Abstract
The barrier function of colonic epithelia is challenged by apoptotic loss of enterocytes. In monolayers of human colonic HT-29/B6 cells, apoptosis induced by camptothecin was assessed by poly-(ADP-ribose)-polymerase (PARP) cleavage, histone ELISA and DNA-specific fluorochrome staining (with 4′,6′-diamidino-2′-phenylindoladihydrochloride (DAPI)). Epithelial barrier function was studied in Ussing chambers by measuring transepithelial conductivity and unidirectional tracer fluxes. The ion permeability associated with single cell apoptoses was investigated with the conductance scanning technique.
The spontaneous rate of apoptotic cells was 3.5 ± 0.3 % with an overall epithelial conductivity of 3.2 ± 0.1 mS cm−2. Camptothecin induced a time- and dose-dependent increase of apoptosis and permeability. With 20 μg ml−1 of camptothecin for 48 h, apoptosis increased 4.1-fold to 14.3 ± 1.5 % and the conductivity doubled to 6.4 ± 1.0 mS cm−2.
While 3H-mannitol flux increased 3.8-fold and 3H-lactulose flux increased 2.6-fold, the flux of 3H-polyethylene glycol 4000 remained unchanged. Hence, the higher permeability was limited to molecules < 4000 Da.
The local epithelial conductivity was higher at the sites of apoptosis than in non-apoptotic areas. With camptothecin the leaks associated with apoptosis became more numerous and more conductive, while in non-apoptotic areas the conductivity remained at control level. Hence, the camptothecin-induced increase in epithelial conductivity reflected the opening of apoptotic leaks and thus the results described, for the first time, epithelial permeability as a function of apoptosis only.
The conductivity of apoptotic leaks contributed 5.5 % to the epithelial conductivity of controls and 60 % to the conductivity of monolayers treated with 20 μg ml−1 of camptothecin. Thus apoptosis increased the contribution of paracellular pathways to the overall epithelial permeability. Under control conditions the paracellular conductivity (Gpara) was smaller than the transcellular (Gtrans), but with 12 % apoptosis, Gpara exceeded Gtrans. By definition, the epithelium became ‘leaky’.
The structure of the intestinal mucosa is maintained by a delicate balance between the (apoptotic) loss of cells and cell regeneration (Potten, 1997). It is disturbed in the inflamed intestine, where the epithelium is exposed to several toxins and pro-inflammatory cytokines with the potential to induce necrosis and apoptosis. In chronic inflammatory bowel disease, the frequency of CD95L-mediated apoptosis is increased which is thought to contribute to the impairment of intestinal barrier function (Sträter et al. 1997). Mesalamine, a standard medication in inflammatory bowel disease, was shown to attenuate peroxynitrite-induced apoptosis in human intestinal epithelial cells (Sandoval et al. 1997). In the case of doxorubicin-induced apoptosis in rat small intestine, Sun and coworkers (1998) showed that agents which induce apoptosis may reduce intestinal barrier integrity.
Among the cytokines that have been identified as able to induce apoptosis in intestinal epithelia are tumor necrosis factor-α (TNFα) and interferon-γ (IFN-γ) (Abreu-Martin et al. 1995; Mullin et al. 1997; Wright et al. 1999; Peralta Soler et al. 1999). The role of TNFα on epithelial barrier permeability in renal LLC-PK1 cells was first described by Mullin & coworkers in 1992. Induction of apoptosis by TNFα is accompanied by alteration of tight junction structure (Schmitz et al. 1999b). Therefore, measurement of the overall epithelial conductivity alone cannot prove that barrier impairment is caused by apoptosis. By measurement of local epithelial conductivity with the conductance scanning technique, we recently showed that spontaneous and TNFα-induced apoptosis affect barrier function and may facilitate loss of solutes and uptake of antigens (Gitter et al. 2000a). However, the degree by which apoptosis leads to a breakdown in barrier function still needs to be defined.
In the present study we quantitatively assessed the functional influence of epithelial apoptosis using the human colonic epithelial cell line HT-29/B6 and measured the rate of apoptosis, overall and local epithelial conductivity and unidirectional 3H-tracer fluxes. HT-29/B6 cells form a ‘tight’ epithelium with a relation of transcellular over paracellular conductivity (Gtrans/Gpara) of 10:1 (Gitter et al. 2000b). As a selective tool to up-regulate the rate of apoptosis, the topoisomerase-I inhibitor camptothecin was used, which has been shown to be a potent inductor of apoptosis in epithelial cell lines (Shimizu & Pommier, 1997; Wagner et al. 1997).
PARP cleavage, histone ELISA and DNA-specific fluorochrome staining (DAPI) were used as independent methods for detection of apoptosis. Ussing experiments were performed to measure epithelial conductivity and unidirectional fluxes of 3H-tracers of different molecular weights. Moreover, the conductance scanning technique (Gitter et al. 1997) allowed direct measurement of the local epithelial ion permeability associated with a single site of apoptosis and in non-apoptotic areas. Thus, combining detection of apoptosis with electrophysiological techniques, we determined the relation of the rate of apoptosis and permeability in a model of tight epithelium.
METHODS
Cell culture experiments
Experiments were performed on HT-29/B6 cells, sub-cloned from the human colon carcinoma cell line HT-29 (Kreusel et al. 1991), which grow as highly differentiated polarised monolayers. In order to limit the biological variance of properties, we only used cells from the 26th passage. HT-29/B6 cells were routinely cultured in 25 cm2 culture flasks in RPMI 1640 (Biochrom, Berlin) containing 2 % stabilised l-glutamine and supplemented with 10 % FCS at 37 °C in an atmosphere of 95 % O2 and 5 % CO2. For electrophysiological measurements, cells were seeded on Millicell PCF filters (effective area 0.6 cm2, Millipore) with an average concentration of 7 × 105 cells cm−2. Three filters were placed together into one conventional culture dish (o.d. 60 mm) filled with 10 ml of culture medium. Confluence of the monolayers was reached after 7 days.
Induction and detection of apoptosis
On day 7, confluent monolayers of HT-29/B6 cells were incubated with the topoisomerase-I inhibitor camptothecin, added to the basolateral side at varying concentrations for 48 h. Maximal camptothecin concentration was restricted to 20 μg ml−1 due to its limited solubility in RPMI medium. Another drug with the potential to induce apoptosis (1 μm staurosporine) was also used.
Apoptosis was assessed by several independent methods: poly-(ADP-ribose)-polymerase (PARP) cleavage, enzyme-linked immunosorbent assay (ELISA) with detection of cytosolic oligonucleosome-bound DNA (histone ELISA) and DNA-specific fluorochrome staining (with DAPI). To detect cytotoxic effects of camptothecin, a lactate dehydrogenase (LDH) assay was performed in cell culture supernatants and cell lysates.
PARP cleavage
Cells grown on filter supports were treated with camptothecin (20 μg ml−1) for 48 h or staurosporine (1 μm) for 24 h. Analysis of PARP proteolysis was assessed by resuspending cells in sample buffer (62.5 mm Tris pH 6.8, 6 m urea, 10 % glycerol, 2 % SDS, 5 %β-mercaptoethanol, 0.05 % bromophenol blue). Samples were boiled for 5 min and loaded onto a 10 % SDS-polyacrylamide gel. After electrophoresis and transfer to a PVDF membrane, PARP and its cleavage product were detected with a mouse monoclonal antibody (Oncogene Research Products, Cambridge, UK). Camptothecin- and staurosporine-treated HL-60 cells (promyelocytic leukemia cells) were used as a positive control for apoptosis-specific PARP cleavage (Shimizu & Pommier, 1997).
Detection of cytosolic oligonucleosome-bound DNA with ELISA (histone ELISA)
Another filter was used for quantitative measurement of apoptosis with an ELISA (Boehringer Mannheim) that detects histone-associated DNA fragments enriched in the cytoplasm. The test was performed according to the manufacturer's instructions. Absorbance of treated and control cells was measured at 405 nm. Each test kit was run with a positive and negative control.
DNA-specific fluorochrome staining (with DAPI)
After measurements in the Ussing chamber, filters were resected, fixed in 4 % formalin and embedded in paraffin. Paraffin blocks were cut into 3 μm slices. After incubation with 1 μg ml−1 4′,6′-diamidino-2′-phenylindoladihydrochloride (DAPI; Kapuscinski, 1995), diluted to 1:1000 for 15 min at 37 °C cells were evaluated by fluorescence microscopy (× 40). In the living tissue, apoptosis led to a typical rosette pattern of the rearranging neighbour cells. The typical apoptotic changes comprise condensation of chromatin, its compaction along the periphery of the nucleus, and segmentation of the nucleus. Rate of apoptosis was determined as the percentage of apoptotic nuclei per visual field. We excluded the possibility that relevant parts of the monolayer were lost by a phenomenon called ‘floating’ during induction of apoptosis (Desjardins & MacManus, 1995) with DAPI staining that was also conducted in supernatants.
Cytotoxicity assay
A LDH assay (Madara & Stafford, 1989) was performed to investigate cytotoxic effects of camptothecin. The LDH content of cell supernatants was determined as well as the overall LDH content after lysis with 4 % Triton X-100 for 20 min in controls and cells treated with camptothecin.
Measurement of ion permeability
As a measure of epithelial ion permeability, the overall conductivity (G, mS cm−2) of the monolayers was determined in Ussing chambers specially designed for insertion of Millicell filters (Kreusel et al. 1991; Schulzke et al. 1992; Schmitz et al. 1996). Unidirectional tracer flux measurements from mucosa to serosa (m → s) were performed under short-circuit conditions with 3H-mannitol, 3H-lactulose or 3H-polyethylene glycol (PEG) 4000 (Biotrend, Cologne, Germany). Here, the medium also contained non-labelled tracer molecules, 10 mmol l−1 mannitol, 20 mmol l−1 lactulose or 1 mmol l−1 PEG 4000, respectively. Four 15 min flux periods were analysed after addition of camptothecin. Samples were taken from the mucosal and serosal side, and radioactivity was counted using a Tri-Carb 2100TR Liquid Scintillation Analyzer (Packard, Meriden, CT, USA). Fluxes were calculated with the standard formula described by Schultz & Zalusky (1964).
Conductance scanning
This method allows determination of the spatial distribution of conductivity in flat epithelia (Gitter et al. 2000a). The monolayers were mounted horizontally between the two half-chambers of the conductance scanning apparatus described previously (Gitter et al. 1997). The cells were viewed through a ×40 water-immersion objective lens (Zeiss, Oberkochen, Germany). Alternating electric current (AC, 0.3 mA cm−2, 24 Hz) was clamped across the epithelium and the electric field generated in the mucosal bath solution was measured with a probe at a constant distance of 25 μm above the epithelial surface. The probe was positioned in the centre of the microscope's visual field by means of a mechanical micromanipulator. The probe consisted of a pair of microelectrodes, which were connected to a differential amplifier and an AC bridge system with synchronous demodulation. Control experiments, as described previously (Gitter et al. 1997), excluded the possibility that the data were affected by amplitude or frequency of the current applied. The position of the probe in relation to the cells was adjusted by moving the experimental chamber with a precise, manually controlled, electrically driven micromanipulator (Model 5171, Eppendorf, Hamburg, Germany). The distance to the surface was determined by lifting the epithelium until it barely touched the probe, which induced a characteristic disturbance in the electric signal. At each point recorded from, two measurements were made and averaged.
The local current density was calculated from the electric field measured with the probe and the specific resistivity of the bath solution. The spatial distribution of current density was even above non-apoptotic areas far from apoptoses, but peaked above apoptoses. Non-apoptotic conductivity was determined from the division of current density above non-apoptotic areas by the transepithelial voltage. Since the current density rose toward apoptoses, the current associated with a single apoptosis was computed by spatial integration of the current density exceeding the non-apoptotic current density. From this current and the transepithelial voltage, the conductance associated with the single apoptosis, was determined. The arithmetic mean of conductances associated with apoptosis, multiplied by the density of apoptoses yielded the apoptotic conductivity. In this manner, the overall epithelial conductivity was differentiated into the non-apoptotic epithelial conductivity which is the conductivity of homogeneous non-apoptotic areas of the epithelium, and the apoptotic conductivity which is the conductivity of all apoptoses in the area investigated. The corresponding density of apoptoses was counted using conventional light microscopy in four filters.
Statistical analysis
Results are given as means ±s.e.m.. Significance was tested by means of a Student's two-tailed t test. P < 0.05 was considered significant.
RESULTS
Detection of apoptosis
Apoptosis as detected by PARP cleavage
Camptothecin (Fig. 1B) and staurosporine (Fig. 1C) treatment of HT-29/B6 monolayers resulted in a cleavage of PARP into the specific 85 kDa fragment whereas in untreated cells no cleavage was detected. The largest PARP signal was seen in camptothecin- or staurosporine-treated HL-60 cells which were used as a positive control for apoptosis.
Figure 1. Poly-(ADP-ribose)-polymerase (PARP) cleavage in HT-29/B6 monolayers treated with camptothecin (20 μg ml−1, 48 h) or staurosporine (1 μm, 24 h).
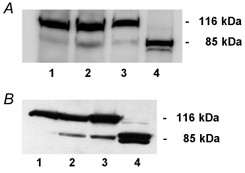
Lysates of cells grown on filter supports were subjected to immunoblotting with anti-PARP antibodies; HL-60 cells served as a positive control for apoptosis. A: lane 1, untreated HT-29/B6 cells; lane 2, treated with camptothecin; lane 3, untreated HL-60 cells; lane 4, treated with camptothecin. B: lane 1, untreated HT-29/B6 cells; lane 2, treated with staurosporine; lane 3, untreated HL-60 cells; lane 4, treated with staurosporine. The upper band shows uncleaved PARP, the lower band cleaved PARP, which indicates apoptosis. Results in both panels are representative of three independent experiments.
Apoptosis as detected by histone ELISA
Histone ELISA allowed quantitative evaluation of apoptosis. After incubation with 20 μg ml−1 camptothecin initial absorbance values (A405nm/A490nm) increased from 0.04 ± 0.01 to 1.78 ± 0.16 after 48 h and to 2.36 ± 0.07 after 72 h (P < 0.001, n = 4; Fig. 2A). Camptothecin treatment dose dependently increased apoptotic rate in HT-29/B6 cell monolayers (Fig. 2B). Minimum effective concentration of camptothecin was 2 μg ml−1 with a most effective concentration at 20 μg ml−1.
Figure 2. Time- and dose-dependence of camptothecin-induced DNA fragmentation during apoptosis.
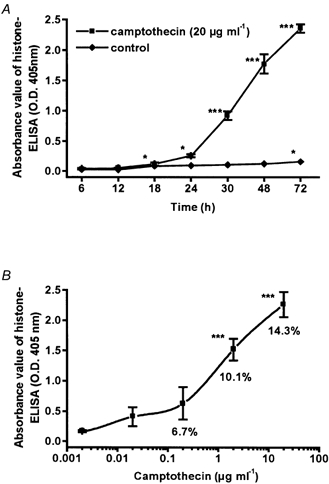
Direct cytosolic nucleosome-bound DNA was detected by histone ELISA in HT-29/B6 cells grown to confluence in 24-well dishes. Data represent means ±s.e.m. (*P < 0.05, ***P < 0.001) of 4 independent experiments (n = 4), each carried out in triplicate. A, time course of cells treated with camptothecin and controls. B, dose-response curve; cells were incubated for 48 h. Values for apoptotic nuclei (percentage of total, control = 3.5 %) were obtained by DAPI staining (n = 6).
Apoptosis as detected by DAPI staining
For measuring the apoptotic rate in HT-29/B6 monolayers the DNA-specific fluorochrome DAPI was used. Typical morphological changes are shown in Fig. 3. After treatment with 20 μg ml−1 camptothecin for 48 h the apoptotic rate was increased from 3.5 ± 0.3 % in controls to 14.3 ± 1.5 % (P < 0.01, n = 8, Fig. 3B). After induction of apoptosis with 1 μm staurosporine, the apoptotic rate was increased from 3.1 ± 0.2 % in controls to 9.7 ± 0.8 % after 6 h (Fig. 3C) and to 23.2 ± 1.3 % after 24 h of incubation (P < 0.001, n = 7).
Figure 3. DAPI staining of HT-29/B6 cell monolayers in controls, after camptothecin (20 μg ml−1 for 48 h) and after staurosporine treatment (1 μm for 6 h).
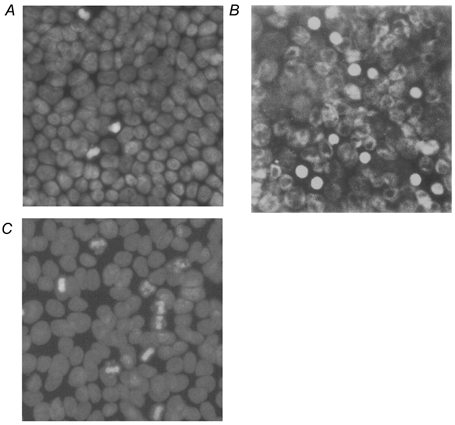
A, untreated cells with spontaneous apoptosis (3.5 %). B and C, increased rate of apoptosis induced by camptothecin (14.3 %;B) or staurosporine (9.7 %, C). Condensed chromatin fragments and segmentation of the nuclei are visible.
In supernatants of HT-29/B6 cells 2.2 ± 0.4 cells per visual field (n = 10) were found after treatment with camptothecin which was not significantly different from control values (2.8 ± 0.4 cells per visual field; n = 10) indicating that in HT-29/B6 monolayers apoptotic cells did not undergo ‘floating’ after camptothecin treatment.
Effect of camptothecin and staurosporine on conductivity
While constant in controls, the conductivity increased continuously during incubation with camptothecin as shown in Fig. 4A. After an incubation period of 7 days with 2 μg ml−1 camptothecin, the conductivity increased from 2.9 ± 0.1 to 9.8 ± 0.5 mS cm−2, equivalent to a reduction of transepithelial resistance from 345 ± 5.0 to 102 ± 5.0 Ω cm2 (P < 0.01, n = 6). After treatment with 20 μg ml−1 camptothecin for 72 h conductivity increased even more from 2.8 ± 0.1 to 21.3 ± 0.5 mS cm−2 (P < 0.01, n = 10). In addition, the change in conductivity caused by camptothecin was dose dependent (Fig. 4B). A minimum effect was reached at 0.2 μg ml−1 and a maximum at 20 μg ml−1, paralleling the dose-dependent effect of camptothecin on apoptosis as detected by histone ELISA and DAPI staining.
Figure 4. Time- and dose-dependence of the camptothecin effect on conductivity (expressed as percentage of control).
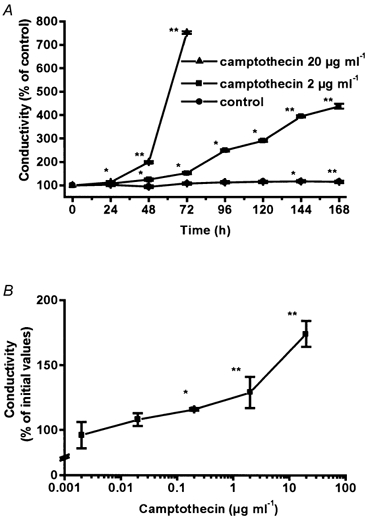
Data represent means ±s.e.m. (*P < 0.05, **P < 0.01). A, time course of untreated cells (from 3.0 to 2.6 mS cm−2 after 48 h, n = 10), and cells incubated with 2 μg ml−1 (from 2.9 to 3.8 mS cm−2 after 48 h, n = 6), or 20 μg ml−1 (from 2.8 to 5.6 mS cm−2 after 48 h, n = 10) camptothecin. B, dose dependency; cells were treated with camptothecin for 48 h. Changes in conductivity are expressed as percentage of initial values (n = 10).
Staurosporine (1 μm) increased transepithelial conductivity from 3.2 ± 0.1 to 6.1 ± 0.1 mS cm−2 after 6 h (P < 0.01, n = 4) and 18.0 ± 0.1 mS cm−2 after 12 h of incubation (P < 0.001, n = 4).
Fluxes of 3H-mannitol, 3H-lactulose and 3H-PEG 4000
Camptothecin increased the mucosa-to-serosa fluxes of paracellular tracer molecules concomitant with the increase in ion permeability (Fig. 5). The flux of 3H-mannitol increased 3.8-fold from 56 ± 6 in controls to 216 ± 69 nmol h−1 cm−2 (n = 8) and that of 3H-lactulose increased 2.6-fold from 89 ± 3 nmol h−1 cm−2 in controls to 231 ± 13 nmol h−1 cm−2 (n = 8) in cells treated with 20 μg ml−1 camptothecin for 48 h. This indicates an increased permeability in the paracellular route. By contrast, the flux of 3H-PEG 4000 remained unchanged (from 10.8 ± 0.5 to 10.5 ± 1.1 nmol h−1 cm−2, n = 4). The short-circuit current was not changed by addition of camptothecin (-4.67 ± 0.05 versus -6.10 ± 2.08 μA cm−2 in control, n.s., n = 8) pointing against an increase in transcellular conductivity due to activation of active transport sites.
Figure 5. Unidirectional mucosa-to-serosa fluxes of different 3H-labelled tracers in HT-29/B6 cells treated with camptothecin for 48 h versus controls.
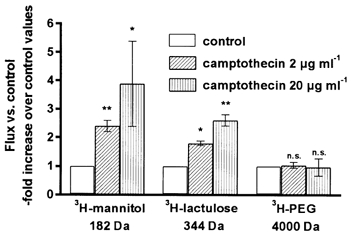
Data represent means ±s.e.m. (*P < 0.05, **P < 0.01).
LDH assay
An LDH assay was performed to investigate cytotoxic effects of camptothecin. In untreated controls LDH release was 1.1 ± 1.5 %. This value did not significantly change after addition of 2 μg ml−1 camptothecin for 48 h (1.1 ± 1.7 %, n.s., n = 6) and 72 h (1.5 ± 0.1 %, n.s., n = 10). When incubated with camptothecin at a concentration of 20 μg ml−1, LDH release did not significantly change compared with controls after 48 h (1.4 ± 0.1 %, n.s., n = 6) but was increased after 72 h (11.2 ± 1.1 %, P < 0.05, n = 6). This indicates that 2 μg ml−1 camptothecin did not induce cell lysis at all which is, for example, observed during necrosis, but incubation with 20 μg ml−1 camptothecin increased LDH release after 72 h. In order to verify that this assay is sensitive enough to detect cell lysis, HT-29/B6 cells were also treated with IFN-γ (1000 U l−1) as a positive control. After 72 h of incubation with IFN-γ, LDH release was increased to 26.7 ± 4.8 % (P < 0.01, n = 6).
Conductance scanning of single cell apoptosis
The apparent local conductivity, i.e. local current density divided by transepithelial voltage, is shown in a typical recording along a line between the apoptotic cell and a non-apoptotic area (Fig. 6). As the current density was measured 25 μm above the mucosal surface, the current associated with apoptosis was determined by integration of the bell-shaped distribution of the local transepithelial current. Thus our recordings differentiated between (a) the current through areas of non-apoptotic cells exhibiting a planar spatial distribution, and (b) the current associated with sites of apoptosis exhibiting a bell-shaped distribution forming a maximum above the apoptotic cell. In the experiments, the overall epithelial conductivity doubled from 3.24 ± 0.07 mS cm−2 to 6.37 ± 0.97 mS cm−2 (P < 0.01, n = 20, Fig. 7A) after treatment with 20 μg ml−1 camptothecin. This was not due to an increase in non-apoptotic epithelial conductivity, which was not altered by addition of camptothecin (2.53 ± 0.35 vs. 3.06 ± 0.07 mS cm−2, n.s., n = 11, Fig. 7A), but was the result of an increase in apoptotic conductivity which increased 22-fold (from 0.17 ± 0.02 to 3.84 ± 1.03 mS cm−2, P < 0.01, n = 11, Fig. 7A). This increase in apoptotic conductivity was caused, on the one hand, by a higher density of apoptoses, which increased from 24.4 ± 4.6 to 103.0 ± 9.2 mm−2 (P < 0.01, n = 4), and, on the other hand, by the higher conductances associated with apoptosis, which were dramatically increased compared with that in untreated cell monolayers (0.72 ± 0.23 versus 0.05 ± 0.02 μS, P < 0.01, n = 11, Fig. 7B).
Figure 6. Conductance scanning of single cell apoptosis.

Apparent local conductivity (local current density measured with the mobile probe, divided by the transepithelial voltage) along a line between an apoptosis (arrow) and a non-apoptotic area with homogeneous conductivity. HT-29/B6 cells were treated with 20 μg ml−1 camptothecin for 48 h.
Figure 7. Contribution of apoptoses to epithelial conductivity.

A, non-apoptotic conductivity of HT-29/B6 monolayers as well as apoptotic conductivity of apoptotic rosettes as obtained by conductance scanning (means ±s.e.m., n = 11). The sum of both non-apoptotic and apoptotic epithelial conductivity represents the overall epithelial conductivity. B, conductance of single apoptoses: left, control (n = 20); right, camptothecin, 20 μg ml−1 (n = 11). Horizontal bars represent mean values.
Thus, apoptosis contributed 60 % to overall epithelial conductivity if induced by 20 μg ml−1 camptothecin, compared with a 5.5 % contribution in controls.
Correlation of the rate of apoptosis with apoptotic conductivity
The percentage of apoptotic cells correlated with the apoptotic conductivity which increased from 0.17 ± 0.02 (control) to 2.78 ± 0.7 mS cm−2 (20 μg ml−1 camptothecin, Fig. 8A), and thus also with the overall epithelial conductivity (Fig. 8B). With a higher rate of apoptosis, the conductivity associated with apoptosis increased exponentially (Fig. 8A) along with the mean conductance of apoptoses (see above).
Figure 8. Correlation of the rate of apoptosis with apoptotic epithelial conductivity and overall conductivity in HT-29/B6 monolayers.
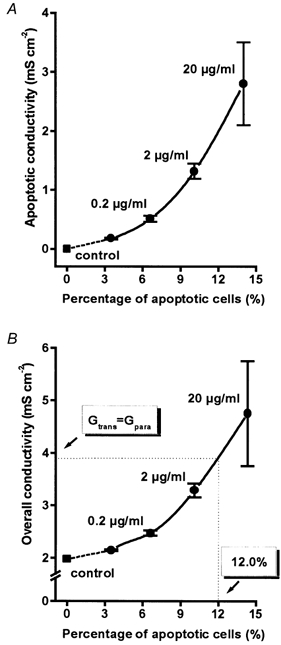
Apoptosis was induced by camptothecin at a concentration of between 0 and 20 μg ml−1 and was quantified as the percentage of apoptotic cells by DAPI staining. Values for 0 % apoptosis were measured in non-apoptotic areas of control monolayers. A, apoptotic conductivity increased exponentially (r2= 0.998, P = 0.001) with the rate of apoptosis, because not only the rate but also the mean conductance of apoptosis increased. B, likewise, overall epithelial conductivity increased with the rate of apoptosis. Under control conditions the paracellular conductivity (Gpara) was smaller than the transcellular (Gtrans), but with 12 % apoptosis, Gpara surpassed Gtrans and, by definition, the epithelium became ‘leaky’.
Since the non-apoptotic conductivity did not change with camptothecin treatment, the increase in overall epithelial conductivity (Fig. 8B) reflected the increase in apoptotic conductivity. The constant conductivity of non-apoptotic epithelium suggests that the transcellular conductivity is not affected by camptothecin. Leaks at the site of apoptosis provide a paracellular route of permeation. Thus, under control conditions as well as with camptothecin, the transcellular conductivity, which is 9/10 of the overall conductivity of HT-29/B6 cells (Gitter et al. 2000b), was about 2 mS cm−2. By interpolation of the data with 2 and 20 μg ml−1 camptothecin (Fig. 8B) it was shown that at an apoptotic rate of 12 % the overall epithelial conductivity was twice the transcellular conductivity, and thus the paracellular conductivity equalled the transcellular conductivity. Hence, the originally ‘tight’ epithelium turned to a ‘leaky’ epithelium.
DISCUSSION
Studying the functional influence of apoptosis in native intestinal epithelia is hampered by the complex structure of crypt and surface epithelia covering several layers of subepithelial tissues (Sträter et al. 1995; Potten, 1996). The conductance scanning technique allowed a direct measurement of the local conductance of single cell apoptoses. However, these experiments are difficult to perform in native epithelia. For these reasons, we used the established human colonic cell line HT-29/B6 (Kreusel et al. 1991) which forms stable, confluent monolayers for more than a week and exhibits transport and barrier functions typical of human colonic epithelium. However, this cancer cell line is not an exact model of the native epithelium (Wang et al. 2001), and the transformation process may have altered its response to apoptotic stimuli. For instance, HT-29 cells contain a p53 mutation (Rodrigues et al. 1990), which may affect their response to apoptotic stimuli (Shao et al. 1997; Zhang et al. 2000). Moreover, it was shown that HT-29 cells do not express the anti-apoptotic bcl-2 gene (Ossina et al. 1997; O'Connell et al. 2000), which is found in human colonic epithelium (Iimura et al. 2000). Although signal transduction processes of apoptotic and anti-apoptotic pathways are mutated in HT-29/B6 cells, the effect of apoptosis induced by camptothecin (50 μm, 24 h) is similar to that of mouse colonocytes derived from isolated murine crypts (Wenzel et al. 2000). Therefore, we believe that the HT-29/B6 model is suitable for studying functional consequences of camptothecin-induced apoptosis, although further studies are needed to investigate these effects in non-transformed colonic cells.
Apoptosis in the gastrointestinal tract
Epithelial apoptosis plays a central role in the regulation of cell number in gastrointestinal epithelia whereas cell loss by non-apoptotic exfoliation seems to be of minor importance (Hall et al. 1994). Whether or not apoptosis impairs intestinal barrier integrity has been discussed controversially. The elimination of cells from the normal epithelium is followed by a physiological rearrangement of tight junctions with maintenance of the macromolecular barrier (Madara, 1990). Therefore, it was assumed that apoptosis of isolated epithelial cells occurs without relevant disruption of epithelial integrity (Jones & Gores, 1997). However, apoptosis evoked not only under pathological conditions but also that occurring during normal epithelial turnover, is associated with a barrier defect. This was first described by Gitter and coworkers by direct measurement of local ion permeability which demonstrates increases in conductivity (leaks) at the site of spontaneous and TNFα-induced apoptosis (Gitter et al. 2000a). As these experiments were performed in a cell culture system and not in native epithelium, care must be taken before our findings are applied to human native tissues. However, the results expand current concepts regarding the functional characteristics of spontaneous and induced apoptosis. Impairment of the epithelial barrier may result in passive loss of ions and water into the lumen of the intestine with the consequence of leak-flux diarrhoea (Rask-Madsen & Brix Jensen, 1973). The apoptotic event is followed by a tissue remodelling process which shows a rosette pattern of rearranging neighbour cells (Peralta Soler et al. 1996; Gitter et al. 2000a). However, the quantitative contribution of apoptotic events to epithelial barrier function remains to be elucidated.
The rate of spontaneous and camptothecin-induced apoptosis
In the fixed and DAPI-stained tissue of untreated control monolayers of HT-29/B6 cells we found a 3.5 % rate of apoptosis, which is higher than the 1.4 % of apoptosis described in both normal crypts of mice (Fazeli et al. 1997) and human jejunal mucosa (Moss et al. 1996) but is somewhat lower than the 6.2 % of spontaneous apoptosis in the human colon cell line VACO-330 (Wang et al. 1995) and the approximately 10 % in Caco-2 cells (Giovanni et al. 2000). The distribution of apoptosis in human colonic crypts was evaluated semiquantitatively by Sträter and coworkers (1995). We determined the rate of apoptosis in DAPI-stained human colonic tissues and obtained 1 % of apoptosis (data not shown).
Camptothecin is a potent inductor of apoptosis in epithelial cell lines (Shimizu & Pommier, 1997; Wagner et al. 1997). In HT-29/B6 cells, 20 μg ml−1 of camptothecin increased the rate of apoptosis 4.1-fold to 14.3 %. Under this condition the probability is much higher that apoptotic cells come into direct contact with one another with the potential to disturb conventional rosette formation (Peralta Soler et al. 1996). For a hexagonal array of cells with a probability P of each cell to be apoptotic, the proportion of apoptotic cells with apoptotic neighbours is P - P (1 - P)6 or 0.67 % for P = 3.5% and 8.63 % for P = 14.3%. Thus the direct neighbourhood of apopototic cells may contribute to the overproportional decrease in barrier function. This may be relevant for pathological conditions such as coeliac disease, where human enterocyte apoptosis is dramatically increased up to > 20 % (Moss et al. 1996), colorectal adenomas in mice after γ-irradiation where apoptosis reaches 17 % (Fazeli et al. 1997) or graft versus host disease (Stüber et al. 1999).
Permeability for ions and molecules of different size
The increase in conductivity was caused by increases in the number as well as the mean conductance associated with single apoptoses. Non-apoptotic conductivity did not change after addition of camptothecin. By contrast, in TNFα-treated HT-29/B6 monolayers the non-apoptotic conductivity also increased (Gitter et al. 2000a). This was explained by an effect of TNFα on the tight junctions of non-apoptotic epithelium, demonstrated by freeze fracture electron microscopy (Schmitz et al. 1999a). Hence, unlike TNFα, camptothecin is a selective tool for induction of apoptosis. Our results, showing that barrier defects at the site of apoptosis lead to an increase in epithelial conductivity, corroborate the hypothesis that apoptosis causes barrier dysfunction in intestinal epithelia (Gitter et al. 2000a).
The present findings show that camptothecin affects apoptosis with a concomitant increase of both conductivity and mucosa-to-serosa fluxes of the paracellular markers 3H-mannitol (cross-sectional diameter 0.6 nm; Lane et al. 1996) and 3H-lactulose. These results are in contrast with the effect of TNFα on CACO-2 BBE cells, causing a decrease in resistance without an increase in mannitol flux (Marano et al. 1998), but are in accordance with the effect of TNFα on LLC-PK1 cells where both incidence of apoptosis and transepithelial mannitol flux was increased (Mullin et al. 1997).
The cross sectional diameter of 1.2 nm for 3H-PEG 4000 was twice that of mannitol (Lane et al. 1996). Considering its size, the flux of PEG 4000 was relatively high; this feature has also been observed by others (Bjarnason et al. 1995). More importantly, in contrast to mannitol and lactulose the flux of PEG 4000 was unchanged after addition of camptothecin. Thus, the size of potentially noxious agents penetrating apoptosis-related leaks in the mucosal barrier to molecules must be smaller than 1.2 nm. Neither bacteria nor viruses can penetrate the leaks caused by apoptosis induced by camptothecin. As the permeability to molecules of 4000 Da was unaltered, it is unlikely that molecules of a size sufficient to possess antigenic properties can pass through the damaged epithelium. By contrast, smaller molecules, e.g. haptens, might penetrate through the apoptotic leaks.
Recent findings indicate that transcytosis provides a major route for uptake of protein antigens in sensitised animals (Berin et al. 1997; Yang et al. 2000). We have not investigated transcytosis, but the cell cultures we used were not sensitised. Moreover, transcytotic transfer is slow compared with the current pulses used in our experiments and would, therefore, not affect the conductance measured.
Functional implications of apoptosis
The present findings not only refute the dogma stating an absence of apoptotic leaks (Jones & Gores, 1997), but also give a measure of the barrier impairment associated with apoptosis. In untreated HT-29/B6 monolayers the ratio of trans- and paracellular conductivity (Gtrans/Gpara) is about 10:1 (Gitter et al. 2000b) in accordance with the definition of a ‘tight’ epithelium. By interpolation of our data it can be concluded that the formerly ‘tight’ colonic epithelium became ‘leaky’ at 12 % apoptosis (Gtrans= Gpara) if we assume that apoptotic conductivity contributed to Gpara. This value may be used to describe the functional change in colonic epithelium induced by apoptosis.
Topoisomerase inhibitors are under intensive investigation as a therapeutic drug against a variety of tumors (Kollmannsberger et al. 1999). The derivative of camptothecin, irinotecan (CPT-11), is clinically effective against several epithelial cancers (Ikuno et al. 1995). A major side effect is diarrhoea, which is assumed to be the result of intestinal barrier disturbance (Ewesuedo & Ratain, 1997). Since the present study shows that camptothecin impairs intestinal barrier function by induction of apoptosis without induction of secretion, it appears reasonable to assume that this mechanism is the main cause of irinotecan-induced diarrhoea.
Location of the conductive changes induced by apoptosis
The bell-shaped current peak above the apoptotic cell (Fig. 6) may reflect conductance increase of (i) the cell membranes of the apoptotic cell, and/or (ii) the tight junctions between the apoptotic cell and the surrounding ‘rosette’ cells, and/or (iii) the tight junctions between neighbouring rosette cells. The apical cell membrane of the apoptotic cell remains optically intact during apoptosis. The tight junctions between the apoptotic cell and the surrounding rosette cells and to a lesser extent also the tight junctions between neighbouring rosette cells underlie a geometrical change during apoptosis. Epithelial tight junctions are able to adapt to a changing geometry within seconds, e.g. during peristaltic contraction. Here, the changes took several hours but the dying apoptotic cell may have lost this capability of adaptation.
On the other hand, this may not be a serious challenge for the intact rosette cells. If apoptosis is induced by TNFα, tight junctions of the non-apoptotic area also open (Gitter et al. 2000a), suggesting that in this case tight junctions between neighbouring rosette cells are also altered. However, during camptothecin-induced apoptosis the conductance of the non-apoptotic area was unchanged (Fig. 7A). In addition, if one single cell in the non-apoptotic area was experimentally destroyed, a very similar bell-shaped current peak above the deleted cell is found (data not shown).
It was beyond the scope of the present study to determine the subcellular localisation of the conductance changes induced by apoptosis, but taken together the findings suggest that the tight junctions between apoptotic cell and surrounding cells are the main cause for the increased conductivity, although a contribution of the tight junctions between rosette cells cannot be ruled out.
Conclusion
Our study presents, for the first time, epithelial permeability as a function of apoptosis. By conductance scanning, camptothecin was shown to be a selective inductor of apoptosis. It increased apoptotic rate and caused a dramatic increase of conductance associated with apoptosis. Thus, ion permeability increased with the percentage of apoptotic cells. Quantitatively, the apoptoses contributed 5.5 % to the overall epithelial conductivity under control conditions and 60 % to the overall epithelial conductivity after 48 h treatment with 20 μg ml−1 camptothecin. By definition, an epithelium is ‘tight’ if the paracellular conductivity is lower than the transcellular one (Gpara< Gtrans), and otherwise ‘leaky’ (GparaGtrans). Hence, the formerly ‘tight’ epithelium becomes ‘leaky’ at 12 % apoptosis. Our flux experiments lead to the hypothesis that the size of potentially noxious agents in our model of colonic epithelial cells undergoing apoptosis is limited to molecules smaller than 4000 Da. Nevertheless, this means that apoptotic events have to be considered of great functional relevance in intestinal diseases associated with barrier impairment, e.g. in inflammatory bowel disease or coeliac sprue, because they provoke loss of solutes and invasion of small molecules.
Acknowledgments
This study was supported by DFG Schu 559/6-3 and 7-1 and the Sonnenfeld-Stiftung Berlin.
References
- Abreu-Martin MT, Vidrich A, Lynch DH, Targan SR. Divergent induction of apoptosis an IL-8 secretion in HT-29 cells in response to TNFα ligation of Fas antigen. Journal of Immunology. 1995;155:4147–4154. [PubMed] [Google Scholar]
- Berin MC, Kiliaan AJ, Yang PC, Groot JA, Taminiau JA, Perdue MH. Rapid transepithelial antigen transport in rat jejunum: Impact of sensitization and the hypersensitivity reaction. Gastroenterology. 1997;113:856–864. doi: 10.1016/s0016-5085(97)70180-x. [DOI] [PubMed] [Google Scholar]
- Bjarnason I, Macpherson A, Hollander D. Intestinal permeability: An overview. Gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- Desjardins LM, MacManus JP. An adherent cell model to study different stages of apoptosis. Experimental Cell Research. 1995;216:380–387. doi: 10.1006/excr.1995.1048. [DOI] [PubMed] [Google Scholar]
- Ewesuedo RB, Ratain MJ. Topoisomerase I inhibitors. Oncologist. 1997;2:359–364. [PubMed] [Google Scholar]
- Fazeli A, Stehen RG, Dickinson SL, Bautista D, Dietrich WF, Bronson RT, Bresalier RS, Lander ES, Costa J, Weinberg RA. Effects of p53 mutations on apoptosis in mouse intestinal and human colonic adenomas. Proceedings of the National Academy of Sciences of the USA. 1997;94:10199–10204. doi: 10.1073/pnas.94.19.10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini C, Sanchez M, Straface E, Scazzocchio B, Silano M, De Vincenzi M. Induction of apoptosis in Caco-2 cells by wheat gliadin peptides. Toxicology. 2000;145:63–71. doi: 10.1016/s0300-483x(99)00223-1. [DOI] [PubMed] [Google Scholar]
- Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNFα-induced single-cell apoptosis. FASEB Journal. 2000a;14:1749–1753. doi: 10.1096/fj.99-0898com. [DOI] [PubMed] [Google Scholar]
- Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Trans/paracellular, surface/crypt, and epithelial/ subepithelial resistances of mammalian colonic epithelia. Pflügers Archiv. 2000b;439:477–482. doi: 10.1007/s004249900202. [DOI] [PubMed] [Google Scholar]
- Gitter AH, Bertog M, Schulzke JD, Fromm M. Measurement of paracellular epithelial conductivity by conductance scanning. Pflügers Archiv. 1997;434:830–840. doi: 10.1007/s004240050472. [DOI] [PubMed] [Google Scholar]
- Hall PA, Coates PJ, Ansari B, Hopwood DJ. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. Journal of Cell Science. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- Iimura M, Nakamura T, Shinozaki S, Iizuka B, Inoue Y, Suzuki S, Hayashi N. Bax is downregulated in inflamed colonic mucosa of ulcerative colitis. Gut. 2000;47:228–235. doi: 10.1136/gut.47.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuno N, Soda H, Watanabe M, Oka M. Irinotecan (CPT-11) and characteristic mucosal changes in the mouse ileum and cecum. Journal of the National Cancer Institute. 1995;87:1876–1883. doi: 10.1093/jnci/87.24.1876. [DOI] [PubMed] [Google Scholar]
- Jones BA, Gores GJ. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. American Journal of Physiology. 1997;273:G1174–1188. doi: 10.1152/ajpgi.1997.273.6.G1174. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J. DAPI: a DNA-specific fluorescent probe. Biotechnic and Histochemistry. 1995;70:220–233. doi: 10.3109/10520299509108199. [DOI] [PubMed] [Google Scholar]
- Kollmannsberger C, Mross K, Jakob A, Kanz L, Bokemeyer C. Topotecan-A novel topoisomerase I inhibitor: pharmacology and clinical experience. Oncology. 1999;56:1–12. doi: 10.1159/000011923. [DOI] [PubMed] [Google Scholar]
- Kreusel KM, Fromm M, Schulzke JD, Hegel U. Cl-secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6) American Journal of Physiology. 1991;261:C574–582. doi: 10.1152/ajpcell.1991.261.4.C574. [DOI] [PubMed] [Google Scholar]
- Lane ME, O'Driscoll CM, Corrigan OI. The relationship beetween rat intestinal permeability and hydrophilic probe size. Pharmaceutical Research. 1996;13:1554–1558. doi: 10.1023/a:1016091915733. [DOI] [PubMed] [Google Scholar]
- Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. Journal of Membrane Biology. 1990;116:177–184. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- Madara JL, Stafford J. Interferon-γ directly affects barrier function of cultured intestinal epithelial monolayers. Journal of Clinical Investigation. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marano CW, Lewis SA, Garulacan LA, Peralta Soler A, Mullin JM. Tumor necrosis factor-α increases sodium and chloride conductance across the tight junction of CACO-2 BBE, a human intestinal epithelial cell line. Journal of Membrane Biology. 1998;161:263–274. doi: 10.1007/s002329900333. [DOI] [PubMed] [Google Scholar]
- Moss SF, Attia L, Scholes JV, Walters JR, Holt PR. Increased small intestinal apoptosis in coeliac disease. Gut. 1996;39:811–817. doi: 10.1136/gut.39.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin JM, Laughlin KV, Marano CW, Russo LM, Peralta Soler A. Modulation of tumor necrosis factor-induced increase in renal (LLC-PK1) transepithelial permeability. American Journal of Physiology. 1992;263:F915–924. doi: 10.1152/ajprenal.1992.263.5.F915. [DOI] [PubMed] [Google Scholar]
- Mullin JM, Marano CW, Laughlin KV, Nuciglio M, Stevenson BR, Soler AP. Different size limitations for increased transepithelial paracellular solute flux across phorbol ester and tumor necrosis factor-treated epithelial cell sheets. Journal of Cellular Physiology. 1997;171:226–233. doi: 10.1002/(SICI)1097-4652(199705)171:2<226::AID-JCP14>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- O'Connell J, Bennett MW, Nally K, O'Sullivan GC, Collins JK, Shanahan F. Interferon-γ sensitizes colonic epithelial cell lines to physiological and therapeutic inducers of colonocyte apoptosis. Journal of Cellular Physiology. 2000;185:331–338. doi: 10.1002/1097-4652(200012)185:3<331::AID-JCP3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ossina NK, Cannas A, Powers VC, Fitzpatrick PA, Knight JD, Gilbert JR, Shekhtman EM, Tomei LD, Umansky SR, Kiefer MC. Interferon-γ modulates a p53-independent apoptotic pathway and apoptosis-related gene expression. Journal of Biological Chemistry. 1997;272:16351–16357. doi: 10.1074/jbc.272.26.16351. [DOI] [PubMed] [Google Scholar]
- Peralta Soler A, Marano CW, Bryans M, Miller RD, Garulacan LA, Mauldin SK, Stamato TD, Mullin JM. Activation of NF-χB is necessary for the restoration of the barrier function of an epithelium undergoing TNFα-induced apoptosis. European Journal of Cell Biology. 1999;78:56–66. doi: 10.1016/s0171-9335(99)80007-7. [DOI] [PubMed] [Google Scholar]
- Peralta Soler A, Mullin JM, Knudsen KA, Marano CW. Tissue remodeling during tumor necrosis factor-induced apoptosis in LLC-PK1 renal epithelial cells. American Journal of Physiology. 1996;270:F869–879. doi: 10.1152/ajprenal.1996.270.5.F869. [DOI] [PubMed] [Google Scholar]
- Potten CS. What is an apoptotic index measuring? A commentary. British Journal of Cancer. 1996;74:1743–1748. doi: 10.1038/bjc.1996.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. Epithelial cell growth and differentiation II. Intestinal apoptosis. American Journal of Physiology. 1997;273:G253–257. doi: 10.1152/ajpgi.1997.273.2.G253. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen J, Brix Jensen P. Electrolyte transport capacity and electrical potentials of the normal and the inflamed human rectum in vivo. Scandinavian Journal of Gastroenterology. 1973;8:169–175. [PubMed] [Google Scholar]
- Rodrigues NR, Rowan A, Smith ME, Kerr IB, Bodmer WF, Gannon JV, Lane DP. p53 mutations in colorectal cancer. Proceedings of the National Academy of Sciences of the USA. 1990;87:7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval M, Liu X, Mannick EE, Clark DA, Miller MJS. Peroxynitrite-induced apoptosis in human intestinal epithelial cells is attenuated by mesalamine. Gastroenterology. 1997;113:1480–1488. doi: 10.1053/gast.1997.v113.pm9352850. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Benzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999a;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis-factor-alpha (TNFα) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. Journal of Cell Science. 1999b;112:137–146. doi: 10.1242/jcs.112.1.137. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Fromm M, Bode H, Scholz P, Riecken EO, Schulzke JD. Tumor necrosis factor-α induces Cl− and K+ secretion in human distal colon driven by prostaglandin E2. American Journal of Physiology. 1996;271:G669–674. doi: 10.1152/ajpgi.1996.271.4.G669. [DOI] [PubMed] [Google Scholar]
- Schultz SG, Zalusky R. Ion transport in isolated rabbit ileum. I. Short-circuit current and Na fluxes. Journal of General Physiology. 1964;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulzke JD, Fromm M, Bentzel CJ, Zeitz M, Menge H, Riecken EO. Ion transport in the experimental short bowel syndrome of the rat. Gastroenterology. 1992;102:497–504. doi: 10.1016/0016-5085(92)90096-h. [DOI] [PubMed] [Google Scholar]
- Shao RG, Cao CX, Pommier Y. Activation of PKCα downstream from caspases during apoptosis induced by 7-hydroxystaurosporine or the topoisomerase inhibitors, camptothecin and etoposide, in human myeloid leukemia HL60 cells. Journal of Biological Chemistry. 1997;272:31321–31325. doi: 10.1074/jbc.272.50.31321. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Pommier Y. Camptothecin-induced apoptosis in p53-null human leukemia HL60 cells and their isolated nuclei: effects of the protease inhibitors Z-VAD-fmk and dichloroisocoumarin suggest an involvement of both caspases and serine proteases. Leukemia. 1997;11:1238–1244. doi: 10.1038/sj.leu.2400734. [DOI] [PubMed] [Google Scholar]
- Sträter J, Koretz K, Günthert AR, Möller P. In situ detection of enterocytic apoptosis in normal colonic mucosa and in familial adenomatous polyposis. Gut. 1995;37:819–825. doi: 10.1136/gut.37.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sträter J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, Krammer PH, Möller P. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology. 1997;113:160–167. doi: 10.1016/s0016-5085(97)70091-x. [DOI] [PubMed] [Google Scholar]
- Stüber E, Büschenfeld A, Von Freier A, Arendt T, Fölsch UR. Intestinal crypt cell apoptosis in murine acute graft versus host disease is mediated by tumor necrosis factor α and not by the FasL-Fas interaction: effect on pentoxifylline on the development of mucosal atrophy. Gut. 1999;45:229–235. doi: 10.1136/gut.45.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Wang X, Wallen R, Deng X, Du X, Hallberg E, Andersson R. The influence of apoptosis on intestinal barrier integrity in rats. Scandinavian Journal of Gastroenterology. 1998;33:415–422. doi: 10.1080/00365529850171053. [DOI] [PubMed] [Google Scholar]
- Wagner S, Beil W, Westermann J, Logan RPH, Bock CT, Trautwein C, Bleck JS, Manns MP. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology. 1997;113:1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hung C, Koh D, Cheong D, Hooi SC. Differential expression of Hox A5 in human colon cancer cell differentiation: A quantitative study using real-time RT-PCR. International Journal of Oncology. 2001;18:617–622. doi: 10.3892/ijo.18.3.617. [DOI] [PubMed] [Google Scholar]
- Wang CY, Eshleman JR, Willson JK, Markowitz S. Both transforming growth factor-beta and substrate release are inducers of apoptosis in a human colon adenoma cell line. Cancer Research. 1995;55:5101–5105. [PubMed] [Google Scholar]
- Wenzel U, Kuntz S, Brendel MD, Daniel H. Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells. Cancer Research. 2000;60:3823–3831. [PubMed] [Google Scholar]
- Wright K, Kolios G, Westwick J, Ward SG. Cytokine-induced apoptosis in epithelial HT-29 cells is independent of nitric oxide formation. Evidence for an interleukin-13-driven phosphatidylinositol-3-kinase-dependent survival mechanism. Journal of Biological Chemistry. 1999;274:17193–17201. doi: 10.1074/jbc.274.24.17193. [DOI] [PubMed] [Google Scholar]
- Yang PC, Berin MC, Yu LCH, Conrad DH, Perdue MH. Enhanced intestinal transepithelial antigen transport in allergic rats is mediated by IgE and CD23 (FcεRII) Journal of Clinical Investigation. 2000;106:879–886. doi: 10.1172/JCI9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Patchett SE, Farthing MJ. Topoisomerase I inhibitor (camptothecin)-induced apoptosis in human gastric cancer cells and the role of wild-type p53 in the enhancement of its cytotoxicity. Anticancer Drugs. 2000;11:757–764. doi: 10.1097/00001813-200010000-00013. [DOI] [PubMed] [Google Scholar]


