Abstract
It was previously shown that expressed in Xenopus oocyte the mouse (mAE1) and the trout (tAE1) anion exchanger behave differently: both elicit anion exchange activity but only tAE1 induces a transport of organic solutes correlated with a chloride channel activity. The present data, obtained by measurement of Xenopus oocyte membrane permeability and conductance, provide evidence that tAE1 also induces a large increase in Na+ and K+ permeability inhibited by several AE1 inhibitors.
This inhibition does not result from an effect on the driving force for electrodiffusion but represents a direct effect on the cation pathway.
As a control, expression of cystic fibrosis transmembrane conductance regulator (CFTR) having, once stimulated by 3-isobutyl-1-methylxanthine (IBMX), the same anion conductance magnitude as tAE1 did not induce any cation movement.
Chloride exchange, channel activity and cation transport induced by anion exchanger expression are inhibited by free or covalently bound H2DIDS as well. This covalent inhibition is reversed by the point mutation of Lys-522, the covalent binding site of H2DIDS to the protein. These data reveal that tAE1 itself acts both as an anion exchanger and as a channel of broad selectivity.
All results obtained by expression of AE1 isoforms in Xenopus oocytes and those obtained in erythrocytes are consistent with the proposal that, in nucleated erythrocytes, tAE1 functions as the swelling-activated osmolyte anion channel involved in cell volume regulation. In contrast AE1 from mammalian red cells, which do not regulate their volume, lacks swelling-activated osmolyte channel properties.
tAE1 illustrates the ability of a specific transport system to be a multifunctional protein exhibiting other transport functions when submitted to regulation.
Many cells, when swollen, activate membrane transport systems that mediate the release of ions and small organic molecules (amino acids such as taurine, polyols such as sorbitol and inositol), with the result that cells undergo a regulatory volume decrease (RVD). There is mounting evidence (Strange et al. 1996; Kirk, 1997) that transport of these structurally unrelated organic molecules is mediated by a single pathway having the characteristics of an anion-selective channel. The cation permeability of such channels is generally low but can greatly vary between cell types, raising the possibility that a significant contribution of the volume regulatory efflux of KCl from some cell types occurs via these channels. The molecular identity of these volume regulatory channels, however, remains an open question (Kirk, 1997).
Fish red blood cells have proved to be a useful model for studying swelling-activated transport systems involved in volume regulation. These cells can adopt different regulatory patterns depending on how their volume has been altered (isosmotic or hypotonic swelling), showing that cells are responding to more than just simple volume enlargement (Motais et al. 1991). After hypotonically induced swelling, they undergo a large RVD, characterised by the loss of both KCl and taurine, but which is partly counteracted by an entry of Na+ down its electrochemical gradient (Garcia-Romeu et al. 1991). There is also a simultaneous activation of a transport of polyols (Kirk et al. 1992) which is, however, inefficient in terms of volume regulation due to a very low level of polyols in these cells. There is convincing evidence (Lewis et al. 1996; Kirk 1997; Guizouarn & Motais, 1999) that all these solutes, including cations, share a common anion channel displaying considerable similarities to those described in other cells quoted above. Consistent with that, it has been shown with patch-clamp techniques that a DIDS-sensitive Cl− conductance is reversibly activated when trout red cells are hypotonically swollen (Egée et al. 1997). The anion channel is ‘switched on’, not by the volume increase, but exclusively by the decrease in intracellular ionic strength (Guizouarn & Motais, 1999; authors’ unpublished observations). Moreover taurine has been shown to move through the channel as a zwitterion (Guizouarn et al. 2000).
From pharmacological data, a role has been proposed for the band 3 protein in the swelling-activated response of fish red blood cells either as a swelling-activated channel (Goldstein & Musch, 1994) or as a regulatory protein coupling the activity of transport systems to the volume-sensing mechanisms (Motais et al. 1991, 1992; Garcia-Romeu et al. 1991). Band 3 is a major constituent of the red cell membrane and is known to operate as an electroneutral anion exchanger (AE1).
Expression in Xenopus oocytes of trout red cell anion exchanger (tAE1) elicits not only an anion exchange activity as expected but also the simultaneous appearance of an anion conductance, and a transport of taurine and sorbitol (Fiévet et al. 1995, 1998; Motais et al. 1996), all of them similarly affected by AE1 inhibitors, suggesting that tAE1 forms a channel through which organic osmolytes can move. In other words tAE1 might carry on multiple functions which are revealed when the protein is expressed in a heterologous system but which are normally involved in cell volume regulation. This suggestion is reinforced by the data obtained with a homologous AE1 isoform, mAE1, from mouse erythrocyte: when expressed in oocytes, mAE1 induces Cl−-Cl− exchange as expected but neither channel activity nor transport of taurine or sorbitol. It is consistent with the fact that anucleated mammalian red blood cells when hypotonically swollen do not regulate their volume and lack swelling-activated osmolyte channel activity.
In the work reported here, since swelling of erythrocyte activates a channel-mediated cation transport, we have studied the capacity of tAE1 to induce in oocytes a transport of Na+ and K+. Then, using electrophysiology and site-directed mutagenesis, we have investigated the extent to which channel activity and cation transport were both dependent on tAE1. These peculiar properties of tAE1 were compared to the mouse red cell anion exchanger mAE1, which induces neither anion conductance nor taurine and sorbitol transport. The results support the view that tAE1, when expressed in oocytes, serves a dual transport function: anion exchanger and channel for neutral and charged solutes. Comparison of data from AE1 expression in oocytes and from volume regulation in erythrocytes indicates that, in fish red blood cells, tAE1 acts as the swelling-activated channel.
METHODS
Production of cRNA
cDNAs encoding the transporters tAE1 and mAE1 were subcloned in pSP64poly(A) and pSPT19, respectively. tAE1(z-), the tAE1 ‘Z loop’ deletion mutant, lacks residues 551-574 as previously described (Fiévet et al. 1995). In contrast to tAE1, this tAE1(z-) mutant is sensitive to DIDS and H2DIDS when expressed in Xenopus oocytes. Site-directed mutagenesis of tAE1(z-) on Lys-522 was carried out using the QuikChange site-directed mutagenesis kit (Stratagene); Codon AAG (Lys) was substituted for AGG (Arg) in a specific primer 5′-GAGACCTTCAGCAGGCTCGGCAAG-3′. cRNAs were obtained using a commercial transcription kit (Ambion).
Taking of oocytes
Xenopus laevis were cooled on 0.2 % MS222-containing ice until complete anaesthesia was achieved and maintained covered with ice during the surgery according to the procedure recommanded by our ethics committee. The surgery consisted of removing about five ovarian lobes containing oocytes. After surgery, the animals were placed in cold water between 0 and 4 °C to recover from anaesthesia, monitored for 3 h and then placed back in their aquarium. After the final collection of oocytes the Xenopus were killed with a lethal dose of MS222.
Oocyte injection
Collected oocytes were washed in modified Barth's saline (MBS; composition (mm): NaCl, 85; KCl, 1; NaHCO3, 2.4; MgSO4, 0.82; Ca(NO3)2, 0.33; CaCl2, 0.41; Hepes, 10; NaOH, 4.5; pH 7.4; supplemented with penicillin, 10 U ml−1, and streptomycin, 10 μg ml−1). After washing with MBS, defolliculation was obtained by 16 h incubation at 18 °C in MBS containing 1.3 mg ml−1 collagenase (SERVA) followed by 30 min incubation in Ca2+-free MBS. Stage V-VI oocytes were then injected with 50 nl of 80 ng μl−1 cRNA and maintained at 18 °C in MBS. Comparison of a great number of data obtained with water-injected or uninjected oocytes showed no difference regarding conductance, and Rb+, Li+ and Cl− permeabilities. Therefore in the presented experiments control oocytes refer to uninjected oocytes.
Electrophysiology
Electrophysiological parameters were measured at room temperature as described previously (Fiévet et al. 1995), using the two-electrode voltage clamp technique with a TEV 200 amplifier (Dagan, Minneapolis, MN) monitored by computer through Digidata 1200 A/D converter/PC clamp software (Axon Instruments Inc., Foster City, CA, USA).
Determination of oocyte ion content
Five oocytes were quickly washed three times in 7.5 ml of milliQ water (Millipore) and dried on aluminium foil for 7 h at 80 °C after removing excess extracellular fluid. Dried oocytes were weighed to determine dry cell solids. Intracellular ions were extracted by suspending dried oocytes in 4 ml of milliQ water overnight at 4 °C. Perchloric acid (80 μl of 70 % v/v) was then added to the suspension. After centrifugation at 30 000 g for 10 min the clear supernatant was saved for analyses of cations. Measurements of sodium and potassium were done with a flame spectrophotometer (Eppendorf). Results were expressed as micromoles per gram dry cell solids.
Influx measurements
For chloride influx measurements, eight oocytes were incubated at 18 °C in 80 μl MBS containing 36Cl− (Amersham) with a specific activity of 360 d.p.m. (nmol chloride)−1. After a 15 min incubation, the oocytes were washed twice in ice-cold MBS and transferred individually into counting vials. This washing procedure took less than 30 s. The volume of extracellular fluid dropped with each oocyte being variable, it was quickly removed and 20 μl of 20 % SDS was added before vortexing. Radioactive chloride uptake by each oocyte was determined after scintillation counting with an external standard procedure to correct for quenching. The incubation medium was counted in duplicate on 5 μl aliquots, using the same protocol to determine the specific activity in each experiment. Chloride uptake was calculated as the mean of the eight values and expressed as picomoles per minute per oocyte. Rubidium influx measurements were carried out as chloride influx in NO3-MBS (MBS in which NaCl and KCl were substituted by NaNO3 and KNO3, respectively) supplemented with 86RbCl with a specific activity of 36 000 c.p.m. nmol−1 for 1 h (tAE1-induced Rb uptake is linear up to 4 h). Lithium influx measurements were performed by atomic absorption spectrometry: eight oocytes were incubated at 18 °C in a Li-MBS (NaCl and KCl replaced by LiNO3 and KNO3, respectively, and Hepes-NaOH replaced by Tris-HNO3, 15 mm, pH 7.4). After 1.5 h incubation (uptake is linear up to 6 h), the oocytes were rapidly washed 3 times in ice-cold milliQ water (plus ouabain, 10−4m) and transferred individually into microcentrifuge tubes. Extracellular water was quickly removed and oocytes were dried at 95 °C for 3 min in a block heater, then oocytes were incubated for 10 min at 95 °C in 50 μl of 0.1 m NaOH and finally 250 μl of milliQ water was added to each oocyte. Intracellular lithium content was determined by graphite furnace atomic absorption spectrometry (Perkin Elmer AAS 3110).
Covalent binding of H2DIDS
H2DIDS was covalently bound to AE1 protein by incubation in MBS of oocytes expressing tAE1(z-) or the mutant K522R (8 oocytes in 5 ml MBS + 5 × 10−5m H2DIDS, 18 °C for 120 min). Then the unreacted H2DIDS was washed away (8 washes with MBS + 1 % bovin serum albumin (BSA), followed by an additional eight washes with MBS without BSA). All other inhibitors were used without any preincubation, including H2DIDS as reversible inhibitor.
Chemicals
Niflumic acid, bumetanide, ouabain, aminobenzoic acid ethyl ester (MS222), 3-isobutyl-1-methylxanthine (IBMX) and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) were obtained from Sigma Chemical Co. (St Louis, MO, USA); H2DIDS was from CEN Saclay (France); 5-nitro-2(3-phenylpropylamino) benzoic acid (NPPB) was kindly provided by Dr R. Greger (Physiological Institute, University of Freiburg) and N-(4-azido-2-nitrophenyl)-2-aminoethylsulfonate (NAP-taurine) was kindly provided by Dr P. A. Knauf (University of Rochester Medical Center, NY, USA). cDNA coding for CFTR was a gift from Dr Lingueglia (IPMC, Sophia-Antipolis, France).
RESULTS
Cation permeability by Xenopus oocytes expressing red blood cell anion exchangers, AE1s
Both mouse (mAE1) and trout (tAE1) red blood cell anion exchangers have been functionally expressed in oocytes (Fiévet et al. 1995). At day 5 post-cRNA injection they elicited a quite similar chloride exchange activity (initial rates of 15 min 36Cl− uptake: 346 ± 9 and 278 ± 16 pmol min−1 oocyte−1, respectively), but whereas in oocytes expressing mAE1, cell sodium and potassium contents remained practically unchanged, oocytes expressing tAE1 showed a 40 % drop of cell potassium and a 245 % increase of sodium content (Fig. 1A). If ouabain was present in saline to inhibit the Na+-K+ pump, a complete reversal of cation contents of oocytes expressing tAE1 was observed as early as 2 days after cRNA injection: oocytes had become low-K+, high-Na+ cells (Fig. 1B). A very similar drastic alteration of oocyte cation contents occurred when, in the presence of ouabain plus bumetanide, external chloride was replaced by nitrate (Fig. 1C): thus the alteration in cation contents did not involve activation of cation pathways such as Na+-K+-2Cl− and/or KCl cotransporters, both transport systems being inhibited by NO3− substitution and bumetanide being a selective inhibitor of the former. Taken together, these results suggest that expression of tAE1 in oocyte membrane induces robust diffusive movements of cations which occur independently, since in the absence of external sodium (N-methyl-d-glucamine substitution), potassium loss was not significantly affected (not shown). As illustrated in Fig. 1A and B, mAE1 in contrast did not induce such net movements of cation.
Figure 1. Changes of sodium and potassium contents of oocytes expressing mAE1 or tAE1.
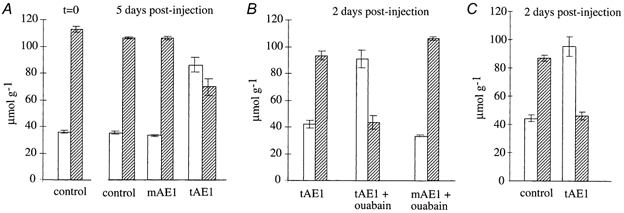
Cation contents are expressed in micromoles per gram of dry cell solids. □, Na+ content;  , K+ content. Control refers to uninjected oocytes. No difference could be detected between water-injected and uninjected oocytes. The bars represent means ±s.e.m.; n = 3. A, cation contents of control oocytes at time zero and 5 days later compared to tAE1- or mAE1-injected oocytes maintained 5 days after cRNA injection in a ouabain-free, normal Cl−-containing saline (MBS). B, cation contents of mAE1- or tAE1-injected oocytes maintained only 2 days after cRNA injection in MBS containing 10−4m ouabain compared to tAE1-injected oocytes maintained for two days in MBS without ouabain. C, cation contents of control and tAE1-injected oocytes maintained 2 days after cRNA injection in a Cl−-free, NO3−-containing saline (NO3-MBS) in the presence of 10−4m ouabain and 10−6m bumetanide.
, K+ content. Control refers to uninjected oocytes. No difference could be detected between water-injected and uninjected oocytes. The bars represent means ±s.e.m.; n = 3. A, cation contents of control oocytes at time zero and 5 days later compared to tAE1- or mAE1-injected oocytes maintained 5 days after cRNA injection in a ouabain-free, normal Cl−-containing saline (MBS). B, cation contents of mAE1- or tAE1-injected oocytes maintained only 2 days after cRNA injection in MBS containing 10−4m ouabain compared to tAE1-injected oocytes maintained for two days in MBS without ouabain. C, cation contents of control and tAE1-injected oocytes maintained 2 days after cRNA injection in a Cl−-free, NO3−-containing saline (NO3-MBS) in the presence of 10−4m ouabain and 10−6m bumetanide.
Measurements in Cl−-free, NO3−-containing saline of unidirectional fluxes of Na+ and K+, using Li+ and Rb+ as respective tracers, confirmed that tAE1 induced a Cl−-independent Na+ and K+ permeability, whereas mAE1 did not (Fig. 2A and B). This Cl−-independent permeability was fully bumetanide insensitive but compounds like niflumic acid, NPPB and NAP-taurine, known to be inhibitors of anion exchangers, greatly inhibited tAE1-induced unidirectional cation fluxes (Fig. 3A) and strongly opposed tAE1-induced alteration of cation contents (Fig. 3B).
Figure 2. Chloride-independent, ouabain-insensitive Rb+ and Li+ influxes in oocytes expressing mAE1 and tAE1.
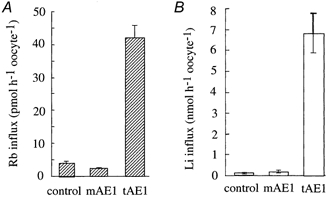
Rb+ influxes (A) and Li+ influxes (B) were measured at day 5 post-cRNA injection, in a Cl−-free, NO3−-containing saline (NO3-MBS for Rb+ influx and Li-MBS for Li+ influx) in the presence of ouabain (10−4m). The bars represent means ±s.e.m.; n = 8.
Figure 3. Effects of inhibitors on tAE1-induced Rb+ (A) and Li+ influxes (B) and on the changes in Na+ and K+ contents (C).
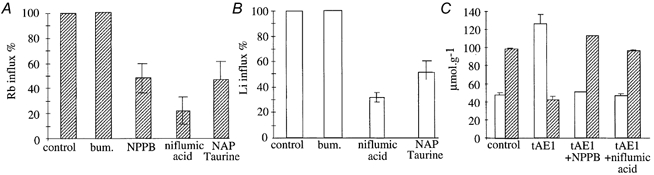
Influxes of Rb+ (A) and Li+ (B) in tAE1-expressing oocytes were measured at day 5 post-injection in a Cl−-free, NO3−-containing saline (NO3-MBS for Rb+ influx or Li-MBS for Li+ influx) in the presence of ouabain 10−4m (control) and the following inhibitors: bumetanide, 10−6m; NPPB, 10−4m; NAP-taurine, 10−3m, or niflumic acid, 10−4m. Bars represent means ±s.e.m.; n = 8. C, □, Na+ content;  , K+ content. tAE1-injected oocytes were maintained 2 days after cRNA injection in Cl−-containing saline (MBS) in the presence of ouabain 10−4m and bumetanide 10−6m and either NPPB, 10−4m, or niflumic acid, 10−4m. Bars represent means ±s.e.m.; n = 3. Control refers to uninjected oocytes maintained for 2 days in MBS with 10−4m ouabain and 10−6m bumetanide.
, K+ content. tAE1-injected oocytes were maintained 2 days after cRNA injection in Cl−-containing saline (MBS) in the presence of ouabain 10−4m and bumetanide 10−6m and either NPPB, 10−4m, or niflumic acid, 10−4m. Bars represent means ±s.e.m.; n = 3. Control refers to uninjected oocytes maintained for 2 days in MBS with 10−4m ouabain and 10−6m bumetanide.
Relationship between tAE1-induced cation permeability and chloride conductance
It has been previously shown that the level of tAE1 expression in Xenopus oocytes increases between days 1 and 6 post-cRNA injection and the membrane potential (Vm) of tAE1-injected oocytes was shifted to about -30 mV and -20 mV 1 day and 6 days post-injection, respectively (Vm of control oocytes is -56.8 ± 1.5 mV, n = 71). That depolarisation is due to the fact that tAE1 generates a chloride conductance across the oocyte membrane, the channel activity increasing as a function of the level of tAE1 expression (Fiévet et al. 1995). Thus by performing measurements at different days post-injection a wide range of tAE1 expression levels, quantified by Cl− fluxes, can be obtained. Figure 4A and B shows the relationship between tAE1-induced chloride conductance and tAE1-induced Rb+ and Li+ unidirectional fluxes, respectively. Clearly K+ and Na+ transports (labelled by Rb+ and Li+) increase as a function of chloride conductance. Note that NPPB, niflumic acid and NAP-taurine, which inhibit Na+ and K+ transport, (Fig. 3A and B) also affect the tAE1-induced chloride conductance (Fiévet et al. 1995). As illustrated in Table 1, even after NPPB and niflumic acid treatment, which inhibits about half of chloride conductance, the membrane potential remained practically unchanged. It, means that the chloride conductive permeability remains much greater than the cation permeability and still largely controls membrane potential. It must be outlined that the mouse anion exchanger mAE1, which did not induce Na+ and K+ transport (Fig. 2A and B), also does not generate chloride conductance whatever its level of expression (Fiévet et al. 1995).
Figure 4. Correlation between tAE1-induced cation fluxes and tAE1-induced anion conductance.
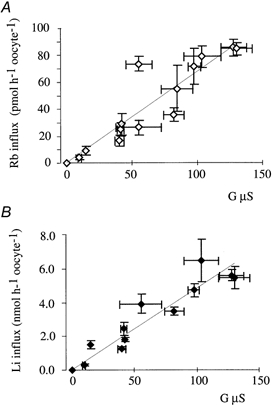
A, Rb+ influx and tAE1-induced anion conductance. B, Li+ influx and tAE1-induced anion conductance. Data point are means ±s.e.m.; n = 8. Conductance, Li+ and Rb+ influxes were measured in parallel on the same batch of tAE1-injected oocytes 1, 2, 3, 4, 5, 6 and 7 days after cRNA injection. Expression level increasing with time, it was thus possible to obtain varying anion conductances. Conductance of control oocytes (uninjected) was also measured as well as Li+ and Rb+ influxes. It was constant over time. G (μS) plotted against Li+ or Rb+ influx represents the difference in anion conductance between tAE1-induced conductance and control oocytes. Li+ and Rb+ influxes represent the difference in fluxes between tAE1-injected oocytes and control oocytes.
Table 1.
Effect of inhibitors on membrane potential (Vm) and membrane conductance (G) of oocytes expressing tAE1 or tAE1(z-). Membrane potential and conductance of control oocytes are given for comparison
| Inhibitors | n | Vm(mV) | G(μS) |
|---|---|---|---|
| Control (none) | 71 | −56.80 ± 1.50 | 0.58 ± 0.02 |
| tAE1 | |||
| None | 5 | −24.62 ± 1.09 | 48.68 ± 2.41 |
| NPPB (10−4 M) | 5 | −25.04 ± 0.97 | 22.51 ± 1.49 |
| Niflumic acid(10−4 M) | 5 | −23.17 ± 1.12 | 22.09 ± 1.71 |
| tAE1(z-) | |||
| None | 6 | −35.95 ± 2.05 | 6.69 ± 1.27 |
| Cov. H2DIDS | 8 | −37.58 ± 1.03 | 2.80 ± 0.31 |
Data are means ± S.E.M. Cov. H2DIDS, covalently bound H2DIDS.
Na+ and K+ transport could occur by electrodiffusion through some endogenous cation channel(s) and then the partial depolarisation of oocyte due to the tAE1-induced Cl− conductance could explain the observed increase in cation permeability: the greater the chloride conductance, the greater the cation permeability. Then, generation of any anion conductance in oocytes is expected to similarly induce an increase in ouabain- and bumetanide-resistant cation fluxes. Two different methods were used to test this possibility. First we created in oocytes a Cl− channel by expressing CFTR stimulated by IBMX. Oocytes expressing unstimulated CFTR have a background current with a conductance of 5.3 ± 1.5 μS (n = 8), which is significantly greater than that of control oocytes (0.70 ± 0.05 μS, n = 67). But stimulation of CFTR with IBMX elicited a large increase in membrane conductance (152.5 ± 17 μS; n = 8). However such a large IBMX-induced anion conductance generated neither Rb+ (Fig. 5A) nor Li+ (Fig. 5B) permeability increase. By contrast, with an induction of a 4-times-smaller anion conductance (38.5 ± 7.9 μS; n = 8), oocytes expressing tAE1 showed a large increase in both Rb+ (Fig. 5A) and Li+ (Fig. 5B) permeability. In the second method, using the two-electrode voltage clamp technique, the membrane potential Vm of four control oocytes (uninjected) was shifted from the resting value (-56.8 ± 1.5 mV, n = 71) and clamped at a holding potential of -20 mV, corresponding to that of high Cl−-conductive tAE1-expressing oocytes. Measurements of unidirectional Rb+ and Li+ fluxes in such conditions did not reveal any increase of cation permeability (data not shown). Thus the cation permeability increase seems not to be linked to the appearance of any anion conductance but is strictly related to the anion conductance resulting from expression of tAE1.
Figure 5. Cl−-independent influx of Rb+ (A) and Li+ (B) in oocytes displaying a large anion conductance due to expression of CFTR or tAE1.
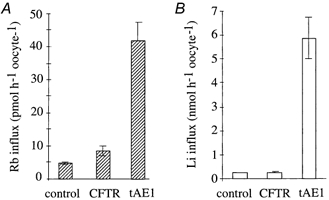
Influxes were measured in a Cl−-free, NO3−-containing saline in the presence of 10−4m ouabain, 10−3m IBMX and 10−3m amiloride. Amiloride was added to inhibit the endogenous cAMP-dependent Na+(Li)-H+ exchanger. CFTR stimulated with IBMX elicited a large increase in membrane conductance (152.5 μS) but generated neither a Rb+ nor a Li+ permeability increase. In contrast, tAE1 elicited a smaller anion conductance (38.5 μS) but generated both a Rb+ and a Li+ permeability increase. It is notewothy that IBMX did not affect tAE1-induced conductance and cation permeability. The bars represent means ±s.e.m.; n = 8.
To further characterise this tAE1 dependence of cation permeability we analysed the functional consequences of site-directed mutagenesis on tAE1.
Relation to anion exchange protein
The stilbenedisulfonate derivatives DIDS and H2DIDS are both known as potent reversible and irreversible inhibitors of mammalian red blood cell anion exchangers (Cabantchick & Greger, 1992). At physiological pH, irreversible inhibition is due to covalent binding of these compounds with a lysine residue (human Lys-539; mouse Lys-558) located close to the extracellular medium in the 5th putative transmembrane helix (Cabantchick & Rothstein, 1974; Passow, 1986). In trout red blood cells anion exchange is also fully inhibited by reversible and irreversible DIDS or H2DIDS binding (Baroin et al. 1984; Romano & Passow, 1984). Curiously, however, when expressed in Xenopus oocyte, tAE1 lost that DIDS (and H2DIDS) sensitivity whereas mAE1 kept it (Fiévet et al. 1995) suggesting that tAE1 conformation differs in oocytes and red cells. To recover the DIDS or H2DIDS sensitivity of tAE1 expressed in Xenopus oocyte, a mutant termed tAE1(z-) was constructed. This mutant tAE1 is characterised by the deletion of 24 amino acids (aa 551-574) within the extracellular loop (called Z loop) connecting the 5th and 6th spans of the protein, thus reducing by half the length of this loop (Fiévet et al. 1995). The length of this extracellular loop is the major difference between tAE1 and mAE1 transmembrane domains; it is twice the length in the tAE1 (Z loop) than in the mAE1 (m loop).
Figure 6A shows that tAE1(z-) elicited a smaller chloride exchange activity than the wild-type tAE1 and generated a significant anion conductance across the oocyte membrane (Fig. 6B). Despite its low level of expression tAE1(z-) also induced a measurable flux of Li+. Note that, as with the wild-type, the Li+ flux increased as a function of the anion conductance induced by tAE1(z-) and inhibition of conductance by NPPB or niflumic acid was associated with an inhibition of Li+ transport (data not shown). In experiments shown in Fig. 7, tAE1(z-) expressing oocytes were incubated in H2DIDS-containing saline for 120 min and then the unreacted H2DIDS was washed away (see Methods) before measuring permeability parameters. As illustrated, covalently bound H2DIDS inhibited very significantly and quite similarly (60-70 %) chloride exchange activity, anion conductance and Li+ permeability of tAE1(z-)-injected oocytes. It is important to note that covalent binding of H2DIDS, which strongly inhibited anion conductance, did not significantly affect membrane potential (Table 1).
Figure 6. Comparison of transport properties of tAE1 and tAE1(z-), a deleted mutant characterized by a Z loop reduction.
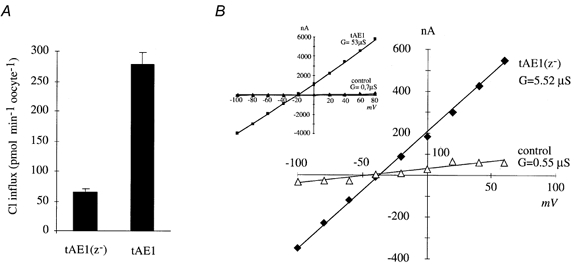
A, chloride exchange activity of tAE1 and tAE1(z-). Bars represent means ±s.e.m.; n = 15. B, current-voltage relationship of tAE1(z-) (in inset tAE1)-expressing oocytes. Measurements at day 5 post-injection.
Figure 7. Effect of covalently bound H2DIDS on tAE1(z-) transport properties.
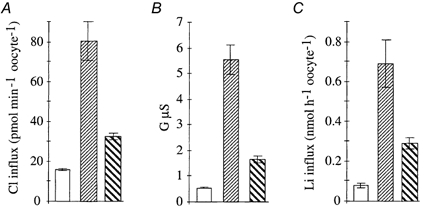
□, control;  , tAE1(z-);
, tAE1(z-); , tAE1(z-) + covalent H2DIDS. Bars represent means ±s.e.m.; n = 8. A, chloride exchange activity; B, anion conductance; C, lithium influx. Measurements at day 5 post-injection.
, tAE1(z-) + covalent H2DIDS. Bars represent means ±s.e.m.; n = 8. A, chloride exchange activity; B, anion conductance; C, lithium influx. Measurements at day 5 post-injection.
Conversion of mouse AE1 Lys-558 to Asn by site-directed mutagenesis prevents the covalent reaction of the H2DIDS with mAE1 expressed in Xenopus oocytes (Bartel et al. 1989a,b). It is expected that the covalent binding of H2DIDS on tAE1(z-) occurs with the Lys-522 which is the counterpart of the mouse Lys-558. Thus the trout Lys-522 was converted to Arg by site-directed mutagenesis to prevent covalent binding of H2DIDS on tAE1(z-). This mutant, termed (K522R), was then tested for its transport capacities. As shown in Fig. 8, the mutant was functionally expressed and as tAE1(z-), it expressed Cl− exchange activity, anion conductance and Li+ permeability. Moreover its transport capacities were partly inhibited by reversible H2DIDS. In contrast all these transport properties remained fully unchanged after a treatment normally producing an irreversible, covalent binding of H2DIDS. In conclusion, a point mutation in the anion exchange protein which controls the chloride exchange activity similarly controls other transport functions associated with the protein expression, i.e. channel activity and cation transport.
Figure 8. Covalent H2DIDS treatment and transport properties of the mutant K522R.
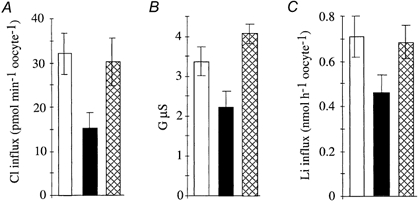
In K522R, the Lys-522 has been converted to Arg by site-directed mutagenesis, thus preventing covalent binding of H2DIDS. □, K522R; ▪, K522R in presence of 5 × 10−5m H2DIDS;  , covalent binding of H2DIDS to K522R. Measurements at day 5 post-injection. Bars represent means ±s.e.m.; n = 8. A, chloride exchange activity; B, anion conductance; C, lithium influx.
, covalent binding of H2DIDS to K522R. Measurements at day 5 post-injection. Bars represent means ±s.e.m.; n = 8. A, chloride exchange activity; B, anion conductance; C, lithium influx.
DISCUSSION
In oocyte is the cation permeability mediated by tAE1?
We have shown in this report that when two different red blood cell AE1 anion exchangers are expressed in Xenopus oocyte the trout (tAE1), but not the mouse (mAE1), exchanger generated so marked an increase in Na+ and K+ permeabilities that in 2 days (in the presence of ouabain to inhibit the Na+-K+ pump), the oocyte was transformed into a low K+, high Na+ cell. Our data showed that this tAE1-induced increase in cation permeability does not take place through the activation of endogenous Na+-K+-2Cl− and KCl cotransport systems but is strictly related to the anion conductance generated when tAE1 is expressed in the oocyte: the greater the channel activity, the greater the cation transport (Fig. 4). Moreover, blockade of the anion conductance with NPPB or niflumic acid inhibited cation permeability. That could be explained by assuming that Na+ and K+ movements are mediated by a cation channel(s) pre-existing in the oocyte membrane (endogenous channel) and are electrically linked to tAE1-induced Cl− conductance. The observation that mAE1, which does not create an anion conductance when expressed in oocyte (Fiévet et al. 1995), did not generate cation permeability is consistent with this interpretation. Several pieces of evidence, however, argue against such a simple explanation. First expression in control oocyte membrane of an anion conductance, entirely unrelated to tAE1, did not induce a cation permeability (Fig. 5). Second, if cation transport occurred only by electrodiffusion and the membrane conductance for cation remained constant, cell depolarisation would be expected to have opposite effects on unidirectional fluxes, increasing efflux and decreasing influx. However K+ efflux (measured as net flux), K+ influx (measured as Rb+ flux) and Na+ influx (measured as Li+ influx) were all greatly stimulated (Fig. 1 and Fig. 2). Thus the increase in cation permeability observed in our experiments obviously reflects an increase in conductance for cations. Three explanations can be considered: (1) tAE1 is bifunctional, as shown for a number of ABC transporters (Higgins 1995), activating endogenous anion and cation channels independently of its normal exchanger activity; (2) tAE1, independently of its normal exchanger activity, functions as an anion channel and simultaneously behaves as an activator of endogenous cation channel(s); (3) tAE1, independently of its normal exchanger activity, functions as an anion channel that permits the passage of cations.
We have previously shown that the anion exchanger tAE1 does not activate an endogenous anion channel but would be able to function itself as a channel, through which different structurally unrelated compounds (amino acids like taurine, electroneutral solutes like sorbitol and urea) can move (Fiévet et al. 1995, 1998). The conversion by site-directed mutagenesis of Lys-522 to Arg in tAE1(z-) eliminated the ability of H2DIDS to bind covalently to the protein. Cl− exchange and anion conductance activities, which were similarly (50-60 %) inhibited by covalent H2DIDS in tAE1(z-) (Fig. 7), were both fully recovered after conversion of Lys to Arg (Fig. 8). Thus the introduction of a point mutation in the protein shows that exchange and channel activities are directly linked and associated to tAE1.
The question remains to know whether tAE1 acts as an anion channel which also conducts monovalent cations or whether it simultaneously behaves as an activator of endogenous cation channel(s). We observed that the covalent binding of H2DIDS to tAE1(z-) and the treatment of the wild-type tAE1 with the reversible inhibitors NPPB and niflumic acid induced a large decrease of the anion conductance but had no significant effect on the membrane potential (Table 1). In other words, the inhibitory effect of these drugs on cation fluxes is not via a decrease in the driving force for diffusional fluxes (i.e. the membrane potential) but is due to direct action on the cation permeability pathway. Since we have shown for H2DIDS, that this inhibitory effect on cation flux was related to a specific binding of the drug on the exchange protein (Fig. 7 and Fig. 8), these results indicate that cation permeability is mediated by the protein tAE1. These data cannot completely rule out the possibility that H2DIDS, by interacting with tAE1, inhibits to the same extent both the channel function of tAE1 and a putative capacity of tAE1 to activate an endogenous cation channel(s). Nevertheless, the simplest hypothesis compatible with the data would be that tAE1 can act as a channel permitting net flow of K+ and Na+. It may appear contradictory to consider cation transport by an anion exchanger. However, some investigators studying ionic disorders of red cells, have previously provided partial or indirect evidence for mediation of cation movements through the red cell anion exchanger AE1 in the hereditary diseases South-East Asian ovalocytosis (SAO) and drepanocytosis (Joiner et al. 1986; Bruce et al. 1999). Moreover, reinvestigating a long known phenomenon (Mond 1927; Donlon & Rothstein, 1969), the massive increase in cation flux that occurs when human red cells are suspended in solutions of very low ionic strength, Jones & Knauf (1985) found these fluxes are sensitive to DIDS and other anion exchange inhibitors and that there is no selectivity of Na+ over K+. A careful analysis of the results led the authors to suggest that some changes of structure in the anion transport protein could alter its normally high selectivity for anions over cations, permitting the protein to act as a channel mediating net flow of cations. The reversal of membrane potential, which occurs when red cells are suspended in media of low ionic strength, could be responsible for a structural perturbation altering AE1 selectivity: indeed several authors have reported a non-selective increase in Na+ and K+ permeability when the inside of human red blood cell is made positive by different manoeuvres (Kracke & Dunham, 1987; Halperin et al. 1989).
It seems difficult to understand how a carrier-type transport mechanism could permit net flow of cations. However models of AE1 suggest that it may form an aqueous channel through the membrane which is blocked by a gating mechanism that imparts to the system its high anion/cation selectivity and one-for-one exchange properties (Knauf, 1979; Passow, 1986). Thus AE1 could mediate a net anion flow as well as anion exchange, with the net flux involving a different protein conformation from the anion exchange (Knauf et al. 1983; Passow, 1986). The existence of this net anion diffusion through AE1 suggests that a similar mechanism might explain the cation flux increase described above: (1) when conformation of hAE1 is strongly altered by either exposure to unusual conditions (red cells suspended in media of very low ionic strength) or mutation and modified interactions to cytoskeleton (SAO cells) or modified interactions to haemoglobin (sickle cells); (2) when the trout protein tAE1 is expressed in oocyte, a very different environment from the high cholesterol containing red cell membrane, revealing a constitutive transport function which is supposed to be normally a regulated one appearing in response to cell swelling.
In summary, the present data are consistent with the view that the trout red cell anion exchanger tAE1, when expressed in oocyte, has a dual transport function, anion exchange and channel function, the channel having a significant permeability to a wide variety of both charged and uncharged solutes.
In red cells is tAE1 the volume regulatory channel?
A large increase in Na+ and K+ permeability is observed in fish red cells submitted to an hypotonic swelling. This cation permeability is inhibited by anion transport blockers and it has been shown that these blockers interfere with the cation transport itself and not through a decrease in Cl− conductance (Garcia-Romeu et al. 1991). In fish erythrocytes, autoradiography of SDS gel electrophoresis of membrane proteins showed that [3H]DIDS bound covalently to the anion exchanger (Motais et al. 1992). This covalent binding of DIDS to anion exchanger blocks the transport of taurine, cations (Garcia-Romeu et al. 1991, 1996; Motais et al. 1992) and polyols (Guizouarn & Motais, 1999), indicating that all these volume regulatory transports are dependent on an anion exchanger. However, the possibility could not be ruled out that in erythrocytes, DIDS also binds covalently to a minor protein in addition to AE1. Expression of tAE1 in oocytes showing that H2DIDS inhibits both cation transport and anion condutance gave compelling evidence that in fish red cells the anion exchanger acts as the channel responsible for the volume regulatory efflux of ions and organic solutes.
These multiple transport functions of AE1 are very interesting per se but also as an example of specific transport systems that may serve other functions when submitted to regulation, or under pathological circumstances. These data also outlined the fact that a transporter can display properties normally considered to be associated with a channel, a fact already suggested for some transporters: Na+-K+ pump (Gadsby et al. 1993; Higelmann 1994), the phosphate exchanger from chloroplast (Schwarz et al. 1994) and the excitatory amino acid transporters (Fairman et al. 1995; Wadiche et al. 1995). Whether some members of the AE family can also be involved in swelling-activated osmolyte channel activity in mammalian epithelial cells and how the tAE1-associated channel can accept neutral, zwitterionic and ionic solutes are the subject of ongoing research.
Acknowledgments
We are grateful to Dr P. A. Knauf for providing the NAP-taurine and Dr E. Lingueglia for providing the CFTR cDNA.
References
- Baroin A, Garcia-omeu F, Lamarre T, Motais R. Hormone-induced cotransport with specific pharmacological properties in erythrocytes of rainbow trout. Journal of Physiology. 1984;350:137–157. doi: 10.1113/jphysiol.1984.sp015193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D, Hans H, Passow H. Identification by site-directed mutagenesis of Lys-558 as the covalent attachment site of H2DIDS in the mouse erythroid band 3 protein. Biochimica et Biophysica Acta. 1989a;985:355–358. doi: 10.1016/0005-2736(89)90427-6. [DOI] [PubMed] [Google Scholar]
- Bartel D, Lepke S, Layh-Schmitt G, Legrum B, Passow H. Anion transport in oocytes of Xenopus laevis induced by expression of mouse erythroid band 3 protein-encoding cRNA and of a cRNA derivative obtained by site-directed mutagenesis at the stilbene disulfonate binding site. EMBO Journal. 1989b;8:3601–3609. doi: 10.1002/j.1460-2075.1989.tb08533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce LJ, Ring SM, Ridgwell K, Reardon DM, Seymour CA, Van Dort HM, Low PS, Tanner MJA. South-east Asian ovalocytic (SAO) erythrocytes have a cold sensitive cation leak: implications for in vitro studies on stored SAO red cells. Biochimica et Biophysica Acta. 1999;1416:258–270. doi: 10.1016/s0005-2736(98)00231-4. [DOI] [PubMed] [Google Scholar]
- Cabantchick ZI, Greger G. Chemical probes for anion transporters of mammalian cell membranes. American Journal of Physiology. 1992;262:C803–827. doi: 10.1152/ajpcell.1992.262.4.C803. [DOI] [PubMed] [Google Scholar]
- Cabantchick ZI, Rothstein A. Membrane proteins related to anion permeability of human red blood cells. Localisation of disulfonic stilbene binding sites in proteins involved in permeation. Journal of Membrane Biology. 1974;15:207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Donlon JA, Rothstein A. The cation permeability of erythrocytes in low ionic stregth media of various tonicities. Journal of Membrane Biology. 1969;1:37–52. doi: 10.1007/BF01869773. [DOI] [PubMed] [Google Scholar]
- Egée S, Harvey BJ, Thomas S. Volume-activated DIDS-sensitive whole-cell chloride currents in trout red blood cells. Journal of Physiology. 1997;504:57–63. doi: 10.1111/j.1469-7793.1997.057bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Fiévet B, Gabillat N, Borgese F, Motais R. Expression of band 3 anion exchanger induces chloride current and taurine transport: structure-function analysis. EMBO Journal. 1995;14:5158–5169. doi: 10.1002/j.1460-2075.1995.tb00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiévet B, Perset F, Gabillat N, Guizouarn H, Borgese F, Ripoche P, Motais R. Transport of uncharged organic solutes in Xenopus oocytes expressing red cell anion exchangers (AE1s) Proceedings of the National Academy of Sciences of the USA. 1998;95:10996–11001. doi: 10.1073/pnas.95.18.10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby DC, Rakowski RF, de Weer P. Extracellular access to the Na+,K+ pump: pathway similar to ion channel. Science. 1993;260:100–103. doi: 10.1126/science.7682009. [DOI] [PubMed] [Google Scholar]
- Garcia-omeu F, Borgese F, Guizouarn H, Fiévet B, Motais R. A role for the anion exchanger AE1 (band 3 protein) in cell volume regulation. Cellular and Molecualr Biology. 1996;42:985–994. [PubMed] [Google Scholar]
- Garcia-omeu F, Cossins AR, Motais R. Cell volume regulation by trout erythrocytes: characteristics of the transport systems activated by hypotonic swelling. Journal of Physiology. 1991;440:547–567. doi: 10.1113/jphysiol.1991.sp018724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L, Musch MW. Volume-activated amino acid transport and cell signaling in skate erythrocytes. Journal of Experimental Zoology. 1994;268:133–138. [Google Scholar]
- Guizouarn H, Motais R. Swelling activation of transport pathways in erythrocytes: effects of Cl−, ionic strength and volume changes. American Journal of Physiology. 1999;276:C210–220. doi: 10.1152/ajpcell.1999.276.1.C210. [DOI] [PubMed] [Google Scholar]
- Guizouarn H, Motais R, Garcia-omeu F, Borgese F. Cell volume regulation: the role of taurine loss in maintaining membrane potential and cell pH. Journal of Physiology. 2000;523:147–154. doi: 10.1111/j.1469-7793.2000.t01-1-00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JA, Brugnara C, Tosteson M, Van Ha T, Tosteson D. Voltage-activated cation transport in human erythrocytes. American Journal of Physiology. 1989;257:C986–996. doi: 10.1152/ajpcell.1989.257.5.C986. [DOI] [PubMed] [Google Scholar]
- Higelmann DW. Channel-like function of the Na+,K+ pump probed at microsecond resolution in giant membrane patches. Science. 1994;263:1429–1432. doi: 10.1126/science.8128223. [DOI] [PubMed] [Google Scholar]
- Higgins CF. The ABC of channel regulation. Cell. 1995;82:693–696. doi: 10.1016/0092-8674(95)90465-4. [DOI] [PubMed] [Google Scholar]
- Joiner CH, Platt OS, Lux SE. Cation depletion by the sodium pump in red cells with pathologic cation leaks. Sickle cells and xerocytes. Journal of Clinical Investigation. 1986;78:1487–1496. doi: 10.1172/JCI112740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JS, Knauf PA. Mechanism of the increase in cation permeability of human erythrocytes in low-chloride media. Involvement of the anion transport protein capnophorin. Journal of Clinical Investigation. 1985;86:721–738. doi: 10.1085/jgp.86.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk K. Swelling-activated organic osmolyte channels. Journal of Membrane Biology. 1997;158:1–16. doi: 10.1007/s002329900239. [DOI] [PubMed] [Google Scholar]
- Kirk K, Ellory JC, Young JD. Transport of organic substrates via a volume-activated channel. Journal of Biological Chemistry. 1992;267:23475–23478. [PubMed] [Google Scholar]
- Knauf PA. Erythrocyte anion exchange and the band 3 protein: transport kinetics and molecular structure. Current Topics in Membrane Transport. 1979;12:249–363. [Google Scholar]
- Knauf PA, Mann N, Kalwas JE. Net chloride transport across the human erythrocyte membrane into low cloride media: evidence against a slippage mechanism. Biophysical Journal. 1983;41:164a. (abstract) [Google Scholar]
- Kracke GR, Dunham PB. Effect of membrane potential on furosemide-inhibitable sodium influxes in human red blood cells. Journal of Membrane Biology. 1987;98:117–124. doi: 10.1007/BF01872124. [DOI] [PubMed] [Google Scholar]
- Lewis RA, Bursell JDH, Kirk K. Anion-selectivity of the swelling-activated osmolyte channel in eel erythrocytes. Journal of Membrane Biology. 1996;149:103–111. doi: 10.1007/s002329900011. [DOI] [PubMed] [Google Scholar]
- Mond R. Umkehr der Anionpermeabilitat der roten Blutkorperchen in eine elektive Durchlassigkeit für Kationen. Ein Beitrag zur Analyse der Zellmembranen. Pfügers Archiv Gesellschaft für Physiologie. 1927;217:618–630. [Google Scholar]
- Motais R, Fiévet B, Borgese F, Garcia-omeu F. Some functional properties of band 3 protein in nucleated red cells. Progress in Cell Research. 1992;2:253–262. [Google Scholar]
- Motais R, Fiévet B, Borgese F, Garcia-omeu F. Association of the band 3 protein with a volume-activated anion and amino acid channel: a molecular approach. Journal of Experimental Biology. 1996;200:361–367. doi: 10.1242/jeb.200.2.361. [DOI] [PubMed] [Google Scholar]
- Motais R, Guizouarn H, Garcia-omeu F. Red cell volume regulation: the pivotal role of ionic strength in controlling swelling-dependent transport systems. Biochimica et Biophysica Acta. 1991;1075:169–180. doi: 10.1016/0304-4165(91)90248-f. [DOI] [PubMed] [Google Scholar]
- Passow H. Molecular aspects of band 3 protein-mediated anion transport across the red blood cell membrane. Reviews in Physiology, Biochemistry and Pharmacology. 1986;103:62–223. doi: 10.1007/3540153330_2. [DOI] [PubMed] [Google Scholar]
- Romano L, Passow H. Characterization of anion transport system in trout red blood cell. American Journal of Physiology. 1984;246:C330–338. doi: 10.1152/ajpcell.1984.246.3.C330. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Gross A, Steinkamp T, Flugge UI, Wagner R. Ion channel properties of the reconstituted chloroplast triose phosphate/phosphate translocator. Journal of Biological Chemistry. 1994;269:29481–29489. [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume sensitive anion channels. American Journal of Physiology. 1996;270:C711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Amara SG, Kavanaugh MP. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]


