Abstract
The γ subunit is a specific component of the plasmalemmal Na+,K+-ATPase. Like structurally related single-spanning membrane proteins such as cardiac phospholemman, Mat-8 and renal CHIF, large ion conductances are activated when γ subunits are expressed in Xenopus oocytes.
Here we report critical properties of the γ-activated conductance. The γ-activated conductance showed non-selective cationic and anionic permeation, and extremely slow kinetics, with an activation time constant > 1 s following steps to -100 mV.
The γ-activated conductance was inhibited by extracellular divalent ions including Ba2+ (Ki= 0.7 mm) and Ca2+ (Ki= 0.4 mm).
2-Deoxyglucose (MW ∼180), inulin (MW ∼5000) and spermidine (MW ∼148) efflux could occur through the γ-activated conductance pathway, indicating a large pore diameter. In contrast, dextran-70 (MW ∼70 000) did not pass through the γ-activated channel, indicating an upper limit to the pore size of ∼50 Å (5 nm).
Similar conductances that are permeable to large molecules were activated by extreme hyperpolarization (> -150 mV) of uninjected oocytes.
We conclude that the Na+,K+-ATPase γ subunits activate Ca2+- and voltage-gated, non-selective, large diameter pores that are intrinsically present within the oocyte membrane.
The Na+,K+-ATPase is a plasma membrane protein responsible for maintaining the high intracellular K+ and low intracellular Na+ concentrations that are characteristic of most mammalian cells. The enzyme consists of two non-covalently linked subunits: a 110 kDa multispanning membrane protein termed the α subunit, and a smaller glycosylated polypeptide of 40-60 kDa termed the β subunit. Although all catalytic functions of the enzyme have been assigned to the α subunit, both subunits are required for the functional expression of normal Na+,K+-ATPase activity (Blanco & Mercer, 1998). A third protein, termed the γ subunit, has also been identified in purified preparations of the enzyme. The γ subunit is a small, single-spanning membrane protein that is associated with the α and β subunits (Forbush et al. 1978; Reeves et al. 1980; Mercer et al. 1993; Kim et al. 1997), and modulates ion transport associated with the αβ complexes (Beguin et al. 1997; Therien et al. 1997, 1999; Arystarkhova et al. 1999). Interestingly, the human γ subunit induces large ion conductances when expressed in Xenopus oocytes, and increases Na+ and K+ uptake when expressed in Sf-9 insect cells (Minor et al. 1998). The γ subunit belongs to a gene family of small membrane proteins that includes cardiac phospholemman, Mat-8 and renal CHIF (Sweadner & Rael, 2000). These proteins also induce large ion conductances when expressed in Xenopus oocytes (Moorman et al. 1992; Morrison et al. 1995; Attali et al. 1995). Like members of the γ subunit gene family, the single-spanning membrane protein minK (iSK) also induces ion currents (Takumi et al. 1988; Attali et al. 1993). However, divergent properties have been ascribed to all these conductances, and controversy remains regarding whether these single-spanning membrane proteins are forming the ion conductance pathway, or modulating conductances intrinsic to the oocyte. MinK protein and its homologues clearly modulate the pore properties and gating of K+-selective conductances by interaction with members of the Kv superfamily of K+ channel proteins (Takumi et al. 1988; Goldstein & Miller, 1991; Barhanin et al. 1996; Sanguinetti et al. 1996; Wang et al. 1996). However, minK has also been reported to activate non-specific conductances in oocytes (Attali et al. 1993; Shimbo et al. 1995; Ben-Efraim et al. 1996). Phospholemman, which bears the most similarity to the Na+,K+-ATPase γ subunit, is also reported to generate chloride-selective or non-selective channels when expressed in oocytes (Moorman et al. 1992; Shimbo et al. 1995) or when the purified protein is reconstituted into bilayers (Moorman et al. 1995). More recently, unusual properties of switched cation-anion selectivity have also been reported using bilayers (Kowdley et al. 1997). Other single-spanning membrane proteins, such as the influenza B virus NB protein, have also been reported to activate endogenous oocyte conductances, by shifting the voltage dependence to less hyperpolarized potentials (Shimbo et al. 1995). Indeed, it is becoming increasingly apparent that heterologous expression of membrane proteins in Xenopus oocytes may modify or induce endogenous currents. For example, expression of several different, functionally distinct membrane proteins in Xenopus oocytes induces an endogenous, hyperpolarization-activated current (Tzounopoulos et al. 1995). Recent results suggest that this current is an endogenous hyperpolarization-activated current that is composed of a Ca2+ current accompanied by a Ca2+-activated Cl− current (Kuruma et al. 2000). We have further examined the electrophysiological and pharmacological properties of the current activated by the γ subunit in Xenopus oocytes (Iγ). The experiments support the hypothesis that the Na+,K+-ATPase γ subunit induces a current by shifting the activation of a Ca2+- and voltage-gated, non-selective, large diameter pore that is endogenous to the oocyte membrane.
METHODS
cDNAs
A cDNA representing the human γ subunit (GenBank accession no. X86400) was used for these studies. This cDNA contains an extended 5′ sequence leading to an expanded N-terminal domain. The protein encoded by this cDNA consists of 97 residues with a predicted molecular weight of 10 690. To determine whether the extended N-terminal sequence is responsible for the channel-inducing properties of the human γ subunit, PCR was used to generate a cDNA in which the first 31 residues were deleted (human γ 32-97). This cDNA results in a γ subunit that starts at a methionine that corresponds to the initiation methionine of the rat γ subunit. The cDNA coding for the rat γ subunit (rat γa) was obtained from R. Blostein (McGill University). For subcloning, PCR was used to generate a Bgl II restriction site starting just 5′ of the initiation methionine.
cRNA preparation and oocyte expression
cDNAs encoding the γ subunits were subcloned into pXOV-60. This vector is a derivative of pSP64 (Promega Corp., Madison, WI, USA) and contains promoter elements for Xenopus globin that promote high levels of expression in Xenopus oocytes. Capped cRNA was generated using an SP6 mMessage mMachine in vitro transcription kit according to the manufacturer's instructions (Ambion, Austin, TX, USA). Stage V-VI Xenopus oocytes were isolated by partial ovariectomy under tricaine anaesthesia from frogs that were humanely killed after the final collection. Oocytes were defolliculated by treatment with 1 mg ml−1 collagenase (Type 1A, Sigma) in 0 mm Ca2+ ND96 (see below) for 1 h. From 2 to 24 h after defolliculation, oocytes were pressure injected with ∼50 nl of cRNA (1-100 ng μl−1). Oocytes were maintained at room temperature in ND96 solution (96 mm NaCl, 2 mm KCl, 1 mm MgCl2, 5 mm Na-Hepes, pH 7.5) containing 2 mm Ca2+ and supplemented with penicillin (100 units ml−1) and streptomycin (100 μg ml−1) for 1-2 days prior to recording. All procedures were in accordance with the recommendations of the Animal Studies Committee of Washington University.
Membrane preparation, immunoprecipitation and immunoblot analysis
Two days after injection, 90 oocytes injected with γ cRNA or water were washed in 5 ml of TBSA buffer (150 mm NaCl, 10 mm magnesium acetate, 20 mm Tris-Cl, pH 7.6, and 1 mm phenylmethylsulfonyl fluoride (PMSF)). The oocytes were resuspended in 0.5 ml of the TBSA buffer containing 10 % (w/v) sucrose and homogenized with seven strokes in a Dounce glass homogenizer. The lysate was layered onto a step gradient consisting of 5 ml of 50 % (w/v) sucrose and 5 ml of 20 % (w/v) sucrose in 50 mm NaCl, 10 mm magnesium acetate, 20 mm Tris-Cl, pH 7.6, and 1 mm PMSF. The gradient was centrifuged at 15 000 g for 30 min at 4 °C. The material down to the 20 %-50 % interface was removed and discarded. The membranes at the 20 %-50 % interface were removed and placed in a microfuge tube. The membranes were centrifuged at 107 000 g in a Beckman TLA100 centrifuge using a TLA-100.2 rotor. The pellet was solubilized in 0.5 ml HBS (150 mm NaCl, 25 mm Hepes, pH 7.5) containing 1 % (w/v) 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid (CHAPS). For immunoprecipitations, 5 μl of anti-γ subunit polyclonal antibody (γ969; Minor et al. 1998) and 50 μl of goat anti-rabbit antibody-coated magnetic beads (BioMag; PerSeptive Diagnostics, Cambridge, MA, USA) were added. After overnight incubation on a rotating stand at 4 °C, beads were isolated by holding the tubes to a magnet. The supernatant was aspirated, and the beads were washed 3 times in 1 % CHAPS in HBS. Precipitated proteins were eluted by resuspending the washed beads in sample buffer (100 mm Tris-HCl pH 6.8, 2 % SDS, 33 % glycerol, 100 mm DTT) and incubating for 10 min at 65 °C. The eluted proteins were separated by SDS-PAGE (10 % polyacrylamide gel) and transferred to polyvinylidene difluoride (PDVF) membrane (Immobilon-P; Millipore, Bedford, MA, USA). As a control, membranes from Sf-9 insect cells expressing the human γ subunit were included. The blot was blocked in Blotto (5 % (w/v non-fat dried milk, 0.1 % sodium azide in HBS) for 2 h at room temperature or overnight at 4 °C. Anti-γ subunit polyclonal antibody (1:100) in Blotto was bound at 37 °C for 1-2 h on a rocking table. After three 10 min washes in 150 mm NaCl, 25 mm Tris, pH 7.4 in 0.1 % Tween-20, goat anti-rabbit HRP-conjugated antibody (ICN) was added in Blotto. Proteins were detected by chemiluminescence as described by the supplier (Pierce, Rockford, IL, USA).
Electrophysiology
Ionic currents were measured using the two-electrode voltage clamp technique in a small chamber (200 μl) mounted on the stage of a binocular microscope (Nikon Instruments). The chamber was connected through agar bridges to the current-sensing headstage of the voltage clamp amplifier (OC-725 Oocyte Clamp, Warner Instruments Corp.) and constantly perfused with a laminar flow of bathing solution supplied by one of five reservoirs connected to a manifold at the inlet to the chamber. Experiments were performed at room temperature (19-22 °C). Currents were recorded in ND96 or KD98 solution (98 mm KCl, 1 mm MgCl2, 5 mm K-Hepes, pH 7.5). Microelectrodes were pulled from thin-walled capillary glass (World Precision Instruments, New Haven, CT, USA) on a horizontal puller (Sutter Instruments, Novato, CA, USA) and tips were mechanically broken to bring electrode resistance to 0.5-2 MΩ when filled with 3 m KCl. To compensate for large membrane potential errors when large membrane currents are flowing, the tip of the current-passing microelectrode was carefully placed near the centre of the oocyte in order to conduct spherically symmetrical current flow. pCLAMP software and a Digidata 1200 converter (Axon Instruments) were used to generate voltage pulse and collect data. Data were normally filtered at 5 kHz, digitized at 22 kHz (Neurocorder, Neurodata, NY, USA) and stored on videotape. When necessary, data were redigitized into a microcomputer using Axotape software (Axon Instruments) for analysis using Clampfit (Axon Instruments) and Excel (Microsoft Inc.) software.
Release of [3H]spermidine, 2-[3H]deoxyglucose, [3H]inulin and [3H]dextran from oocytes expressing γ channels
Oocytes were injected with ∼50 nl of 60 μm[3H]spermidine (specific activity, 15 Ci mmol−1) in 5 mm unlabelled spermidine, or with ∼50 nl of 20 μm[3H]deoxyglucose (specific activity, 5 Ci mmol−1) in 1 mm unlabelled deoxyglucose, or with 10 μm[3H]inulin (specific activity, 10 Ci mmol−1) in 1 mm unlabelled inulin or dextran (MW 70 000, [OCH2(CHOH)5-]n; specific activity, 20 mCi g−1; American Radiolabeled Chemicals, Inc.), at least 1 h before efflux measurements. Samples were counted in a Packard 1600TR scintillation analyser. Radioactivity in samples ranged from 5 to 1000 times background level.
RESULTS
Ionic conductances (Iγ) induced by the Na+,K+-ATPase γ subunit
Injection of oocytes with the cRNA coding for the human γ subunit (human γ 1-97) resulted in the appearance of large conductances activated by depolarization to > +100 mV or hyperpolarization to > -100 mV in either ND96 or KD98 solutions (Minor et al. 1998). There was a negligible shift in the reversal potential when the extracellular solution was switched from high [Na+] to high [K+] and the current amplitude was unaffected (not shown). In the following experiments we only investigated hyperpolarization-activated γ currents. This is because in oocytes, depolarization also activates endogenous Ca2+-activated chloride channels and, for unknown reasons, maintained depolarization was detrimental to the oocyte membrane. To further characterize the ion conductances induced by the expression of the γ subunit, the whole-cell current and current-voltage relationships from oocytes expressing the γ subunit with the extended N-terminus (human γ 1-97), the human γ subunit with the 31 N-terminal residues removed (human γ 32-97) or the rat γ subunit (rat γa), and from a control oocyte were determined. As shown in Fig. 1A, membrane hyperpolarization from a holding potential of -10 mV to -140 mV evoked slowly activating inward currents. The first step in the pulse protocol was to the most negative potential (-140 mV; indicated by an arrow in the human γ 1-97 recording). As shown in the records, this did not typically demonstrate any instantaneous current. However, following activation, the de-activation kinetics at the holding potential of -10 mV were extremely slow (> 5 min for full recovery). It was thus impractical to wait for full recovery at the holding potential before each test pulse. With a subsequent inter-pulse interval of 30 s, the conductance was therefore already partially activated at the holding potential (although there was no current because this is approximately the reversal potential, Vrev). Therefore, an instantaneous current, followed by the time-dependent development of more complete activation, was seen during subsequent hyperpolarizing test pulses. As shown in Fig. 1A, essentially identical currents were obtained for the human γ subunit lacking the first 31 N-terminal amino acids and the rat γ subunit. For all properties determined, the human γ 1-97 and human γ 32-97 subunits gave similar results. Thus, residues within this N-terminal region are not responsible for the induction of channel activity. Interestingly, in contrast to our previous results (Minor et al. 1998), rat γ cDNA devoid of any 5′ untranslated sequence could also induce such ionic currents. Previous failures to induce currents in Xenopus oocytes seem to have been a result of the presence of 5′ untranslated sequences within the rat γ cDNA. These new results demonstrate that the γ-induced channel activity in Xenopus oocytes is not limited to the human subunit and suggest that the induction of ion conductances by γ may be a common feature of the subunit.
Figure 1. Non-selective ion conductances induced by human γ subunit expression in Xenopus oocytes.
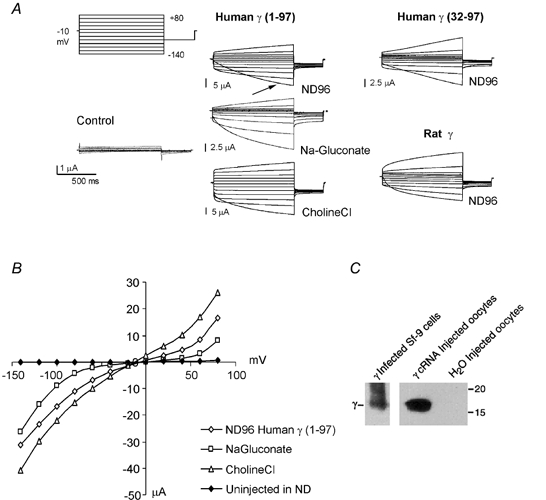
A, currents recorded under voltage clamp, from an uninjected Xenopus oocyte (Control), and from an oocyte expressing the human γ subunits with (Human γ (1-97)) and without the extended N-terminal sequence (Human γ (32-97)) or the rat γ subunit (Rat γ). The membrane was stepped from a holding potential of -10 mV to voltages between -140 and +80 mV, for 2 s, every 30 s. The records shown are a typical first set of currents after turning on the voltage clamp in ND96 for control, or human γ subunit-expressing oocytes. The arrow in the human γ 1-97 recording indicates the first pulse from -10 to -140 mV. Also shown are currents from the human γ subunit-expressing (human γ 1-97) oocyte after switching the solution first to one in which 90 mm Cl− was replaced by 90 mm gluconate−, and then after replacing 90 mm Na+ from ND96 with 90 mm choline+. B, pseudo-steady-state current-voltage relationships from the records shown in A. C, expression of the γ subunit (human γ 1-97) in Xenopus oocytes. Proteins from oocytes injected with human γ subunit cRNA or water were immunoprecipitated with a γ subunit-specific polyclonal antibody. Immunoprecipitated proteins were separated by SDS-PAGE (10 % polyacrylamide gel), transferred to membrane and probed with the same antibody. For comparison, the human γ subunit (γ 1-97) from Sf-9 insect cells infected with a recombinant γ baculovirus is shown. Positions of molecular mass standards (15 and 20 kDa) are shown on the right.
During hyperpolarization, currents did not reach true steady state, even for pulses lasting as long as 30 s (not shown). The pseudo-steady-state I-V relationships from the end of the pulse are shown in Fig. 1B. The activated currents appear to be non-selective, since replacement of Cl− by gluconate− did not qualitatively alter the currents, nor shift the reversal potential, suggesting that Iγ is not a specific Cl− current. Replacement of Na+ by choline+ also did not significantly change the amplitude or time dependence of the γ current (Fig. 1A), indicating a high permeability to cations.
To verify that the injection of γ cRNA results in the expression of γ subunit polypeptides, proteins from γ cRNA- or water-injected control Xenopus oocytes were immunoprecipitated with a γ subunit-specific polyclonal antibody (γ969). The immunoprecipitated proteins were separated by SDS-PAGE, transferred to PVDF membrane and probed with the γ subunit-specific antibody. As shown in Fig. 1C, only oocytes injected with the γ cRNA expressed γ polypeptides.
Effect of extracellular Ba2+ and Ca2+ on Iγ
As shown in Fig. 2, extracellular divalent Ba2+ and Ca2+ ions inhibited the γ-induced current. Figure 2A shows representative currents recorded from an oocyte in control ND96 solution, and in the presence of 10 mm Ca2+ or Ba2+. As suggested by pseudo-steady-state current-voltage relationships, the inhibition was weakly or non-voltage dependent (data not shown). We examined the dose- response relationship for these two ions using a single hyperpolarizing pulse to -100 mV (Fig. 2B). The dose-response curve for Ba2+ inhibition indicates a very steep relationship with an empirically determined Hill coefficient of 5.5 and a Ki,Ba (Ba2+ concentration causing half-maximal inhibition) of 0.7 mm. The dose-response relationship for extracellular Ca2+ was considerably shallower, with a Hill coefficient of 0.9, and a Ki,Ca of 0.4 mm.
Figure 2. Divalent cations cause voltage-independent inhibition of Iγ.
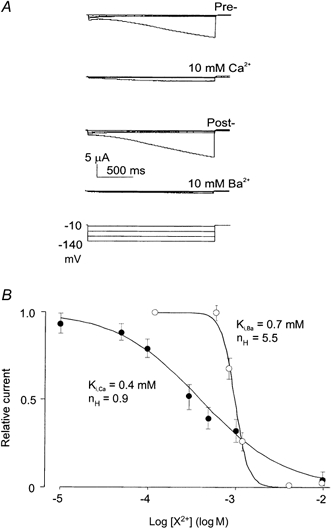
A, currents recorded under voltage clamp, from an oocyte expressing human γ subunits. The membrane was stepped from a holding potential of -10 mV to voltages between -20 and -140 mV, for 2 s, every 30 s. The records are from a single oocyte that was sequentially exposed to ND96 (pre-control) solution, then ND96 + 10 mm Ca2+, then post-control solution, then 10 mm Ba2+. B, pseudo-steady-state dose-response relationships for inhibition of Iγ by Ca2+ and Ba2+ in ND96 solution, at -100 mV. Data points show mean currents in the presence of Ca2+ (•) or Ba2+ (○) normalized to pre-control currents in the absence of Ca2+ (mean ±s.e.m., n = 11) or Ba2+ (mean ±s.e.m., n = 8), respectively. Curves are least squares fits of the Hill equation (relative current = 100/(1 + [X2+/Ki] nH)), where X2+ is Ca2+ or Ba2+.
Iγ conducts large molecules and trivalent spermidine
Given the finding that choline ions may permeate the γ-activated conductance pathway, we investigated the permeability of the γ-induced channel to larger molecules. Figure 3 illustrates the typical protocol employed in these studies. Oocytes, injected with 2-[3H]deoxyglucose, were first held at the zero current potential (typically -10 to -20 mV). After 10 min the bathing solution was aspirated and counted. The oocyte was then clamped to -10 mV and repeatedly stepped to -100 mV for 10 s every minute. The bathing solution was aspirated and counted after this period (Fig. 3A). The protocol was repeated in the presence of 5 mm Ca2+ and then again in zero Ca2+, ensuring that changes in efflux rate were reversible and not simply a result of progressive changes caused by the long duration of the voltage clamp (> 30 min). Activation of Iγ using this protocol stimulated deoxyglucose efflux 5- to 50-fold above background (Fig. 3B), and this efflux was blocked by extracellular Ca2+. The small rise in the amplitude of the deoxyglucose efflux in successive periods that is evident in control oocytes probably reflects the leakage caused by the electrodes.
Figure 3. [3H]deoxyglucose efflux through γ-activated conductance pathway.
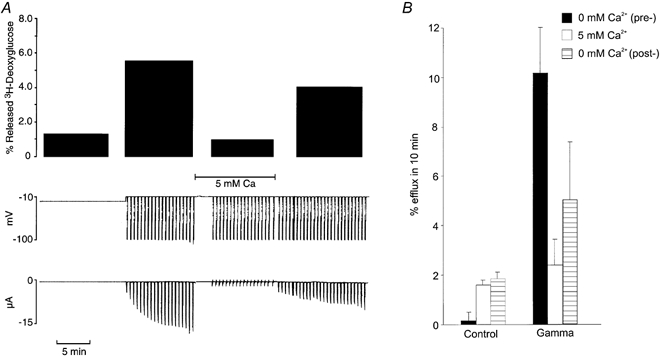
A, slow time base records of membrane potential (mV) and membrane current (μA) from an oocyte clamped first at the zero current potential for 10 min, then at a holding potential of -10 mV and stepped to -100 mV for 10 s every minute for 10 min in zero Ca2+, then again in 5 mm Ca2+, then again in 0 mm Ca2+. The top panel shows [3H]deoxyglucose released during successive 10 min periods. B, Ca2+ inhibitable deoxyglucose efflux through the γ-activated conductance pathway. Averaged results from experiments like those shown in A in control and human γ subunit-injected oocytes (Gamma). Bar graphs show percentage efflux of [3H]deoxyglucose in 10 min (±s.e.m., n = 12) from clamped oocytes sequentially exposed to zero Ca2+ (pre-control), 5 mm Ca2+, then zero Ca2+ (post-control).
Given the ability of deoxyglucose to permeate the γ-induced conductance pathway, we were interested in the size of the conductive pores. Using essentially the same protocol as for deoxyglucose efflux, we tested whether the large fructose polymer inulin (MW ∼5000) was also permeable through the γ-induced pore (Fig. 4). Again, when the γ-induced conductance was activated by repeated hyperpolarizing steps for 10 min, there was a 5- to 50-fold increase in inulin efflux over control, and the inulin efflux was inhibited by extracellular Ca2+. As shown in Fig. 4, in oocytes expressing ROMK1 (Kir1.1, an inwardly rectifying K+ channel), there was no increase in inulin efflux during hyperpolarization. Thus, the increase in inulin efflux is not simply a result of an increase in membrane conductance since comparable ionic conductances were generated in oocytes expressing the ROMK1 channel and the human γ subunit (in these experiments, ROMK1 current at -100 mV was -10.5 ± 2.7 μA, n = 5; human γ-induced current at -100 mV was -12.8 ± 1.5 μA, n = 8). We utilized essentially the same protocol to examine [3H]dextran efflux (Fig. 5). In contrast to inulin, dextran-70 (MW ∼70 000) efflux was not stimulated by activation of the γ-induced conductance in γ-expressing oocytes (Fig. 5). This indicates an upper limit to the pore size of ∼50 Å (5 nm; Saito et al. 2000).
Figure 4. Ca2+-inhibitable inulin efflux through the γ-activated conductance pathway.
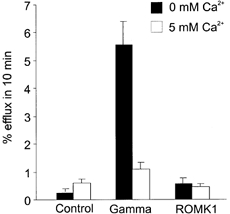
Averaged results from experiments similar to those shown in Fig. 3 in voltage-clamped (-10 mV holding potential, step to -100 mV for 10 s every minute) control and human γ-injected oocytes for inulin efflux. Bar graphs show percentage efflux of [3H]inulin in 10 min (±s.e.m., n = 11). Oocytes were sequentially exposed to zero Ca2+, then 5 mm Ca2+.
Figure 5. Dextran-70 does not permeate the γ-stimulated conductance pathway.
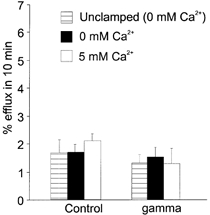
Averaged results from experiments similar to those shown in Fig. 3 in control and human γ-injected oocytes. Bar graphs show percentage efflux of [3H]dextran-70 in 10 min (±s.e.m., n = 11). Oocytes were sequentially exposed to zero Ca2+ (unclamped), then were voltage clamped (-10 to -100 mV for 10 s every minute) in zero Ca2+, then in 5 mm Ca2+.
Consistent with efflux through a membrane channel, spermidine efflux from Xenopus oocytes is a simple electrodiffusive process that is inhibitable by Ba2+ and Ca2+ ions (Sha et al. 1996). We repeated the above protocols to examine [3H]spermidine efflux from oocytes expressing the human γ subunit. Spermidine release was also stimulated when the γ-induced conductance was active. However, the stimulation of spermidine efflux was only about 1/3 of that for deoxyglucose or inulin at comparable γ-activated current density (Fig. 6). This is consistent with the trivalent nature of spermidine, since cationic efflux will be slowed under hyperpolarization (see Sha et al. 1996), although given the voltage protocol employed, it is difficult to predict the difference quantitatively.
Figure 6. Large molecule fluxes are proportional to the γ-induced conductance.
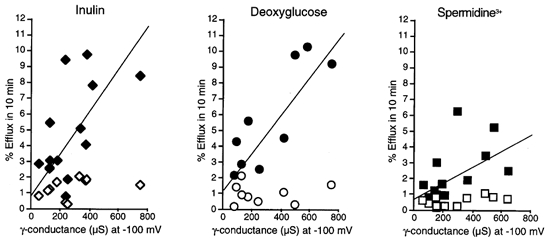
Results from individual experiments like those shown in Figs 3–5. Percentage efflux in 10 min is plotted versusγ conductance (chord conductance from pseudo-steady-state current at -100 mV). Open symbols show efflux measured under non-activated conditions (10 min at a membrane potential of -10 mV); filled symbols show efflux measured during subsequent voltage clamping (10 min repeated clamps from -10 to -100 mV for 10 s every minute) in zero Ca2+. Data shown for [3H]inulin, [3H]deoxyglucose and [3H]spermidine release. The straight lines are linear least squares approximations (Microsoft Excel).
Properties of endogenous hyperpolarization-activated conductances
Shimbo et al. (1995) have previously suggested that the single-spanning membrane proteins activate conductances that are integral to the oocyte membrane. Support for this view is provided by studies demonstrating that currents similar in size and with similar divalent cation sensitivities to those induced by these proteins can be activated by extreme hyperpolarizations (> -150 mV) in uninjected oocytes (Shimbo et al. 1995). Accordingly, we investigated the possibility that the γ-induced conductances are also endogenous to the oocyte. As shown in Fig. 7A, we could induce similar large inward currents that were sensitive to extracellular Ba2+ and Ca2+ in uninjected oocytes at -160 mV. Significantly, these experiments also show that the endogenous conductance pores permit inulin efflux that is comparable at -160 mV to that induced at -100 mV in the γ-expressing oocytes (Fig. 7B).
Figure 7. [3H]inulin efflux through hyperpolarization-activated conductances in control oocytes.
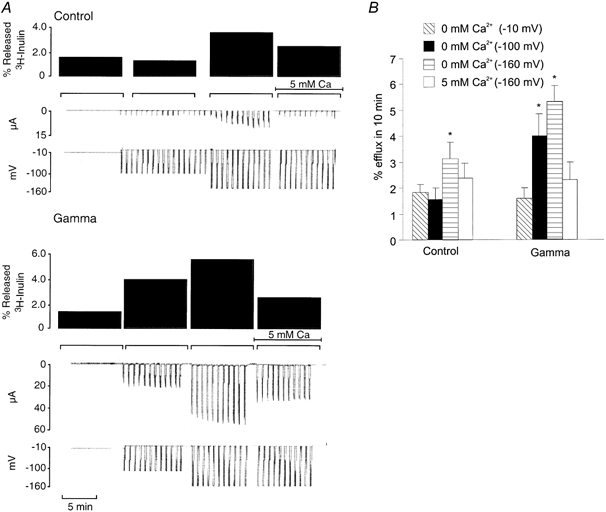
A, slow time base records of membrane potential (mV) and membrane current (μA), from oocytes clamped first at -10 mV for 10 min, then at a holding potential of -10 mV and stepped to -100 mV for 10 s every minute for 10 min in zero Ca2+, then stepped to -160 mV for 10 s every minute for 10 min, first in zero Ca2+, then in 5 mm Ca2+. B, Ca2+-inhibitable [3H]inulin efflux through hyperpolarization-activated conductances in control oocytes. Averaged results from experiments like those shown in A in control and γ-injected oocytes. Bar graphs show percentage efflux of [3H]inulin in 10 min (±s.e.m., n = 6). Oocytes were sequentially exposed to zero Ca2+ (clamped at -10 mV), then were voltage clamped (-10 to -100 mV for 10 s every minute) in zero Ca2+, then clamped to -160 mV for 10 s every minute first in zero Ca2+, then in 5 mm Ca2+. The differences are statistically significant; * P < 0.05 from oocytes in zero Ca2+ clamped at -10 mV (paired t test, SigmaPlot, Jandel Scientific).
DISCUSSION
Ionic nature and external cation dependence of the γ subunit-induced conductance
Large ionic conductances reversing around -10 mV were activated by hyperpolarization to -100 mV in oocytes expressing the human γ subunit (Fig. 1). Interestingly, the human γ subunit without the extended N-terminal sequence also induced channel activity, indicating that these residues are not required for channel activity. Moreover, in contrast to previous results, the rat γ subunit also induced channel activity in Xenopus oocytes. It appears that 5′ untranslated sequences within the rodent cDNA prevent the expression of the γ subunit in the oocytes. Thus, the ability of the γ polypeptide to induce ionic conductances may be a common property of the subunit. Replacement of Cl− with gluconate−, or Na+ with choline+ did not significantly alter the nature or reversal potential of the expressed conductance, indicating non-selectivity. In contrast, a clear ionic dependence was seen for external divalent cations, which significantly inhibited the conductance in an essentially voltage-independent manner (Fig. 2). However, the nature of the inhibitory process is not clear. The steep apparent dose dependence of Ba2+ inhibition (Hill coefficient > 5) of the γ-induced conductance is not consistent, in any simple way, with a pore-blocking mechanism. Moreover, such a mechanism seems unlikely given the large diameter of the γ-induced pores (see below). However, this divalent ion dependency is a useful pharmacological parameter and, as discussed below, gives added strength to the idea that the γ-induced conductance is through an endogenous pathway that can also be activated by very strong hyperpolarization in uninjected oocytes.
The size of γ subunit-induced pores
Using 3H-labelled 2-deoxyglucose (MW ∼180) or inulin (MW ∼5000) as a tracer, efflux measurements indicated that relatively large molecules can pass through the γ-activated permeation pathway. The linear dependence of the deoxyglucose and inulin fluxes on the amplitude of Iγ (Fig. 6) is consistent with the efflux being directly through this pathway, not an indirect consequence of Iγ reaching a threshold for activation of a distinct efflux mechanism. Further support for this hypothesis is provided by the spermidine efflux studies. Spermidine is trivalent, and under the hyperpolarizing voltage protocol employed here, electrodiffusive efflux should be lower (between 1- and 3-fold) than for an uncharged species. In accordance with this, although there was still an approximately linear relationship between γ-induced conductance and spermidine efflux, the slope was less than half that seen for inulin or deoxyglucose (Fig. 6). Inulin is a polysaccharide of 30 fructose monomers linked in a long chain. At room temperature, crystalline inulin is a semi-hydrate with an orthorhombic structure and a P212121 space group. Its cell parameters are: a = 1.670 nm, b = 0.965 nm, c (chain axis) = 1.44 nm. In the hydrated form, the crystals have the same orthorhombic structure and a P212121 space group. The cell parameters remain very similar to those of the semi-hydrated structure: a = 1.670 nm, b = 0.980 nm, c (chain axis) = 1.47 nm. (data from I. André, J. L. Putaux, H. Chanzy, K. Mazeau & F. R. Taravel; http://www.cermav.cnrs.fr/ posters_virutels/putaux/ inuline/ PosterECG1_main.html). This would place the minimal pore diameter for the γ-induced conductance at greater than 1 nm. Another larger, non-metabolized molecule, dextran-70 (MW ∼70 000), did not pass through the γ-induced conductance pathway (Fig. 5). Dextran-70 is a glucose polymer, with an estimated diameter of the globular molecule in solution of 5 nm, giving an upper limit estimate for the pore size.
The nature of the γ subunit-induced conductance
Large ion conductances are also induced in oocytes by structurally similar single-spanning membrane proteins such as cardiac phospholemman (Moorman et al. 1992), Mat-8 (Morrison et al. 1995), renal CHIF (Attali et al. 1995), and minK (IsK, Takumi et al. 1988; Attali et al. 1993). However, different properties have been ascribed to such conductances, and there is no consistent picture as to whether these proteins are directly forming the ion conductances, or modulating conductances intrinsic to the oocyte. The well-characterized minK protein and its homologues clearly generate K+-selective conductances by interaction with members of the Kv superfamily of K+ channel proteins, modulating both the pore properties and the gating (Takumi et al. 1988; Goldstein & Miller, 1991; Barhanin et al. 1996; Sanguinetti et al. 1996; Wang et al. 1996). CHIF induces a K+-specific current in oocytes (Attali et al. 1995). Phospholemman, which is most similar to the Na+,K+-ATPase γ subunit, generates non-selective, or Cl−-selective channels in oocytes (Moorman et al. 1992; Shimbo et al. 1995), and unusual properties of switched cation-anion selectivity have been reported in bilayers (Moorman et al. 1995; Kowdley et al. 1997). Taken together, these studies are most consistent with phospholemman directly forming channels.
Conversely, various single-spanning membrane proteins, such as IsK (minK), phospholemman and the NB protein of influenza B virus, appear to open endogenous, hyperpolarization-activated, non-selective conductances by shifting the voltage dependence of the channels to less hyperpolarized potentials (Attali et al. 1993; Shimbo et al. 1995). These slowly activating conductances are similar to the γ-induced conductances reported here, both kinetically and in regard to their sensitivities to divalent ions (Shimbo et al. 1995). However, neither the currents activated by these proteins in the studies of Shimbo et al. (1995), nor those activated by the human γ subunit in our studies match the properties of the many hyperpolarization-activated Cl− channels or outward-rectifying Cl− currents that have been described in Xenopus oocytes (Peres & Bernardini, 1983; Parker & Miledi, 1988; Ackerman et al. 1994; Kowdley et al. 1994; Buyse et al. 1997). Stretch-activated, non-selective, conductances have also been described in Xenopus oocyte membranes (Yang & Sachs, 1990). Could these form the conductance pathway activated by γ and other single-spanning membrane proteins? As with stretch-activated conductances, Gd3+ inhibits the γ-induced conductance (data not shown), although this is not a very specific probe. Attempts to manipulate the open probability of stretch-activated channels in intact oocytes were unsuccessful.
Both endogenously (Zhang et al. 1998) and exogenously (Paul et al. 1991; Ebihara & Steiner, 1993; Trexler et al. 1996) expressed gap junction hemi-channel currents have been recorded in Xenopus oocytes. Some of the properties we have described for the γ channels, namely non-selectivity and relatively large diameter pores that are inactivated by external Ca2+, are reminiscent of the properties of expressed hemi-gap-junctional channels (DeVries & Schwartz, 1992; Trexler et al. 1996; Zhang et al. 1998). These non-selective, large diameter pores might also be considered as candidate proteins for the γ-activated and phospholemman-activated conductance pathways. However, there are some distinct differences between the observed conductances. For example, gap-junctional channels appear to activate instantaneously following voltage-clamp steps, then inactivate (Zampighi et al. 1999), which is kinetically distinct from the γ-activated conductance. This reduces the appeal of hemi-channels as possible mediators of the γ-activated conductance, although it is conceivable that novel kinetics could result from the association of the gap-junctional channels with the γ subunit.
There is increasing evidence that the γ subunit can modify Na+,K+-ATPase function. It has been shown that the γ subunit can lower the affinity of the Na+,K+-ATPase for K+ (Beguin et al. 1997; Arystarkhova et al. 1999) and Na+ (Arystarkhova et al. 1999), increase the apparent affinity of the enzyme for ATP (Therien et al. 1999) and influence the binding of the specific Na+,K+-ATPase inhibitor ouabain (Fontes et al. 1999). Through its association with the other Na+,K+-ATPase subunits, the γ subunit may help regulate or modify enzymatic activity, which in turn may be important in adapting Na+,K+-ATPase function to the requirements of each cell. Our findings demonstrate that in Xenopus oocytes the γ polypeptide can activate non-selective pores in the membrane that mediate the passage of molecules of > 1 nm diameter. It is unknown whether this property of the γ subunit extends to cells other than Xenopus oocytes. However, in certain cells such a conductance could play a role in the molecular mechanisms that drive ion transport. For example, in the mouse preimplantation embryo, the γ subunit is an important determinant of the trans-trophectodermal ion transport that drives fluid transport during blastocoel formation (Jones et al. 1997).
The properties we have described for the γ-induced conductance, namely non-selective, large diameter pores that are inhibited by external Ca2+ and Ba2+, are similar to those reported for currents induced by several other single-spanning membrane proteins in oocytes, and of hemi-gap-junctional channels in a variety of cells. We conclude that γ subunits induce non-selective channel activity by shifting the voltage dependence of intrinsic membrane conductances within the oocyte. The identification of the proteins responsible for the intrinsic membrane conductance and the nature of their activation by the γ subunit await further study.
Acknowledgments
This work was supported by National Institutes of Health grants (HL54171 to C.G.N. and DK45181 to R.W.M.), a National Institutes of Health Cardiovascular Training Grant (to Q.S.) and the George M. O'Brien Kidney and Urological Diseases Center at Washington University School of Medicine.
References
- Ackerman MJ, Wickman KD, Clapham DE. Hypotonicity activates a native chloride current in Xenopus oocytes. Journal of General Physiology. 1994;103:153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arystarkhova E, Wetzel RK, Asinovski NK, Sweadner KJ. The gamma subunit modulates Na+ and K+ affinity of the renal Na,K-ATPase. Journal of Biological Chemistry. 1999;274:33183–33185. doi: 10.1074/jbc.274.47.33183. [DOI] [PubMed] [Google Scholar]
- Attali B, Guillemare E, Lesage F, Honore E, Romey G, Lazdunski M, Barhanin J. The protein IsK is a dual activator of K+ and Cl− channels. Nature. 1993;365:850–852. doi: 10.1038/365850a0. [DOI] [PubMed] [Google Scholar]
- Attali B, Latter H, Rachamine N, Garty H. A corticosteroid-induced gene expressing an [IsK-like] K+ channel activity in Xenopus oocytes. Proceedings of the National Academy of Sciences of the USA. 1995;92:6092–6096. doi: 10.1073/pnas.92.13.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Beguin P, Wang X, Firsov D, Puoti A, Claeys D, Horisberger JD, Geering K. The gamma subunit is a specific component of the Na,K-ATPase and modulates its transport function. EMBO Journal. 1997;16:4250–4260. doi: 10.1093/emboj/16.14.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Efraim I, Shai Y, Attali B. Cytoplasmic and extracellular IsK peptides activate endogenous K+ and Cl− channels in Xenopus oocytes. Evidence for regulatory function. Journal of Biological Chemistry. 1996;271:8768–8771. doi: 10.1074/jbc.271.15.8768. [DOI] [PubMed] [Google Scholar]
- Blanco G, Mercer RW. Isozymes of the Na,K-ATPase: Heterogeneity in structure, diversity in function. American Journal of Physiology. 1998;275:F633–650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- Buyse G, Voets T, Tytgay J, De Greef C, Droogmans G, Nilius B, Eggermont J. Expression of human pICln and ClC-6 in Xenopus oocytes induces an identical endogenous chloride conductance. Journal of Biological Chemistry. 1997;272:3615–3621. doi: 10.1074/jbc.272.6.3615. [DOI] [PubMed] [Google Scholar]
- Devries SH, Schwartz EA. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. Journal of Physiology. 1992;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L, Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. Journal of General Physiology. 1993;102:59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes CF, Lopes FE, Scofano HM, Barrabin H, Nørby JG. Stimulation of ouabain binding to Na,K-ATPase in 40 % dimethyl sulfoxide by a factor from Na,K-ATPase preparations. Archives of Biochemistry and Biophysics. 1999;366:215–223. doi: 10.1006/abbi.1999.1198. [DOI] [PubMed] [Google Scholar]
- Forbush B, Kaplan JH, Hoffman JF. Characterization of a new photoaffinity derivative of ouabain: labeling of the large polypeptide and of a proteolipid component of the Na,K-ATPase. Biochemistry. 1978;17:3667–3676. doi: 10.1021/bi00610a037. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Miller C. Site-specific mutations in a minimal voltage-dependent K+ channel alter ion selectivity and open-channel block. Neuron. 1991;7:403–408. doi: 10.1016/0896-6273(91)90292-8. [DOI] [PubMed] [Google Scholar]
- Jones DH, Davies TC, Kidder GM. Embryonic expression of the putative gamma subunit of the sodium pump is required for acquisition of fluid transport capacity during mouse blastocyst development. Journal of Cell Biology. 1997;139:1545–1552. doi: 10.1083/jcb.139.6.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Lee Y, Lee IA, Kang HB, Choe YK, Choe IS. Cloning and expression of human cDNA encoding Na+,K+-ATPase gamma-subunit. Biochimica et Biophysica Acta. 1997;1350:133–135. doi: 10.1016/s0167-4781(96)00219-9. [DOI] [PubMed] [Google Scholar]
- Kowdley GC, Ackerman SJ, Chen Z, Szabo G, Jones LR, Moorman JR. Anion, cation, and zwitterion selectivity of phospholemman channel molecules. Biophysical Journal. 1997;72:141–145. doi: 10.1016/S0006-3495(97)78653-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowdley GC, Ackerman SJ, John JE, Jones LR, Moorman JR. Hyperpolarization-activated chloride currents in Xenopus oocytes. Journal of General Physiology. 1994;103:217–230. doi: 10.1085/jgp.103.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruma A, Hirayama Y, Hartzell HC. A hyperpolarization- and acid-activated nonselective cation current in Xenopus oocytes. American Journal of Physiology. 2000;279:C1401–1413. doi: 10.1152/ajpcell.2000.279.5.C1401. [DOI] [PubMed] [Google Scholar]
- Mercer RW, Biemesderfer D, Bliss DPJr, Collins JH, Forbush B. Molecular cloning and immunological characterization of the γ polypeptide, a small protein associated with the Na,K-ATPase. Journal of Cell Biology. 1993;121:579–586. doi: 10.1083/jcb.121.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor NT, Sha Q, Nichols CG, Mercer RW. The γ subunit of the Na,K-ATPase induces cation channels. Proceedings of the National Academy of Sciences of the USA. 1998;95:6521–6525. doi: 10.1073/pnas.95.11.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman JR, Ackerman SJ, Kowdley GC, Griffin MP, Mounsey JP, Chen Z, Cala SE, O'Brian JJ, Szabo G, Jones LR. Unitary anion currents through phospholemman channel molecules. Nature. 1995;377:737–740. doi: 10.1038/377737a0. [DOI] [PubMed] [Google Scholar]
- Moorman JR, Palmer CJ, John JE, Durieux ME, Jones LR. Phospholemman expression induces a hyperpolarization-activated chloride current in Xenopus oocytes. Journal of Biological Chemistry. 1992;267:14551–14554. [PubMed] [Google Scholar]
- Morrison BW, Moorman JR, Kowdley GC, Kobayashi YM, Jones LR, Leder P. Mat-8, a novel phospholemman-like protein expressed in human breast tumors, induces a chloride conductance in Xenopus oocytes. Journal of Biological Chemistry. 1995;270:2176–2182. doi: 10.1074/jbc.270.5.2176. [DOI] [PubMed] [Google Scholar]
- Parker I, Miledi R. A calcium-independent chloride current activated by hyperpolarization in Xenopus oocytes. Proceedings of the Royal Society. 1988;233:B191–199. doi: 10.1098/rspb.1988.0018. [DOI] [PubMed] [Google Scholar]
- Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. Journal of Cell Biology. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres A, Bernardini G. A hyperpolarization-activated chloride current in Xenopus laevis oocytes. Pflügers Archiv. 1983;399:157–159. doi: 10.1007/BF00663914. [DOI] [PubMed] [Google Scholar]
- Reeves AS, Collins JH, Schwartz A. Isolation and characterization of (Na,K)-ATPase proteolipid. Biochemical and Biophysical Research Communications. 1980;95:1591–1598. doi: 10.1016/s0006-291x(80)80080-5. [DOI] [PubMed] [Google Scholar]
- Saito M, Korsmeyer SJ, Schlesinger PH. BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nature Cell Biology. 2000;2:553–555. doi: 10.1038/35019596. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Sha Q, Romano C, Lopatin AN, Nichols CG. Spermidine release from Xenopus oocytes. Electrodiffusion through a membrane channel. Journal of Biological Chemistry. 1996;271:3392–3397. doi: 10.1074/jbc.271.7.3392. [DOI] [PubMed] [Google Scholar]
- Shimbo K, Brassard DL, Lamb RA, Pinto LH. Viral and cellular small integral membrane proteins can modify ion channels endogenous to Xenopus oocytes. Biophysical Journal. 1995;69:1819–1829. doi: 10.1016/S0006-3495(95)80052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweadner KJ, Rael K. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics. 2000;68:41–56. doi: 10.1006/geno.2000.6274. [DOI] [PubMed] [Google Scholar]
- Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988;242:1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Therien AG, Goldshleger R, Karlish SJD, Blostein R. Tissue-specific distribution and modulatory role of the gamma subunit of the Na,K-ATPase. Journal of Biological Chemistry. 1997;272:32628–32634. doi: 10.1074/jbc.272.51.32628. [DOI] [PubMed] [Google Scholar]
- Therien AG, Karlish SJD, Blostein R. Expression and functional role of the gamma subunit of the Na,K-ATPase in mammalian cells. Journal of Biological Chemistry. 1999;274:12252–12256. doi: 10.1074/jbc.274.18.12252. [DOI] [PubMed] [Google Scholar]
- Trexler EB, Bennett MVL, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proceedings of the National Academy of Sciences of the USA. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Maylie J, Adelman JP. Induction of endogenous channels by high levels of heterologous membrane proteins in Xenopus oocytes. Biophysical Journal. 1995;69:904–908. doi: 10.1016/S0006-3495(95)79964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KW, Tai KK, Goldstein SA. MinK residues line a potassium channel pore. Neuron. 1996;16:571–577. doi: 10.1016/s0896-6273(00)80076-8. [DOI] [PubMed] [Google Scholar]
- Yang XC, Sachs F. Characterization of stretch-activated ion channels in Xenopus oocytes. Journal of Physiology. 1990;431:103–122. doi: 10.1113/jphysiol.1990.sp018322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi GA, Loo DD, Kreman M, Eskandari S, Wright EM. Functional and morphological correlates of connexin50 expressed in Xenopus laevis oocytes. Journal of General Physiology. 1999;113:507–524. doi: 10.1085/jgp.113.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McBride DWJr, Hamill OP. The ion selectivity of a membrane conductance inactivated by extracellular calcium in Xenopus oocytes. Journal of Physiology. 1998;508:763–776. doi: 10.1111/j.1469-7793.1998.763bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


