionomycin, followed by 3 mm calcium saline with 10 μm ionomycin.
Electrophysiological recordings
After cells were loaded with the membrane-permeant form of indo-1 for 20 min, a sample of cells were transferred to a perfusion chamber and placed on the stage of a Nikon Diaphot microscope. No substance was used to attach cells to the bottom of the chamber. Indo-1 containing saline was exchanged for catfish saline containing agents that enhanced the voltage-gated calcium current and blocked voltage-gated sodium and potassium currents. This saline consisted of 110 mm NaCl, 4 mm KCl, 1 mm MgCl2, 10 mm CaCl2, 1 μm Bay K8644, 15 mm dextrose, 2 mm Hepes, 10 mm 4-aminopyridine, 0.01 mm tetrodotoxin (pH adjusted to 7.4).
Catfish cone horizontal cells were easily identified based on their characteristic morphology and membrane properties (Shingai & Christensen, 1986). Targeted cells were voltage clamped using patch pipettes made from borosilicate glass according to the method described by Hamill et al. (1981). All pipettes were pulled using a two-stage Narishige (Tokyo) vertical microelectrode puller. When measured in normal catfish saline, electrode resistances ranged between 4 and 10 MΩ. Electrodes were not fire polished or bevelled and contained 120 mm CsCl, and 20 mm TEA to block potassium channels, 2 mm MgCl2, 1 mm ATP and 11 mm Hepes (pH adjusted to 7.4). No calcium, EGTA or BAPTA was added to the patch electrode solution under normal conditions. In a few experiments, voltage clamped cells were loaded with membrane-permeant BAPTA, BAPTA AM, to buffer calcium released from intracellular stores or cells were voltage-clamped using electrodes containing EGTA or BAPTA in the recording pipette solution.
Once a cone horizontal cell was patched, the membrane potential was held at −70 mV. To elicit the voltage-gated calcium current, two different stimulus protocols were followed. In some experiments, the membrane potential of the cell was changed from the holding potential of −70 mV to +50 mV in a rampwise manner over a 500 ms period (Sullivan & Lasater, 1992). Under saline conditions that enhanced the voltage-gated calcium current and blocked other voltage-gated currents, this stimulus protocol resulted in a calcium current-voltage (I-V) curve. In other experiments, the membrane potential of an isolated voltage-clamped catfish cone horizontal cell was held at the resting membrane potential of −70 mV. The membrane potential was subsequently changed from −70 mV to +50 mV using a series of 10 mV depolarization steps.
In this study, we used low frequency activation of the voltage-gated calcium current for durations up to 500 ms since activation of the calcium channel under these conditions produced no increases of [Ca2+]i measured from loaded horizontal cells. Longer durations of calcium channel activation produced significant increases of [Ca2+]i that were not utilized in this study. In addition, low frequency stimulation of the isolated cells prevented rundown of the calcium current. Using the stimulus protocol outlined in this study, rundown of the calcium current did not occur for a minimum of 20 min.
The following measurements were obtained after obtaining current responses from either stimulus protocol: (1) the peak calcium current amplitude, (2) the membrane potential where an inward calcium current could be detected, and (3) the membrane potential corresponding to the peak calcium current amplitude. When using a ramp stimulus protocol in caffeine experiments, the membrane potential corresponding to detection of an inward calcium current was calculated as the membrane potential corresponding to 5 % of the peak current amplitude.
Recordings were obtained using an Axon Instruments Axopatch 200A (Foster City, CA, USA). Series resistance and capacitative artifacts were compensated for using the amplifier controls. Series resistance was adjusted to greater than 90 %. Leakage currents were not corrected for in these studies as data were only collected from cells with relatively small leakage currents that did not change throughout the course of the experiment (less than 20 pA). A small junctional potential was measured (less than 4 mV) and was therefore not compensated for. Data collection was controlled by a personal computer in conjunction with the Digidata 1200 data acquisition board. Digitization and analysis of the data were carried out using the Axon Instruments suite of programs. Data were filtered at 1 kHz (-3 dB) and sampled at 5 kHz.
Drug application
Drug agents such as kainate, caffeine, BAPTA AM, l-glutamate, 2-amino-7-phosphonoheptanoate (AP-7), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 2-amino-2-methyl-4-phosphonoburanoic acid/α-methyl-AP4 (MAP-4), RS-1-aminoindan-1,5-dicarboxycyclopropyl-glycine (AIDA), calmidazolium, KN-62 and ryanodine were applied to voltage-clamped cells via a gravity fed perfusion system (Valveback, AutoMate Scientific) and subsequently washed away using control Ringer solution. The complete exchange of different solutions occurred within 2 s. Other agents, such as ruthenium red, d-myo-inositol-1,4,5-trisphosphate hexasodium (IP3), EGTA, W-7, CaM kinase II inhibitor 281-302 and heparin were allowed to diffuse into the cell through the recording pipette solution. All drugs were obtained from Sigma/RBI. In this study, agonist doses were chosen after preliminary experiments to find the effective saturating concentration. Antagonist concentrations were chosen by their relative effectiveness against the intracellular calcium store agonist used.
As previously mentioned, catfish cone horizontal cells contain a calcium permeable AMPA receptor that has affinity for agonists including l-glutamate, AMPA and kainate (Linn & Christensen, 1992; Eliasof & Jahr, 1997). In this study, we chose to use the glutamate agonist, kainate, instead of AMPA, to activate the ionotropic glutamate receptor due to AMPA desensitization of the channel. Kainate activates the channel without desensitization.
RESULTS
Caffeine raised [Ca2+]i and modulated the voltage-gated calcium current
Previous studies on isolated teleost horizontal cells have demonstrated that cone horizontal cells contain non-mitochondrial intracellular calcium stores sensitive to calcium and agents such as caffeine (Linn & Christensen, 1992). Figure 1 illustrates caffeine-induced changes of intracellular calcium in a catfish cone horizontal cell and the corresponding caffeine-induced effect on voltage-gated calcium current activity. In Fig. 1A, fluorescence images were obtained before, during and after caffeine application from a voltage-clamped cone horizontal cell loaded with the calcium fluorescence indicator indo-1. Fluorescence ratios were obtained from the areas boxed in red. Calcium concentrations were calculated from the averaged signal obtained from these ratios using the equation by Grynkiewicz et al. (1985; see Methods) and illustrated in Fig. 1B. Before application of 10 mm caffeine, the resting [Ca2+]i was 80 nm (Fig. 1B, left). During a brief 10 s exposure to 10 mm caffeine, [Ca2+]i transiently increased to 1.6 μm before returning to control levels (Fig. 1B, middle). The right panel represents an image obtained 2 min after caffeine application (Fig. 1A and B, right). The transient response to caffeine was typical of responses recorded from 21 other dye-filled, voltage-clamped cone horizontal cells, where the [Ca2+]i increased to an average of 1.6 μm after caffeine application (Table 1). This increased [Ca2+]i with caffeine was repeatable in the same cell several times if 1-3 min was allowed between caffeine applications, similar to results obtained from Linn & Christensen (1992).
Figure 1. Intracellular calcium and membrane current responses evoked by caffeine.
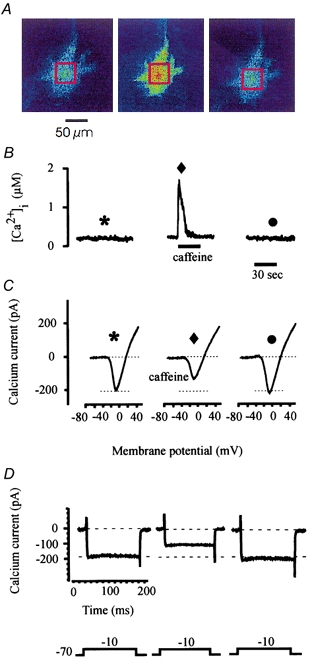
A, fluorescence ratio images obtained from a voltage-clamped horizontal cell loaded with indo-1 before, during and after a 30 s application of 10 mm caffeine. The red box in each image demonstrates the area that was averaged and ratioed. The cell was held at a holding potential of −70 mV to obtain these images. B, calculated calcium concentrations corresponding to the fluorescence images shown in A. C, current traces were elicited before, during and after caffeine application using the rampwise stimulus protocol. The symbol above each calcium current corresponds to symbols illustrated in B and indicates when each calcium current was obtained. D, calcium currents elicited using a stepwise stimulus protocol.
Table 1.
Calcium released from intracellular stores is linked to a reduction of the voltage-gated calcium current in catfish cone horizontal cells
| n | Increase in [Ca2+]i (μM) | Decrease in Ca2+ current amplitude (%) | |
|---|---|---|---|
| Caffeine | 22 | 1.6 ± 0.3* | 33.2 ± 5.2* |
| Caffeine + BAPTA | 6 | 0.4 ± 0.2 | 5.0 ± 3.2 |
| Caffeine + Ruth. red | 6 | 0.3 ± 0.2 | 4.8 ± 3.8 |
| Ruthenium red | 6 | 0.2 ± 0.2 | 6.2 ± 3.4 |
| Caffeine + ryanodine† | 7 | 0.3 ± 0.3 | 7.1 ± 3.9 |
| Caffeine + Ruthenium red + heparin | 6 | 0.3 ± 0.3 | 2.1 ± 1.8 |
| Caffeine + ryanodine + heparin† | 5 | 0.2 ± 0.2 | 8.2 ± 4.1 |
| Caffeine + heparin | 6 | 1.8 ± 0.4* | 33.1 ± 4.8* |
| Caffeine + Ruthenium red | 5 | 0.4 ± 0.2 | 5.3 ± 2.8 |
| Caffeine + calmidazolium | 8 | 1.7 ± 0.3* | 4.9 ± 4.2 |
| Caffeine + W-7 | 5 | 1.5 ± 0.4* | 10.5 ± 5.1 |
| Caffeine + KN-62 | 7 | 1.5 ± 0.2* | 7.0 ± 6.5 |
| Caffeine + CaM kinase II inhibitor | 8 | 1.7 ± 0.3* | 5.2 ± 3.9 |
Data are means ± S.D.
Responses obtained from cells that were pretreated with ryanodine and depleted of intracellular stores.
Significant change compared to control levels (P < 0.01).
The effect of caffeine on the sustained voltage-gated calcium current in catfish cone horizontal cells is shown in Fig. 1C. Calcium current activity was elicited from an indo-1-loaded cell using the ramp stimulus protocol described in Methods. The symbol above each calcium current corresponds to symbols illustrated in Fig. 1B and indicates when each calcium current was obtained. Under control conditions, inward calcium current was detected at a membrane potential of −31 mV and peaked at −9 mV measuring -203 pA (asterisk). Following caffeine application, during the period of elevated [Ca2+]i, peak voltage-gated calcium current decreased by 35.1 % (Fig. 1C) corresponding to the large transient increase in [Ca2+]i (Fig. 1B; diamond). There was no change detected in the calcium current's time course. This was typical of results obtained from 21 other voltage-clamped cone horizontal cells where application of 10 mm caffeine for 30 s resulted in an average decrease of 33.2 % in the voltage-gated calcium current, corresponding to the increase of [Ca2+]i (Table 1). The right panel of Fig. 1C demonstrates an I-V relationship obtained 2 min after caffeine perfusion and demonstrates recovery of the voltage-gated calcium current following caffeine application (circle). In Fig. 1D, the membrane potential of the same indo-1-loaded voltage-clamped catfish cone horizontal cell was changed using the stepwise stimulus protocol described in Methods. Step current traces were obtained immediately after obtaining each I-V relationship shown in Fig. 1C. The left current trace was obtained under control conditions. Characteristic of the L-type calcium current described in teleost cone horizontal cells (Lasater, 1992; Sullivan & Lasater, 1992), the calcium current elicited in catfish cone horizontal cells was sustained and voltage dependent. The centre current trace shown in Fig. 1D was obtained during the large transient increase of [Ca2+]i due to caffeine application. Similar to results obtained using the rampwise stimulus protocol, caffeine caused a decrease of the peak sustained current by 33.1 % when compared to control conditions. Recovery of this effect is shown as the right current trace in Fig. 1D, which was obtained immediately after obtaining the recovery I-V relationship illustrated in Fig. 1C. Similar results using the stepwise stimulus protocol were obtained from 15 other voltage-clamped catfish cone horizontal cells, measuring a mean decrease of 31.3 ± 5.7 % (s.d.) compared to control conditions. Activation of the voltage-gated calcium channel for up to 500 ms using either the rampwise stimulus protocol or the stepwise stimulus protocol failed to produce a detectable change in bulk [Ca2+]i and therefore did not account for or contribute to the caffeine-induced changes of [Ca2+]i (see Methods).
The time course of caffeine's effect on the amplitude of the voltage-gated calcium current is demonstrated in Fig. 2. In Fig. 2A, 10 mm caffeine was perfused over an indo-1-loaded voltage-clamped catfish cone horizontal cell. The change of [Ca2+]i over 2.5 min is illustrated. Similar to results demonstrated in Fig. 1, caffeine produced a one-time large transient increase of [Ca2+]i in the cell that lasted 20 s. Data points shown in Fig. 2B illustrate the measured peak calcium current amplitude recorded at 5 s intervals throughout the course of the experiment. In response to caffeine application, the peak sustained calcium current decreased by 30.1 %. Unlike the [Ca2+]i response, the voltage-gated calcium current did not fully recover to control levels for 90 s. This relationship between the time course of the [Ca2+]i response and the calcium current response was similar in 35 other indo-1-loaded voltage-clamped cells where caffeine caused a mean transient response for [Ca2+]i that lasted 18.5 ± 2.4 s (s.d.) and a mean response of the voltage-gated calcium current that lasted 88.6 ± 5.9 s (s.d.). These results suggest that caffeine's effect may not be a direct action of calcium on the voltage-gated channel itself.
Figure 2. Time course of calcium's effect.
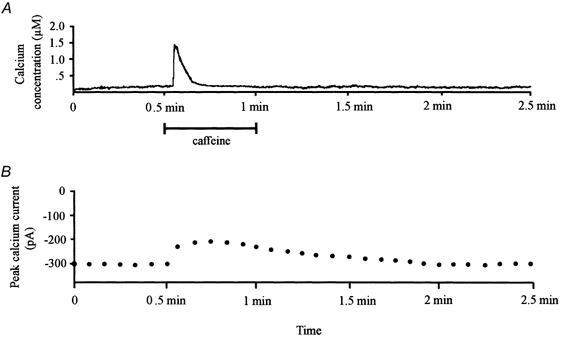
A, time course of the intracellular calcium concentration obtained from an indo-1-loaded catfish cone horizontal cell for a period of 2.5 min; 10 mm caffeine was applied to the cell for 30 s. B, series of data points representing the peak calcium current amplitude obtained over the same time period from the same cell. Calcium currents were elicited using the rampwise stimulus protocol.
However, it is also possible that caffeine itself has a direct effect on the L-type calcium current in catfish cone horizontal cells. To address this issue, caffeine was applied in the absence and presence of the fast calcium buffer, BAPTA AM. Under control conditions, application of 10 mm caffeine to a voltage-clamped catfish cone horizontal cell elicited a transient increase of [Ca2+]i (Fig. 3A) and a corresponding decrease of the L-type calcium current by 31.1 % compared to control conditions (Fig. 3C). The results illustrated in Fig. 3B were obtained from the same cell after treatment with 5 mm BAPTA AM. In the presence of the calcium buffer, the change of [Ca2+]i due to caffeine was significantly reduced from a peak increase of 1.5 μm[Ca2+]i to a peak of 0.5 μm[Ca2+]i. In addition, in the presence of BAPTA AM, the decrease of the L-type calcium current normally associated with caffeine application was virtually eliminated (Fig. 3D) compared to the reduction that occurred in the absence of BAPTA (Fig. 3C). These results were typical of data obtained from six voltage-clamped catfish cone horizontal cells (Table 1). This supports the hypothesis that the suppression of the voltage-gated calcium current in catfish cone horizontal cell is directly or indirectly due to calcium release from intracellular calcium stores and is not a direct effect of caffeine.
Figure 3. Effect of BAPTA on [Ca2+]i and calcium current activity.
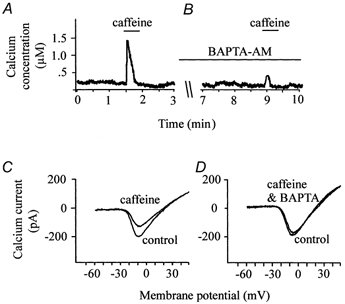
A and B, effect of caffeine on [Ca2+]i in the absence (A) and presence (B) of the calcium chelator BAPTA. C and D, calcium currents elicited at different times throughout the course of the experiment. In C, two superimposed current traces are shown that were obtained before and after application of caffeine in the absence of BAPTA. D shows two superimposed current traces obtained before and after application of caffeine in the presence of BAPTA.
Ruthenium red blocks the caffeine response
Previous studies, using both cardiac and skeletal muscle, have shown that ruthenium red inhibits calcium release from sarcoplasmic reticulum (Oyamada et al. 1993). When 2 μm ruthenium red was allowed to diffuse into voltage-clamped catfish cone horizontal cells through the recording pipette, we found that caffeine failed to increase [Ca2+]i and failed to reduce the voltage-gated calcium current compared to control conditions (Table 1).
Besides inhibition of calcium release from sarcoplasmic reticulum, ruthenium red has also been found to have a variety of other effects in muscle preparations including inhibition of calcium uptake in mitochondria (Broekemeier et al. 1994), inhibition of the Ca2+-ATPase in heart sarcolemma (Gupta et al. 1989), inhibition of calcium-dependent cyclic nucleotide phosphodiesterase (Masuoka et al. 1990), binding to capsaicin receptors (Amann & Lembeck, 1989) and inhibition of L-type calcium and sodium currents in isolated heart cells (Malecot et al. 1998). To ensure that ruthenium red by itself did not affect [Ca2+]i or L-type calcium current activity in catfish cone horizontal cells, fluorescence ratios were obtained over 5 min from indo-1-loaded catfish cone horizontal cells patched with an electrode containing 2 μm ruthenium red in the patch pipette solution. No caffeine was applied during this period of time and the membrane potential was held at −70 mV. We found that ruthenium red had no direct effect on [Ca2+]i or calcium current activity over a 5 min period of time under these conditions (Table 1). Taken together, these results support the hypothesis that ruthenium red's effect is due to a direct effect on ryanodine-sensitive intracellular stores and is not due to a direct action on intracellular stores or on the voltage-gated calcium channels.
Ryanodine's effect on calcium release
As in the sarcoplasmic reticulum of muscle tissue, the plant alkaloid ryanodine binds to the calcium channel protein that is activated by caffeine or calcium (Sutko et al. 1986). Following binding, ryanodine either locks the channel in a subconductance state that causes the inhibition of both calcium- and caffeine-mediated calcium release due to prevention of calcium accumulation by internal stores (Bezprozvanny et al. 1991), or induces the blockade of the endoplasmic reticulum calcium channel (McPherson et al. 1991). To determine if ryanodine can inhibit release of calcium from intracellular calcium stores in catfish cone horizontal cells, indo-1-loaded, voltage-clamped catfish cone horizontal cells were exposed to 10 μm ryanodine. As shown in Fig. 4A, after the addition of ryanodine, only the first caffeine application was able to induce the [Ca2+]i transient, while a second one failed to release calcium. This property reflects the use-dependent interaction of endoplasmic reticulum calcium channels with ryanodine and is typical of ryanodine effects reported in other preparations (Usachev et al. 1993). The complete block of caffeine-induced [Ca2+]i transients was obtained from six other indo-1-loaded catfish cone horizontal cells (Table 1). This effect could not be reversed after 20 min of washout.
Figure 4. Ryanodine's block of caffeine-induced response.
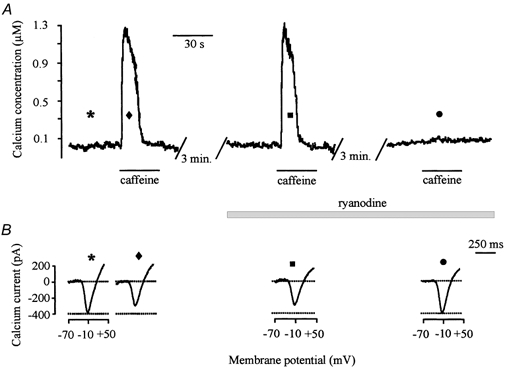
A, 10 mm caffeine was applied to an indo-1-loaded, voltage-clamped horizontal cell for 30 s before addition of 10 μm ryanodine. After washout and incubation in ryanodine, only the first caffeine application was able to induce the [Ca2+]i transient, while a second one failed to release calcium. B, voltage-gated calcium currents were elicited using the rampwise stimulus protocol during the course of the experiment. The symbols above each calcium current correspond to the symbols illustrated in A and indicate when each calcium current was obtained.
As illustrated in Fig. 4B, ryanodine's block of calcium release from ryanodine-sensitive intracellular stores eliminated caffeine's inhibitory effect on the voltage-gated calcium current. The symbol shown above each calcium current in Fig. 4B corresponds to the symbols shown in Fig. 4A and indicates when the voltage-gated calcium currents were obtained during the course of the experiment. As demonstrated before, in the presence of 10 mm caffeine, the voltage-gated calcium current was significantly reduced compared to the control amplitude (diamond vs. asterisk). However, in the presence of ryanodine, a second dose of caffeine failed to release calcium from the ryanodine-sensitive stores and the amplitude of the voltage-gated calcium current returned to control levels (circle). These results demonstrate that caffeine, by itself, does not affect calcium channel activity and support the hypothesis that calcium released from the ryanodine-sensitive intracellular store is needed for inhibition of the voltage-gated calcium current. Similar results were obtained from six other voltage-clamped catfish cone horizontal cells (Table 1).
IP3 intracellular stores
Another principal intracellular calcium store found in neurons has been shown to be sensitive to IP3 (Berridge, 1997), which is increased by hydrolysis of the lipid precursor, phosphatidylinositol 4,5-bisphosphate. IP3 acts on specific receptors that have been well characterized using molecular, pharmacological and immunocytochemical techniques (Micci & Christensen, 1996; Berridge, 1997). In catfish cone horizontal cells, previous immunocytochemical studies have provided evidence of IP3 receptors (Micci & Christensen, 1996). Due to considerable evidence which demonstrates that the IP3 receptor found on IP3-sensitive intracellular stores is also sensitive to the level of cytosolic calcium (Bezprozvanny et al. 1991; Iino & Endo, 1992), and due to the finding that AMPA receptors can activate second messenger cascades in some systems (Kawai & Sterling, 1999; Hayashi et al. 1999) control experiments were performed to determine whether IP3-sensitive intracellular stores contributed to the caffeine response or to the effect of caffeine on calcium current activity. Figure 5A demonstrates the change of [Ca2+]i that occurred when 50 μm IP3 was allowed to diffuse into an indo-1-loaded voltage-clamped catfish cone horizontal cell. In the presence of IP3, [Ca2+]i gradually increased in the cell for 5 min following patch formation. Figure 5B demonstrates the effect of another loaded cell dialysed with 100 μg ml−1 heparin along with 50 μm IP3. In several preparations, including catfish cone horizontal cells, heparin has been found to inhibit IP3-mediated calcium release from IP3-sensitive intracellular stores (Ghosh et al. 1988; Guillemette et al. 1989). Although heparin eliminated the IP3-mediated increase of [Ca2+]i (Fig. 5B), it had no effect on the magnitude or time course of the caffeine-induced response (Fig. 5C; Table 1).
Figure 5. IP3-sensitive intracellular stores do not affect the caffeine-induced response.
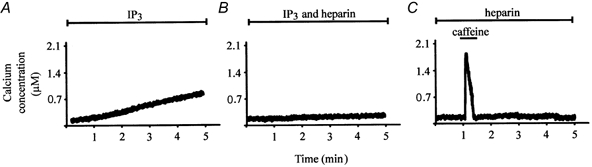
A, effect that 50 μm IP3 has on [Ca2+]i in an indo-1-loaded voltage-clamped catfish cone horizontal cell. IP3 was allowed to diffuse into the cell through the recording pipette solution. However, if the cell was also dialysed with 100 mg ml−1 heparin, IP3‘s effect was blocked (B). C, a typical caffeine response obtained in the presence of 100 mg ml−1 heparin.
To rule out the possibility that ruthenium red or ryanodine has an effect on release of calcium from IP3-sensitive stores, the previously described ruthenium red and ryanodine experiments were repeated in catfish cone horizontal cells dialysed with 100 mg ml−1 heparin. We found no difference between the results obtained in the presence or absence of heparin. Heparin had no effect on ruthenium red's block of the caffeine-induced response and had no effect on the use-dependent inhibition of the ryanodine-sensitive intracellular store caused by 10 μm ryanodine (Table 1). Taken together, these control experiments suggest that the caffeine-induced increase of [Ca2+]i and corresponding inhibition of the voltage-gated calcium current do not involved calcium release from IP3-sensitive intracellular stores.
Physiological activation
The results reported in the above sections demonstrate that calcium released from ryanodine-sensitive intracellular calcium stores decrease the voltage-gated calcium current amplitude in catfish cone horizontal cells. However, we have not addressed the issue of what physiological stimulus could cause a release of calcium from the intracellular store to create the increased [Ca2+]i. We propose that activation of glutamate receptors is linked to this process. In vertebrates, the excitatory neurotransmitter released from photoreceptors onto cone horizontal cells is l-glutamate. Previous studies done by Linn & Christensen (1992) have demonstrated that permeation of calcium through calcium-permeable ionotropic glutamate channels in catfish cone horizontal cells triggers CICR from ryanodine-sensitive intracellular stores. Figure 6 illustrates that activation of these calcium-permeable ionotropic glutamate receptors with the non-NMDA glutamate receptor agonist kainate also resulted in a decrease of the voltage-gated calcium current activity. In Fig. 6A, fluorescence ratios were obtained from an indo-1-loaded voltage-clamped catfish cone horizontal cell before, during and after application of 20 μm kainate. As shown in Fig. 6A, in the presence of kainate, [Ca2+]i increased to a maximum of 1.2 μm and remained elevated until kainate was removed. Associated with the kainate-induced increase of [Ca2+]i was a significant decrease of the voltage-gated calcium current amplitude (Fig. 6C), which persisted well after the kainate-induced transient increase of [Ca2+]i and persisted after the kainate-induced inward current fully recovered. Recovery of the kainate-induced inward current took an average of 34.3 s following kainate removal (n = 11, s.d. 4.2 s), whereas a significant decrease of calcium current amplitude was measured for an average of 92.5 s after kainate washout (n = 11, s.d. 5.2 s). These results were typical of data obtained from 10 other loaded voltage-clamped catfish cone horizontal cells (Table 2) and support the hypothesis that activation of ionotropic glutamate receptors in catfish cone horizontal cells is linked to inhibition of the voltage-gated calcium current via calcium release from ryanodine-sensitive intracellular stores.
Figure 6. KA's effect on [Ca2+]i and calcium current activity.
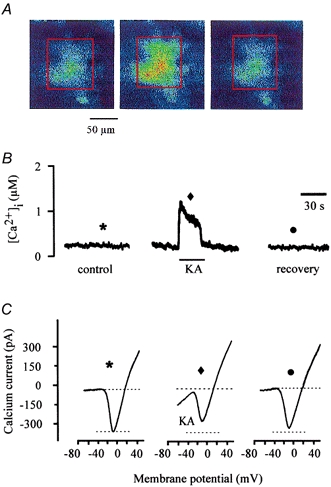
A, fluorescence ratio images obtained from a voltage-clamped cone horizontal cell loaded with indo-1 before, during and after application of KA. B, the calculated calcium concentrations corresponding to the fluorescence images shown in A. The recovery concentration was measured 2 min after caffeine application. C, calcium current activity was elicited before, during and after KA application using a rampwise stimulus protocol. The symbols above each calcium current correspond to the symbols in B and indicate when each calcium current was obtained.
Table 2.
Ionotropic glutamate receptor activation is linked to CICR and modulation of the voltage-gated calcium current in catfish cone horizontal cells
| n | Increase in [Ca2+]i (μM) | Decrease in Ca2+ current amplitude (%) | |
|---|---|---|---|
| KA | 11 | 1.3 ± 0.4* | 29.2 ± 3.5* |
| AMPA | 6 | 1.2 ± 0.3* | 27.2 ± 3.3* |
| L-Glutamate | 8 | 1.1 ± 0.2* | 25.5 ± 5.2* |
| KA + Ruthenium red | 6 | 0.4 ± 0.3 | 4.3 ± 3.9 |
| KA + ryanodine† | 5 | 0.4 ± 0.2 | 3.9 ± 3.0 |
| KA + calmidazolium | 8 | 1.3 ± 0.3* | 13.0 ± 5.2 |
| KA + W-7 | 5 | 1.5 ± 0.4* | 10.5 ± 5.0 |
| KA + KN-62 | 7 | 1.4 ± 0.2* | 9.1 ± 3.8 |
| KA + CaM kinase II inhibitor | 8 | 1.4 ± 0.3* | 7.3 ± 3.5 |
Data are means ± S.D.
Responses obtained from cells that were pretreated with ryanodine and depleted of intracellular stores.
Significant change compared to control levels (P < 0.01).
Previous pharmacological and electrophysiological studies have provided evidence that the non-NMDA ionotropic glutamate receptor on catfish cone horizontal cells is an AMPA receptor (Eliasof & Jahr, 1997). Unlike the sustained inward current elicited by kainate, activation of this receptor by l-glutamate or AMPA elicits a desensitizing biphasic current response consisting of a transient component followed by a maintained inward current component. Because current responses varied depending on the agonist, we repeated the above experiments using AMPA or l-glutamate in the presence of the NMDA antagonist AP-7, and metabotropic glutamate receptor antagonists including AIDA (200 μm) and MAP-4 (500 μm). AMPA (50 μm) inhibited the voltage-gated calcium current by 27.22 ± 4.34 % (n = 6, mean ± s.d.) while 100 μml-glutamate inhibited the high voltage-activated calcium current amplitude by 25.5 ± 5.22 % (n = 8, mean ± s.d.). These results suggest that the natural transmitter is capable of significantly reducing voltage-gated calcium current activity through activation of AMPA receptors found on catfish cone horizontal cells.
Dose-response effects
In an effort to determine the concentration range over which KA was active, several concentrations of KA were used on other loaded, voltage-clamped cells. Figure 7 illustrates the effects of various concentrations of KA on [Ca2+]i and on calcium current amplitude. As shown in this figure, concentrations of KA as low as 10 μm produced an increase in [Ca2+]i, compared to control conditions. Maximal responses were recorded when 50 μm KA was perfused over voltage-clamped cells for 30 s. Concentrations above 50 μm produced an increase in [Ca2+]i that cells did not often recover from and resulted in a loss of the voltage-clamp seal 30 % of the time. Figure 7B shows that concentrations of KA, as low as 10 μm, inhibit calcium current amplitude. Peak inhibition of the calcium current occurred in the presence of 50 μm KA. The IC50 calculated from the dose-response curve was 18 μm.
Figure 7. Effect of KA is dose dependent.
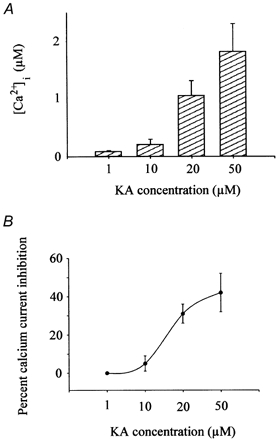
A, peak calcium current amplitudes were obtained from indo-1-loaded voltage-clamped horizontal cells before and after 30 s exposures to various concentrations of KA. Bars show normalized change of [Ca2+]i that occurred under these conditions. Bars show means from 5-20 cells and error bars represent standard deviations. B, percentage of calcium current inhibition that occurred in the presence of various KA concentrations. The membrane potentials of between 8 and 25 indo-1-loaded voltage-clamped cone horizontal cells were changed in a rampwise manner to elicit calcium current activity. The mean percentage difference in calcium current peak amplitude was then plotted as a data point. Points were curve fitted using the Hill equation.
Permeation of calcium through KA-gated channels
Figure 8 demonstrates that calcium permeation through KA-gated channels is linked to changes of [Ca2+]i (Fig. 8A) and modulation of the voltage-gated calcium current (Fig. 8B). To obtain the figure bars, voltage-gated calcium currents were elicited from indo-1-loaded, voltage-clamped cone horizontal cells before and after application of 20 μm KA under a variety of conditions. Looking at the results in Fig. 8A from left to right, we found that 20 μm KA increased [Ca2+]i to an average of 1.25 μm in the presence of high calcium (10 mm). This intracellular calcium increase was reduced when KA was applied in normal 3 mm calcium and almost abolished in the presence of 0.3 mm extracellular calcium. When 20 μm KA was applied in the presence of the competitive KA receptor antagonist CNQX (2 μm), 80 % of the KA-induced [Ca2+]i was blocked compared to control responses. The far right bar in Fig. 8A illustrates the effect of KA when 11 mm EGTA or 5 mm BAPTA was allowed to diffuse into indo-1-loaded voltage-clamped cells through the recording pipette solution. In the presence of the calcium chelating agent EGTA, the increase of [Ca2+]i due to KA was reduced by a mean of 82.2 % compared to control values. Identical results were obtained if the fast calcium chelator, BAPTA, was included in the recording pipette solution instead of EGTA. These results confirm that the increase of [Ca2+]i after KA application is the result of calcium permeation through KA ligand-gated channels.
Figure 8. The role of calcium in KA's effect.
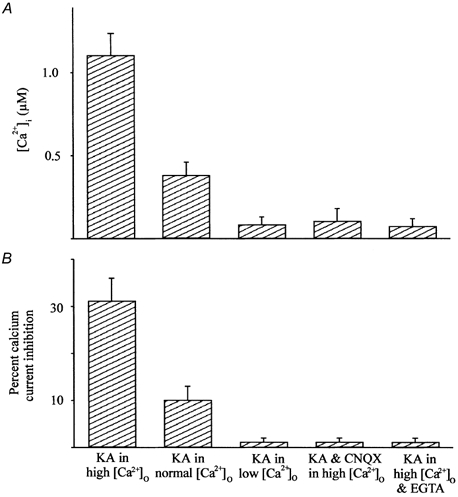
The 3 left bars illustrate the [Ca2+]i (A) and percentage calcium current inhibition (B) measured in the presence of KA in high extracellular calcium catfish saline (10 mm), in normal extracellular catfish saline (3 mm) and in low calcium extracellular catfish saline (0.3 mm). The right 2 bars represent the mean [Ca2+]i obtained when 20 μm KA was applied in the presence of 2 μm CNQX and when KA was applied to cells loaded with 11 mm EGTA. The bars show means from 10-20 cells and error bars represent standard deviations.
Increases in [Ca2+]i due to KA are strongly associated with calcium current inhibition. We found that reduced extracellular calcium concentrations decreased voltage-gated calcium current amplitude (left three bars in Fig. 8B). In addition, Fig. 8B demonstrates that calcium current inhibition was also nearly abolished if KA was applied in the presence of CNQX, or if EGTA or BAPTA was allowed to diffuse into cells through the recording pipette solution. Similar to results obtained in Fig. 8A, these results demonstrate that inhibition of the voltage-gated calcium current is linked to calcium permeation through calcium-permeable KA channels. Furthermore, there is a strong association between KA-induced changes of [Ca2+]i and calcium current inhibition due to CICR from intracellular stores.
Ruthenium red inhibits KA's effect
As previously mentioned, in catfish cone horizontal cells, calcium permeation through KA channels has been found to produce CICR from ryanodine-sensitive intracellular stores (Linn & Christensen, 1992). Previous studies have shown that ruthenium red and ryanodine inhibit these ryanodine-sensitive stores in a dose-dependent manner (Rousseau et al. 1987; Baylor et al. 1989). We found that KA's effect on [Ca2+]i and calcium current activity was inhibited when 2 μm ruthenium red was allowed to diffuse into voltage-clamped cone horizontal cells through the recording pipette solution (Table 2), or when ryanodine-sensitive intracellular stores were depleted using 10 μm ryanodine (Fig. 8, see below).
Depletion of ryanodine-sensitive intracellular stores
Depletion of the intracellular stores was produced by incubating cells in 10 μm ryanodine. Similar to results shown in Fig. 4, in the presence of ryanodine, the first application of caffeine elicited a significant increase of [Ca2+]i (Fig. 9A, asterisk). However, caffeine failed to increase [Ca2+]i when intracellular stores were depleted (Fig. 9A, square). To determine if kainate's inhibition of catfish cone horizontal cells was dependent on CICR from ryanodine-sensitive intracellular stores, kainate was applied to the same cell after intracellular calcium stores were depleted (Fig. 9A, arrow). With depleted stores, KA elicited a significantly smaller change of [Ca2+]i compared to responses obtained when intracellular stores were not depleted.
Figure 9. Depletion of ryanodine-sensitive intracellular stores.
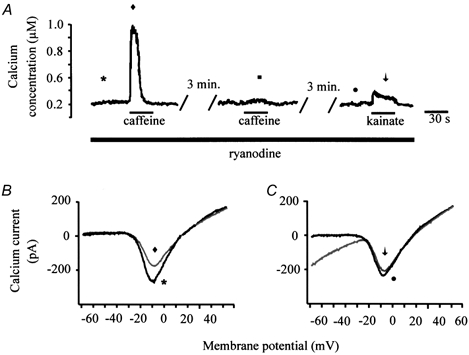
A, in the presence of 10 μm ryanodine, caffeine elicited a large transient increase of [Ca2+]i in response to the first application of 10 mm caffeine (left). A second application of caffeine produced no response (middle). Under these depleted conditions, kainate only evoked a small increase of [Ca2+]i (right) compared to conditions where ryanodine was absent. B and C, current traces obtained at various times throughout the experiment shown in A. The symbols next to each calcium current correspond to the symbols in A and indicate when each calcium current was obtained. The left two superimposed current traces were taken before (asterisk) and after (diamond) application of caffeine in the ryanodine-treated cell. The right two superimposed current traces were obtained from the same cell before (circle) and after (arrow) application of kainate.
When CICR was inhibited with ryanodine, kainate also failed to modulate the voltage-gated calcium current. The two superimposed current traces illustrated in Fig. 9B were obtained under control conditions (asterisk) and after application of caffeine (diamond) from the same cell used to generate the data shown in Fig. 9A. In the ryanodine-treated cell, the first application of caffeine produced a typical decrease of voltage-gated calcium current amplitude. However, after depletion of the intracellular stores, kainate failed to significantly affect the calcium current amplitude (Fig. 9C, circle compared to arrow). Similar results were obtained from four other ryanodine-treated cells (Table 2). Taken together, these results support the hypothesis that kainate-induced CICR is involved in inhibition of the voltage-gated calcium current in catfish cone horizontal cells.
Calmodulin pathway
The results presented above link the reduction of the voltage-gated calcium current in catfish cone horizontal cells with an increase of [Ca2+]i due to CICR from ryanodine-sensitive intracellular stores. But how does release of calcium from intracellular calcium stores modulate a voltage-gated calcium channel? One possibility is that calcium, by itself, can act as a second messenger (Hidaka & Yokokura 1996). Calcium ions can bind to a variety of proteins to alter function and can alter nerve cell activity by modifying characteristics of voltage-gated ion channels (Chad & Eckert, 1986; Scott et al. 1991). However, calcium can also bind to calmodulin (CaM), a ubiquitous calcium-binding protein, and activate a family of Ca2+-CaM-dependent protein kinases to mediate protein phosphorylation of voltage-gated channels (Wolff & Bromstrom 1979; Tanaka & Hidaka 1980).
Two groups of Ca2+-CaM-dependent protein kinases have been discovered. CaM kinase I/IV, II and IV are considered multifunctional enzymes, whereas MLCK, phosphorylase kinase, and CaM kinase III are assigned to specific enzymes (Schulman, 1993). It is widely accepted that CaM kinase II is highly involved in neuronal activities, such as neurotransmitter synthesis and release, neuronal plasticity and memory (Schulman, 1993). Therefore, we investigated whether increased [Ca2+]i modulated the voltage-gated calcium current in catfish cone horizontal cells by forming the Ca2+-CaM complex to activate CaM kinase II. Various calmodulin antagonists and CaM kinase II inhibitors were applied to voltage-clamped cone horizontal cells before and after application of KA.
Two different calmodulin antagonists, calmidazolium and W-7 were used in separate inhibition studies. Preliminary studies found that high concentrations of calmidazolium (10-100 μm) by itself produced an inward current when the cell's membrane potential was held at −70 mV. High concentrations of calmidazium also acted to decrease the voltage-gated calcium current amplitude when the membrane potential was changed to elicit calcium current activity. This result is consistent with other studies that found direct binding of calmidazolium on L-type calcium channels to inhibit the current (Greenberg et al. 1987; Nakazawa et al. 1993). However, we found that preincubation of voltage-clamped cells in a relatively low concentration of calmidazolium (1 μm) did not affect the cell's resting membrane potential, or directly affect the voltage-gated calcium current amplitude. Therefore, in this study, 1 μm calmidazolium was used in inhibition studies using KA. The effect of 1 μm calmidazolium on calcium current activity is illustrated in Fig. 10. Figure 10A illustrates four current traces obtained from a single voltage-clamped cell (from left to right) under control conditions, during 20 μm KA application, after washout and during a second dose of KA in the presence of 1 μm calmidazolium. In the presence of calmidazolium, KA's inhibition of the calcium current was significantly reduced. The first application of KA perfused under control conditions decreased the peak calcium current amplitude by 33.2 %. After recovery, a second application of KA in the presence of 1 μm calmidazolium decreased the peak calcium current amplitude by only 9.5 %. Calmidazolium decreased KA's effect on calcium current activity. This effect of calmidazolium was typical of results obtained from seven other voltage-clamped cells (Table 2).
Figure 10. Involvement of calmodulin and CaM-dependent protein kinase II.
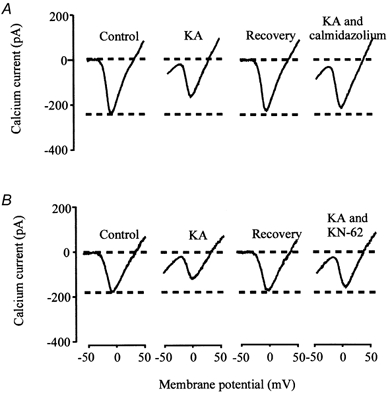
The 4 current traces shown in A were obtained from a single voltage-clamped cone horizontal cell. Calcium current was evoked in control conditions, during KA application, after recovery and during another KA application in the presence of 1 μm calmidazolium. The 4 current traces shown in B were obtained from another voltage-clamped cone horizontal cell. Calcium currents were evoked in control conditions, during KA application, after recovery and during another KA application in the presence of 1 μm KN-62.
Another calmodulin antagonist, W-7, was allowed to diffuse into voltage-clamped cells through the recording pipette solution. Fifty micromolar W-7 had no apparent direct effect on the resting membrane current or voltage-gated calcium current activity when applied in this manner. However, in the presence of W-7, KA's inhibition of the calcium current amplitude was significantly diminished (Table 2). These results using calmodulin antagonists suggest that KA's effect on calcium current activity involves calmodulin.
To determine if calmodulin's action was through activation of CaM protein kinase II, two CaM kinase II inhibitors were used in separate studies. A CaM kinase II inhibitor commonly used in second messenger studies is KN-62, which has high selectivity for CaM kinase II relative to other protein kinases, such as kinase A, kinase C or myosin light chain kinase (Tokumitsu et al. 1990). Like experiments using calmidazolium, we found a dose-dependent effect of KN-62. Bath application of KN-62, greater than 1 μm (10-100 μm), significantly reduced the voltage-gated calcium current by itself, without application of KA. Similar results have been reported from studies that found that KN-62 directly interacts with L-type calcium channels to inhibit the current (Greenberg et al. 1987; Guodong et al. 1992). However, direct inhibition of the catfish calcium current did not occur if the cells were incubated in 1 μm KN-62. In addition, 1 μm KN-62 had no effect on the resting membrane current. This concentration was therefore used for KA inhibition experiments in this study. The results from these studies are represented in Fig. 10B. Four current traces were obtained from a single voltage-clamped cell (from left to right) under control conditions, during 20 μm KA application, after washout and during a second application of KA in the presence of 1 μm KN-62. In the presence of KN-62, KA's inhibition of the calcium current was significantly reduced: from a decrease of 33.0 % under control condition to 6.5 % in the presence of KN-62. Similar results were obtained from six other voltage-clamped cells (Table 2).
Another CaM kinase II inhibitor, CaM kinase II inhibitor 281-302, had no apparent direct effects on resting membrane currents or voltage-gated calcium currents when allowed to diffuse into the cells through the recording pipette solution. However, in the presence of 1 μm CaM kinase II inhibitor 281-302, KA's effect on peak calcium current amplitude dropped from a mean inhibition of 31.5 % under control condition to 9.8 % (Table 2).
Although these calmodulin antagonists and CaM kinase II inhibitors virtually eliminated KA's inhibition of the calcium current, they had no significant effect on the change of [Ca2+]i due to KA application (Table 2). In addition, these inhibitors prevented the effect of caffeine on the calcium current without preventing the caffeine-induced increase in [Ca2+]i (Table 2). Taken together, these results support the hypothesis that calcium permeation through AMPA channels on catfish cone horizontal cells triggers a large transient increase of [Ca2+]i due to CICR from ryanodine-sensitive intracellular stores. Calcium released from these stores binds to calmodulin, activates CaM protein kinase II and directly or indirectly modulates calcium current activity.
DISCUSSION
Intracellular calcium store release
In this study, we have looked at the effect of calcium released from the ryanodine-sensitive intracellular calcium store on the sustained voltage-gated calcium current found in catfish cone horizontal cells. Fluorescence data provided evidence of calcium release from the ryanodine-sensitive intracellular store that was sensitive to caffeine, calcium and ryanodine. In the presence of 10 mm caffeine, this ryanodine-sensitive store released calcium in a transient manner from a resting [Ca2+]i of ≈100 nm to between 1.2 and 1.9 μm. Release of calcium was blocked in the presence of EGTA, BAPTA and ruthenium red, and when low concentrations of ryanodine were allowed to bind to the open calcium release channel. The effects of caffeine, ruthenium red and ryanodine were identical in the presence or absence of heparin.
Inhibition of calcium current
The increase of [Ca2+]i from intracellular stores in catfish cone horizontal cells corresponded with a significant decrease in the peak amplitude of the voltage-gated calcium current. We found that the calcium current amplitude decreased by a mean of 33.2 % in the presence of caffeine-induced increased [Ca2+]i. This reduction of voltage-gated calcium current amplitude was virtually eliminated when caffeine was applied in the presence of EGTA, BAPTA, or ruthenium red, when ryanodine blocked the calcium release channel and when ryanodine-sensitive stores were depleted of calcium.
In addition, the results presented in this study provide evidence that CICR is necessary for modulation of voltage-gated calcium current activity to occur. When kainate was applied in the presence of agents that blocked CICR, modulation of calcium channel activity was eliminated. Therefore, calcium permeation through the calcium-permeable AMPA receptor is not sufficient to trigger events leading to calcium channel modulation.
Could the large transient increase of [Ca2+]i itself be sufficient to cause the change of calcium current amplitude by changing calcium's driving force? We find this very unlikely for the following reasons. Although a ≈10-fold elevation in [Ca2+]i would have a significant effect on the equilibrium potential for calcium, it typically has little effect on the experimentally observed reversal potential due to the permeability to monovalent cations. Also, the Goldman-Hodgkin-Katz theory predicts little change in inward currents between 0.1 μm and 1 μm[Ca2+]i as the inward currents depend primarily on [Ca2+]o (Hille, 1992). In addition, results obtained from two different experiments performed in this study would argue that reduced calcium current does not result from a reduction in driving force on calcium. One of these experiments demonstrated that calcium current reduction outlasts the increase of [Ca2+]i due to caffeine (Fig. 2). Another experiment demonstrated that an increase of [Ca2+]i did not lead to a significant reduction of the calcium current if calmodulin antagonists or CaM kinase II inhibitors were present (Table 2).
Physiological link
In this study, we have provided evidence that activation of AMPA receptors is a likely physiological trigger for initiation of CICR and modulation of calcium current activity. This is based on the findings that kainate, l-glutamate and AMPA mimic responses measured in the presence of agents that caused release of calcium from intracellular stores. One may ask why the AMPA receptor was targeted for this study? Catfish cone horizontal cells have been found to contain distinct ionotropic glutamate receptors as well as two types of metabotropic glutamate receptors (O'Dell & Christensen, 1989; Eliasof & Jahr, 1997; Linn & Gafka, 1999; Gafka et al. 1999). Previous studies done by Dixon et al. (1993) found that l-glutamate application to isolated catfish cone horizontal cells reduced calcium current activity. However, these studies were done in the absence of glutamate receptor subtype antagonists and it was uncertain which type of glutamate receptor was responsible for the resulting decrease of calcium current activity. Therefore, one of the purposes of this study was to identify which glutamate receptor subtype was responsible for glutamate's effect. In cortical neurons, activation of multiple types of metabotropic glutamate receptors was found to inhibit the voltage-gated calcium channels present in these cells (Choi & Lovinger, 1996). However, we ruled out metabotropic glutamate receptors as a possible candidate in the catfish since previous studies by Linn & Gafka (1999) demonstrated that activation of the metabotropic glutamate receptors on catfish cone horizontal cells were not responsible for inhibition of the voltage-gated calcium current (Linn & Gafka, 1999; Gafka et al. 1999). Other studies have linked activation of ionotropic glutamate receptors to inhibition of calcium channel currents (Zeilhofer et al. 1993; Shen & Slaughter, 1998). Therefore, ionotropic receptors were analysed in this study, specifically targeting the AMPA receptor. Further studies in the lab are currently looking at the effect of NMDA receptor activation on voltage-gated channel activity in catfish cone horizontal cells. The existence of multiple glutamate receptor subtypes in retinal horizontal cells may be a mechanism used to regulate information concerning various stimulus conditions.
Pathway linked through calmodulin and CaM kinase II
These results strongly link the reduction of the voltage-gated calcium currents with an increase of [Ca2+]i due to activation of ionotropic glutamate receptors and release of calcium from the ryanodine-sensitive intracellular calcium stores. But how does the release of calcium from intracellular stores modulate a voltage-gated calcium channel? In this study, we have provided electrophysiological and pharmacological evidence that calmodulin activation of CaM kinase II is a possible pathway involved in the modulation of the voltage-gated calcium channel.
However, besides calcium-dependent protein kinases, it is well known that Ca2+-CaM also binds to neuronal nitric oxide synthase (NOS) and can stimulate guanylyl cylase activity to form cGMP (Wang et al. 1998). NO itself can serve as an intra- or intercellular messenger (Garthwaite & Boulton, 1995) or cGMP can effect a diversity of biological responses including modulation of agonist-gated currents in retinal cells (McMahan & Ponomareva, 1996; Wexler et al. 1998). Since it is well known that ion channel properties can be modulated by protein phosphorylation, we are currently in the process of investigating which pathways link the release of calcium from intracellular calcium stores to modulation of the voltage-gated calcium channel in catfish cone horizontal cells through calmodulin and CaM kinase II.
Physiological considerations
In this study we reported that calcium released from ryanodine-sensitive intracellular calcium stores decreased the voltage-gated calcium current measured in catfish cone horizontal cells. We also provided evidence that activation of ionotropic AMPA glutamate receptors located on isolated catfish cone horizontal cells can act as a physiological stimulus to trigger release of calcium from intracellular stores.
Although these results occur in isolated cells, how likely is it that desensitizing AMPA receptors can trigger CICR in vivo? As pointed out by Eliasof & Jahr (1997), the relative role of the AMPA receptor in catfish cone horizontal cells depends on the relative rates of release, clearance and uptake of glutamate from the synapse. If the rate of release is faster than the combined rates of clearance and uptake, glutamate concentrations would be sufficient to desensitize most of the AMPA receptors in the synapse. This would be likely to occur in the dark, when transmitter release from the photoreceptors is the greatest. However, rapid glutamate uptake systems have been shown to exist in glial cells (Brew & Attwell, 1987), as well as in photoreceptors (Tachibana & Kaneka, 1988; Eliasof & Werblin, 1993). Rapid glutamate uptake would serve to lower the neurotransmitter concentration to a level that does not cause significant desensitization. To substantiate that AMPA receptor activation triggers CICR in the catfish horizontal cells, further experiments need to be performed in an in vivo or slice preparation. These experiments are currently underway.
Based on the findings from this study, we propose the following scenario. In the dark, catfish photoreceptors release the neurotransmitter l-glutamate onto bipolar and horizontal cells. On the horizontal cells, l-glutamate binds to ionotropic glutamate receptors and calcium influx occurs through calcium-permeable AMPA receptor channels. The permeation of calcium through these channels triggers CICR from ryanodine-sensitive intracellular stores (Linn & Christensen, 1992). A large increase of [Ca2+]i directly or indirectly affects the calcium channel to modify channel activity. We do not believe that calcium ions themselves are responsible for inhibition of the voltage-gated calcium current as previous studies have demonstrated that these channels are not inactivated by calcium (Lasater, 1992; Sullivan & Lasater, 1992), although we cannot rule out the possibility that they may be inactivated by elevated [Ca2+]i more slowly than the previously described calcium-dependent inactivation in other types of cells (Von Gersdorff & Matthews, 1996). However, we have provided evidence that the calcium effect involves calmodulin and CaM kinase II to modulate calcium current activity.
These results present another mechanism used by retinal neurons to regulate intracellular calcium concentrations and connect all three of the major sources of calcium fluctuation together in a single pathway to regulate calcium channel activity (i.e. agonist-gated channels, voltage-gated channels and calcium release from intracellular stores). Modulation of calcium levels in this way will have profound physiological consequences for several aspects of retinal processing.
Acknowledgments
The authors thank Dr John Cork for imaging assistance, Dr David Linn for helpful discussions throughout the course of this study and L. Brewer for technical assistance. This study was supported by National Eye Institute grant EY-11133 awarded to C.L.L.
References
- Amann R, Lembeck F. Ruthenium red selectively prevents capsaicin-induced nociceptor stimulation. European Journal of Pharmacology. 1989;161:227–229. doi: 10.1016/0014-2999(89)90849-2. [DOI] [PubMed] [Google Scholar]
- Baylor S M, Hollingworth S, Marshall M W. Effects of intracellular ruthenium red on excitation-contraction coupling in intact frog skeletal muscle fibres. Journal of Physiology. 1989;408:617–635. doi: 10.1113/jphysiol.1989.sp017480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M J. Elementary and global aspects of calcium signalling. Journal of Physiology. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich B E. Bell-shaped calcium-response curves of IP3 and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Brew H, Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature. 1987;327:707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Broekemeier K M, Krebsbach R J, Pfeiffer D R. Inhibition of the mitochondrial calcium uniporter by pure and impure ruthenium red. Molecular and Cellular Biochemistry. 1994;139:33–40. doi: 10.1007/BF00944201. [DOI] [PubMed] [Google Scholar]
- Chad J, Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. Journal of Physiology. 1986;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lovinger D M. Metabotropic glutamate receptor modulation of voltage-gated calcium channels involves multiple receptor subtypes in cortical neurons. Journal of Neuroscience. 1996;16:36–45. doi: 10.1523/JNEUROSCI.16-01-00036.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D B, Takahashi K-I, Copenhagen D R. L-glutamate suppresses HVA calcium current in catfish horizontal cells raising intracellular proton concentration. Neuron. 1993;11:267–277. doi: 10.1016/0896-6273(93)90183-r. [DOI] [PubMed] [Google Scholar]
- Eliasof S, Jahr C E. Rapid AMPA receptor desensitization in catfish cone horizontal cells. Visual Neuroscience. 1997;14:13–18. doi: 10.1017/s0952523800008713. [DOI] [PubMed] [Google Scholar]
- Eliasof S, Werblin F. Characterization of the glutamate transporter in retinal cones of the tiger salamander. Journal of Neuroscience. 1993;13:402–411. doi: 10.1523/JNEUROSCI.13-01-00402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafka A C, Vogel K S, Linn C L. Evidence of metabotropic glutamate receptor subtypes found on catfish horizontal and bipolar retinal neurons. Neuroscience. 1999;90:1403–1414. doi: 10.1016/s0306-4522(98)00512-0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Boulton C L. Nitric oxide signaling in the central nervous system. Annual Review of Physiology. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Greenberg M E. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Ghosh T K, Elis P S, Mullaney J M, Ebert C L, Gill D L. Competitive, reversible, and potent antagonism of inositol 1,4,5,-trisphosphate activated calcium release by heparin. Journal of Biological Chemistry. 1988;263:11075–11079. [PubMed] [Google Scholar]
- Gray-Keller M P, Detwiler P B. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994;13:849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Greenberg D A, Carpenter C L, Messing R O. Interaction of calmodulin inhibitors and protein kinase C inhibitors with voltage-dependent calcium channels. Brain Research. 1987;404:401–404. doi: 10.1016/0006-8993(87)91403-x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien R Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Guillemette G, Lamontagne S, Boulay G, Mouillac B. Differential effects of heparin on inositol 1,4,5-trisphosphate binding, metabolism, and calcium release activity in the bovine adrenal cortex. Molecular Pharmacology. 1989;35:339–344. [PubMed] [Google Scholar]
- Guodong L, Hidaka H, Wollheim C B. Inhibition of voltage-gated calcium channels and insulin secretion in HIT cells by the Ca2+/CaM-dependent protein kinase II inhibitor, KN-62: Comparison with antagonists of calmodulin and L-type calcium channels. Molecular Pharmacology. 1992;42:489–498. [PubMed] [Google Scholar]
- Gupta M P, Dixon I M, Zhao D, Dhalla N S. Influence of ruthenium red on rat heart subcellular calcium transport. Canadian Journal of Cardiology. 1989;5:55–63. [PubMed] [Google Scholar]
- Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Umemori H, Mishina M, Yamamoto T. The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. Nature. 1999;397:72–76. doi: 10.1038/16269. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Yokokura H. Molecular and cellular pharmacology of a calcium/calmodulin-dependent protein kinase II (CaM kinaseII) inhibitor, KN-62, and proposal of CaM kinase phosphorylation cascades. Advances in Pharmacology. 1996;36:193–219. doi: 10.1016/s1054-3589(08)60583-9. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates Inc.; 1992. [Google Scholar]
- Iino M, Endo M. Calcium-dependent immediate feedback control of IP3-induced calcium release. Nature. 1992;360:76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Kawai F, Sterling P. AMPA receptor activates a G-protein that suppresses a cGMP-gated current. Journal of Neuroscience. 1999;19:2954–2959. doi: 10.1523/JNEUROSCI.19-08-02954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E M. Membrane properties of distal retinal neurons. Progress in Retinal Research. 1992;11:215–246. [Google Scholar]
- Levitan I B. Modulation of ion channels in neurons and other cells. Annual Review of Neuroscience. 1988;11:119–136. doi: 10.1146/annurev.ne.11.030188.001003. [DOI] [PubMed] [Google Scholar]
- Linn C L, Gafka A C. Activation of metabotropic glutamate receptors modulates the voltage-gated sustained calcium current in a teleost horizontal cell. Journal of Neurophysiology. 1999;81:425–434. doi: 10.1152/jn.1999.81.2.425. [DOI] [PubMed] [Google Scholar]
- Linn C P, Christensen B N. Excitatory amino acid regulation of intracellular calcium in isolated catfish cone horizontal cells measured under voltage- and concentration-clamp conditions. Journal of Neuroscience. 1992;12:2156–2164. doi: 10.1523/JNEUROSCI.12-06-02156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D, Madison D V, Poenie M, Reuter H, Tsien R W, Tsien R Y. Imaging cytosolic Ca2+ transients arising from Ca2+ channels in sympathetic neurons. Neuron. 1988;1:355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- McMahon D G, Ponomareva L V. Nitric oxide and cGMP modulate retinal glutamate receptors. Journal of Neurophysiology. 1996;76:2307–2315. doi: 10.1152/jn.1996.76.4.2307. [DOI] [PubMed] [Google Scholar]
- McPherson P S, Kim Y K, Valdivia H, Knudson C M, Takehura H, Franzini-Armstrong C, Coronado R, Campbell K P. The brain ryanodine receptor: a caffeine-sensitive calcium release channel. Neuron. 1991;7:17–25. doi: 10.1016/0896-6273(91)90070-g. [DOI] [PubMed] [Google Scholar]
- Malecot C O, Bito V, Argibay J A. Ruthenium red as an effective blocker of calcium and sodium currents in guinea-pig isolated ventricular heart cells. British Journal of Pharmacology. 1998;124:465–472. doi: 10.1038/sj.bjp.0701854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuoka H, Ito M, Nakano T, Naka M, Tanaka T. Effects of ruthenium red on activation of calcium-dependent cyclic nucleotide phosphodiesterase. Biochemical and Biophysical Research Communications. 1990;169:315–322. doi: 10.1016/0006-291x(90)91470-d. [DOI] [PubMed] [Google Scholar]
- Micci M A, Christensen B N. Distribution of the inositol trisphosphate receptor in the catfish retina. Brain Research. 1996;720:139–147. doi: 10.1016/0006-8993(95)01463-2. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Higo K, Abe K, Tanaka Y, Saito H, Matsuki N. Blockade by calmodulin inhibitors of calcium channels in smooth muscle from rat vas deferens. British Journal of Pharmacology. 1993;109:137–141. doi: 10.1111/j.1476-5381.1993.tb13543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neering I R, McBurney R N. Role for microsomal calcium storage in mammalian neurones. Nature. 1984;309:158–160. doi: 10.1038/309158a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M C, Fox A P, Tsien R W. Three types of calcium channels. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- O'Dell T, Christensen B N. Horizontal cells isolated from catfish retina contain two types of excitatory amino acid receptors. Journal of Neurophysiology. 1989;61:1097–1109. doi: 10.1152/jn.1989.61.6.1097. [DOI] [PubMed] [Google Scholar]
- Oyamada H, Iino M, Endo M. Effects of ryanodine on the properties of calcium release from the sarcoplasmic reticulum in skinned skeletal muscle fibres of the frog. Journal of Physiology. 1993;470:335–348. doi: 10.1113/jphysiol.1993.sp019861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E, Smith J S, Meissner G. Ryanodine modifies conductance and gating behavior of single calcium release channel. American Journal of Physiology. 1987;253:364–368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Witkovsky P. Dependence of photoreceptor glutamate release on a dihydropyridine-sensitive calcium channel. Neuroscience. 1997;78:1209–1216. doi: 10.1016/s0306-4522(96)00678-1. [DOI] [PubMed] [Google Scholar]
- Schulman H. The multifunctional Ca2+/calmodulin-dependent protein kinase. Current Opinion in Cell Biology. 1993;5:247–253. doi: 10.1016/0955-0674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Scott R H, Pearson H A, Dolphin A C. Aspects of vertebrate neuronal voltage-activated calcium currents and their regulation. Progress in Neurobiology. 1991;36:485–520. doi: 10.1016/0301-0082(91)90014-r. [DOI] [PubMed] [Google Scholar]
- Shen W, Slaughter M M. Metabotropic and ionotropic glutamate receptors regulate calcium channel currents in salamander retinal ganglion cells. Journal of Physiology. 1998;510:815–828. doi: 10.1111/j.1469-7793.1998.815bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai R, Christensen B N. Excitable properties and voltage-sensitive ion conductances of horizontal cells isolated from catfish (Ictalurus punctatus) retina. Journal of Neurophysiology. 1986;56:32–49. doi: 10.1152/jn.1986.56.1.32. [DOI] [PubMed] [Google Scholar]
- Sullivan J M, Lasater E M. Sustained and transient calcium currents in horizontal cells of the white-bass retina. Journal of General Physiology. 1992;99:85–107. doi: 10.1085/jgp.99.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko J L, Thompson L J, Kort A A, Lakatta E A. Comparison of effects of ryanodine and caffeine on rat ventricular myocardium. American Journal of Physiology. 1986;250:86–95. doi: 10.1152/ajpheart.1986.250.5.H786. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Kaneka A. L-glutamate-induced depolarization in solitary photoreceptors: A process that may contribute to the interaction between photoreceptors in situ. Proceedings of the National Academy of Sciences of the USA. 1988;85:5315–5319. doi: 10.1073/pnas.85.14.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Hidaka H. Hydrophobic regions function in calmodulin-enyzme(s) interactions. Journal of Biological Chemistry. 1980;255:11078–11080. [PubMed] [Google Scholar]
- Tokumitsu H, Chijiwa T, Hagiwara M, Mizutani A, Terasawa M, Hidaka H. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]−4-phenylpiperazine, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. Journal of Biological Chemistry. 1990;265:4315–4320. [PubMed] [Google Scholar]
- Usachev Y, Shmigol A, Pronchuk N, Kostyuk P, Verkhratsky A. Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience. 1993;57:845–859. doi: 10.1016/0306-4522(93)90029-f. [DOI] [PubMed] [Google Scholar]
- Von Gersdorff H, Matthews G. Calcium-dependent inactivation of calcium current in synaptic terminals of retinal bipolar neurons. Journal of Neuroscience. 1996;16:115–122. doi: 10.1523/JNEUROSCI.16-01-00115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y G, Rechenmacher C E, Lipsius S L. Nitric oxide signaling mediates stimulation of L-type calcium current elicited by withdrawal of acetylcholine in cat atrial myocytes. Journal of General Physiology. 1998;111:113–125. doi: 10.1085/jgp.111.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler E M, Stanton P K, Nawy S. Nitric oxide depresses GABAA receptor function via coactivation of cGMP-dependent kinase and phosphodiesterase. Journal of Neuroscience. 1998;18:2342–2349. doi: 10.1523/JNEUROSCI.18-07-02342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow R L. Bifurcation analysis of nonlinear retinal horizontal cell models. I. Properties of isolated cells. Journal of Neurophysiology. 1989;62:738–749. doi: 10.1152/jn.1989.62.3.738. [DOI] [PubMed] [Google Scholar]
- Wolff D J, Brostrom C O. Properties and functions of the calcium-dependent regulator protein. In: Greengard P, Robison G A, editors. Advances in Cyclic Nucleotide Research. New York: Raven Press; 1979. pp. 27–88. [PubMed] [Google Scholar]
- Zeilhofer H U, Muller T H, Swandulla D. Inhibition of high voltage-activated calcium currents by L-glutamate receptor-mediated calcium influx. Neuron. 1993;10:879–887. doi: 10.1016/0896-6273(93)90203-4. [DOI] [PubMed] [Google Scholar]


