Abstract
A recombinant adenovirus was generated with the human ρ1 GABAC receptor subunit (adeno-ρ). Patch-clamp and antibody staining were employed to confirm functional expression of recombinant ρ1 receptors after infection of human embryonic kidney cells (HEK293 cell line), human embryonic retinal cells (911 cell line), dissociated rat hippocampal neurons and cultured rat hippocampal slices.
Standard whole-cell recording and Western blot analysis using ρ1 GABAC receptor antibodies revealed that recombinant ρ1 receptors were expressed in HEK293 and 911 cells after adeno-ρ infection and exhibited properties similar to those of ρ1 receptors after standard transfection.
Cultured rat hippocampal neurons (postnatal day (P)3-P5) did not show a native GABAC-like current. After adeno-ρ infection, however, a GABAC-like current appeared in 70-90 % of the neurons.
Five days after infection, expression of GABAC receptors in hippocampal neurons significantly decreased native GABAA receptor currents from 1200 ± 300 to 150 ± 70 pA (n = 10). The native glutamate-activated current was unchanged.
Hippocampal slices (P8) did not show a native GABAC-like current, although recombinant ρ1 receptors could be expressed in cultured hippocampal slices after adeno-ρ infection.
These data indicate that an adenovirus can be used to express recombinant GABAC receptors in hippocampal neurons. This finding could represent an important step towards the gene therapy of CNS receptor-related diseases.
The ligand-gated chloride channels including ρ GABAC and α, β, γ, ε, π, δ and θ GABAA receptors are key elements in the tonic and synaptic inhibitory signalling in the CNS (Cutting et al. 1991; MacDonald & Olsen, 1994; Wang et al. 1994). Unlike GABAC receptors, GABAA receptors are reversibly blocked by bicuculline and modulated by barbiturates and benzodiazepines (Polenzani et al. 1991; Shimada et al. 1992). GABAA receptors are widely distributed in the retina, spinal cord, hippocampus, cerebellum, superior colliculus, thalamus and other brain regions (Houser et al. 1988; Zimprich et al. 1991; Feigenspan & Bormann, 1994; MacDonald & Olsen, 1994). GABAC receptors are widely expressed in the retina, with lower levels in the brain and spinal cord (Strata & Cherubini, 1994; Zhang et al. 1995; Enz et al. 1996; Koulen et al. 1998; Lukasiewicz & Shields, 1998).
Numerous CNS diseases such as epilepsy, hepatic encephalopathy, spinocerebellar degeneration and dementia may be associated with a functional abnormality of GABAergic transmission (Cossart et al. 2001). A potential method to treat these abnormalities is the delivery of the DNA coding for functional GABA receptors into the disease-affected tissue. The human adenovirus (serotypes 2, 5) is a potentially powerful gene-delivery vehicle in that it satisfies the following stringent criteria: (i) high level of transduction, (ii) high insert capacity, (iii) wide variety of cell targets, (iv) amplification to very high titres, (v) non-oncogenic, and (vi) replication deficient (Douglas & Curiel, 1997; Krasnykh et al. 2000). The prime receptor for the human adenovirus (serotypes 2, 5) was shown to be similar to that for coxsackie B virus and has therefore been termed the coxsackie/adenovirus receptor (CAR) (Roelvink et al. 1998). Biochemical analysis of CAR revealed that it is a 46 kDa glycoprotein widely distributed in human fibroblasts, glia, and to a lesser extent in the differentiated respiratory epithelium, mature skeletal muscle and human lymphocytes (Zabner et al. 1997; Walters et al. 1999; Nalbantoglu et al. 1999; Hidaka et al. 1999). Less is known about CAR distribution in neuronal cells.
In this study we have used adenovirus serotype 5 to deliver DNA encoding the ρ1 GABAC receptor subunit into neuronal hippocampal cells. Recombinant adenovirus containing the ρ1 subunit (adeno-ρ) was produced under the human cytomegalovirus (CMV) promoter. Recombinant ρ1 GABAC receptors were expressed in 70-90 % of cultured hippocampal neurons after adeno-ρ infection. Patch-clamp analysis of GABA-activated current revealed that the ρ1 receptors had similar properties to ρ1 receptors expressed using standard transfection methods in non-neuronal cells. This finding could represent an important step towards the gene therapy of CNS receptor-related diseases.
METHODS
Molecular biology
The human ρ1 subunit and rat α1, β2 and γ2 subunits were obtained from cDNA libraries via the polymerase chain reaction as described previously (Amin et al. 1994; Amin & Weiss, 1994). The cDNA of the ρ1 subunit was excised using BamH I and Xba I restriction enzymes and inserted in the pShuttle CMV vector using Bgl II and Xba I ligation sites. Recombinant adenovirus containing ρ1 under the control of the human CMV promoter was produced using the QuantumAdEasy kit (Quantum Biotechnologies, Quebec, Canada) and has been termed adeno-ρ. Adeno-ρ was propagated in 109 HEK293 cells and was purified by centrifugation in a CsCl gradient according to Quantum protocols. The titre of infectious viral particles of adeno-ρ determined by plaque assay after large-scale purification was 2 × 1011 plaque-forming units (PFU) ml−1. Dialysed adeno-ρ was aliquoted and stored at -80 °C.
Transfection
HEK293 cells were transfected with ρ1 and/or α1, β2 and γ2 subunits in the pCDNA3 vector using Fugene 6 (Roche, Indianapolis, IN, USA) as described by the manufacturer. α1, β2 and γ2 were cotransfected at a cDNA ratio of 1:1:2 with a total of 4 μg of cDNA per 35 mm dish. For the case of the cotransfection of ρ1, α1, β2 and γ2, the cDNA ratio was 1:1:1:2 with a total of 5 μg of cDNA per 35 mm dish. In all cases, 1 μg of green fluorescent protein (GFP) was included for visualization of transfected cells.
Primary culture of hippocampal neurons and cell infection
For preparation of dissociated neurons, Sprague-Dawley rats at stage P3-P5 (Harlan, Indianapolis, IN, USA) were rapidly decapitated after cervical dislocation, and the hippocampi were removed from the brain and dissected free of meninges in cooled (6 °C), oxygenated, phosphate-buffered saline (PBS) containing Ca2+ and Mg2+. This procedure, as well as the procedure for obtaining hippocampal slices (described below), were carried out under the guidelines and approval of the UAB Institutional Animal Care and Use Committee. The hippocampi were then transferred into Ca2+, Mg2+-free PBS, cut into small pieces and incubated with 0.3 % (w/v) protease from Aspergillus oryzae (Type XXIII; Sigma, St Louis, MO, USA) and 0.1 % (w/v) DNase (Type I, Sigma) for 20 min at 25 °C. The tissue was washed and triturated. After a brief centrifugation, the cell pellet was resuspended in culture medium (minimal essential medium (MEM), Gibco BRL, Gaithersburg, MD, USA), supplemented with 10 % NU serum (Fischer Scientific, Pittsburgh, PA, USA), penicillin (5 U ml−1) and streptomycin (5 μg ml−1), and plated at a density of 8-10 (× 104) cells cm−2 on glass coverslips coated with poly-l-lysine. Cells were used after 10-14 days in culture.
Adeno-ρ was used at a concentration of 100 PFU cell−1 for the neuronal cultures, and in the range of 2 to 100 PFU cell−1 for the HEK293 and 911 cell lines. GABA-activated currents were recorded from 12 h to 5 days after infection.
Organotypic hippocampus slice culture and slice infection
Stage P7 Sprague-Dawley rats (Harlan) were cervically dislocated and rapidly decapitated. Hippocampal slices (200-400 μm thick) were prepared with a custom-designed wire slicer and maintained in vitro on Millicell-CM filter inserts (Millipore, Bedford, MA, USA) in a 36 °C, 5 % CO2, humidified (99 %) incubator (Stoppini et al. 1991). The concentration of horse serum (Gibco) in the culture medium was reduced from 20 to 10 % at 6 days in vitro. Over the next 2 days, serum was reduced to 5 % and then 0 %. The culture medium was completely exchanged every 3 days. The best infection of slices was observed in serum-free medium.
Electrophysiology
Experiments were performed at room temperature (20-24°C) using the whole-cell recording patch-clamp technique as previously described (Filippova et al. 1999). The holding potential was −50 mV. The external recording solution contained (mm): NaCl, 160; KCl, 3.5; glucose, 10; CaCl2, 2; and Hepes, 10 (pH 7.4). In some experiments TTX (1 μm), dl-2-amino-5-phosphonopentanoic acid (dl-APV, 10 μm), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μm) or bicuculline (30-50 μm) was added to the external bath solution to decrease spontaneous synaptic activity. The recording pipettes (borosilicate glass) had resistances of 3-5 MΩ when filled with internal solution containing (mm): CsCl, 150; CaCl2, 0.25; EGTA, 1.1 (free Ca2+, 5 × 10−8m); Hepes, 10; and Mg-ATP, 4 (pH 7.2). GABA, glycine and glutamate were applied to the cells through a double-barrelled perfusion system. In some experiments, bicuculline (30-50 μm) was added in the GABA-containing solution. In order to determine the GABAA and GABAC current amplitudes in native neurons infected with adeno-ρ, the amplitude of the GABAA current was calculated by subtraction of the GABAC current amplitude (activated by 20 μm GABA in the presence of 50 μm bicuculline) from the current activated by 300 μm GABA without bicuculline. In the case of the ρ1 and ρ1-α1β2γ2 coexpression studies in HEK293 cells, the amplitude of the GABAA current was calculated by subtraction of the GABAC current amplitude (activated by 10 μm GABA in the presence of 20 μm bicuculline) from the current activated by 200 μm GABA without bicuculline.
Dose-response relationships were fitted with the following Hill equation using a non-linear least-squares method:
where I is the peak current response at a given concentration of agonist (A), Imax is the maximum current response, EC50 is the concentration of agonist with half-maximal activation, and nH is the Hill coefficient. Data were compared statistically by Student's t test. Statistical significance was determined at the 5 % level. All results are presented as means ± s.e.m.
N-terminal GABAC receptor antibodies
N-terminal ρ1 GABAC receptor antibodies were raised against the GABAC receptor ρ1 subunit by synthesizing a fusion protein corresponding to the ρ1 N-terminal region (positions 14-191) with a 6 His tag on the N-terminus. The specific oligonucleotide primers used for the N-terminal fusion protein were as follows.
Forward primer (position 42-67): 5′-CCA CGC GGA TCC GGC CAC TGA A A G CAGAATGCACTGG-3′
Reverse primer (position 573-538): 5′-GAC TGA GCC CAA GCT TCT A C A TTGCAGTTACTGTAACCCTGAGACTATAGAGCAC-3′
The PCR product was subcloned, using BamH I and Hind III sites added to the primers, into the bacterial expression vector pQE-30 (Qiagen Inc., Valencia, CA, USA). The 6His fusion protein was expressed in Epicurian Coli BL21-Gold(DE3) pLysS cells (Stratagene, La Jolla, CA, USA) and was purified from urea-solubilized inclusion bodies by Ni-NTA chromatography (Qiagen) and then refolded. Mice were injected with the antigen and the serum was purified using an antigen-coupled, cyanogen bromide-activated column (Sigma). The mice were humanely killed at the end of the procedure, which was carried out under the guidelines and approval of the UAB Institutional Animal Care and Use Committee.
Gel electrophoresis and Western blot analysis
HEK293 cells expressing ρ1 were lysed in cell culture lysis reagent (Promega, Madison, WI, USA). The concentration of total cell protein was determined using a DC protein assay kit (Bio-Rad, Hercules, CA, USA) with bovine serum albumin as a standard. Total cell protein from HEK293 cells and bacterial fusion proteins were separated by SDS-PAGE, transferred to Hybond-P membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and detected using the ECL+Plus Western blotting detection system (Amersham Pharmacia Biotech). The following dilutions ofantibody were used: anti-N-terminal ρ1 GABAC receptor antibody, 1:500 for HEK293 cell lysates and 1:2000 for E. coli fusion proteins; secondary sheep anti-mouse Ig horseradish peroxidase-linked antibody (Amersham Life Sciences), 1:2000.
Immunocytochemistry and laser scanning confocal microscopy
Slices or primary hippocampal cells were fixed overnight in 4 and 2 % paraformaldehyde in PBS solution, respectively, rinsed in PBS, incubated in blocking solution (10 % horse serum, 2 % bovine serum albumen in PBS), and then incubated overnight at 4 °C in primary antibody (anti-N-terminal ρ1 GABAC receptor antibody, 1:200). Following washout of the primary antibody with PBS solution, slices or cells were incubated overnight with the secondary antibody (Texas Red-conjugated antibody, Amersham Life Sciences) and mounted. Imaging was performed with a laser scanning confocal microscope (LSCM; Olympus Fluoview, Mellville, NY, USA). In some cases, to aid cell visualization, the membrane-permeable red fluorescent dye Ro31-8222 (Roche Molecular Biochemicals) was added to the external solution. Appropriate controls lacking primary and secondary antibodies were performed, and background fluorescence was adjusted for each experiment.
Drugs
The following drugs were used for the experiments: bicuculline, GABA, glutamate, glycine (all from Sigma), CNQX, dl-APV5, 3-aminopropylphosphonic acid (3-APA), (2S)(+)-5,5-dimethyl-2-morpholineacetic acid (SCH 50911) and trans-4-aminocrotonic acid (TACA) (all from Tocris, Ballwin, MO, USA).
RESULTS
Characterization of recombinant ρ1 receptors in HEK293 cells after adeno-ρ infection
HEK293 cells were infected with adeno-ρ at a concentration of 2-10 PFU cell−1. To confirm expression of recombinant ρ1 receptors, N-terminal ρ1 GABAC receptor antibodies were used in a Western blot analysis performed on HEK293 cells. Figure 1A demonstrates that the N-terminal ρ1 antibody recognized a bacterially synthesized N-terminal fusion protein of the human ρ1 subunit at a concentration of 20 and 2 ng. N-terminal ρ1 GABAC receptor antibodies did not recognize any specific proteins from untransfected HEK293 cells (Fig. 1B, lane 1). However, we observed specific signals of the expected size for the ρ1 subunit of GABAC receptors in HEK293 cells 24 h after adeno-ρ infection at two different titres, 2 and 10 PFU cell−1 (Fig. 1B, lanes 2 and 3, respectively).
Figure 1. Properties of recombinant ρ1 receptors expressed in HEK293 cells after adeno-ρ infection.
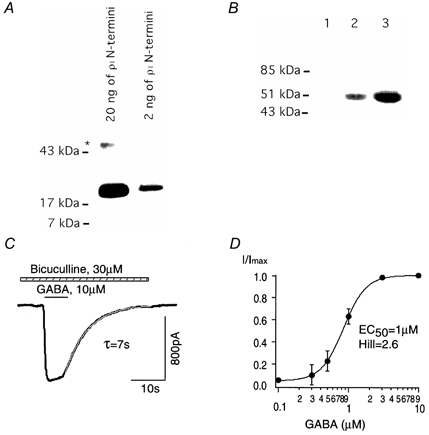
A, Western blot with N-terminal ρ1 GABAC receptor antibodies, which recognized a bacterially synthesized N-terminal fusion protein. The band indicated by the asterisk probably represents non-specific staining of an unidentified protein. B, Western blot from uninfected HEK293 cells (lane 1) and HEK293 cells infected with adeno-ρ (2 and 10 PFU cell−1, lanes 2 and 3, respectively). Note the specific band of the ρ1 subunit of the GABAC receptor (50 kDa) in lanes 2 and 3. C, whole-cell current evoked at a holding potential of −50 mV with GABA (10 μm) in the presence of bicuculline (30 μm) from HEK293 cells after adeno-ρ infection. Decay of the current upon GABA removal was well described by a single exponential component with a time constant (τ) of 7 s. D, mean dose-response relationship for GABA-activated current fitted with a Hill equation (n = 4).
Figure 1C illustrates current activated by GABA application (10 μm) in the presence of bicuculline (30 μm) from HEK293 cells 24 h after adeno-ρ infection. The GABA-evoked current showed no desensitization, had a linear current-voltage relationship, and was insensitive to bicuculline (not shown). The time constant of decay upon GABA removal (deactivation) was 8 ± 1 s (Fig. 1C, n = 6). The GABAC receptor antagonist 3-APA (300 μm) completely and reversibly blocked this current (n = 3). Figure 1D is the mean dose-response relationship best fitted with the Hill equation yielding an EC50 of 1 ± 0.3 μm, and a Hill coefficient of 2.6 ± 0.4 (n = 4). These properties of recombinant ρ1 GABAC receptors after adeno-ρ infection are indistinguishable from those of ρ1 receptors in HEK293 cells after standard transfection protocols (Filippova et al. 1999).
Pharmacological properties of current recorded from hippocampal neurons in culture before and after adeno-ρ infection
Before expressing ρ1 subunits, we first characterized the native ligand-activated current in uninfected neurons. Based on these studies, we divided uninfected neurons into three groups according to the properties of the ligand-activated current (Fig. 2Ai-iii). The first group (Fig. 2Ai) represented about 14 % (6/44 cells) of the cultured neurons tested and had GABAA receptors; that is, a GABA-activated current which was inhibited by bicuculline and had strong desensitization at high GABA concentrations (> 100 μm). The second group (Fig. 2Aii) represented 63 % (28/44 cells) of the neurons and had GABAA receptors similar to group i, but also exhibited a glutamate-activated current. Note that in both groups, GABA (10 μm) in the presence of bicuculline (30 μm) did not activate a current, confirming the absence of native GABAC-like receptors. The third group of cells represented 23 % of the population (10/44 cells). In addition to the GABAA and glutamate currents as in group ii, these neurons contained a glycine-activated current (Fig. 2Aiii). Moreover, GABA (10 μm) in the presence of bicuculline (30 μm) induced a current with an amplitude of 40-100 pA, a linear current-voltage relationship and fast deactivation (τ < 1 s; Fig. 2C). Unlike a GABAC receptor current, this current was not blocked by the GABAC competitive antagonist 3-APA (300 μm; Fig. 2B). In fact, we observed a slight potentiation of this current in the presence of 3-APA and the GABAB antagonist SCH 50911. A similar potentiation was observed with 3-APA alone (data not shown). Our results suggest that rat hippocampal neurons do not express a classic GABAC-like current, although we have not yet identified the receptors responsible for the bicuculline-insensitive, GABA-activated current in neurons from group iii.
Figure 2. Characteristics of ligand-activated current from hippocampal neurons in culture before and after adeno-ρ infection.
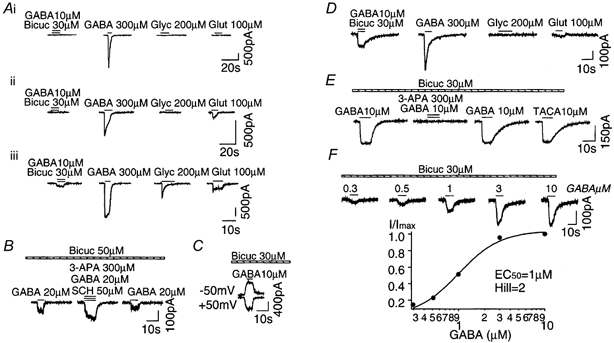
Ai-iii, examples of ligand-activated currents from uninfected neurons at a holding potential of −50 mV. Note that GABA (10 μm) in the presence of bicuculline (Bicuc; 30 μm) did not activate current from group i and ii neurons, but evoked a small current from group iii neurons. B, 3-APA (300 μm) did not block the bicuculline-insensitive current from neurons in group iii. SCH 50911 (SCH; 50 μm) was present to block GABAB receptors. C, the bicuculline-insensitive current from group iii neurons had a linear current-voltage relationship. D, examples of ligand-activated currents from neurons after adeno-ρ infection. Note that GABA (10 μm) in the presence of bicuculline (30 μm) evoked a GABAC-like current. E, 3-APA (300 μm) completely and reversibly blocked the bicuculline-insensitive current that appeared after adeno-ρ infection. F, dose-response relationship of bicuculline-insensitive current after adeno-ρ infection. Glyc, glycine; Glut, glutamate.
Two to three days after adeno-ρ infection, GABA (10 μm), in the presence of bicuculline (30 μm), induced a current with a linear current-voltage relationship, no desensitization and a slow deactivation rate (τ = 6 ± 2 s, n = 24) in all three types of cell. Figure 2D shows currents from a type ii neuron after infection. Figure 2E demonstrates that 3-APA (300 μm) completely and reversibly blocked the bicuculline-insensitive GABA-activated current (n = 8), and the GABA agonist TACA (10 μm) evoked a current with the same amplitude as GABA (10 μm) with or without bicuculline (30 μm). To estimate the dose-response relationship, we applied GABA at different concentrations in the presence of bicuculline (30 μm; Fig. 2F). The dose-response relationship was best fitted with a single Hill equation yielding an EC50 of 1 ± 0.4 μm, and a Hill coefficient of 2.2 ± 0.4 (n = 4). These results confirm that recombinant ρ1 receptors were expressed in hippocampal neurons after adeno-ρ infection, and had similar properties to ρ1 receptors after infection of HEK293 cells (Fig. 1C and D).
Level and time course of expression of recombinant ρ1 GABAC receptors
To estimate the percentage of cells that can be infected with adenovirus, we infected HEK293 cells, 911 cells, and hippocampal neurons with an adenovirus containing GFP (adeno-GFP). For cell visualization, the membrane-permeable red fluorescent dye Ro31-8222 was added to the external solution 5 min prior to examination. Figure 3A (left) shows a dissociated hippocampal neuron infected with adeno-GFP (top, green fluorescence) and treated with Ro31-8222 dye (middle, red fluorescence). The two images are merged in the bottom panel of Fig. 3A. Confocal scanning microscopy revealed that 70-90 % of the neurons in the hippocampal culture had green fluorescence and, thus, this percentage of cells could be potentially targeted by adenovirus (Fig. 3B). In the case of the HEK293 and 911 cell lines, all cells had green fluorescence and showed a cytopathic effect (Fig. 3C).
Figure 3. Level and time course of expression of ρ1 GABAC receptors in neurons and in HEK293 and 911 cell lines after adeno-ρ infection.
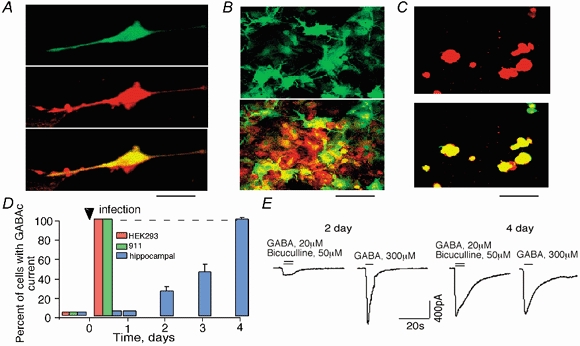
A, cultured neurons were infected with adeno-GFP and visualized with the red fluorescent dye Ro31-8222. Images of a single neuron using a confocal scanning microscope. The bottom image shows the merging of the green and red channels. B, same as in A but a group of neurons is shown. C, images of HEK293 cells after adeno-GFP infection and treatment with Ro31-8222. Note the 100 % co-localization of red and green fluorescence. Scale bars: 50 μm in A and C, 100 μm in B. D, percentage of cells expressing recombinant ρ1 receptors after adeno-ρ infection of HEK293 and 911 cell lines and hippocampal neurons over time. E, examples of GABA-activated currents on the second and fourth days after adeno-ρ infection of hippocampal cells. Note that on the fourth day, the GABAA current was greatly diminished. Only a GABAC-like current with the characteristic slow decay time was present.
To estimate the percentage of infectible neurons that express recombinant ρ1 GABAC receptors, we co-infected neuronal cells with both adeno-GFP and adeno-ρ. From 12 h to 4 days after infection, we analysed the current amplitude of recombinant ρ1 GABAC receptors activated by GABA (20 μm) in the presence of bicuculline (50 μm) and found that the amplitude of the GABAC current increased over this time course. Four days after infection, 100 % of the infected cells (about 90 % of all neurons) contained a GABAC current (Fig. 3D). In the HEK293 and 911 cell lines, 100 % of the cells expressed recombinant ρ1 GABAC receptors 12 h after infection (Fig. 3D).
Figure 3E presents examples of GABAC- and GABAA-activated currents in the hippocampal neurons 2 and 4 days after infection. The amplitude of the recombinant GABAC current on the second day of infection was 100-400 pA, which was 4- to 10-fold less than that of the typical GABAA current (1-2 nA). However, 4 days post-infection, the GABAC current amplitude reached 0.8-1.5 nA, while the GABAA current decreased to < 300 pA. The mean ratio between the GABAC and GABAA current amplitudes was 0.16 ± 0.05 (n = 10) and 8 ± 1 (n = 10) on the second and fourth days post-infection, respectively. We did not observe a significant change in the amplitude of the glutamate-activated current after ρ1 receptor expression (290 ± 70 and 210 ± 40 pA before and at 4 days post-adeno-ρ infection, respectively; n = 5).
We also observed a decrease in the expression level of α1β2γ2 GABAA receptors transfected into HEK293 cells following infection with adeno-ρ. Seventy-two hours after transfection and 12 h after adeno-ρ infection, the amplitude of the α1β2γ2 GABAA current was 335 ± 150 pA (n = 5) as compared to 1486 ± 600 pA (n = 5) without adeno-ρ infection. A qualitatively similar finding was observed when, rather than adeno-ρ infection, HEK293 cells were transfected with both ρ1 GABAC and α1β2γ2 GABAA receptors. In this case, the GABAA current was 100 ± 80 pA (n = 4) as compared to 1486 ± 600 pA (n = 5) with α1β2γ2 expression alone.
Recombinant ρ1 GABAC receptors expressed in cultured hippocampal slices after adeno-ρ infection
Cultured hippocampal slices were co-infected with both adeno-GFP and adeno-ρ. Three days after infection, slices were fixed and primary N-terminal ρ1 GABAC receptor and secondary Texas Red-conjugated antibodies antibodies were used for visualization of ρ1 GABAC receptors. Control non-infected slices did not exhibit specific antibody staining (not shown). However, we observed bright red fluorescence confirming the presence of ρ1 receptors on the cell surface 3 days after adeno-ρ infection (Fig. 4B). Moreover, we observed a strong correlation between green and red fluorescence (Fig. 4A-C) suggesting that adeno-GFP and adeno-ρ infected the same neurons.
Figure 4. Recombinant ρ1 receptors expressed in cultured hippocampal slices after adeno-ρ infection.
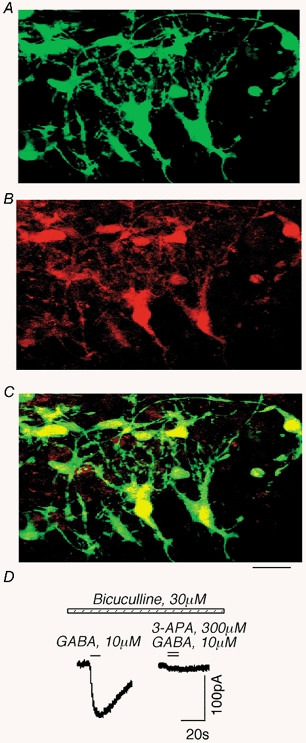
A-C, images of cultured hippocampal slices after co-infection with adeno-GFP and adeno-ρ using a confocal scanning microscope. A, GFP fluorescence. B, staining with N-terminal ρ1 GABAC receptor antibodies (red). C, co-localization of GFP and ρ1. Scale bar, 50 μm. D, example of a recombinant GABAC current in the slice after adeno-ρ infection. The current was blocked by 3-APA, an antagonist of GABAC receptors.
To confirm the functional expression of ρ1 receptors, we employed the patch-clamp technique in the whole-cell recording configuration. In uninfected slices, we did not observe a native GABAC-like current (5/5 cells). However, in adeno-ρ1-infected neurons, GABA (10 μm) in the presence of bicuculline (30 μm) induced a current with a linear current-voltage relationship, and slow decay time (τ > 10 s; 4 of 4 cells). As expected for GABAC receptors, the current was reversibly blocked by 3-APA (300 μm; n = 4; Fig. 4D). Thus, recombinant ρ1 receptors were expressed in hippocampal slices after adeno-ρ infection.
DISCUSSION
We have demonstrated that recombinant ρ1 GABAC receptors could be expressed in hippocampal neurons after adeno-ρ infection and these receptors exhibited properties similar to those of recombinant ρ1 GABAC receptors previously described in HEK293 cells (Filippova et al. 1999). Furthermore, the hippocampal neurons expressed ρ1 receptors regardless of their existing complement of ligand-activated receptors.
The distribution of ρ1 subunits has been identified by RT-PCR and in situ hybridization in the retina, superior colliculus, dorsal lateral geniculate nucleus and visual cortex (Boue-Grabot et al. 1998). In our experiments, we did not observe specific immunostaining of hippocampal neurons using N-terminal ρ1 GABAC receptor antibodies, suggesting either the absence, or very low levels, of native GABAC receptors in the hippocampus. Overall, based on patch-clamp experiments and immunostaining, we conclude that ρ1 GABAC receptors are not evident in the rat hippocampus at P4-P8. The presence of other subunit combinations (ρ2, ρ3) of GABAC receptors cannot be confirmed or eliminated. Considering the native bicuculline-insensitive current that was not blocked by 3-APA (Fig. 2Aiii), it is possible that GABAC receptor subunits interact with GABAA or glycine subunits, and form GABA-activated receptors with unexpected characteristics.
An interesting observation in our study was that expression of ρ1 GABAC receptors after adeno-ρ infection of hippocampal neurons diminished functional expression of GABAA receptors. Functional coassembly of the ρ1(T314A) GABAC subunit with the γ2 GABAA receptor subunit was recently confirmed (Pan et al. 2000). Moreover, the authors observed a dramatic decrease in the functional expression of GABAA and glycine receptors after co-expression with ρ1 GABAC receptors in Xenopus oocytes. It is possible that in native neurons, an interaction of recombinant ρ1 subunits with native γ2 GABAA subunits could replace GABAA receptors with ρ1-γ2 chimeric receptors. If this occurs, the interaction would not be evident functionally as the proposed wild-type ρ1-γ2 receptor has properties indistinguishable from those of ρ1 alone (Pan et al. 2000). Another possibility for the decrease in the GABAA receptor current after expression of GABAC receptors is that the GABAC receptors monopolize the translational machinery of the cells. This explanation seems unlikely, since the amplitude of the glutamate-activated current was unchanged after GABAC receptor expression.
During the review of our manuscript, a report appeared that also demonstrated expression of GABAC receptors by infection with an adenovirus containing the ρ1 subunit (Cheng et al. 2001) In addition to documenting functional expression, the authors demonstrated that expression of GABAC receptors eliminated neuronal hyperactivity and delayed the neuronal death induced by chronic blockade of glutamate receptors. One interesting difference between the two studies was that these authors noted an increased expression of GABAA receptors after infection with the ρ1 subunit as opposed to our observed decrease in GABAA expression. A possible explanation for this difference could be the choice of promoter. While we employed a CMV promoter, Cheng et al. (2001) used a promoter from Rous sarcoma virus (RSV). The promoter affects cell-type specificity, temporal patterns of expression and absolute expression levels (Smith et al. 2000). Comparison of β-galactosidase or GFP expression under the control of different viral promoters in hippocampal neurons demonstrated a high expression level in pyramidal neurons and low expression in granule cells with CMV with the opposite pattern with the RSV promoter (Smith et al. 2000). Furthermore, expression under the CMV promoter peaked rapidly and remained high, whereas the RSV promoter produced lower levels of β-galactosidase that began to decrease after several days in culture. These findings are confirmed with ρ1 as the highest level of expression Chen et al. (2001) could obtain with the RSV promoter without observing gross morphological abnormalities was an infection of 10-20 % of the cells, whereas we estimated ≈70-90 % of the cells expressed GABAC-like currents with the CMV promoter. The choice of the expression vector could be an important consideration in the control of the levels of GABAC receptors as well as the desired spatial and temporal expression pattern within the central nervous system.
The successful example of the employment of adenovirus in the treatment of cystic fibrosis as well as carcinomas of various organs, including the lung, bladder, ovary and liver, is already well documented (Wilson, 1995; Eck et al. 1996). The high infectional capabilities of adeno-ρ with respect to hippocampal neurons opens the way for gene therapy in the treatment of CNS-related diseases such as epilepsy. If the goal is to decrease neuronal excitability, the delivery of GABAC receptors composed of ρ1 subunits may be an optimal choice for several reasons. First, they have a higher sensitivity for GABA compared to GABAA receptors. Second, ρ1 receptors do not desensitize and they demonstrate a slow rate of deactivation upon agonist removal. Both of these factors could increase the efficiency of tonic inhibition of neurons in the presence of low concentrations of extracellular GABA. Finally, the structure-function relationship of ρ1 receptors has been well characterized allowing the use of custom-made ρ1 mutants with preferred properties.
Acknowledgments
The authors would like to thank the UAB High Resolution Imaging Facility for the use of the confocal microscope. The work was supported by NS40027.
Natalia Filippova and Anna Sedelnikova contributed equally to this work.
References
- Amin J, Dickerson I M, Weiss D S. The agonist binding site of the γ-aminobutyric acid type A channel is not formed by the extracellular cysteine loop. Molecular Pharmacology. 1994;45:317–323. [PubMed] [Google Scholar]
- Amin J, Weiss D S. Homomeric rho 1 GABA channels: activation properties and domains. Receptors and Channels. 1994;2:227–236. [PubMed] [Google Scholar]
- Boue-Grabot E, Roudbaraki M, Bascles L, Tramu G, Bloch B, Garret M. Expression of GABA receptor rho subunits in rat brain. Journal of Neurochemistry. 1998;70:899–907. doi: 10.1046/j.1471-4159.1998.70030899.x. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Kulli J C, Yang J. Suppression of neuronal hyperexcitability and associated delayed neuronal death by adenoviral expression of GABAC receptors. Journal of Neuroscience. 2001;21:3419–3428. doi: 10.1523/JNEUROSCI.21-10-03419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch J, Merchan-Perez A, Felipe J, Esclapez M, Bernard C, Ben-Ari Y. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nature Neuroscience. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- Cutting G R, Lu L, O'Hara B F, Kasch L M, Montrose-Rafizadeh C, Donovan D M, Shimada S, Antonarakis S E, Guggino W B, Uhl G R. Cloning of the gamma-aminobutyric acid (GABA) rho 1 cDNA: a GABA receptor subunit highly expressed in the retina. Proceedings of the National Academy of Sciences of the USA. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J T, Curiel D T. Adenoviruses as vectors for gene therapy. Science and Medicine. 1997;4:44–53. [Google Scholar]
- Eck S, Alavi J, Alavi A, Davis A, Hackney D, Judy K, Mollman J, Phillips P, Wheeldon E, Wilson J. Treatment of advanced CNS malignancies with the recombinant adenovirus H5 101RSVTK: a phase I trial. Human Gene Therapy. 1996;7:1465–1482. doi: 10.1089/hum.1996.7.12-1465. [DOI] [PubMed] [Google Scholar]
- Enz R, Brandstatter J H, Wassle H, Bormann J. Immunocytochemical localization of the GABAC receptor rho subunits in the mammalian retina. Journal of Neuroscience. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Differential pharmacology of GABAA and GABAC receptors on rat retinal bipolar cells. European Journal of Pharmacology. 1994;288:97–104. doi: 10.1016/0922-4106(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Filippova N, Dudley R, Weiss D S. Evidence for phosphorylation-dependent internalization of recombinant human ρ1 GABAC receptors. Journal of Physiology. 1999;518:385–399. doi: 10.1111/j.1469-7793.1999.0385p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka C, Milano E, Leopold P L, Bergelson J M, Hackett N R, Finberg R W, Wickham T J, Kovesdi I, Roelvink P, Crystal R G. CAR-dependent and CAR-independent pathways of adenovirus vector-mediated gene transfer and expression in human fibroblasts. Journal of Clinical Investigation. 1999;103:579–587. doi: 10.1172/JCI5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser C R, Olsen R W, Richards J G, Mohler H. Immunohistochemical localization of benzodiazepine/GABAA receptors in the human hippocampal formation. Journal of Neuroscience. 1988;8:1370–1383. doi: 10.1523/JNEUROSCI.08-04-01370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P, Brandstatter J H, Enz R, Bormann J, Wassle H. Synaptic clustering of GABA(C) receptor rho-subunits in the retina. European Journal of Neuroscience. 1998;10:115–127. doi: 10.1046/j.1460-9568.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- Krasnykh N V, Douglas J T, Beusechem V W. Genetic targeting of adenoviral vectors. Molecular Therapy. 2000;1:391–405. doi: 10.1006/mthe.2000.0062. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz P D, Shields C R. Different combinations of GABAA and GABAC receptors confer distinct temporal properties to retinal synaptic responses. Journal of Neurophysiology. 1998;79:3157–3167. doi: 10.1152/jn.1998.79.6.3157. [DOI] [PubMed] [Google Scholar]
- MacDonald R L, Olsen R W. GABAA receptor channels. Annual Review of Neuroscience. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu J, Pari G, Karpati G, Holland P C. Expression of the primary coxsakie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Human Gene Therapy. 1999;10:1009–1019. doi: 10.1089/10430349950018409. [DOI] [PubMed] [Google Scholar]
- Pan Z, Zhang D, Zhang X, Lipton S. Evidence for coassembly of mutant GABACρ1 with GABAAγ2S, glycine α1 and glycine α2 receptor subunits in vitro. European Journal of Neuroscience. 2000;12:3137–3145. doi: 10.1046/j.1460-9568.2000.00198.x. [DOI] [PubMed] [Google Scholar]
- Polenzani L, Woodward R, Miledi R. Expression of mammalian γ-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proceedings of the National Academy of Sciences of the USA. 1991;88:4318–4322. doi: 10.1073/pnas.88.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelvink P W, Lizonova A, Lee J G, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. The coxsackievirus-adenovirus receptor protein can function as cellular attachment protein for adenovirus serotypes from subgroup A, C, D, E, and F. Journal of Virology. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Cutting G, Uhl G R. γ-Aminobutyric acid A or C receptor?γ-Aminobutyric acid rho 1 receptor RNA induces bicuculline-, barbiturate-, and benzodiazepine-insensitive γ-aminobutyric acid responses in Xenopus oocytes. Molecular Pharmacology. 1992;41:683–687. [PubMed] [Google Scholar]
- Smith R L, Traul D L, Schaack J, Clayton G H, Staley K J, Wilcox C L. Characterization of promoter function and cell-type-specific expression from viral vectors in the nervous system. Journal of Virology. 2000;74:11254–11261. doi: 10.1128/jvi.74.23.11254-11261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strata F, Cherubini E. Transient expression of a novel type of GABA response in rat CA3 hippocampal neurones during development. Journal of Physiology. 1994;480:493–503. doi: 10.1113/jphysiol.1994.sp020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs L-A, Muller D. A simple method for organotypic cultures of nervous tissue. Journal of Neuroscience Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. Journal of Biological Chemistry. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- Wang T L, Guggino W B, Cutting G R. A novel gamma-aminobutyric acid receptor subunit (rho 2) cloned from human retina forms bicuculline-insensitive homooligomeric receptors in Xenopus oocytes. Journal of Neuroscience. 1994;14:6524–6531. doi: 10.1523/JNEUROSCI.14-11-06524.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. Gene therapy for cystic fibrosis: Challenges and future directions. Journal of Clinical Investigation. 1995;96:2547–2554. doi: 10.1172/JCI118318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. Journal of Clinical Investigation. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Pan Z-H, Zhang X, Brideau A D, Lipton S A. Cloning of a γ-aminobuturic acid type C receptor subunit in rat retina with a methionine residue critical for picrotoxinin channel block. Proceedings of the National Academy of Sciences of the USA. 1995;92:11756–11760. doi: 10.1073/pnas.92.25.11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich F, Zezula J, Sieghart W, Lassmann H. Immunohistochemical localization of the α1-, α2- and α3-subunit of the GABAA receptor in the rat brain. Neuroscience Letters. 1991;127:125–128. doi: 10.1016/0304-3940(91)90910-l. [DOI] [PubMed] [Google Scholar]


