Abstract
The present study tested the hypothesis that motion sickness affects thermoregulatory responses to cooling in humans.
Ten healthy male volunteers underwent three separate head-out immersions in 28 °C water after different preparatory procedures. In the ‘control’ procedure immersion was preceded by a rest period. In the ‘motion sickness’ procedure immersion was preceded by provocation of motion sickness in a human centrifuge. This comprised rapid and repeated alterations of the gravitational (G-) stress in the head-to-foot direction, plus a standardized regimen of head movements at increased G-stress. In the ‘G-control’ procedure, the subjects were exposed to similar G-stress, but without the motion sickness provocation.
During immersion mean skin temperature, rectal temperature, the difference in temperature between the forearm and 3rd digit of the right hand (ΔTforearm-fingertip), oxygen uptake and heart rate were recorded. Subjects provided ratings of temperature perception, thermal comfort and level of motion sickness discomfort at regular intervals.
No differences were observed in any of the variables between control and G-control procedures. In the motion sickness procedure, the ΔTforearm-fingertip response was significantly attenuated, indicating a blunted vasoconstrictor response, and rectal temperature decreased at a faster rate. No other differences were observed.
Motion sickness attenuates the vasoconstrictor response to skin and core cooling, thereby enhancing heat loss and the magnitude of the fall in deep body temperature. Motion sickness may predispose individuals to hypothermia, and have significant implications for survival time in maritime accidents.
Hypothermia is a recognized hazard to the survival of victims in maritime accidents. Anecdotal observations have attributed the apparent predisposition of seasick individuals to hypothermia to their inactivity, and to vasomotor changes associated with motion sickness (Golden, 1973).
It is difficult to reconcile the suggestion of a possibly greater heat loss in motion sick individuals with the initial signs of motion sickness, namely facial pallor and cold sweating. Whilst the sweating response, which under these circumstances is not initiated for thermoregulatory purposes, enhances heat loss from the skin and may result in core cooling, facial pallor would suggest vasoconstriction, which if generalized would reduce heat loss in motion sick individuals.
The reports of a fall in body, skin and mouth temperature (reviewed by Money, 1970) in motion sick subjects, would suggest that the thermoregulatory responses associated with motion sickness favour heat loss. This hypothesis may be tenable, if (a) the often reported facial pallor does not reflect an overall peripheral vasoconstriction, but only a vasomotor change in the facial region, (b) the motion sickness-induced sweating response is sufficient to initiate cooling of deep body temperature, or (c) there is a decrease in metabolic heat production due to either an inhibition of thermoregulatory shivering, or just simply due to the reduced volitional activity of motion sick victims.
The present study tested the hypotheses that (a) the effects of motion sickness persist following the removal of the stimulus inducing motion sickness and (b) motion sickness affects thermoregulation, and consequently predisposes survivors of maritime accidents to hypothermia. We investigated the shivering and vasomotor responses of subjects immersed in tepid (28 °C) water. This temperature was used to simulate the conditions in which motion sickness may have the greatest impact on survivability. That is, where thermal balance is achievable, and cold stress is not so great as to result in drowning or hypothermia in a short period of time. For example, appropriately clothed survivors in a life raft, floating in 0-2 °C water with air temperatures ranging from -5 to 4 °C, maintain skin temperature at about 28 °C for over 20 h (Veghte, 1972).
METHODS
Subjects
Subjects were informed of the risks associated with the experimental protocol and gave their informed consent prior to participating in the study. The study was performed according to the Declaration of Helsinki and the procedures were approved by the Karolinska Institutet Ethics Committee. Ten healthy male subjects participated in the study. Their mean age was 32.5 years (range, 22-45 years), mean height 1.76 m (range, 1.70-1.86 m) and mean weight 75.2 kg (range, 66-92 kg).
Protocol
Subjects participated in three experimental procedures separated by at least 72 h. The order in which the procedures were conducted was alternated among subjects. For an individual subject, all procedures were performed at the same time of the day. In each procedure, the swim-suited subjects were immersed to the manubrium in 28 °C water for 80 min. In the control procedure the immersion was preceded by a 10 min rest period. In the motion sickness procedure subjects were rendered motion sick prior to immersion by exposing them to intermittent rapid changes in gravitational (G) stress in the head-to-foot direction (+Gz), in combination with a regimen of standardized head movements in a human centrifuge. To control for any confounding G-induced effects (i.e. not derived from motion sickness), subjects undertook a third procedure (G-control) with the head stable during centrifugation. Thus, they were not rendered motion sick in this condition. The three pre-immersion procedures are detailed below.
Motion sickness
Following brief familiarization, subjects were instrumented with a 5-lead ECG and were seated in the gondola of a human centrifuge (Asea, Sweden). The centrifugation commenced with a 2 min accommodation period at idle speed (+1.4 Gz). Thereafter, the subjects were subjected to a series of 20 s exposures at +2.5 Gz, whereby the gondola was accelerated from +1.4 to +2.5 Gz at a rate of 3 G s−1. During the 20 s period at +2.5 Gz, subjects turned their head slowly to the right and left, and looked up and down when instructed by the experimenters. Once the procedure was completed they were decelerated at 3 G s−1 from +2.5 to +1.4 Gz and remained at +1.4 Gz for 20 s prior to the next manoeuvre. This procedure was repeated for a maximum of 20 manoeuvres. Following each manoeuvre the subjects provided a rating of their motion sickness on a 5-point scale. The motion sickness provocation was terminated once a rating of 3 (very nauseous) was reported by the subjects. During the provocation, the subject was monitored via closed circuit television and ECG.
G-control
Following a 2 min accommodation period at +1.4 Gz, subjects were accelerated to +2.5 Gz at a rate of 0.05 G s−1 and remained at this level for 5 min. Thereafter they were decelerated to +1.4 Gz at a rate of 0.05 G s−1. After 5 min at +1.4 Gz the centrifugation was terminated. During the centrifugation phase of the G-control procedure, subjects were instructed not to move their heads.
Control
In the control procedure subjects were not exposed to elevated G-stress prior to immersion.
The immersion procedure and instrumentation during the immersion in 28 °C water was identical in all three procedures. Prior to immersion, subjects rested supine in 28 °C air for 10 min and resting values were recorded during the last 5 min. During this period, evaporative sweating rate from the forehead (Esw, expressed in g m−2 min−1) was measured using a ventilated capsule. Flow meters at the inlet and outlet of the capsule (Perflow Instruments Ltd, London, UK) ensured a constant flow of air of 1 l min−1, and that no air leaked from the capsule. The temperature and relative humidity of the air entering and exiting the capsule was measured with thermistors and resistance hygrometers, respectively (model Smart Reader 2, temperature and relative humidity logger, ACR Systems Inc., Canada). Evaporative sweating rate was estimated from the difference in water vapour content of the outflowing and inflowing air, adjusting for the skin surface covered by the capsule (467 mm2).
Following the rest period subjects stepped into the immersion tank and sat down. During the immersion, rectal temperature (Tre) was measured with a rectal thermistor (Yellow Springs Instruments (YSI), Yellow Springs, USA) placed in a protective sheath and inserted 10 cm beyond the anal sphincter. Mean skin temperature (Tsk) was derived from the unweighted average of skin temperature recorded with skin thermistors (YSI Model 409AC) at the chest, thigh and calf. During the immersion, the subject's right hand rested on a ledge positioned slightly above the water's surface.
The difference in the skin temperature between the forearm and the tip of the third finger (ΔTforearm-fingertip) of the non-immersed arm was measured; this is recognized as a measure of peripheral vasomotor tone (Sessler et al. 1988). During the immersions subjects breathed through a low resistance breathing valve (Hans Rudolf, MO, USA) via an oro-nasal mask. Inspiratory minute volume (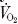 ) was measured with a turbine ventilation module (KL Engineering, USA). Expired air was directed via respiratory hosing to an 8 l Plexiglas mixing box. A sample of the expired air was drawn continuously from the mixing box at a rate of 0.2 l min−1 and analysed for oxygen (Applied Electrochemistry model S-3A/I oxygen analyser, Pittsburgh, PA, USA) and carbon dioxide (Beckman model LB-2, carbon dioxide analyser, Fullerton, CA, USA) contents. All temperature and cardiorespiratory measurements were recorded at 1 min intervals with a data acquisition system (Biopac Systems Inc., Santa Barbara, CA, USA) connected to a computer (Apple, Cupertino, USA). On-line analysis was provided by AcqKnowledge software (Biopac Systems Inc.). Oxygen uptake (
) was measured with a turbine ventilation module (KL Engineering, USA). Expired air was directed via respiratory hosing to an 8 l Plexiglas mixing box. A sample of the expired air was drawn continuously from the mixing box at a rate of 0.2 l min−1 and analysed for oxygen (Applied Electrochemistry model S-3A/I oxygen analyser, Pittsburgh, PA, USA) and carbon dioxide (Beckman model LB-2, carbon dioxide analyser, Fullerton, CA, USA) contents. All temperature and cardiorespiratory measurements were recorded at 1 min intervals with a data acquisition system (Biopac Systems Inc., Santa Barbara, CA, USA) connected to a computer (Apple, Cupertino, USA). On-line analysis was provided by AcqKnowledge software (Biopac Systems Inc.). Oxygen uptake (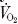 expressed in l min−1) were calculated at 1 min intervals during the rest and immersion periods. Electrocardiogram was continuously monitored using pre-gelled electrodes placed in the precordial 5-lead arrangement and the heart rate (HR) recorded.
expressed in l min−1) were calculated at 1 min intervals during the rest and immersion periods. Electrocardiogram was continuously monitored using pre-gelled electrodes placed in the precordial 5-lead arrangement and the heart rate (HR) recorded.
At 5 min intervals, subjects provided subjective ratings of their temperature perception (7 point scale: 1, cold; 2, cool; 3, slightly cool; 4, neutral; 5, slightly warm; 6, warm; 7, hot), thermal comfort (4 point scale: 1, comfortable; 2, slightly uncomfortable; 3, uncomfortable; 4, very uncomfortable) and motion sickness (5 point scale: 0, no discomfort; 1, slight discomfort/mild nausea; 2, discomfort/nausea; 3, very nauseous/almost vomiting; 4, extremely nauseous/vomiting).
Plasma glucose levels were assessed from capillary blood samples with a blood glucose analyser (Model One Touch Profile, Life Scan, Malmö, Sweden) before and after centrifugation, to account for any G-induced alterations in blood glucose (Daligcon & Oyama, 1985). To ensure that the observed thermoregulatory responses were not affected by changes in blood glucose levels (Passias et al. 1996), the latter were also assessed before and after immersion.
Thermoregulatory and cardiorespiratory data during the immersion phase were analysed with a repeated measures ANOVA, with the procedure (control, G-control, and motion sickness) as the main factor. The Tukey-Kramer post hoc analysis was used to compare the means of the responses between procedures. The Kruskall-Wallis non-parametric test was used to analyse the subjective ratings of temperature, thermal comfort and motion sickness. The 5 % level (P < 0.05) was chosen as statistically significant.
RESULTS
The motion sickness provocation in the centrifuge induced substantial nausea in eight subjects, two subjects felt no or minimal discomfort following the provocation. The median motion sickness score was 3 (very nauseous/almost vomiting; range, 0.5-3) following the motion sickness provocation. The control centrifugation (G-control) induced a slight feeling of discomfort (0.5) in only one subject, all other subjects reported a motion sickness rating of zero. The subjects commonly reported a rapid decline of the nausea upon termination of the provocation. The median subjective motion sickness rating decreased from 3 at the end of the provocation to 1 just prior to immersion, approximately 15 min after the provocation. The sensation of motion sickness continued to subside over the duration of the immersion.
The mean ±s.e.m. pre-immersion forehead sweating rate (Esw) was 0.8 ± 0.2 in control and 1.3 ± 0.2 g m−2 min−1 in G-control. The difference in Esw between these two conditions was not significant. In contrast, the mean Esw following motion sickness provocation (2.6 ± 0.5 g m−2 min−1) was significantly higher (P < 0.01) than the Esw observed in control and G-control. Subjects who experienced nausea during the motion sickness provocation were sweating profusely upon completion of the provocation. Sweating did not appear to be limited to any particular area of the body.
Upon immersion in the 28 °C water bath mean skin temperature (Tsk) in control decreased rapidly from a pre-immersion value of 33.5 ± 0.1 °C to an asymptotic value of 28.3 ± 0.1 °C. The Tsk response was identical for all three conditions (Fig. 1A). The decrease in rectal temperature (Tre) during immersion was similar in control and G-control (0.7 ± 0.1 °C, Fig. 1B), but was 29 % greater (P < 0.001) in motion sickness (0.9 ± 0.1 °C).
Figure 1.
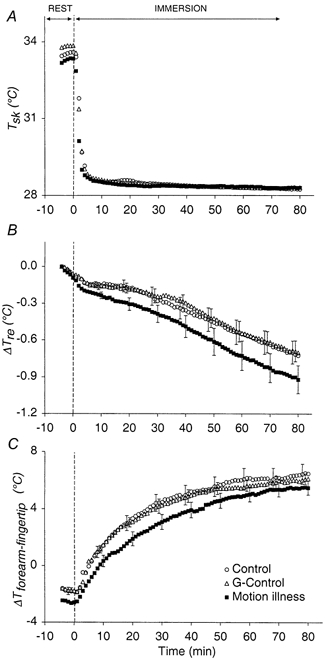
The mean ±s.e.m. responses of average unweighted skin temperature (Tsk) (A), change in rectal temperature relative to pre-immersion values (ΔTre) (B) and the difference in the temperature between the forearm and fingertip (ΔTforearm-fingertip) (C) - an indication of peripheral vasomotor tone, during rest (from minute -10 to 0) and immersion (from minute 1 to 80) in the three experimental procedures (N = 10). For clarity, the standard error bars are plotted for each procedure at 10 min intervals, commencing with minute -2 for control, minute -1 for G-control and minute 0 for motion sickness.
Figure 1C depicts the vasomotor response during the immersion (ΔTforearm-fingertip). The negative values prior to immersion indicate that the cutaneous vasculature was vasodilated. Upon immersion, there was a non-linear increase in ΔTforearm-fingertip indicating a progressive vasoconstriction of the peripheral vessels. There was no difference in the ΔTforearm-fingertip response in control and G-control. However, the ΔTforearm-fingertip response was significantly lower (P < 0.001) in motion sickness.
In all conditions, HR, 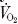 exhibited similar instantaneous increases upon immersion, followed by a slow return to control values around minute 20 (Fig. 2). Thereafter
exhibited similar instantaneous increases upon immersion, followed by a slow return to control values around minute 20 (Fig. 2). Thereafter 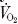 gradually increased during the remainder of the immersion. There was no difference in the resting and immersion responses of HR,
gradually increased during the remainder of the immersion. There was no difference in the resting and immersion responses of HR, 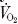 between the three conditions. Since the shivering response is reflected in the
between the three conditions. Since the shivering response is reflected in the 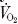 observed in the three experimental conditions confirms that the shivering response, and thus the endogenous heat produced by the shivering muscles, was similar in all three experimental conditions.
observed in the three experimental conditions confirms that the shivering response, and thus the endogenous heat produced by the shivering muscles, was similar in all three experimental conditions.
Figure 2.
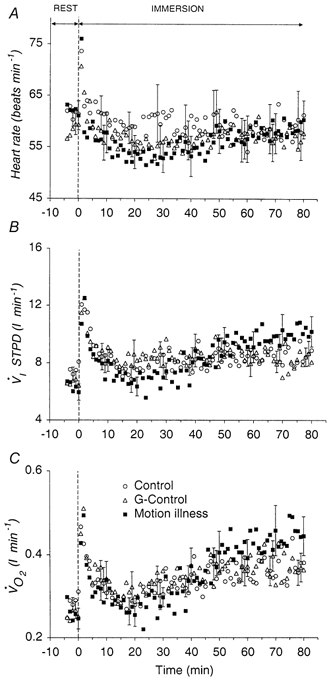
Mean ±s.e.m. responses of heart rate (HR) (A), minute ventilation 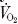 ) (C) during rest (minute 0) and immersion (minutes 1-80) in the three experimental procedures (N = 10). For clarity, the standard error bars are plotted for each procedure at 10 min intervals, commencing with minute -2 for control, minute -1 for G-control and minute 0 for motion sickness.
) (C) during rest (minute 0) and immersion (minutes 1-80) in the three experimental procedures (N = 10). For clarity, the standard error bars are plotted for each procedure at 10 min intervals, commencing with minute -2 for control, minute -1 for G-control and minute 0 for motion sickness.
All subjects were euglycaemic throughout the three experimental procedures (plasma glucose levels were in the range 3.9-4.5 mmol l−1).
Ratings of temperature perception and thermal comfort were similar in the three conditions. During the 5 min pre-immersion rest period, subjects felt neutral and comfortable. During the immersion, and with decreasing Tre, subjects reported being slightly cool and slightly thermally uncomfortable.
DISCUSSION
The present study demonstrates that motion sickness, which upon immersion was rated as only mild, significantly attenuated the cold-induced vasoconstrictor response, as reflected by the lower ΔTforearm-fingertip at any given ΔTre (Fig. 3). The greater fall in Tre during the motion sickness immersion was not due to a reduction in heat production; the oxygen uptake, indicative of the shivering response, was similar in all three procedures. The greater magnitude of cooling during motion sickness procedure was most probably due to the resultant greater heat loss from the skin surface. Since there were no differences in the ΔTforearm-fingertip and Tre responses between control and G-control, it may be concluded that the responses observed in motion sickness were not due to any confounding factors associated with the G-stress, and they are attributable solely to the provoked motion sickness. Thus, in certain conditions, the presence of motion sickness could increase the likelihood of the occurrence of hypothermia or its severity.
Figure 3.
ΔTforearm-fingertip response (N = 10) during the immersion phase in the three procedures plotted as a function of change in rectal temperature relative to pre-immersion values.
The relative contributions of decreasing core and skin temperature are evident in Fig. 3. In control and G-control, the increase in ΔTforearm-fingertip, which reflects vasoconstriction, in response to a small change in Tre from pre-immersion values is largely due to the sudden drop in Tsk upon immersion. Thereafter the gradual increase in ΔTforearm-fingertip is mediated mainly by the continued decline in Tre, as Tsk is held constant (Fig. 1). In contrast, the initial primarily skin-mediated ΔTforearm-fingertip response is attenuated in motion sickness, as is the latter core cooling-mediated increase in ΔTforearm-fingertip.
Facial pallor is a common sign in motion sickness and has previously been regarded as evidence of peripheral vasoconstriction in response to increased sympathetic activity (Money, 1970). However, facial pallor may not be indicative of generalized vasoconstriction since it has been shown that motion sickness increases skin blood flow in the forehead, but reduces skin blood flow in the fingertip (Kolev et al. 1997). Furthermore, Cui et al. (1999) did not observe a motion sickness-induced reduction in skin blood flow in the dorsum of the foot, despite a concomitant increase in skin sympathetic activity. Though there appears to be a regional disparity in the complex peripheral vascular response to motion sickness, the present study clearly demonstrates a blunted cold-induced vasoconstrictor response.
It cannot be excluded that the profuse sweating observed in the subjects following the motion sickness provocation is indicative of active vasodilatation. There is mounting evidence of a neural and/or humoral coupling between sweating and active vasodilatation emanating from central thermoregulatory structures (Johnson & Proppe, 1996; Sugenoya et al. 1998). Regardless of the mechanism involved, it should not be surprising that reduced vascular resistance accompanies profuse sweating, as seen in the present study.
The sweating measured during the G-control and motion sickness procedures, following exposure to the centrifuge will, in part, have been due to the physical effort associated with these exposures. However, the greater levels of sweating observed in the motion sickness procedure may be attributed solely to motion sickness. The observation that this sweating was not confined to the regions of the apocrine sweat glands is new. Since the subjects in the present study were immersed to the manubrium in water, evaporative skin cooling could not occur over most of the body. However, in an air environment, the combination of sweating and reduced cold-induced vasoconstriction may enhance cooling and, as a consequence, the differences between the Tre responses of motion sick and non-motion sick subjects may be more pronounced.
Motion sickness was not induced during the immersion in the present study, but provoked during the 20 min pre-immersion centrifugation. Despite the substantial and rapid decrease in motion sickness on cessation of centrifugation, the provocation was sufficient to have physiological consequences during the immersion phase. It is possible that the observed attenuation of the vasoconstrictor response, and its consequences for deep temperature, would have been greater had the motion sickness provocation been maintained during the immersion. Such circumstances are encountered in survival scenarios at sea.
Acknowledgments
This study was supported by the Swedish Defence Material Administration. The authors are indebted to Dr Frank St. C. Golden for valuable discussions.
References
- Cui J, Iwase S, Mano T, Kitazawa H. Responses of sympathetic outflow to skin during caloric stimulation in humans. American Journal of Physiology. 1999;276:R738–744. doi: 10.1152/ajpregu.1999.276.3.R738. [DOI] [PubMed] [Google Scholar]
- Daligcon BC, Oyama J. Hyper-G stress-induced hyperglycemia in rats mediated by glucoregulatory hormones. Aviation, Space, and Environmental Medicine. 1985;56:37–42. [PubMed] [Google Scholar]
- Golden F, St C. Death after rescue from immersion in cold water. Journal of the Royal Naval Medical Service. 1973;59:5–8. [PubMed] [Google Scholar]
- Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. Handbook of Physiology. In: Fregly MJ, Blatteis CM, editors. Environmental Physiology. New York: Oxford University Press; 1996. pp. 215–243. section 4. [Google Scholar]
- Kolev OI, Möller C, Nilsson G, Tibbling L. Responses in skin microcirculation to vestibular stimulation before and during motion sickness. Canadian Journal of Neurological Sciences. 1997;24:53–57. doi: 10.1017/s0317167100021090. [DOI] [PubMed] [Google Scholar]
- Money KE. Motion sickness. Physiological Reviews. 1970;50:1–39. doi: 10.1152/physrev.1970.50.1.1. [DOI] [PubMed] [Google Scholar]
- Passias TC, Meneilly GS, Mekjavic IB. Effect of hypoglycemia on thermoregulatory responses. Journal of Applied Physiology. 1996;80:1021–1032. doi: 10.1152/jappl.1996.80.3.1021. [DOI] [PubMed] [Google Scholar]
- Sessler D, Olofsson CI, Rubinstein EH. The thermoregulatory threshold in humans during nitrous oxide-fentanyl anesthesia. Anesthesiology. 1988;69:357–364. doi: 10.1097/00000542-198809000-00012. [DOI] [PubMed] [Google Scholar]
- Sugenoya J, Iwase S, Mano T, Sugiyama Y, Ogawa T, Nishiyama T, Nishimura N, Kimura T. Vasodilator component in sympathetic nerve activity destined for the skin of the dorsal foot of mildly heated humans. Journal of Physiology. 1998;507:603–610. doi: 10.1111/j.1469-7793.1998.603bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veghte JH. Cold sea survival. Aerospace Medicine. 1972;43:506–511. [PubMed] [Google Scholar]


