Abstract
In chickens, low Na+ diets markedly decrease the hexose transport in the rectal segment of the large intestine; transport in the ileum shows a lower, but significant reduction and transport in the jejunum is unaffected. These effects involve both apical (SGLT1) and basolateral (GLUT2) hexose transporters.
The role of the renin-angiotensin-aldosterone axis (RAAS) in the epithelial response to Na+ intake was studied in chickens fed high-NaCl (HS) and low-NaCl (LS) diets. The Vmax of α-methyl-D-glucoside and D-glucose were determined in vesicles from the brush-border (BBMVs) and basolateral (BLMVs) membranes, respectively. The binding of phlorizin to BBMV and cytochalasin B to BLMV were used as indicators of the abundance of SGLT1 and GLUT2, respectively.
In HS-adapted chickens, the serum concentration of aldosterone (means ± S.E.M.) was 35 ± 5 pg ml−1 (n = 6) and that of renin was 20 ± 2 ng ml−1 (n = 3). In LS-fed birds, these values were 166 ± 12 pg ml−1 (n = 6) and 122 ± 5 ng ml−1 (n = 3), respectively. Administration of captopril, the inhibitor of the angiotensin-converting enzyme (ACE), to LS-chickens lowered the aldosterone serum concentration without affecting the renin concentration. Captopril also prevented the reduction of apical and basolateral hexose transport in ileum and rectum characteristic of the intestinal response to LS adaptation.
Administration of the aldosterone antagonist spironolactone to LS-adapted chickens did not affect the serum concentrations of aldosterone, but prevented the effects of LS intake on hexose transport in both apical and basolateral membranes. This suggests that the effects of aldosterone are mediated by cytosolic mineralcorticoid receptors.
Administration of exogenous aldosterone to HS-fed birds induced hexose transport and binding properties typical of the LS-adapted animals. These findings support the view that aldosterone, besides its primary role in controlling intestinal Na+ absorption, can also modulate the expression of apical and basolateral glucose transporters in the chicken distal intestine.
Unlike mammals, certain regions of the large intestine of chickens can co-transport glucose and amino acids with Na+ (Lind et al. 1980; Moretó & Planas, 1989; Ferrer et al. 1994; Bindslev et al. 1997). This function is observed in animals fed commercial diets, typically containing relatively high amounts of NaCl. When chickens are switched to diets containing low levels of NaCl, the RAAS is activated to control several homeostatic adaptations: a significant increase in the short-circuit current in the ileum, caecum and rectum (Skadhauge, 1983; Grubb & Bentley, 1987; Amat et al. 1988), and higher expression of the Na+-H+ exchanger isoform 2 (NHE2) and the epithelial Na+ channel (ENaC) (Goldstein et al. 1997; Donowitz et al. 1998). Interestingly, adaptation to LS diets also reduces the capacity of the large intestine to transport glucose and amino acids (Lind et al. 1980).
We have studied the effects of Na+ intake on the intestinal transport of sugars in chickens for several years. When birds are fed a LS diet, the capacity to accumulate hexoses by jejunal enterocytes is partly reduced (Jaso et al. 1995), whereas this capacity is completely abolished in enterocytes from the rectum (Lind et al. 1980; Árnason & Skadhauge, 1991; Jaso et al. 1995). In the small intestine, in the proximal caecum and in the rectum, glucose is absorbed by a Na+-dependent phlorizin-sensitive SGLT1-type transporter present in the brush-border membrane (Amat et al. 1996; Bindslev et al. 1997; Garriga et al. 1999a). Western blot analysis of this transporter has shown that chicken SGLT1 has an apparent molecular mass of 75 kDa (Garriga et al. 1999b), similar to that described for mammals (Bindslev et al. 1997). The basolateral membrane has a Na+-independent, phloretin and cytochalasin B-sensitive GLUT2-type transporter that has been characterised by functional and binding studies (Kimmich & Randles, 1975; Garriga et al. 1997).
In a previous study on hexose kinetics across apical and basolateral membranes, it was demonstrated that during LS adaptation there is a dramatic reduction in the number of specific transporters in the rectum and the ileum, but not in the jejunum (Garriga et al. 1999a). These effects are rapidly reversed by resalination. Indeed, LS-adapted chickens given a single oral dose of saline showed the HS diet profile of intestinal glucose transport within 4 h of NaCl administration (Garriga et al. 2000). Birds fed LS diets have a serum aldosterone concentration that is 5- to 8-fold higher than animals fed HS diets (Skadhauge, 1983; Árnason et al. 1986; Grubb & Bentley, 1987; Rosenberg & Hurwitz, 1987; Bindslev et al. 1997; Garriga et al. 1999a). Furthermore, when LS-adapted chickens are resalinated, the serum aldosterone concentration drops even below the concentration representative of the HS condition (Skadhauge, 1993; Garriga et al. 2000). Studies on whether dietary NaCl affects other blood hormones indicate that the concentration of circulating corticosterone is not modified by Na+ intake (Skadhauge, 1983; Árnason et al. 1986; Rosenberg & Hurwitz, 1987), while arginine-vasotocin (Arad et al. 1986vs.Skadhauge, 1983) and prolactin (Arad et al. 1986; Árnason et al. 1986) show conflicting results. Aldosterone is, therefore, the best candidate for the signal that controls the number of apical and basolateral glucose transporters in the distal chicken intestine. However, evidence that aldosterone is directly responsible for these changes is lacking.
Here we pursue this question further. We determined the hexose transport function in the apical and basolateral membranes, and the number of transporters at different stages of the RAAS response. In view of the limitations of Western blot analysis of chicken apical SGLT1 (Garriga et al. 1999b) and in the absence of specific antibodies to quantify the chicken basolateral GLUT2, we used a probe-binding approach to quantify both transporters. This technique enables accurate quantification of the transporters and, together with the kinetic results, an estimation of turnover numbers (Cheeseman & Maenz, 1989; Garriga et al. 1999a, 2000). The results support the hypothesis that aldosterone is directly involved in the control of glucose transport in the distal intestinal segments of the chicken, acting through mineralocorticoid receptors.
METHODS
Animals
Male White Leghorn chickens (Gallus gallus domesticus L.) were obtained from a commercial farm (Gibert, Tarragona, Spain) on the day of hatching and housed in stainless-steel cages in standard temperature and humidity conditions, and 18-6 h light-dark cycle. They were fed a commercial diet (Gallina Blanca Purina, Barcelona, Spain) and water ad libitum. When they were 10 weeks old, they were given a 14 day diet consisting of wheat and barley (1:1), with the following contents (in g (kg diet)−1): crude protein, 107.9; lipid, 20.5; carbohydrate, 626.4; crude fibre, 100.5; Na+, 0.013; K+, 0.413; and Cl−, 0.346. The metabolisable energy content was 13.2 MJ kg−1. Drinking water contained added NaCl to yield a final concentration of either 0.015 mm NaCl (LS diet) or 150 mm NaCl (HS diet) as used in previous studies (Garriga et al. 1999a, 2000).
The experimental design was based on seven groups (10-15 animals per group): (1) high Na+ diet (HS diet), (2) low Na+ diet (LS diet), (3) HS diet + 100 μm captopril, (4) LS diet + 100 μm captopril, (5) HS diet + 50 μm spironolactone, (6) LS diet + 50 μm spironolactone, and (7) HS diet + osmotic pump delivering aldosterone.
Captopril was used to inhibit the angiotensin-converting enzyme and thus reduce the conversion of angiotensin I to angiotensin II (Brunner et al. 1978). Spironolactone is a mineralcorticoid that binds cytosolic mineralocorticoid receptors with higher affinity than aldosterone (Atwill et al. 1965). Osmotic pumps were implanted in HS animals to deliver aldosterone in order to stimulate the secondary hyperaldosteronism characteristic of the LS condition, which has proved effective in rats (Pácha & Pohlová, 1995) and birds (Grubb & Bentley, 1987).
Chickens were killed in the morning, without previous withdrawal of food, by cervical dislocation and were then bled. The intestinal segments were removed, immediately flushed with ice-cold saline containing 0.2 mm phenylmethanesulfonyl fluoride (PMSF), 0.41 μm LiN3 and 0.1 mm benzamidine, and opened lengthways. The mucosa was scraped, frozen in liquid nitrogen and stored at -80 °C.
The Ethical Committee of the University of Barcelona, in accordance with the Spanish regulations for the use and handling of experimental animals, approved the animal manipulation and experimental procedures.
Implantation of osmotic pumps delivering aldosterone in vivo
Alzet mini-osmotic pumps (model 2002, Alza Corporation, Palo Alto, CA, USA) were used. The pump reservoir was filled in sterile conditions with a solution of aldosterone in propylene glycol at the appropriate concentration to guarantee a daily pumping rate of approximately 15 μg aldosterone (kg body wt)−1. Preliminary experiments showed that the hyperaldosteronism induced at lower delivery rates was lower than that of the LS condition. For the subcutaneous implantation of pumps, 5 mg (kg body wt)−1 of diazepam was administered intramuscularly and 30 min later the animals were anaesthetised by the intravenous administration of sodium pentobarbital (20 mg (kg body wt)−1). An incision was then made in the nape of the neck and a small pocket was formed by spreading apart the subcutaneous connective tissues. The pump was inserted into the pocket with the flow moderator pointing away from the incision, which was then closed with sutures.
Serum determinations
Blood was sampled by vein puncture between 09.00 and 11.00 h and the serum was stored at -20 °C. The renin concentration was determined by radioimmunoassay with a commercial kit (SB-REN, CIS Bio International, Cambridge, UK). The serum concentration of aldosterone was determined with the Diasorin kit (Vercelli, Italy) and the serum concentrations of Na+ and K+ were measured by flame photometry (Corning 400, Halstead, Essex, UK).
Brush-border membrane vesicle preparation
BBMVs were prepared by MgCl2 precipitation (Garriga et al. 1999a). The vesicle suspension was obtained in a medium containing 300 mm mannitol, 0.1 mm MgSO4, 0.41 μm LiN3 and 20 mm Hepes-Tris (pH 7.4), with a protein concentration of 20-30 mg ml−1.
Basolateral membrane vesicle preparation
BLMVs were prepared following Garriga et al. (1997). The vesicles were suspended in a medium containing 300 mm mannitol, 20 mm Hepes-Tris (pH 7.5), 0.1 mm MgSO4 and 0.41 μm LiN3, with a protein concentration of 10-20 mg ml−1.
Enzyme and protein determinations
The activity of the ouabain-sensitive Na+-K+-activated ATPase (Na+-K+-ATPase, EC 3.6.1.3) was routinely assayed as a marker of the basolateral membrane following Colas & Maroux (1980). Sucrase (α-d-glucohydrolase, EC 3.2.1.48), the marker of the brush-border membrane, was assayed according to Messer & Dahlqvist (1966). The protein content was determined using the Coomassie brilliant blue method with bovine serum albumin as standard (Bradford, 1976).
Transport assays
The uptake of α-methyl-d-glucoside (αGlc1Me) and d-glucose (d-Glc) was measured at 37 °C by a rapid filtration technique (Garriga et al. 1999a). We have used αGlc1Me in the BBMV studies because it is specific for the apical SGLT1 isoform thus avoiding the possible influence of basolateral membrane contamination. For the kinetic studies of the basolateral GLUT2 isoform, we used d-Glc in the absence of Na+ to avoid any influence of apical contamination. To perform the αGlc1Me transport assays in short-circuit conditions, the vesicles were preloaded with 200 mm mannitol, 50 mm KCl, 20 mm Hepes-Tris (pH 7.4), 0.1 mm MgSO4 and 0.41 μm LiN3. Valinomycin was added to the incubation medium at a final concentration of 45 μm in order to render the vesicles permeable to K+. The substrate concentrations used for the kinetic analyses of αGlc1Me and d-Glc uptake by BBMVs and BLMVs were 0.01, 0.05, 0.075, 0.1, 1, 10, 25, 50 and 75 mm, and 0.01, 0.5, 1, 5, 15, 50, 100, 150 and 200 mm, respectively. The osmolality of intra- and extravesicular media was kept constant at 320 mosmol kg−1 by adjusting the total sugar concentration with mannitol.
Phlorizin binding measurements
Steady-state phlorizin binding was assayed at 37 °C by following Peerce & Clarke (1990) with some modifications (Garriga et al. 1999a). The density of phlorizin binding sites is expressed in picomoles of phlorizin bound per milligram of protein at 50 μm phlorizin (B50).
Cytochalasin B binding measurements
Steady-state cytochalasin B binding was assayed at 37 °C by the method described by Cheeseman & Maenz (1989) with some modifications (Garriga et al. 1999a). The density of cytochalasin B binding sites was expressed in picomoles of cytochalasin B bound per milligram of protein at 1 μm cytochalasin B (B1).
Chemicals
All unlabelled reagents were from Sigma except those used to determine enzymatic activity, which were from Boehringer (Mannheim, Germany). d-[U-14C]Glucose (specific activity 251 mCi mmol−1), α-methyl-d-[14C]glucoside (specific activity 265 mCi mmol−1), [3H]phlorizin (specific activity 46.4 Ci mmol−1) and [3H]cytochalasin B (specific activity 15 Ci mmol−1) were purchased from New England Nuclear Research Products (Dreieich, Germany). The final activity of labelled substrates in the incubation medium was 0.5-2 μCi ml−1.
Kinetic analysis
Total αGlc1Me and d-Glc fluxes from at least three independent experiments were analysed by non-linear regression using the Biosoft Enzfitter program (Cambridge, UK). As the errors associated with experimental fluxes were roughly proportional to their values it was considered appropriate to apply proportional weighting to the data.
Statistical analysis
Kinetic parameters and binding measurements were compared using Student's t test (P < 0.05).
RESULTS
Animal model
The serum concentrations of renin (6-fold) and aldosterone (5-fold) were higher in LS than in HS groups (Fig. 1), in agreement with previous results (Garriga et al. 1999a, 2000). The serum K+ concentration was significantly higher in the LS group than in HS animals and no differences in the blood concentration of Na+ were observed (results not shown), again confirming previous observations (Garriga et al. 1999a).
Figure 1. Serum aldosterone and renin concentration, and relative changes of maximal transport rates across BBMVs and BLMVs from the jejunum, ileum and rectum.
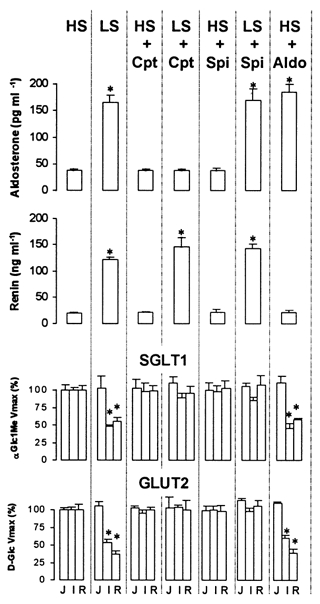
Results of aldosterone and renin are presented as means +s.e.m. (n = 3-7 animals). Maximal transport rates (Vmax) for αGlc1Me and d-Glc are expressed relative to the values of the HS condition and are calculated from data contained in Tables 1 and 2. *P < 0.05 compared with the HS group. Cpt, captopril; Spi, spironolactone; Aldo, aldosterone.
The effects of drug treatment are shown in Fig. 1. Captopril, which inhibits the conversion of angiotensin I into angiotensin II, had no effect on the renin or aldosterone concentrations in HS-fed animals, but prevented hyperaldosteronism in LS animals without affecting the renin concentration. Spironolactone, which competes with aldosterone for the cytosolic mineralocorticoid receptors, had no effect on renin or aldosterone concentrations in either dietary condition. Finally, the chickens fed the HS diet that was supplied with aldosterone by subcutaneous osmotic pumps developed hyperaldosteronism that was similar to that induced by the LS condition, indicating that the protocol used to mimic physiological hyperaldosteronism in HS birds was appropriate.
Characterisation of the membrane vesicles
The membrane purity of both BBMVs and BLMVs was determined by marker enzyme assays. In the final BBMV preparation, sucrase activity was highly enriched and the overall recovery was over 82 % in the three intestinal segments and for all experimental groups. The enrichment factor was between 11.5 and 13.0 (jejunum), 11.3 and 13.1 (ileum), and 11.3 and 12.7 (rectum), without differences between experimental groups in any intestinal segment. The activity of the basolateral marker Na+-K+-ATPase was not enriched (0.81 ± 0.10-fold, n = 42). Membrane orientation was studied according to Del Castillo & Robinson (1982), and results indicated that 92 ± 2 % of the vesicles were oriented outside-out. The intravesicular volume, calculated in equilibrium conditions at 0.1 mmαGlc1Me, was the same in the three intestinal segments and in all experimental groups, with a mean value of 0.63 ± 0.15 μl (mg protein)−1 (n = 42).
In BLMVs, the enrichment factor and the overall recovery of Na+-K+-ATPase activity were high. In jejunal preparations enrichment was between 10.1 and 11.0, in ileal vesicles between 9.8 and 12.0, and in rectal BBMVs between 10.8 and 12.1. The activity of sucrase was not enriched in BLMVs (0.99 ± 0.12-fold, n = 42). The orientation of BLMVs was routinely checked as described previously (Garriga et al. 1997) demonstrating that the vesicle preparation consisted of more than 85 % outside-out vesicles. The intravesicular volume, calculated in equilibrium conditions at 1 mmd-Glc, was 2.07 ± 0.33 μl (mg protein)−1 (n = 42), without statistical differences between intestinal segments and experimental groups.
Transport of αGlc1Me across BBMVs
The kinetic study was carried out in triplicate in the seven experimental groups described above (Table 1). The total fluxes from each experiment were resolved into a saturable (mediated) and a linear component, and the resulting values were subjected to an analysis of variance. This revealed two patterns: the HS pattern, characterised by high mediated hexose transport rates (Vmax) and the LS pattern, characterised by significantly lower Vmax. The jejunum only showed a single pattern, indicating that the transport of αGlc1Me by the apical membrane was not affected by NaCl intake. The Michaelis constants (Km) calculated for all segments and experimental conditions ranged from 1.1 to 1.8 mm without differences between groups. The linear component enabled the calculation of the diffusion constants (Kd) for αGlc1Me, which were between 11.8 and 17.8 nl (mg protein)−1 s−1, without statistical differences between groups. These results show that there is a single apical pathway for the co-transport of glucose in the three intestinal regions, and that dietary NaCl intake does not affect the affinity constant for the glucose analogue αGlc1Me.
Table 1.
Maximal αGlc1Me transport rates and specific phlorizin binding to BBMVs in the 7 groups
| Captopril (50 μM) |
Spironolactone (100 μM) |
Aldosterone (osmotic pump) | |||||
|---|---|---|---|---|---|---|---|
| HS | LS | HS | LS | HS | LS | HS | |
| Jejunum | |||||||
| Vmax | 225 ± 16 | 230 ± 41 | 248 ± 16 | 254 ± 48 | 236 ± 15 | 237 ± 11 | 250 ± 21 |
| B50 | 82 ± 3 | 86 ± 3 | 82 ± 2 | 82 ± 5 | 86 ± 3 | 83 ± 4 | 84 ± 4 |
| Ileum | |||||||
| Vmax | 241 ± 10 | 116 ± 6 * | 237 ± 40 | 215 ± 14 | 226 ± 51 | 205 ± 11 | 110 ± 18 * |
| B50 | 80 ± 3 | 43 ± 3 * | 85 ± 5 | 83 ± 2 | 79 ± 6 | 78 ± 3 | 47 ± 4 * |
| Rectum | |||||||
| Vmax | 125 ± 7 | 69 ± 8 * | 127 ± 11 | 119 ± 12 | 120 ± 10 | 134 ± 18 | 72 ± 3 * |
| B50 | 38 ± 3 | 14 ± 2 * | 36 ± 6 | 38 ± 5 | 42 ± 5 | 40 ± 3 | 15 ± 2 * |
The maximal transport rate constant (Vmax) of αGlc1Me is expressed as pmol (mg protein)−1 s−1 and the specific phlorizin binding, determined using 50 μM phlorizin (B50), is expressed as pmol phlorizin (mg protein)−1. Values are the means ± s.e.m. of 3 separate experiments.
P < 0.05 compared with HS condition. There were no differences in Km and Kd values between groups (see Results).
Adaptation to the LS diet had no effect on apical hexose transport by the jejunum but this was significantly reduced in the ileum and rectum, confirming previous observations (Garriga et al. 1999a, 2000). Captopril had no effect on hexose transport in HS-fed animals, but prevented the changes in Vmax induced by the LS diet. Spironolactone had no effect in the HS group, but prevented the reduction of Vmax characteristic of the LS condition. The subcutaneous administration of aldosterone to HS-fed chickens had the same effect on hexose transport as LS-induced secondary hyperaldosteronism.
Phlorizin binding measurements
The specific binding of phlorizin (B50) for the three intestinal segments and for all experimental conditions is shown in Table 1. The effects of diet and drug treatment on B50 parallel the changes observed in Vmax (Fig. 2A). Both variables show a linear correlation defined by the equation y = 2.5x+ 23.9 (r = 0.990).
Figure 2. Correlation between hexose Vmax and density of transporters.
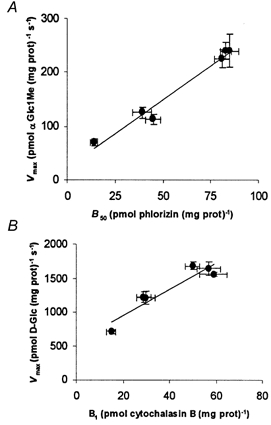
A, αGlc1Me uptake vs. density of phlorizin binding sites in BBMVs. αGlc1Me Vmax is a linear function of the density of specific phlorizin binding (50 μm, B50), and is defined by the equation y = 2.5x+ 23.9 (r = 0.990). B, d-Glc uptake vs. density of cytochalasin B binding sites in BLMVs. d-Glc Vmax is a linear function of the density of specific cytochalasin B binding (1 μm, B1), defined by the equation y = 29.4x+ 561.9 (r = 0.986).
Transport of d-Glc across BLMVs
The initial d-Glc uptake by BLMV was analysed using the same strategy that was used for αGlc1Me uptake by BBMVs (Table 2). The LS adaptation had no effect in the jejunum, but reduced glucose transport in the ileum and the rectum. Drug interaction with the RAAS paralleled the changes observed in the apical membrane, i.e. captopril and spironolactone prevented the effects of secondary hyperaldosteronism, and exogenous aldosterone induced a LS pattern in HS-fed animals. The Michaelis constants (Km) of the seven groups were between 12.9 and 17.0 mm and the diffusion constants (Kd) between 17.3 and 21.6 nl (mg protein)−1 s−1, without statistical differences between groups.
Table 2.
Maximal D-Glc transport rates and specific cytochalasin B binding to BLMVs in the 7 groups
| Captopril (50 μm) |
Spironolactone (100 μm) |
Aldosterone (osmotic pump) | |||||
|---|---|---|---|---|---|---|---|
| HS | LS | HS | LS | HS | LS | HS | |
| Jejunum | |||||||
| Vmax | 1525 ± 32 | 1602 ± 101 | 1498 ± 44 | 1563 ± 12 | 1626 ± 74 | 1702 ± 106 | 1679 ± 34 |
| B1 | 53 ± 6 | 56 ± 5 | 60 ± 6 | 62 ± 5 | 59 ± 4 | 60 ± 4 | 58 ± 2 |
| Ileum | |||||||
| Vmax | 1725 ± 124 | 1234 ± 131 * | 1530 ± 106 | 1627 ± 98 | 1714 ± 34 | 1736 ± 50 | 1194 ± 70 * |
| B1 | 49 ± 1 | 30 ± 2 * | 49 ± 3 | 53 ± 3 | 51 ± 5 | 50 ± 3 | 30 ± 6 * |
| Rectum | |||||||
| Vmax | 1206 ± 75 | 728 ± 40 * | 1234 ± 65 | 1178 ± 115 | 1197 ± 102 | 1303 ± 28 | 692 ± 24 * |
| B1 | 36 ± 2 | 14 ± 1 * | 35 ± 4 | 36 ± 4 | 30 ± 2 | 35 ± 4 | 15 ± 3 * |
D-Glc Vmax is expressed as pmol (mg protein)−1 s−1 and the specific cytochalasin B binding, determined using 1 μM cytochalasin B (B1), is expressed as pmol cytochalasin B (mg protein)−1. Values are the means ± s.e.m. of 3 separate experiments.
P < 0.05 compared with HS. There were no significant differences between Km and Kd values (see Results).
Cytochalasin B binding measurements
Table 2 shows the specific cytochalasin B binding (B1) for all segments and experimental conditions. The changes observed in B1 closely correlate with the maximal transport rates, as shown in Fig. 2B. Both variables are linearly correlated and defined by the equation y = 29.4x+ 561.9 (r = 0.986).
Calculation of turnover numbers for glucose transporters
In a previous paper (Garriga et al. 1999a) we estimated the turnover number for SGLT1 using αGlc1Me Vmax and the maximal specific phlorizin binding constant (Bmax) and obtained a mean SGLT1 turnover number for the jejunum, ileum and rectum of 3 s−1. In the present study, using a phlorizin concentration that saturates 75-100 % of the binding sites of the transporter, we found a value of 2.5 s−1, which is similar. This indicates that the experimental conditions used in the present study allow a good estimation of the turnover number. The statistical analysis showed that there were no differences between experimental groups or intestinal segments. The turnover numbers for GLUT2 were approximately 30 s−1, without differences between segments or experimental conditions.
DISCUSSION
In contrast to the large intestine of mammals, that of birds can transport amino acids and glucose by mechanisms analogous to those present in the small intestine. The small and large intestine of the chicken have a single glucose transporter in the apical membrane, with functional and structural properties compatible with SGLT1 (Amat et al. 1996; Bindslev et al. 1997; Garriga et al. 1999a,b). They also have a GLUT2-like glucose and fructose transporter in the basolateral membrane (Kimmich & Randles 1975; Garriga et al. 1997, 1999a). An interesting feature of the chicken is that a high NaCl-containing (high sodium, HS) diet favours the expression of glucose transporters in the distal segments whereas a low NaCl-containing (low sodium, LS) diet results in reduced hexose accumulation and transport, especially in the rectum (Lind et al. 1980; Árnason & Skadhauge, 1991; Jaso et al. 1995; Garriga et al. 1999a). It has been demonstrated that the decrease in hexose transport in the apical and basolateral membranes of ileal and rectal enterocytes during LS adaptation is due to a reduction in the number of apical (SGLT1) and basolateral (GLUT2) hexose transporters (Garriga et al. 1999a).
The transport rate of hexoses either across the apical membrane in response to changes in dietary composition, or across the basolateral membrane in response to changes in plasma glucose concentration (e.g. in diabetes), can be regulated by signals from the mucosal or the serosal compartment, respectively (Karasov & Diamond, 1983; Solberg & Diamond, 1987; Cheeseman, 1992). Nonetheless, systemic hormones can also regulate the intestinal absorption of nutrients (Ferraris & Diamond, 1997). Thus, in chicken adaptation to dietary NaCl, aldosterone is believed to mediate some (if not all) of the effects of dietary sodium on intestinal glucose transport. This hypothesis is sustained by the correlation between serum aldosterone concentration and hexose transport and accumulation during LS adaptation, and after resalination (Skadhauge, 1983; Jaso et al. 1995; Donowitz et al. 1998; Garriga et al. 2000). However, direct evidence of the involvement of aldosterone in the control of hexose transport had not been reported.
The role of the RAAS system in sodium homeostasis in birds is well established (Wilson, 1984; Skadhauge, 1993). Chickens have granulated juxtaglomerular cells in the kidney and a macula densa that responds to changes in NaCl intake (Christensen et al. 1982). Thus, LS adaptation stimulates renin secretion, which eventually increases the concentration of circulating aldosterone. To study the involvement of RAAS in intestinal hexose transport, we measured serum renin concentration as an indicator of juxtaglomerular activation and determined serum aldosterone concentration. To investigate the contribution of the different levels of RAAS activation, we used captopril to prevent the formation of angiotensin II and aldosterone secretion, and spironolactone to block aldosterone interaction with its specific cytosolic receptors. We also induced hyperaldosteronism in animals with low concentrations of renin and angiotensin II in the blood by the administration of exogenous aldosterone.
Figure 1 shows the correlation of renin and aldosterone concentrations in serum with the hexose transport rates for the different experimental groups. Captopril, a drug that inhibits the angiotensin-converting enzyme (Brunner et al. 1978), affected neither the aldosterone concentration nor the glucose kinetics in the HS group. However, it prevented the establishment of secondary hyperaldosteronism in LS animals without affecting the high circulating renin concentration representative of the LS condition. This supports the view that the chicken RAAS regulates the production of angiotensin II, which in turn controls the secretion of aldosterone by the adrenocortical tissue, as in other animal species (Quinn & Williams, 1988).
Spironolactone is a competitive mineralocorticoid antagonist that binds the intracellular mineralocorticoid receptors (MRs) thus blocking its activation by aldosterone (Atwill et al. 1965). This drug inhibits aldosterone-induced short-circuit current in the rat colon (Jorkasky et al. 1985) and the current induced by LS in the hen coprodeum (Clauss et al. 1987). Our results show that spironolactone has no effects per se on hexose transport since the transport variables measured were not affected when the drug was administered to HS animals. However, when spironolactone was given to LS birds, in spite of the high circulating concentration of renin (hence high angiotensin II) and aldosterone, the expression of apical SGLT1 and basolateral GLUT2 were switched from the profile of the LS condition to that of the HS condition. This indicates that cytosolic MRs participate in the intestinal response to changes in dietary NaCl intake. In rats, the cytosolic mineralocorticoid receptors are expressed throughout the small and large intestine, with higher levels in the distal colon (Fuller & Verity, 1990). In chickens, studies on the regional distribution of aldosterone receptors along the intestine are lacking, although they are predicted to be more abundant in the distal regions, because these segments show the main functional changes in Na+ transport during intestinal adaptation to varying NaCl intakes (Skadhauge, 1993).
The involvement of cellular MRs in the effects of aldosterone on glucose transport received further support using a different experimental approach. This consisted of inducing serum hyperaldosteronism in HS animals (i.e. animals with a physiologically low endogenous aldosterone production) by the exogenous administration of the hormone. In spite of a high NaCl intake and a low renin concentration (hence low angiotensin II), the expression of SGLT1 and GLUT2 in the ileum and the rectum followed the pattern of the LS condition. That is, when the only variable affected was the concentration of circulating aldosterone, the HS glucose transport pattern was switched to the LS pattern. To induce experimental hyperaldosteronism in HS-fed chickens, mini-osmotic pumps were implanted subcutaneously to avoid the oscillations in the serum aldosterone concentration observed when the hormone is administered by intramuscular injections (Clauss et al. 1984). In preliminary experiments, we observed that when pumps delivered aldosterone at a rate of 15 μg (kg body wt)−1 day−1, the concentration of aldosterone in serum (measured 10 and 14 days after pump implantation) was in the range of the values obtained by the diet-induced secondary hyperaldosteronism. Higher rates of aldosterone delivery did not significantly increase the concentration of circulating aldosterone, suggesting that aldosterone metabolism keeps the hormone serum concentration within the range of 150-200 pg ml−1 (0.4-0.6 nm). This might explain the relatively low concentration of aldosterone in the plasma (only 153 pg ml−1) determined by Grubb & Bentley (1987) in chickens with implanted pumps delivering high amounts of aldosterone (128 μg (kg body wt)−1 day−1).
The changes in intestinal glucose absorption in response to dietary Na+ involve at least two mechanisms. First, there is a reduction in the number of apical and basolateral transporters, which decreases Vmax and therefore the absorptive capacity of the distal regions (Garriga et al. 1999a, 2000). Second, there is also a reduction in the membrane electrical potential, at least in enterocytes from the rectum (Jaso et al. 1995), which decreases the electrochemical gradient for Na+ across SGLT1. Both processes reduce hexose accumulation and transport in distal enterocytes. The mechanisms mediating the effects of aldosterone are complex since they involve the transcriptional regulation of genes encoding regulatory proteins that, in turn, control the synthesis or the activation of effector proteins (Verrey, 1990). These regulatory proteins may control the activity of pre-existing effector proteins involved in Na+ transport and increase the synthesis of constitutive elements of the Na+ transport machinery (Garty, 1994; Verrey, 1995; Verrey et al. 2000). The reduction of hexose transport in the ileum and rectum in response to secondary hyperaldosteronism may also be mediated by aldosterone-induced proteins controlling either the expression of genes encoding the hexose transporters or the trafficking of pre-existing hexose transporters from cytoplasmatic vesicles to the cell membrane.
The present study supports the view that, in birds, the distribution of functions between the regions of the intestine is not as well defined as in mammals. For example, in mammals the absorption of hexoses and amino acids is restricted to the small intestine while the large intestine (where aldosterone controls the expression of apical ENaC and NHE, and the basolateral Na+-K+-ATPase) is more involved in the homeostasis of electrolyte and extracellular volume. In the chicken, both functions can be performed in both intestinal regions. Thus, the large intestine, i.e. the rectum and the proximal caecum, can absorb hexoses and amino acids by similar mechanisms to those present in the small intestine (Lind et al. 1980; Moretó & Planas, 1989). Another example is the ileum, which, although it belongs to the small intestine, shares certain functions with the large intestine. For example, it contributes to Na+ homeostasis under the control of aldosterone (Skadhauge, 1983; Grubb & Bentley, 1987).
In conclusion, our results support the view that aldosterone controls the expression of apical and basolateral hexose transporters in the chicken ileum and rectum, acting through cytosolic mineralocorticoid receptors. Both kinds of transporters are affected to the same extent during adaptation to the LS condition, and these effects are rapidly reversed after resalination (Garriga et al. 2000). We suggest that the effects of aldosterone on hexose transport may differ from those described for amino acid transport during LS adaptation because they are partial (Árnason, 1997) and slow, as they require more than 72 h to reach the HS state after resalination (Clauss et al. 1991). We also suggest that the capacity of the small and large intestine of the chicken to share several functions points out that the intestine of birds has a higher functional versatility - hence adaptability - than that of mammals.
Acknowledgments
This study was supported by grants PB96/1255 from the Ministerio de Educación y Cultura and 1999-SGR-00271 from the Generalitat de Catalunya (Spain). Carles Garriga was a recipient of a Col. laboració amb Projectes d'Investigació grant from the Universitat de Barcelona.
References
- Amat C, Planas J M, Díez A, Moretó M. Does chicken rectal adaptation to a low NaCl diet involve changes in paracellular permeability. Comparative Biochemistry and Physiology. 1988;A 91:367–370. [Google Scholar]
- Arad Z, Árnason S S, Chadwick A, Skadhauge E. Osmotic stimuli and NaCl intake in the fowl; release of arginine vasotocin and prolactin. Journal of Comparative Physiology. 1986;B 156:399–406. doi: 10.1007/BF01101102. [DOI] [PubMed] [Google Scholar]
- Árnason S S. Aldosterone and the control of lower intestinal Na+ absorption and Cl− secretion in chickens. Comparative Biochemistry and Physiology. 1997;A 118:257–259. [PubMed] [Google Scholar]
- Árnason S S, Rice G E, Chadwick A, Skadhauge E. Plasma levels of arginine vasotocin, prolactin, aldosterone and corticosterone during prolonged dehydration in the domestic fowl. Journal of Comparative Physiology. 1986;B 156:383–393. doi: 10.1007/BF01101101. [DOI] [PubMed] [Google Scholar]
- Árnason S S, Skadhauge E. Steady-state sodium absorption and chloride secretion of colon and coprodeum, and plasma levels of osmoregulatory hormones in hens in relation to sodium intake. Journal of Comparative Physiology. 1991;B 161:1–14. doi: 10.1007/BF00258740. [DOI] [PubMed] [Google Scholar]
- Atwill W H, Boyarsky S, Glenn J F. Effect of spironolactone on experimental renovascular hypertension. Surgical Forum. 1965;16:494–495. [PubMed] [Google Scholar]
- Bindslev N, Hirayama B A, Wright E M. Na/D-glucose cotransport and SGLT1 expression in hen colon correlates with dietary Na+ Comparative Biochemistry and Physiology. 1997;A 118:219–227. doi: 10.1016/s0300-9629(97)00071-6. [DOI] [PubMed] [Google Scholar]
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunner H R, Gavras H, Turini G A, Waeber B, Cappuis P, McKinstry D N. Long-term treatment of hypertension in man by an orally active angiotensin-converting enzyme inhibitor. Clinical Science and Molecular Medicine. 1978;4:293–295. doi: 10.1042/cs055293s. [DOI] [PubMed] [Google Scholar]
- Cheeseman C. Role of intestinal basolateral membrane in absorption of nutrients. American Journal of Physiology. 1992;263:R482–488. doi: 10.1152/ajpregu.1992.263.3.R482. [DOI] [PubMed] [Google Scholar]
- Cheeseman C, Maenz D D. Rapid regulation of D-glucose transport in basolateral membrane of rat jejunum. American Journal of Physiology. 1989;256:G878–883. doi: 10.1152/ajpgi.1989.256.5.G878. [DOI] [PubMed] [Google Scholar]
- Christensen J A, Morild I, Mikeler E, Bohle A. Juxtaglomerular apparatus in the domestic fowl (Gallus domesticus) Kidney International. 1982;22:S24–35. [PubMed] [Google Scholar]
- Clauss W, Árnason S S, Munck B G, Skadhauge E. Aldosterone induced sodium transport in lower intestine. Effects of varying NaCl intake. Pflügers Archiv. 1984;401:354–360. doi: 10.1007/BF00584335. [DOI] [PubMed] [Google Scholar]
- Clauss W, Biehler K H, Schafer H, Wile N K. Ion transport and electrophysiology of early proximal colon of rabbit. Pflügers Archiv. 1987;408:592–599. doi: 10.1007/BF00581161. [DOI] [PubMed] [Google Scholar]
- Clauss W, Dantzer V, Skadhauge E. Aldosterone modulates electrogenic Cl secretion in the colon of the hen (Gallus domesticus) American Journal of Physiology. 1991;261:R1533–1541. doi: 10.1152/ajpregu.1991.261.6.R1533. [DOI] [PubMed] [Google Scholar]
- Colas B, Maroux S. Simultaneous isolation of brush border and basolateral membrane from rabbit enterocytes. Presence of brush border hydrolases in the basolateral membrane of rabbit enterocytes. Biochimica et Biophysica Acta. 1980;600:406–420. doi: 10.1016/0005-2736(80)90444-7. [DOI] [PubMed] [Google Scholar]
- Del Castillo J R, Robinson J W L. The simultaneous preparation of basolateral and brush-border membrane vesicles from guinea-pig intestinal epithelium, and the determination of the orientation of the basolateral vesicles. Biochimica et Biophysica Acta. 1982;688:45–56. doi: 10.1016/0005-2736(82)90577-6. [DOI] [PubMed] [Google Scholar]
- Donowitz M, Dela Horra C, Calonge M L, Wood I S, Dyer J, Gribble S M, Sánchez de Medina F, Tse C M, Shirazi-Beechey S P, Ilundain A A. In birds, NHE2 is major brush-border Na+/H+ exchanger in colon and is increased by a low-NaCl diet. American Journal of Physiology. 1998;274:R1659–1669. doi: 10.1152/ajpregu.1998.274.6.R1659. [DOI] [PubMed] [Google Scholar]
- Ferraris R P, Diamond J. Regulation of intestinal sugar transport. Physiological Reviews. 1997;77:257–302. doi: 10.1152/physrev.1997.77.1.257. [DOI] [PubMed] [Google Scholar]
- Ferrer R, Gil M, Moretó M, Oliveras M, Planas J M. Hexose transport across the apical and basolateral membrane of enterocytes from different regions of chicken intestine. Pflügers Archiv. 1994;426:83–88. doi: 10.1007/BF00374674. [DOI] [PubMed] [Google Scholar]
- Fuller P J, Verity K. Mineralocorticoid receptor gene expression in the gastrointestinal tract: distribution and ontogeny. Journal of Steroid Biochemistry. 1990;36:263–267. doi: 10.1016/0022-4731(90)90215-e. [DOI] [PubMed] [Google Scholar]
- Garriga C, Moretó M, Planas J M. Hexose transport across the basolateral membrane of the chicken jejunum. American Journal of Physiology. 1997;272:R1330–1335. doi: 10.1152/ajpregu.1997.272.4.R1330. [DOI] [PubMed] [Google Scholar]
- Garriga C, Moretó M, Planas J M. Hexose transport in the apical and basolateral membranes of enterocytes in chickens adapted to high and low NaCl intakes. Journal of Physiology. 1999a;514:189–199. doi: 10.1111/j.1469-7793.1999.189af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga C, Moretó M, Planas J M. Effects of resalination on intestinal glucose transport in chickens adapted to low Na+ intakes. Experimental Physiology. 2000;85:371–378. [PubMed] [Google Scholar]
- Garriga C, Rovira N, Moretó M, Planas J M. Expression of Na+-D-glucose cotransporter in brush-border membrane of the chicken intestine. American Journal of Physiology. 1999b;276:R627–631. doi: 10.1152/ajpregu.1999.276.2.R627. [DOI] [PubMed] [Google Scholar]
- Garty H. Molecular properties of epithelial, amiloride blockable Na channels. FASEB Journal. 1994;8:522–528. doi: 10.1096/fasebj.8.8.8181670. [DOI] [PubMed] [Google Scholar]
- Goldstein O, Asher C, Garty H. Cloning and induction by low NaCl intake of avian intestine Na+ channel subunits. American Journal of Physiology. 1997;272:C270–277. doi: 10.1152/ajpcell.1997.272.1.C270. [DOI] [PubMed] [Google Scholar]
- Grubb B R, Bentley P J. Aldosterone-induced, amiloride-inhibitable short-circuit current in the avian ileum. American Journal of Physiology. 1987;253:G211–216. doi: 10.1152/ajpgi.1987.253.2.G211. [DOI] [PubMed] [Google Scholar]
- Jaso M J, Vial M, Moretó M. Hexose accumulation by enterocytes from the jejunum and rectum of chickens adapted to high and low NaCl intake. Pflügers Archiv. 1995;429:511–516. doi: 10.1007/BF00704156. [DOI] [PubMed] [Google Scholar]
- Jorkasky D, Cox M, Feldman G M. Differential effects of corticosteroids and Na+ transport in rat distal colon in vitro. American Journal of Physiology. 1985;248:G424–431. doi: 10.1152/ajpgi.1985.248.4.G424. [DOI] [PubMed] [Google Scholar]
- Karasov W H, Diamond J M. Adaptive regulation of sugar and amino acid transport by vertebrate intestine. American Journal of Physiology. 1983;245:G443–462. doi: 10.1152/ajpgi.1983.245.4.G443. [DOI] [PubMed] [Google Scholar]
- Kimmich G A, Randles J. A Na+-independent, phloretin-sensitive monosaccharide transport system in isolated intestinal epithelial cells. Journal of Membrane Biology. 1975;23:57–76. doi: 10.1007/BF01870244. [DOI] [PubMed] [Google Scholar]
- Laverty G. Transport characteristics of the colonic epithelium of the Japanese quail (Coturnix coturnix) Comparative Biochemistry and Physiology. 1997;A 118:261–263. doi: 10.1016/s0300-9629(97)00078-9. [DOI] [PubMed] [Google Scholar]
- Lind J, Munck B G, Olsen O. Effects of dietary intake of sodium chloride on sugar and amino acid transport across isolated hen colon. Journal of Physiology. 1980;305:327–336. doi: 10.1113/jphysiol.1980.sp013366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer M, Dahlqvist A. An one-step ultramicro method for the assay of intestinal disaccharidases. Analytical Biochemistry. 1966;14:376–392. doi: 10.1016/0003-2697(66)90280-6. [DOI] [PubMed] [Google Scholar]
- Moretó M, Planas J M. Sugar and amino acid transport properties of the chicken ceca. The Journal of Experimental Zoology. 1989;3:S111–116. doi: 10.1002/jez.1402520518. [DOI] [PubMed] [Google Scholar]
- Pácha J, Pohlová I. Relationship between dietary Na+ intake, aldosterone and colonic amiloride-sensitive Na+ transport. British Journal of Nutrition. 1995;73:633–640. doi: 10.1079/bjn19950065. [DOI] [PubMed] [Google Scholar]
- Peerce B E, Clarke R D. Isolation and reconstitution of the intestinal Na+/glucose cotransporter. Journal of Biological Chemistry. 1990;265:1731–1736. [PubMed] [Google Scholar]
- Quinn S J, Williams G H. Regulation of aldosterone secretion. Annual Review of Physiology. 1988;50:409–426. doi: 10.1146/annurev.ph.50.030188.002205. [DOI] [PubMed] [Google Scholar]
- Rosenberg J, Hurwitz S. Concentration of adrenocortical hormones in relation to cation homeostasis in birds. American Journal of Physiology. 1987;253:R20–24. doi: 10.1152/ajpregu.1987.253.1.R20. [DOI] [PubMed] [Google Scholar]
- Skadhauge E. Intestinal Transport. Berlin: Springer-Verlag; 1983. Temporal adaptation and hormonal regulation of sodium transport in the avian intestine; pp. 284–294. [Google Scholar]
- Skadhauge E. Advances in Comparative and Environmental Physiology. Vol. 16. Berlin: Springer-Verlag; 1993. Basic characteristics and hormonal regulation of ion transport in avian hindguts; pp. 67–93. [Google Scholar]
- Solberg D H, Diamond J M. Comparison of different dietary sugars as inducers of intestinal sugar transporters. American Journal of Physiology. 1987;252:G574–584. doi: 10.1152/ajpgi.1987.252.4.G574. [DOI] [PubMed] [Google Scholar]
- Verrey F. Regulation of gene expression by aldosterone in tight epithelia. Seminars in Nephrology. 1990;10:410–420. [PubMed] [Google Scholar]
- Verrey F. Transcriptional control of sodium transport in tight epithelia by adrenal steroids. Journal of Membrane Biology. 1995;144:93–110. doi: 10.1007/BF00232796. [DOI] [PubMed] [Google Scholar]
- Verrey F, Pearce D, Pfeiffer R, Spindler B, Mastroberardino L, Summa V, Zecevic M. Pleiotropic action of aldosterone in epithelia is mediated by transcription and post-transcription mechanisms. Kidney International. 2000;57:1277–1282. doi: 10.1046/j.1523-1755.2000.00962.x. [DOI] [PubMed] [Google Scholar]
- Wilson J X. The renin-angiotensin system in nonmammalian vertebrates. Endocrine Reviews. 1984;5:45–59. doi: 10.1210/edrv-5-1-45. [DOI] [PubMed] [Google Scholar]


