Abstract
Age-associated loss of skeletal muscle mass and strength can partly be counteracted by resistance training, causing a net synthesis of muscular proteins. Protein synthesis is influenced synergistically by postexercise amino acid supplementation, but the importance of the timing of protein intake remains unresolved.
The study investigated the importance of immediate (P0) or delayed (P2) intake of an oral protein supplement upon muscle hypertrophy and strength over a period of resistance training in elderly males.
Thirteen men (age, 74 ± 1 years; body mass index (BMI), 25 ± 1 kg m−2 (means ± S.E.M.)) completed a 12 week resistance training programme (3 times per week) receiving oral protein in liquid form (10 g protein, 7 g carbohydrate, 3 g fat) immediately after (P0) or 2 h after (P2) each training session. Muscle hypertrophy was evaluated by magnetic resonance imaging (MRI) and from muscle biopsies and muscle strength was determined using dynamic and isokinetic strength measurements. Body composition was determined from dual-energy X-ray absorptiometry (DEXA) and food records were obtained over 4 days. The plasma insulin response to protein supplementation was also determined.
In response to training, the cross-sectional area of m. quadriceps femoris (54.6 ± 0.5 to 58.3 ± 0.5 cm2) and mean fibre area (4047 ± 320 to 5019 ± 615 μm2) increased in the P0 group, whereas no significant increase was observed in P2. For P0 both dynamic and isokinetic strength increased, by 46 and 15 %, respectively (P < 0.05), whereas P2 only improved in dynamic strength, by 36 % (P < 0.05). No differences in glucose or insulin response were observed between protein intake at 0 and 2 h postexercise.
We conclude that early intake of an oral protein supplement after resistance training is important for the development of hypertrophy in skeletal muscle of elderly men in response to resistance training.
Ageing is associated with a progressive reduction of skeletal muscle volume (Lexell et al. 1988) and a concomitant reduction in strength (Grimby & Saltin, 1983; Young et al. 1985; Vandervoort & McComas, 1986; Frontera et al. 1991). This influences the physical performance and thereby the daily function of the elderly. However, resistance training has been shown to counteract the atrophy and loss of strength in this age group (Frontera et al. 1988; Fiatarone et al. 1990; Charette et al. 1991; Welle et al. 1996).
Hypertrophy following resistance training requires net protein synthesis of the myofibrillar proteins, and hence, a maximal stimulation of protein synthesis is favourable for the development of muscle hypertrophy in the elderly. Mixed muscle protein synthesis rate is increased in humans after bouts of resistance training provided the stimulus is of a sufficient magnitude (Chesley et al. 1992). This transient increase in protein synthesis is marked and surpasses the increase in degradation rate, and persists for up to 48 h following an acute exercise bout (Phillips et al. 1997). Evidence exists in favour of the balance between protein synthesis and degradation in skeletal muscle being tipped in favour of protein synthesis by protein intake and hyperaminoacidaemia during rest (Bennet et al. 1989; Biolo et al. 1997; Smith et al. 1998), and net protein balance remains negative after training if individuals remain postabsorptive (Biolo et al. 1995; Phillips et al. 1997). Yet, amino acid supplementation postexercise has been shown to have a synergistic effect upon the muscle contraction-induced augmentation of muscle protein synthesis, when provided both intravenously (Biolo et al. 1997) and orally (Tipton et al. 1999). The stimulation of protein synthesis after bouts of resistance exercise probably follows a specific time course. Thus, it has been observed that protein synthesis is greater 3 h compared to 24 and 48 h postexercise (Phillips et al. 1997). As protein administration is crucial for an optimal effect on net protein synthesis, an early intake of protein after exercise is likely to be important. Recently, it was observed that young individuals had identical acute protein synthesis responses to an amino acid-carbohydrate intake during the first hour following ingestion, irrespective of the supplement being administered 1 or 3 h after resistance exercise (Rasmussen et al. 2000). However, in a resistance training study on rats the timing of a mixed meal ingestion after each training session influenced net protein synthesis over a 10 week training period, as the group that was fed immediately postexercise increased hindlimb muscle mass more than the group fed 4 h later (Suzuki et al. 1999). Yet, it is not known whether ingestion of amino acids immediately postexercise will have a greater effect on the net protein synthesis compared to a later ingestion in elderly males during a period of resistance training.
Hence, the purpose of this study was to investigate the importance of the timing of protein intake after exercise upon the development of muscle hypertrophy and strength during a period of resistance training in elderly individuals. Muscle hypertrophy was evaluated by MRI and from muscle biopsy samples, and muscle strength was determined using both dynamic and isokinetic strength measurements. The acute glucose, insulin and catecholamine responses to exercise and supplementation were also determined for 4 h after training.
METHODS
Subjects and experimental design
Thirteen men (age, 74 ± 1 years (mean ± s.e.m.; range, 70-80 years); height, 178 ± 2 cm (range, 159-190 cm); and BMI, 25 ± 1 kg m−2 (range, 22-32 kg m−2)) volunteered for the study. Subjects had not participated in resistance training within the last 5 years and had no history of metabolic or cardiovascular diseases. All subjects were informed of the nature of the study and the possible risks and discomfort associated with the experimental procedures before they gave their written consent to participate. The study was approved by the Copenhagen Ethics Committee (KF 02-130/97) and was done in accordance with the Declaration of Helsinki.
The subjects completed a 12 week resistance training programme. Strength measurements were conducted prior to (pre-training), after 6 weeks (mid-training) and within 4 days after the training period (post-training). One week prior to the pre-training testing, subjects were familiarised to the strength measurement procedures. Anthropometrical measurements (DEXA scanning), food registration (weighed food records) and muscle cross-sectional area measurements (MRI scanning and muscle biopsy) were performed before (pre-training) and after (post-training) the training period. On two occasions during the period of training acute studies were conducted to measure the insulin response.
All subjects, P-tot (n = 13), were matched in pairs based on body composition and daily protein intake, and randomly assigned to either of two groups, P0 (n = 7) and P2 (n = 6), after the pre-training test round. The odd number is due to one subject's withdrawal from the study due to reasons not associated with the study.
Training
A supervised progressive bilateral resistance training programme was performed 3 times a week for 12 weeks (≈36 training days) in the morning between 08.00 and 10.00 h. All subjects were individually supervised at all training sessions by one of the study investigators. Each bout of training lasted approximately 30 min starting with a 5-10 min warm up on a cycle ergometer (Monark, Sweden). The resistance training consisted of three different concentric strength exercises: leg press (Casall Leg Press), latissimus dorsi (lat) pulldown (Technogym lat machine, Italy) and knee extension (Technogym leg extension R.O.M. (range of movement)), always performed in the described order. The load for the leg exercises increased from 20 repetition maximum (RM) to 12 RM during the first 6 weeks (10-12 repetitions, 3-4 sets), and during the last 6 weeks it remained at 8 RM (8 repetitions, 3-5 sets) to ensure as high an exercise intensity as possible, taking into account the need for graded adaptation in these previously untrained individuals. The load for lat pulldown increased from 20 to 10 RM over the period of training (8-12 repetitions, one set). The load for all exercises was adjusted at every third training session to the exact RM according to the subject's schedule.
Protein supplementation
The supplement consisted of a protein gel (JogMate Protein, Otsuka Pharmaceutical, Japan) containing 10 g protein (from skimmed milk and soybean), 7 g carbohydrate and 3.3 g lipid, corresponding to an energy intake of 420 kJ. The protein gel was dissolved in warm water (approximately 35 °C) before oral ingestion. P0 ingested the protein supplement within 5 min after the termination of each training session and P2 ingested the protein supplement 2 h after each training session. Subjects were not allowed to ingest anything but the protein supplement during the 2 h following training, and after that free access to food was allowed. Further, subjects were instructed to have finished breakfast 1.5 h before the onset of training. The subjects were allowed water ad libitum.
Anthropometrical measurements
For determination of body composition, regional and whole-body dual-energy X-ray absorptiometry (DEXA scanning) was conducted after an overnight fast. Subjects were placed in the supine position and scanned (Lunar DPX-IQ) at medium speed (25 min). Lunar software (Lunar 4.6C) was used to calculate body composition. Subjects were instructed to be normally hydrated at both the pre- and post-training scanning, as muscle mass was indirectly determined and may to some extent be affected by the subject's hydration status (Proctor et al. 1999).
Daily food intake
Self-reported food records (Team Danmark, Denmark) were kept for 4 consecutive days starting on a Sunday to determine daily food intake. Subjects were given verbal and written information and received electronic scales (Tefal, France) with ± 1 g accuracy for use at home.
Strength measurements
Isokinetic strength
Unilateral maximal isokinetic strength was defined as the maximal voluntary torque produced in an isokinetic dynamometer (KinCom, Kintic Communicator, Chattecx Corp., Chattanooga, TN, USA). Knee joint angular velocity was 60 and 180 deg s−1, and the range of motion was 85 to 0 deg (0 deg = full knee extension). Only the concentric strength of knee extension of the right leg was measured as the two legs presumably had been trained equally. The number of subjects with the right leg as the dominant leg did not differ between the two groups. Subjects were familiarised with the apparatus 1 week before the pre-training test.
After a 10 min warm up on the cycle ergometer, subjects were tested seated in a rigid chair with a 90 deg hip flexion and arms crossed. The hip, thigh and lower leg were held in position by straps attached to the chair and lever arm of the dynamometer, respectively. The axis of rotation of the lever arm was aligned with the transversal axis of the knee joint. Gravity correction was measured at an angle of 30 deg in order to avoid passive hamstring strain to contribute to the reference gravity torque.
During testing, verbal encouragement was given, and the subjects were able to follow the force production during the trial. A multiple trial protocol was used, where the voluntary maximal isokinetic torque was defined as being achieved when the subject was unable to produce a higher torque in the two succeeding attempts. This was typically reached within six to eight attempts. All trials were saved for further analysis. The torque produced at each degree of movement was sampled, saved and automatically computed for gravity correction. The torque at 60 deg was chosen a priori for comparison of the voluntary isokinetic strength as peak force is typically close to this angle.
Dynamic training strength
A value of 5 RM (repetition maximum) was used as a measure of dynamic training strength. This was measured with the training apparatus (Technogym leg extension R.O.M.) used and was always carried out after the isokinetic measurements.
Muscle cross-sectional area (CSA)
MR scanning was conducted by a 1.5T Philips ACS-NT scanner within 1 week prior to the 12 week training period and 1 week after the termination of training. Subjects had not been engaged in any testing or training 3 days prior to the MR scanning. Subjects were placed within the stationary, external field in the supine position with the mid-point of the thigh (half-way between trochanter major and epicondylus lateralis) as the point of reference. For determination of CSA, five transaxial images were obtained at mid-thigh in a flip angle of 35 deg with the following parameters: repetition time = 500 ms and echo time = 14 ms. Each data set was obtained with field of view = 200 mm consisting of a 212 pixel matrix. Slice thickness was 6 mm and interslice gap equalled 0.3 mm. The transaxial images were transferred to a Unix Workstation (SPARC station 20, Sun Microsystems, Mountain View, CA, USA) and analysed using the software program Easy Vision 4.2. All four heads of m. quadriceps femoris were outlined manually 3 times for analysis, and CSA was determined as the average of the three analyses.
Biopsies were obtained from m. vastus lateralis after local anaesthesia using the percutaneous needle biopsy technique (Bergström, 1962) with suction. A part of the biopsy was oriented, mounted and immediately frozen in isopentane cooled by liquid nitrogen and kept at -80 °C till further analysis. The rest of the biopsy was frozen directly in liquid nitrogen.
Analysis
ATPase histochemistry
The embedded (Tissue-Tek) biopsy was serially cryosectioned (10 μm) at -25 °C in a cryostat. For identification of fibre types the sections were mounted on glass slides and stained for ATPase activity after preincubation at pH 4.37, 4.60 and 10.3 (Brooke & Kaiser, 1970). The serial sections were visualised and analysed using an Olympus BX40 microscope (Olympus Optical, Tokyo, Japan), a Sanyo high-resolution colour charge-coupled device camera (Sanyo Electronic) and an 8-bit Matrox Meteor Framegrabber (Matrox Electronic Systems, Quebec, Canada), combined with an image-analysing computer program (Tema, Scanbeam, Hadsund, Denmark). Five fibre types were distinguished (1, 1/2a, 2a, 2a/b and 2b) from the staining pattern (Andersen & Aagaard, 2000). For determination of fibre-type distribution 214 ± 11 fibres (mean ± s.e.m.) were analysed per biopsy. For assessment of mean fibre area (MFA) 123 ± 10 fibres were analysed per biopsy.
Electrophoretic separation of myosin heavy chain (MHC) isoforms
SDS-PAGE was conducted to assess the relative expression of MHC isoforms in the biopsies. Twenty serial cryosections (10 μm) from the Tissue-Tek-embedded biopsy were collected in Eppendorf tubes. Denaturation buffer (Fry et al. 1994) was applied to the Eppendorf tubes, which were then heated for 3-5 min at 90 °C. Gels were run at 70 V and at 4 °C for 42-45 h (Andersen & Aagaard, 2000). Subsequently, the gels were Coomassie stained and the relative amount of each MHC isoform (I, IIA and IIX) was quantified using a scanner and densiometric software (Cream, Ke-Men-Tec Aps, Copenhagen, Denmark).
The acute insulin response
Six of the subjects were included in an additional acute study (3 from each training group, P0 and P2). The study was performed twice on two normal training days by use of a crossover design. Subjects arrived at 07.00 h after an overnight fast, and had a catheter inserted in the antecubital vein. The procedures of training and protein intake were identical to those described previously, except that strength exercise for the upper extremities in this case was excluded. Blood samples were collected before and every 30 min during the 4 hours following the training session. After sampling, plasma glucose concentration was analysed immediately (Yellow Springs Instruments, Yellow Springs, OH, USA) and the remaining blood was centrifuged and stored at -80 °C until analysed for plasma adrenaline, noradrenaline and insulin concentration by immunoradiometric (RIA) assays (Pharmacia, Uppsala, Sweden).
Calculations
The results for MFA were based on calculations where normalisation of the fibre-type distribution was performed in order to reduce the influence of the increased degree of heterogeneity observed in the ageing muscle (Lexell & Downham, 1991). Hence, the calculation of MFA of all fibres analysed (MFA-tot) included the MFA and distribution (relative number) of all subtypes. Due to the low number of hybrid fibres (fibre-type 1/2a and 2a/b), these fibres were evenly divided into fibre-type 1, 2a and 2b for calculation of MFA-1, -2a and -2b, respectively.
Insulin availability during the 120 min after the intake of the supplement was assessed as the area under the curve calculated by the trapezoid method.
Statistics
The statistical analysis of the data was performed with SPSS standard version (7.5.3). As the data were non-normally distributed, the dependent variable was tested by non-parametric statistics. Wilcoxon signed-rank test (2-tailed) was applied for dependent statistical comparison between two time points (intra-group). Mann-Whitney U test (2-tailed) was used for independent statistical comparison between two groups (inter-group). The relationship between variables was tested by the Spearman rank-difference correlation method (2-tailed). The significance level was set at P < 0.05.
RESULTS
Anthropometrical measurements
No significant difference was observed between P0 and P2 for any anthropometrical measurement before or after training. However, whole-body lean body mass increased 1.8 ± 0.7 % (P < 0.05) in P0 and decreased 1.5 ± 0.7 % (P < 0.05) in P2 (Table 1).
Table 1.
Anthropometrics and dietary intake
| P0 |
P2 |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Age (years) | 74 ± 1 | 74 ± 1 | 73 ± 1 | 74 ± 1 |
| Weight (kg) | 78 ± 6 | 78 ± 5 | 78 ± 3 | 77 ± 3 |
| BMI (kg m−2) | 26 ± 1 | 26 ± 1 | 25 ± 1 | 25 ± 1 |
| WB fat (%) | 26 ± 3 | 25 ± 2 | 26 ± 2 | 26 ± 3 |
| WB-LBM (kg)† | 54 ± 2 | 55 ± 3* | 54 ± 2 | 53 ± 2* |
| Energy intake (MJ) | 8.6 ± 0.6 | 8.2 ± 0.4 | 9.9 ± 0.5 | 9.1 ± 0.5 |
| Fat (E%) | 38 ± 4 | 34 ± 4 | 37 ± 3 | 39 ± 4 |
| Carbohydrate (E%) | 43 ± 4 | 43 ± 4 | 42 ± 4 | 38 ± 4 |
| Protein (E%) | 14 ± 1 | 15 ± 1 | 14 ± 1 | 15 ± 1 |
| Protein per BW (g kg−1) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Protein per LBM (g kg−1) | 1.4 ± 0.2 | 1.3 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 |
Data are presented as mean values ± s.e.m. for P0 (n = 7) and P2 (n = 6) before (Pre) and after (Post) a 12 week training period.
Significantly different from Pre (P < 0.05).
Significantly larger increase in P0 than in P2. E%, percentage of total energy; WB, whole body; LBM, lean body mass; BW, body weight.
Daily food intake
No significant difference in any dietary parameter was observed between P0 and P2 either before or after training when assessed absolutely and relative to body weight. Further, training did not change dietary habits in either group (Table 1) or in the total of all subjects, P-tot. The protein supplementation was 0.13 ± 0.01 g (kg body weight)−1 in both P0 and P2.
Strength measurements
In P-tot, dynamic training strength (5 RM), and isokinetic 60 and 180 deg s−1 strength increased 42 ± 6, 13 ± 7 and 16 ± 5 % (P < 0.05), respectively, with training. No difference was observed between values for P0 and P2 pre-, mid- or post-training for any of the strength measurements.
In P0, 5 RM, and isokinetic 60 and 180 deg s−1 strength increased (P < 0.05) 47 ± 4, 24 ± 9 and 21 ± 5 %, respectively, with training (Fig. 1), whereas in P2, an increase in strength (P < 0.05) was only observed for 5 RM (36 ± 12 %). Only three and four out of six subjects in P2 showed an increase in isokinetic strength at 60 and 180 deg s−1, respectively. No significant difference in the relative increase in strength was observed between P0 and P2 for any measurement. However, isokinetic strength at 60 deg s−1 tended (P = 0.07) to increase more in P0 than in P2 (24 ± 9 vs. 0 ± 8 %). At 60 deg s−1 peak torque was obtained at 57 ± 1 deg in P-tot with no significant differences between the two groups (P0 55 ± 2 deg and P2 59 ± 2 deg). Changes in peak force were in agreement with torque changes at 60 deg (data not shown).
Figure 1. Strength measurements of knee extension.
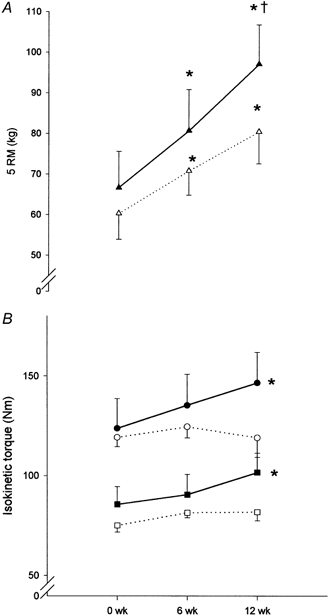
Absolute values of strength in the group ingesting protein immediately postexercise (P0, n = 7, filled symbols) and in the group ingesting protein 2 h postexercise (P2, n = 6, open symbols). A shows dynamic training strength at 5 RM (▴, P0; ▵, P2). B shows isokinetic strength at 60 deg s−1 (•, P0; ○, P2), and isokinetic strength at 180 deg s−1 (▪, P0; □, P2), pre- (0 weeks), mid- (6 weeks) and post-resistance training (12 weeks) for 3 months. * Significantly different from pre-training (P < 0.05); † significantly different from mid-training (P < 0.05). Data are shown as means ± s.e.m.
CSA of m. quadriceps femoris
The CSA of m. quadriceps femoris (CSA-q.f.) increased 4 ± 1 % (P < 0.05) in P-tot from pre- to post-training (54.0 ± 2.9 to 56.0 ± 3.1 cm2). CSA-q.f. increased 7 ± 1 % (P < 0.05) in P0 from pre- to post-training (54.6 ± 0.5 to 58.3 ± 0.5 cm2), whereas no significant change in CSA-q.f. was observed in P2 (53.2 ± 0.2 to 53.3 ± 0.3 cm2; see Fig. 2). Hence, the relative increase in CSA-q.f. from pre- to post-training was larger (P < 0.01) in P0 than in P2.
Figure 2. Cross-sectional area of m. quadriceps femoris (CSA-q.f.).
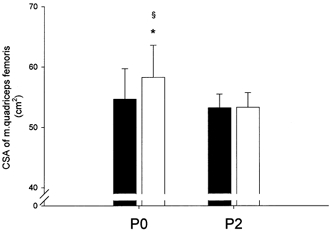
Absolute values of CSA-q.f. pre- (▪) and post-resistance training (□) for 12 weeks in the group ingesting protein immediately postexercise (P0, n = 7) and in the group ingesting protein 2 h postexercise (P2, n = 6). * Significantly different from pre-training (P < 0.05); § significantly larger relative increase in P0 than in P2 (P < 0.01). Bars are means ± s.e.m.
MFA
In P-tot, no significant increase was found in MFA-tot with training (4164 ± 226 to 4589 ± 391 μm2). In P0, MFA-tot increased (P < 0.05) from pre- to post-training (4047 ± 320 to 5019 ± 615 μm2) whereas no significant change was observed in P2 (4300 ± 338 to 4088 ± 415 μm2; see Fig. 3). Hence, MFA-tot increased relatively more in P0 than in P2 (22 ± 6 vs. -5 ± 6 %; P < 0.01).
Figure 3. Mean fibre area (MFA).
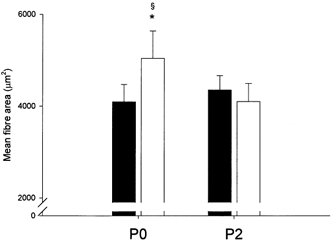
Absolute values of MFA pre- (▪) and post-resistance training (□) for 12 weeks in the group ingesting protein immediately postexercise (P0, n = 7) and in the group ingesting protein 2 h postexercise (P2, n = 6). * Significantly different from pre-training (P < 0.05); § significantly larger relative increase in P0 than in P2 (P < 0.01). Bars are means ± s.e.m.
In P-tot, MFA of type 1 fibres (MFA-1) was larger than MFA of type 2 fibres (MFA-2) before training (P < 0.05), but not after. This was due to the fact that MFA-2 increased (P < 0.05) from pre- to post-training (3552 ± 215 to 4079 ± 360 μm2), whereas MFA-1 did not change significantly (4668 ± 321 to 5012 ± 482 μm2) in response to training. In P0, MFA-1 and MFA-2 increased 18 ± 5 and 29 ± 7 % (4556 ± 462 to 5460 ± 764 μm2 and 3485 ± 316 to 4520 ± 567 μm2), respectively (P < 0.05), thus the relative increase in MFA-2 was larger than in MFA-1 (P < 0.05). In P2, no significant changes of MFA-1 or MFA-2 were observed (4798 ± 480 to 4489 ± 537 μm2 and 3630 ± 314 to 3564 ± 351 μm2, respectively).
In P-tot, neither MFA-2a nor MFA-2b changed significantly with training (3885 ± 228 to 4413 ± 389 μm2 and 2982 ± 205 to 3320 ± 349 μm2, respectively). In P0, MFA-2a increased (P < 0.05) from pre- to post-training (3917 ± 341 to 4910 ± 630 μm2), while no change was observed in P2 (3849 ± 326 to 3835 ± 331 μm2). MFA-2b did not change significantly in either group with training (2849 ± 266 to 3730 ± 516 μm2 in P0, and 3115 ± 332 to 2910 ± 450 μm2 in P2).
MHC isoforms and fibre-type distribution
In P-tot, no change in the distribution of MHC isoforms was observed with training (data not shown). In P0, MHC-I decreased (P < 0.05) 8 ± 3 % from pre- to post-training (63 ± 5 to 57 ± 5 %; see Table 2), and the sum of MHC-IIA and MHC-IIX (MHC-II) increased (P < 0.05) 17 ± 5 % (see Fig. 4). Whereas MHC-IIA increased (P < 0.05) with training in P0 (32 ± 4 to 38 ± 5 %), no significant change was observed in MHC-IIX (see Table 2). In P2, a significant change was only observed in the relative distribution of MHC-IIX, which decreased (P < 0.05; see Table 2).
Table 2.
Distribution of MHC isoforms and fibre types
| P0 |
P2 |
||||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| MHC (%) | MHC-I | 63 ± 5 | 57 ± 5* | 63 ± 5 | 65 ± 4 |
| MHC-IIA | 32 ± 4 | 38 ± 5* | 30 ± 4 | 31 ± 4 | |
| MHC-IIX | 6 ± 2 | 5 ± 5 | 7 ± 2 | 4 ± 1* | |
| Number (%) | Type 1 | 56 ± 6 | 51 ± 5 | 52 ± 4 | 60 ± 3 |
| Type 2a | 29 ± 3 | 34 ± 4* | 30 ± 4 | 29 ± 3 | |
| Type 2b | 15 ± 4 | 15 ± 2 | 18 ± 3 | 11 ± 1* | |
| Area (%) | Type 1 | 62 ± 6 | 56 ± 6* | 58 ± 5 | 65 ± 4 |
| Type 2a | 28 ± 4 | 34 ± 5* | 28 ± 4 | 27 ± 4 | |
| Type 2b | 10 ± 2 | 11 ± 2 | 14 ± 4 | 8 ± 1* | |
Data are presented as mean values ± s.e.m. for P0 (n = 7) and P2 (n = 6) before (Pre) and after (Post) a 12 week training period.
Significantly different from Pre (P < 0.05).
Figure 4. MHC isoforms.
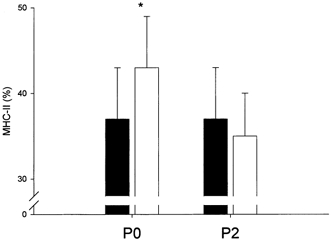
The relative distribution of the MHC isoform MHC-II pre- (▪) and post-resistance training (□) for 12 weeks in the group ingesting protein immediately postexercise (P0, n = 7) and in the group ingesting protein 2 h postexercise (P2, n = 6). * Significantly different from pre-training (P < 0.05). Bars are means ± s.e.m.
In P-tot, no change in the fibre-type distribution (ATPase histochemistry) was observed with training, evaluated on the relative number and area of fibres. In P0 and P2, the effect of the training was equal to the changes of the MHC isoforms, except no significant decrease was observed in the relative number of type 1 fibres (see Table 2).
The results of the two methods applied (gel electrophoresis and histochemistry) correlated significantly for all three MHC isoforms analysed (n = 26 biopsies). The Spearmann correlation coefficient of MHC isoform composition and fibre-type area was r = 0.936 (P < 0.001) for MHC-I:type 1, r = 0.890 (P < 0.001) for MHC-IIa:type 2a and r = 0.804 (P < 0.001) for MHC-IIX:type 2b.
Acute glucose, insulin and catecholamine response
Plasma glucose concentration increased 30 min after intake of the supplement in P2 (P < 0.05) at 150 min, and tended to increase in P0 (P = 0.75) at 30 min (Fig. 5). Correspondingly, plasma insulin concentration increased to peak values 30 min after the respective intakes of the supplement in both P0 (23.2 ± 6.7 μU ml−1 at 30 min) and P2 (18.9 ± 3.0 μU ml−1 at 150 min) with no difference between the groups (Fig. 5). Likewise no difference was observed between P0 and P2 in insulin availability 2 h after the respective intakes of the supplement assessed as the area under the plasma insulin concentration curve (data not shown).
Figure 5. Acute response to resistance exercise and intake of protein supplementation.
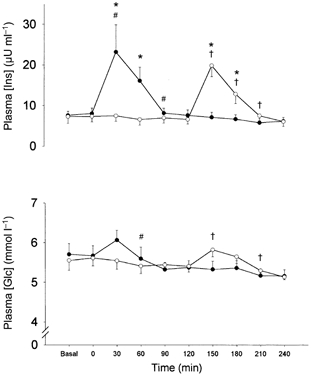
The plasma concentrations of insulin (plasma [Ins]) and glucose (plasma [Glc]) before and during 4 h of recovery from one bout of resistance exercise. •, group ingesting protein immediately postexercise (P0, n = 7); ○, group ingesting protein 2 h postexercise (P2, n = 6). * Significant difference between P0 and P2 at specific time point (P < 0.05); # significantly different from preceding data point in P0; † significantly different from preceding data point in P2.
Plasma adrenaline and noradrenaline concentrations increased with exercise in P0 (to 0.27 ± 0.05 and 4.05 ± 0.46 mmol l−1, respectively) and in P2 (to 0.25 ± 0.04 and 3.88 ± 0.72 mmol l−1, respectively; P < 0.05). At 30 min postexercise plasma adrenaline and noradrenaline concentration had reached basal levels and no differences in plasma adrenaline and noradrenaline concentration were observed between groups at any time point during the 240 min of recovery (data not shown).
DISCUSSION
The major finding of the present study is that the timing of protein intake after resistance training bouts in elderly males is of importance for the development of hypertrophy in skeletal muscle. Thus, over a 12 week resistance training period, the CSA of m. quadriceps femoris and MFA-tot increased by 7 and 22 %, respectively, when protein was ingested immediately after exercise (P0), whereas no significant changes were observed when protein was supplemented 2 h postexercise (P2) (Fig. 2 and Fig. 3). The degree of hypertrophy found in P0 was similar to the findings of other studies investigating the effects of resistance training in the elderly where no specific dietary restrictions were reported (Frontera et al. 1988; Brown et al. 1990; Fiatarone et al. 1990; Welle et al. 1996). In addition to the difference in hypertrophy development between the two groups, it is interesting to note the absence of any detectable hypertrophy in P2 despite 12 weeks of resistance exercise identical to the P0 group. This points to the importance of the early timing of protein intake in recovery from resistance exercise in terms of the amount of net protein synthesis in skeletal muscle. Such a hypothesis somewhat resembles the findings of the importance of early carbohydrate intake for the magnitude of glycogen resynthesis after exercise-induced depletion of muscle glycogen (Ivy et al. 1988).
The increase in MFA was more pronounced (22 %) than that in CSA of the whole muscle (7 %). This is in accordance with the findings of other groups (Frontera et al. 1988) and indicates that a concomitant reduction in the relative amount of non-muscular tissue (fat and connective tissue) to CSA takes place in response to training. This is supported by findings in resistance-trained fragile elderly women, where a 10 % increase in CSA of quadriceps was measured when corrected for fat and connective tissue versus only a 5 % increase when not corrected for this (Harridge et al. 1999). Furthermore, since the angle of pennation has been shown to increase with resistance training in young individuals (Aagaard et al. 2001) it is likely that this contributes to the discrepancy between the two measurements of the relative increase of the muscle mass, and thus to the fact that the anatomical CSA of the muscle (determined by MRI) underestimated the true increase in physiological CSA (determined by muscle biopsy).
The MFA of fibre-type 1 (MFA-1) was found to be larger than MFA-2 in P-tot (as well as in P0) in agreement with previous observations in the elderly (Lexell et al. 1983; Aniansson et al. 1992). However, with training MFA-2 increased significantly more than MFA-1 in P0, and concomitantly the distribution of MHC-II increased in P0 (Fig. 4). These changes with resistance exercise are similar to findings in both young (Andersen & Aagaard, 2000) and elderly individuals (Charette et al. 1991) and could indicate that training induces a larger increment in stress of the type 2 fibres than type 1 fibres compared to normal daily living (Henneman et al. 1965). Further, it was only the area of fibre-type 2a that increased, as MFA-2b determined histochemically was unaffected by training. In addition, lean body mass increased more in P0 than in P2 with training, in agreement with a larger net muscle protein synthesis in P0 over the 12 week period of resistance training compared to P2.
In line with the observed disparity in the degree of hypertrophy between the two groups, the muscle strength was also affected differently by the 12 weeks of training in P0 compared to P2. Thus, isokinetic strength increased at both measured velocities (60 and 180 deg s−1) in P0, whereas no significant increase was observed in P2 at any velocity (see Fig. 1B). However, both groups showed an increase in dynamic training strength assessed as 5 RM (see Fig. 1A), but this is more likely to reflect the neural factors of learning and coordination resulting from training (Rutherford & Jones, 1986). Thus, differences between P0 and P2 were observed only in the isolated non-trained isokinetic knee extensions, where the increase in strength in P0 was in agreement with other studies investigating the effect of 12 weeks of resistance training in the elderly using knee extension as the exercise mode (Frontera et al. 1988; Lexell et al. 1995).
No significant differences were observed between P0 and P2 for any anthropometrical, dietary, muscle or strength parameter before training (see Table 1). Hence, both P0 and P2 fulfilled the recommended daily allowance for energy and protein intake (WHO/FAO/UNU, 1985) and also met the more extensive protein recommendations for the elderly (0.9 g kg−1 day−1; Campbell et al. 1994). Moreover, in the elderly it has been found that the protein requirements probably are not increased above normal dietary intake on non-training days as the myofibrillar protein synthesis was found to be similar in an exercised leg 23 h postexercise whether high-protein (28 E %) or isocaloric low-protein meals (7 E %) were ingested (Welle & Thornton, 1998). Therefore, when subjects are fulfilling the dietary recommendations, it appears only to be necessary to provide protein supplementation on training days in order to maximise the net protein synthesis stimulated by the bout of resistance exercise. However, some controversy exists as to whether or not a daily nutritional supplement combined with resistance training has an additive effect on hypertrophy in the elderly. Whereas one group has observed an additive effect (Meredith et al. 1992), another group did not (Fiatarone et al. 1994). In both studies supplementation was given on top of normal adequate nutrition, however, and furthermore food recordings in both studies were not optimal. In the present study subjects in the two groups received the same controlled protein supplementation per body weight (0.13 g kg−1) and per lean body mass (0.19 g kg−1) after every training bout. Hence, the findings do seem to indicate that the timing of the protein intake is of utmost importance for protein synthesis and muscle hypertrophy.
In the present study muscle protein synthesis was not determined, but it is evident that the resulting hypertrophy after training is a product of an accumulation of net synthesis of structural muscle proteins after each resistance exercise bout. As the synthesis of mixed muscle, myofibrillar and MHC proteins has been shown to increase in response to resistance training in the elderly (Yarasheski et al. 1993; Welle et al. 1999; Hasten et al. 2000), resulting in net protein synthesis over a period of resistance training (Frontera et al. 1988; Brown et al. 1990; Fiatarone et al. 1990; Welle et al. 1996), it might be surprising that in the present study one of the groups, P2, did not show any significant increase in muscle CSA. However, two studies have failed to show an acute response in muscle protein synthesis to resistance exercise (Tipton et al. 1996; Roy et al. 1997), although in these studies, trained subjects were used suggesting that the training stimulus could have been insufficient (Rennie & Tipton, 2000). In the present study all individuals were untrained prior to the programme, and furthermore relative loads at 75 % of RM were used during the last 6 weeks, making it unlikely that loading was insufficient to stimulate muscle protein synthesis. Nevertheless, no hypertrophy was observed in P2 despite the fact that comparable studies with very similar (Hakkinen & Hakkinen, 1995) or slightly heavier training protocols (Frontera et al. 1988; Fiatarone et al. 1990; Welle et al. 1996) have found an increase in muscle mass. However, we have no well-founded reason for believing that these studies were carried out in circumstances in which the subjects ate ‘early’ though the changes in muscle mass resemble our findings in P0. Yet, none of these studies report the dietary habits in association with the training. Alternatively, it could be speculated that the time of day when the training was carried out was important. The diurnal hormonal profile could influence the anabolic response to resistance exercise. Thus, in the present study the subjects always trained in the morning between 08.00 and 10.00 h with no differences in the specific time points between the groups P0 and P2. This was done in order to standardise the conditions, which were best controlled at this time of the day. Hence, the subjects in the present study may have had a disadvantage in training compared with other studies, and this could explain the lack of hypertrophy in P2. Unfortunately, no previous studies report when the training was carried out; further investigations are required to elucidate this point.
In spite of this, the difference in hypertrophy between the two groups suggests that the isolated act of contraction is counteracted by other factors, e.g. delayed food intake. In line with this, Tipton et al. (1999) have shown that postexercise net muscle protein balance is negative when individuals are maintained in the postabsorptive state during recovery, whereas if they ingest protein and achieve hyperaminoacidaemia the protein balance becomes positive. Finally, we are confident that both groups trained properly as the training sessions were always supervised and loads adjusted at every third training session. This is supported by the increased training strength (5 RM), which was observed for both groups.
Although not directly determined in this study we do believe that the protein intake highly stimulated muscle protein synthesis, since in a recent study by Rasmussen et al. (2000) protein synthesis was elevated 3.5 times above pre-intake values when a supplement of only 6 g amino acid with 35 g carbohydrate was ingested, whereas we gave 10 g protein together with 7 g carbohydrate. Furthermore, with ageing the stimulation of protein synthesis by resistance exercise has been shown to be preserved (Welle et al. 1994). However, as the dietary restrictions ended 2 h postexercise the possibility cannot be ruled out that if P2 regularly ingested a meal shortly after the supplement then the maximal effective dose of protein was exceeded, hence the stimulatory effect of the protein supplementation was blunted compared to P0. Yet, the dose-response relationship of ingested protein and protein synthesis remains to be elucidated. Consequently, we suggest that the contraction-induced stimulation of protein synthesis was used to a lesser extent in the formation of muscle protein in P2 compared to P0, provided that the stimulation of the protein synthesis follows a time course with a rapid increase within the first few hours following exercise (Phillips et al. 1997). Moreover, since the amino acid delivery is dependent on blood flow (Biolo et al. 1995), the intracellular amino acid availability in the exercised muscle may have been larger in P0 than in P2, hence favouring an increased anabolic response in P0 as it correlates with intracellular amino acid concentration (Biolo et al. 1995). Interestingly, Rasmussen and co-workers have found that protein synthesis and breakdown are stimulated similarly by protein intake in recovery from resistance exercise whether the protein is ingested 1 or 3 h after the termination of exercise, at least in young individuals when protein synthesis in the hour following intake is compared (Rasmussen et al. 2000). However, a 1 h measurement period may be too short to determine differences that affect muscle protein synthesis for many hours.
Altogether, our findings suggest that the first 2 h of recovery after resistance exercise are important for the net protein synthesis during a strength-training programme evaluated over a period of time, and to optimise the protein synthesis the intramuscular concentration of free amino acids is critical if it is not to be a limiting factor.
In the present study muscle fibre hypertrophy was more pronounced in P0 than in P2 despite identical rises in plasma insulin after the intake of a supplement containing protein and carbohydrate (Fig. 5). However, it may be speculated whether the insulin sensitivity of protein turnover is markedly higher immediately after exercise than 2 h later. This has, however, to our knowledge not been studied in humans. Yet, the importance of hyperinsulinaemia with respect to the present study can be questioned. First of all, the effect of postexercise hyperinsulinaemia has been shown to decrease mixed muscle protein degradation whereas synthesis is unaffected (Biolo et al. 1999) and, presumably, the effect is primarily on lysosomal degradation and not myofibrillar breakdown, which follows on the ubiquitin-proteasome pathway. Second, a recent study on postabsorptive exercise in diabetic/non-diabetic rats observed that insulin only played a permissive role at low concentrations in stimulating protein synthesis (Fedele et al. 2000). Thus, it was concluded that the effect of insulin on protein synthesis was only apparent in the low range of plasma insulin, whereas a further increase in insulin did not enhance net protein synthesis additionally (Fedele et al. 2000).
Finally, we cannot exclude the possibility that our finding of a difference in hypertrophy between the groups is a result of the relatively low number of subjects. However, no subjects were systematic outliers and, furthermore, all subjects in P0 had a larger increase in the relative change of the CSA of the quadriceps than any subject in P2. Additionally, theoretically it cannot be ruled out that exercise-induced changes in tissue and serum levels of anabolic hormones such as growth hormone, cortisol or insulin-like growth factor 1, which were not determined in the present study, could contribute to the difference between P0 and P2.
In conclusion, this study investigated the importance of the timing of protein intake after each exercise bout over 12 weeks of resistance training on morphological and strength characteristics of skeletal muscle in elderly individuals. Based on the findings in the present study it appears that the timing of protein intake after strength training bouts can be important for protein synthesis and hypertrophy of skeletal muscle in elderly individuals, and that this appears not to be related to the hyperinsulinaemia in response to the intake of a protein-carbohydrate supplement. The present findings support the hypothesis that early intake of protein after resistance exercise enhances total muscle mass as well as hypertrophy of single muscle fibres in elderly humans.
Acknowledgments
We thank Annie Høi and Vinnie Tagerup for analysis of blood samples. The study was supported by Otsuka Pharmaceutical (Japan), the Danish Sports Research Council, Team Danmark Research Foundation, the Danish Medical Research Council (9802636) and the Danish National Research Foundation (504-14).
References
- Aagaard P, Andersen J L, Dyhre-Poulsen P, Leffers A-M, Wagner A, Magnusson S P, Halkjær-Kristensen J, Simonsen E B. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. Journal of Physiology. 2001;534:613–623. doi: 10.1111/j.1469-7793.2001.t01-1-00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J L, Aagaard P. Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle and Nerve. 2000;23:1095–1104. doi: 10.1002/1097-4598(200007)23:7<1095::aid-mus13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Aniansson A, Grimby G, Hedberg M. Compensatory muscle fiber hypertrophy in elderly men. Journal of Applied Physiology. 1992;73:812–816. doi: 10.1152/jappl.1992.73.3.812. [DOI] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. American Journal of Physiology. 1999;276:C120–127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Bennet W M, Connacher A A, Scrimgeour C M, Smith K, Rennie M J. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1–13C]leucine. Clinical Science. 1989;76:447–454. doi: 10.1042/cs0760447. [DOI] [PubMed] [Google Scholar]
- Bergström J. Muscle electrolytes in man. Scandinavian Journal of Laboratory Investigation. 1962;14:511–513. [Google Scholar]
- Biolo G, Maggi S P, Williams B D, Tipton K D, Wolfe R R. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. American Journal of Physiology. 1995;268:E514–520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- Biolo G, Tipton K D, Klein S, Wolfe R R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. American Journal of Physiology. 1997;273:E122–129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- Biolo G, Williams B D, Fleming R Y, Wolfe R R. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes. 1999;48:949–957. doi: 10.2337/diabetes.48.5.949. [DOI] [PubMed] [Google Scholar]
- Brooke M H, Kaiser K K. Three “myosin adenosine triphosphatase” systems: the nature of their pH lability and sulfhydryl dependence. Journal of Histochemistry and Cytochemistry. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Brown A B, McCartney N, Sale D G. Positive adaptations to weight-lifting training in the elderly. Journal of Applied Physiology. 1990;69:1725–1733. doi: 10.1152/jappl.1990.69.5.1725. [DOI] [PubMed] [Google Scholar]
- Campbell W W, Crim M C, Dallal G E, Young V R, Evans W J. Increased protein requirements in elderly people: new data and retrospective reassessments. American Journal of Clinical Nutrition. 1994;60:501–509. doi: 10.1093/ajcn/60.4.501. [DOI] [PubMed] [Google Scholar]
- Charette S L, McEvoy L, Pyka G, Snow-Harter C, Guido D, Wiswell R A, Marcus R. Muscle hypertrophy response to resistance training in older women. Journal of Applied Physiology. 1991;70:1912–1916. doi: 10.1152/jappl.1991.70.5.1912. [DOI] [PubMed] [Google Scholar]
- Chesley A, MacDougall J D, Tarnopolsky M A, Atkinson S A, Smith K. Changes in human muscle protein synthesis after resistance exercise. Journal of Applied Physiology. 1992;73:1383–1388. doi: 10.1152/jappl.1992.73.4.1383. [DOI] [PubMed] [Google Scholar]
- Fedele M J, Hernandez J M, Lang C H, Vary T C, Kimball S R, Jefferson L S, Farrell P A. Severe diabetes prohibits elevations in muscle protein synthesis after acute resistance exercise in rats. Journal of Applied Physiology. 2000;88:102–108. doi: 10.1152/jappl.2000.88.1.102. [DOI] [PubMed] [Google Scholar]
- Fiatarone M A, Marks E C, Ryan N D, Meredith C N, Lipsitz L A, Evans W J. High-intensity strength training in nonagenarians. Effects on skeletal muscle. Journal of the American Medical Association. 1990;263:3029–3034. [PubMed] [Google Scholar]
- Fiatarone M A, O'Neill E F, Ryan N D, Clements K M, Solares G R, Nelson M E, Roberts S B, Kehayias J J, Lipsitz L A, Evans W J. Exercise training and nutritional supplementation for physical frailty in very elderly people. New England Journal of Medicine. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- Frontera W R, Hughes V A, Lutz K J, Evans W J. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. Journal of Applied Physiology. 1991;71:644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- Frontera W R, Meredith C N, O'Reilly K P, Knuttgen H G, Evans W J. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. Journal of Applied Physiology. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- Fry A C, Allemeier C A, Staron R S. Correlation between percentage fiber type area and myosin heavy chain content in human skeletal muscle. European Journal of Applied Physiology. 1994;68:246–251. doi: 10.1007/BF00376773. [DOI] [PubMed] [Google Scholar]
- Grimby G, Saltin B. The ageing muscle. Clinical Physiology. 1983;3:209–218. doi: 10.1111/j.1475-097x.1983.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Hakkinen K, Hakkinen A. Neuromuscular adaptations during intensive strength training in middle-aged and elderly males and females. Electromyography in Clinical Neurophysiology. 1995;35:137–147. [PubMed] [Google Scholar]
- Harridge S D, Kryger A, Stensgaard A. Knee extensor strength, activation, and size in very elderly people following strength training. Muscle and Nerve. 1999;22:831–839. doi: 10.1002/(sici)1097-4598(199907)22:7<831::aid-mus4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hasten D L, Pak-Loduca J, Obert K A, Yarasheski K E. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78-84 and 23-32 yr olds. American Journal of Physiology – Endocrinology and Metabolism. 2000;278:E620–626. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter D O. Excitability and inhibitability of motoneurons of different sizes. Journal of Neurophysiology. 1965;28:599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Ivy J L, Katz A L, Cutler C L, Sherman W M, Coyle E F. Muscle glycogen synthesis after exercise: effect of time of carbohydrate ingestion. Journal of Applied Physiology. 1988;64:1480–1485. doi: 10.1152/jappl.1988.64.4.1480. [DOI] [PubMed] [Google Scholar]
- Lexell J, Downham D Y. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathologica. 1991;81:377–381. doi: 10.1007/BF00293457. [DOI] [PubMed] [Google Scholar]
- Lexell J, Downham D Y, Larsson Y, Bruhn E, Morsing B. Heavy-resistance training in older Scandinavian men and women: short- and long-term effects on arm and leg muscles. Scandinavian Journal of Medicine and Science in Sports. 1995;5:329–341. doi: 10.1111/j.1600-0838.1995.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Lexell J, Henriksson-Larsen K, Sjostrom M. Distribution of different fibre types in human skeletal muscles. 2. A study of cross-sections of whole m. vastus lateralis. Acta Physiologica Scandinavica. 1983;117:115–122. doi: 10.1111/j.1748-1716.1983.tb07185.x. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor C C, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. Journal of the Neurological Sciences. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Meredith C N, Frontera W R, O'Reilly K P, Evans W J. Body composition in elderly men: effect of dietary modification during strength training. Journal of the American Geriatrics Society. 1992;40:155–162. doi: 10.1111/j.1532-5415.1992.tb01937.x. [DOI] [PubMed] [Google Scholar]
- Phillips S M, Tipton K D, Aarsland A, Wolf S E, Wolfe R R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. American Journal of Physiology. 1997;273:E99–107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Proctor D N, O'Brien P C, Atkinson E J, Nair K S. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. American Journal of Physiology. 1999;277:E489–495. doi: 10.1152/ajpendo.1999.277.3.E489. [DOI] [PubMed] [Google Scholar]
- Rasmussen B B, Tipton K D, Miller S L, Wolf S E, Wolfe R R. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. Journal of Applied Physiology. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- Rennie M J, Tipton K D. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annual Review of Nutrition. 2000;20:457–483. doi: 10.1146/annurev.nutr.20.1.457. [DOI] [PubMed] [Google Scholar]
- Roy B D, Tarnopolsky M A, MacDougall J D, Fowles J, Yarasheski K E. Effect of glucose supplement timing on protein metabolism after resistance training. Journal of Applied Physiology. 1997;82:1882–1888. doi: 10.1152/jappl.1997.82.6.1882. [DOI] [PubMed] [Google Scholar]
- Rutherford O M, Jones D A. The role of learning and coordination in strength training. European Journal of Applied Physiology. 1986;55:100–105. doi: 10.1007/BF00422902. [DOI] [PubMed] [Google Scholar]
- Smith K, Reynolds N, Downie S, Patel A, Rennie M J. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. American Journal of Physiology. 1998;275:E73–78. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Doi T, Lee S J, Okamura K, Shimizu S, Okano G, Sato Y, Shimomura Y, Fushiki T. Effect of meal timing after resistance exercise on hindlimb muscle mass and fat accumulation in trained rats. Journal of Nutritional Science and Vitaminology. 1999;45:401–409. doi: 10.3177/jnsv.45.401. [DOI] [PubMed] [Google Scholar]
- Tipton K D, Ferrando A A, Phillips S M, Doyle D J, Wolfe R R. Postexercise net protein synthesis in human muscle from orally administered amino acids. American Journal of Physiology. 1999;276:E628–634. doi: 10.1152/ajpendo.1999.276.4.E628. [DOI] [PubMed] [Google Scholar]
- Tipton K D, Ferrando A A, Williams B D, Wolfe R R. Muscle protein metabolism in female swimmers after a combination of resistance and endurance exercise. Journal of Applied Physiology. 1996;81:2034–2038. doi: 10.1152/jappl.1996.81.5.2034. [DOI] [PubMed] [Google Scholar]
- Vandervoort A A, McComas A J. Contractile changes in opposing muscles of the human ankle joint with aging. Journal of Applied Physiology. 1986;61:361–367. doi: 10.1152/jappl.1986.61.1.361. [DOI] [PubMed] [Google Scholar]
- Welle S, Bhatt K, Thornton C A. Stimulation of myofibrillar synthesis by exercise is mediated by more efficient translation of mRNA. Journal of Applied Physiology. 1999;86:1220–1225. doi: 10.1152/jappl.1999.86.4.1220. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C A. High-protein meals do not enhance myofibrillar synthesis after resistance exercise in 62- to 75-yr-old men and women. American Journal of Physiology. 1998;274:E677–683. doi: 10.1152/ajpendo.1998.274.4.E677. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Statt M, McHenry B. Postprandial myofibrillar and whole body protein synthesis in young and old human subjects. American Journal of Physiology. 1994;267:E599–604. doi: 10.1152/ajpendo.1994.267.4.E599. [DOI] [PubMed] [Google Scholar]
- Welle S, Totterman S, Thornton C. Effect of age on muscle hypertrophy induced by resistance training. Journals of Gerontology Series A - Biological Sciences and Medical Sciences. 1996;51:M270–275. doi: 10.1093/gerona/51a.6.m270. [DOI] [PubMed] [Google Scholar]
- Who/Fao/Unu. Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. Geneva: WHO; 1985. WHO Technical Report Series no. 724. [PubMed] [Google Scholar]
- Yarasheski K E, Zachwieja J J, Bier D M. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. American Journal of Physiology. 1993;265:E210–214. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]
- Young A, Stokes M, Crowe M. The size and strength of the quadriceps muscles of old and young men. Clinical Physiology. 1985;5:145–154. doi: 10.1111/j.1475-097x.1985.tb00590.x. [DOI] [PubMed] [Google Scholar]


