Abstract
The mechanisms regulating the flow of sensory signals and their modification by synaptic interactions in the dorsal column nuclei are incompletely understood. Therefore, we examined the interactions between EPSPs evoked by stimulation of dorsal column and corticofugal fibres in the dorsal column nuclei cells using an in vitro slice technique.
Dorsal column EPSPs had briefer durations at depolarised membrane potentials than corticofugal EPSPs. Superfusion of the NMDA receptor antagonist 2d(-)-2-amino-5-phosphonovaleric acid (AP5) did not modify dorsal column EPSPs but reduced corticofugal EPSPs. Application of the AMPA/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) abolished both dorsal column and corticofugal EPSPs in cells held at the resting potential. Therefore, dorsal column EPSPs were mediated by non-NMDA receptors but corticofugal EPSPs revealed both non-NMDA- and NMDA-dependent components.
Paired-pulse stimulation of dorsal column fibres elicited a depression of the second EPSP at pulse intervals of < 50 ms; however, paired-pulse stimulation of corticofugal fibres evoked facilitation of the second EPSP at pulse intervals of < 30 ms. When stimulation of the corticofugal fibres preceded stimulation of the dorsal column fibres, facilitation of the dorsal column EPSP was observed at pulse intervals of < 100 ms. This facilitation was blocked at hyperpolarised membrane potentials or in the presence of AP5, suggesting activation of NMDA receptors. There was a depression of corticofugal EPSPs by previous dorsal column stimulation.
Dorsal column EPSPs were gradually depressed during stimulation with barrages at frequencies of > 10 Hz, while corticofugal EPSPs were facilitated and summated at frequencies > 30 Hz. Hyperpolarisation and application of AP5 prevented the facilitation of corticofugal EPSPs. High frequency stimulation of the corticofugal input elicited a short-lasting AP5-sensitive facilitation of both corticofugal and dorsal column EPSPs. Depolarising current facilitated dorsal column EPSPs but not corticofugal EPSPs.
These results indicate that synaptic interactions include different forms of activity-dependent synaptic plasticity, with the participation of NMDA receptors and probably Ca2+ inflow through voltage-gated channels. These complex synaptic interactions may represent the cellular substrate of the integrative function of the dorsal column nuclei observed in vivo.
Flow of sensory information in subcortical relay stations is controlled by the cooperative action of ascending afferents, carrying sensory information from the periphery, and descending connections from the neocortex. The dorsal column nuclei, which include the gracile and cuneate nuclei, are the first relay station in the somatosensory system; they receive sensory information from the hindlimbs and forelimbs, and project to the somatosensory thalamus. In agreement with the generalised pattern of connectivity described above, neurones of the dorsal column nuclei receive two major excitatory inputs which control their function; (1) ascending somatosensory fibres via the dorsal column, which contact both thalamic projection neurones and inhibitory interneurones (Rustioni & Weinberg, 1989; DeBiasi et al. 1994; Lue et al. 1996); (2) corticofugal descending fibres mainly from cells in the forelimb and hindlimb regions of the sensorimotor cortex and, to a lesser extent, from the second somatosensory area, which run mainly through the pyramidal tract (Jabbur & Towe, 1961; Kuypers & Tuerk, 1964; Valverde, 1966; Weisberg & Rustioni, 1976). Modulatory inputs from the brainstem reticular formation also contact neurones of the dorsal column nuclei (see e.g. Rustioni & Weinberg, 1989).
The ascending dorsal column input terminates mainly on proximal dendrites, whereas the descending corticofugal fibres contact mainly distal dendrites (Walberg, 1966; Rustioni & Sotelo, 1974). There is considerable immunohistochemical evidence to suggest that l-glutamate is the excitatory neurotransmitter released by both dorsal column and corticofugal pathways (Rustioni & Cuénod, 1982; Banna & Jabbur, 1989; Broman, 1994; DeBiasi et al. 1994). Moreover, N-methyl-d-aspartate (NMDA) and non-NMDA receptors have been described in neurones of the dorsal column nuclei (Watanabe et al. 1994; Kus et al. 1995; Popratiloff et al. 1997).
Electrophysiological studies have shown that both corticofugal and dorsal column inputs exert a major influence on the firing activity of the dorsal column nuclei. Natural or electrical stimulation of the peripheral sensory receptive fields evoke action potentials (APs) in neurones of the dorsal column nuclei (e.g. Gordon & Paine, 1960; Gordon & Jukes, 1964a; Dykes et al. 1982). In vivo studies have shown that peripheral stimulation evokes excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs, respectively) in neurones of the dorsal column nuclei (Andersen et al. 1964a, b; Schwartzkroin et al. 1974; Canedo et al. 2000). Stimulation of dorsal column fibre also evokes EPSPs and IPSPs in in vitro preparations (Nuñez & Buño, 1999). In addition, activation of corticofugal inputs not only modifies the activity but also changes the properties of the somatosensory response of neurones of the dorsal column nuclei (Towe & Jabbur, 1961; Gordon & Jukes, 1964b; Cole & Gordon, 1992; Canedo et al. 2000).
The anatomical characteristics and the projection profiles of the corticofugal input onto the subcortical somatosensory relay nuclei are well known, but their contribution to somatosensory information processing is largely unknown, especially at the level of the dorsal column nuclei. A recent report showed that the sensorimotor cortex exerts a selective control of the somatosensory transmission in the dorsal column nuclei (Malmierca & Nuñez, 1998). Indeed, the corticofugal input facilitates sensory responses of gracile neurones with overlapping receptive fields and inhibits sensory responses of cells with separate receptive fields, probably providing a synaptic mechanism for the enhancement of contrast between sensory signals.
The electrophysiological and pharmacological characteristics of the EPSPs evoked by stimulation of the dorsal column have been recently analysed in vitro (Nuñez & Buño, 1999), but the properties of the corticofugal input and especially of the synaptic interactions between dorsal column and corticofugal inputs are incompletely understood. Therefore, analysis of the synaptic interactions between the dorsal column and corticofugal fibres is an essential step in understanding the cellular mechanisms that regulate the flow of sensory information in the dorsal column nuclei. The goal of the present study was to characterise the properties of the corticofugal input and especially of the interactions between dorsal column and corticofugal EPSPs using an in vitro preparation. We show the existence of activity-dependent synaptic plasticity which may be the cellular substrate of the corticofugal modulatory effects observed in vivo on neurones of the dorsal column nuclei.
METHODS
Experiments were performed on sagittal brain slices from 14-day-old Wistar rats, following standard procedures described in detail previously (Nuñez & Buño, 1999). Briefly, animals were anaesthetised with an intraperitoneal injection of sodium pentobarbital (35 mg kg−1) and decapitated immediately after disappearance of the pinch reflex. The brain was rapidly removed, and submerged in a vial containing cold (4 °C) artificial cerebrospinal fluid (ACSF). The composition of the ACSF was as follows (mm): NaCl 124, KCl 2.69, KH2PO4 1.25, MgSO4 2, NaHCO3 26, CaCl2 2 and d-glucose 10. The ACSF was maintained at pH 7.4 by bubbling with ‘carbogen’ (95 % O2+ 5 % CO2). Sagittal slices (300-400 μm) were cut with a vibratome and incubated for at least 1 h in carbogen-bubbled ACSF maintained at room temperature (20-22 °C). Slices containing the dorsal column nuclei were transferred to a recording chamber (with a volume of 2 ml) placed on an inverted microscope stage and maintained at 30-32 °C by means of a feedback-controlled heater. Slices were superfused at a rate of 1 ml min−1 with the gassed ACSF.
The microelectrode tip was positioned under visual guidance on the dorsal column nuclei, according to the Paxinos & Watson (1998) atlas, and cells were impaled with micropipettes (90-120 MΩ) filled with potassium acetate (3 m). Signals were amplified with an Axoclamp 2B amplifier (Axon Instruments Inc.) and the traditional bridge current-clamp method was employed. Synaptic responses were evoked by stimulation of dorsal column and corticofugal fibres with bipolar nichrome electrodes (diameter, 80 μm). Electrodes were placed in the dorsal column and in the collaterals of the pyramidal tract that project to the dorsal column nuclei. A schematic diagram of the localisation of the dorsal column nuclei within the slice and of the position of the stimulating and recording electrodes is shown in Fig. 1. Electrical stimulation was performed with a Grass stimulator/isolation unit (duration, 0.1-0.3 ms; strength, 0.2-5.0 V). In several experiments 50 μm 2d(-)-2-amino-5-phosphonovaleric acid (AP5), 10 μm 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX) and 50 μm picrotoxin were added to the perfusion medium. All chemicals were purchased from Sigma-Aldrich, except AP5 and CNQX which were from RBI. Data were low-pass filtered at 3 kHz, stored with a PCM-videocassette recorder (Cibertec) and analysed using a Pentium-based computer through a TL-1 DMA interface (sampling frequency, 6-12 kHz). The pCLAMP software (Axon Instruments Inc.), which also generated stimulus timing signals and transmembrane current pulses, were used. Single sweeps are shown in the figures except when otherwise indicated. Changes in the amplitude and duration of EPSPs were estimated by calculating the change in EPSP areas (see inset, Fig. 4C) and the non-parametric Wilcoxon test was used to compare duration and slope. The ANOVA test for repetitive measures was applied to compare changes in EPSP area in experimental and control conditions. This analysis was followed by a post-hoc test (Fisher test) and differences were considered statistically significant at the 95 % level (P < 0.05). Data were expressed as means ±s.e.m. Experiments were carried out in accordance with the European Communities Council Directive (86/609/EEC), and all efforts were made to minimise animal suffering and the number of animals used.
Figure 1. Diagram of experimental preparation.
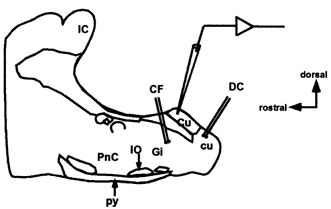
Schematic diagram of the sagittal dorsal column nuclei slice preparation showing placement of recording and stimulating electrodes. Dorsal column (DC) and corticofugal (CF) stimulating electrodes placed at dorsal column fibres and corticofugal fibres, respectively. Cu, cuneate nucleus; cu, cuneate fasciculus; Gi, gigantocellular reticular nucleus; IC, inferior colliculus; IO, inferior olive; PnC, caudal pontine reticular nucleus; py, pyramidal tract.
Figure 4. Homosynaptic paired-pulse effects.
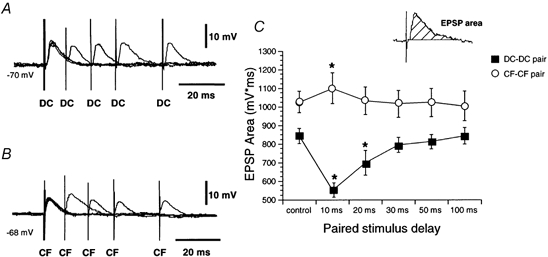
A, average data (n = 4) show a depression of the second dorsal column EPSP (DC) at stimulation intervals < 50 ms. B, average data (n = 4) show a small facilitation of the second corticofugal EPSP (CF) at stimulation intervals of 10-30 ms. C, graph showing mean EPSP areas of the second EPSP as a function of the paired-pulse interval (mean ±s.e.m.). Inset shows the method of measuring the EPSP area. In this and subsequent figures, control values correspond to the area of single EPSPs (*P < 0.05).
RESULTS
In a previous report using similar techniques two types of dorsal column nuclei cells were defined on the basis of their membrane properties and morphological characteristics (Nuñez & Buño, 1999). We found no differences in the EPSPs recorded in both neurone types; thus the 81 neurones selected were analysed together. Neurones showed a stable membrane potential of -71.2 ± 0.8 mV and an input resistance of 47.6 ± 5.7 MΩ and fired overshooting APs with a mean peak amplitude of 78.5 ± 0.7 mV when depolarised above a threshold membrane potential at -50.0 ± 1.0 mV.
Synaptic responses
The EPSPs evoked by stimulation of the dorsal column have been described in detail previously (Nuñez & Buño, 1999). The dorsal column EPSPs had a mean latency and duration of 2.3 ± 0.2 ms and 10.6 ± 2.3 ms, respectively, a rising slope of 3.7 ± 0.5 V s−1 and a peak amplitude that increased gradually with stimulus intensity (Fig. 2A). The EPSPs evoked by stimulation of corticofugal fibres had a similar latency (2.9 ± 0.48 ms) and stimulus intensity- EPSP amplitude relationship, but had a statistically significant longer duration (17.5 ± 1.61 ms; P < 0.05, Wilcoxon test, n = 26) and a slower rising slope (2.7 ± 0.4 V s−1; P < 0.05, Wilcoxon test, n = 26) when held at the resting membrane potential (Fig. 2B). Both dorsal column and corticofugal EPSPs were occasionally followed by an IPSP, and the proportion was similar for both fibre types (14 out of 52 cells, 27 %). Application of the GABAA receptor antagonist picrotoxin (50 μm; n = 9) blocked IPSPs in all tested cells, facilitating AP firing and unmasked a late longer lasting component of the corticofugal EPSP (Fig. 2D), but not the dorsal column EPSP where only an increase in the amplitude of the EPSP was observed (Nuñez & Buño, 1999). The most distinctive difference between dorsal column and corticofugal EPSPs was the voltage dependence of the latter. Stimuli applied at different membrane potentials evoked the expected decrease in amplitude of dorsal column EPSP with depolarisation without change in the time course of the EPSP (Fig. 2A). However, corticofugal EPSPs displayed a late voltage-dependent component that was absent at hyperpolarised potentials but that appeared at the resting level, as suggested by the longer duration of corticofugal EPSPs than dorsal column EPSPs. The voltage-dependent component increased in amplitude and particularly in duration with further depolarisation (arrow in Fig. 2B). Thus, a larger difference between corticofugal and dorsal column EPSP durations was observed at -60 mV (26.6 ± 1.74 ms and 12.1 ± 1.2 ms, respectively; P < 0.01, Wilcoxon test, n = 26; Fig. 2C). At more depolarised membrane potentials, EPSPs from both fibre types triggered APs, but while dorsal column stimulation always evoked a single AP, corticofugal stimulation usually elicited a pair of APs (Fig. 2A and B).
Figure 2. EPSPs evoked by dorsal column and corticofugal stimulation.
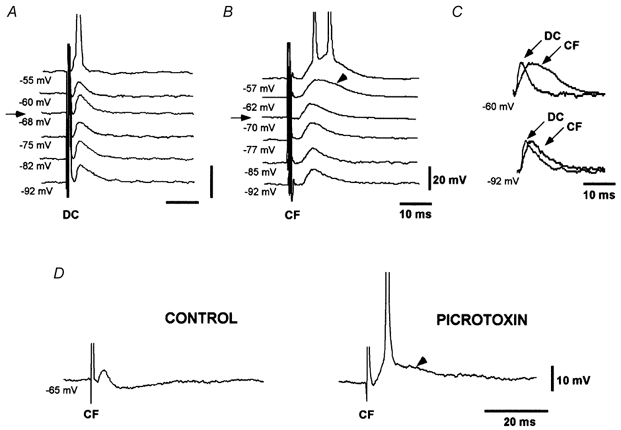
A, dorsal column EPSP (DC) evoked during depolarising and hyperpolarising current pulses show the expected decrease and increase in EPSP amplitude with depolarisation and hyperpolarisation, respectively. B, corticofugal EPSPs (CF) evoked during depolarising and hyperpolarising current pulses increases with both depolarisation and hyperpolarisation. A late slow component was evoked at depolarised membrane potentials (arrowhead). C, scaled and superimposed version of records in A and B at -60 mV (-62 mV in B) and -92 mV. Differences between EPSPs are evident at depolarised membrane potentials. D, picrotoxin (50 μm) blocked the IPSP and unmasked a late longer lasting component of the corticofugal EPSP (arrowhead). In this and subsequent figures, the membrane potential is indicated below each record. Arrows in A and B indicate the resting membrane potential. Spikes are truncated.
To characterise the glutamatergic receptor types mediating the evoked EPSPs in neurones of the dorsal column nuclei (see Introduction) the GABAA inhibition was blocked with picrotoxin (50 μm) and the NMDA or non-NMDA receptor antagonists AP5 or CNQX, respectively, were applied.
The dorsal column EPSPs were not affected by AP5 (50 μm; n = 10), but were reversibly blocked by CNQX in all cases (10 μm; n = 8; Fig. 3A). Superfusion with AP5 reduced the corticofugal EPSPs in a voltage-dependent manner. Inhibition by AP5 was small or even absent at hyperpolarised membrane potentials (< 10 % of the EPSP area), but the corticofugal EPSPs were markedly reduced (30-50 % of the EPSP area) at depolarised membrane potentials (n = 4; middle trace in Fig. 3B). The reduction induced by AP5 was particularly apparent during the late voltage-dependent decaying phase of the corticofugal EPSP and in cells at a membrane potential more depolarised than -70 mV (right-hand trace in Fig. 3B). Adding CNQX (10 μm) to the solution containing AP5 abolished the corticofugal EPSPs (right-hand trace in Fig. 3B). Therefore, dorsal column EPSPs were mediated via activation of non-NMDA receptors while the corticofugal EPSP resulted from the activation of both non-NMDA and NMDA receptors.
Figure 3. Pharmacological characterisation of dorsal column and corticofugal EPSPs.
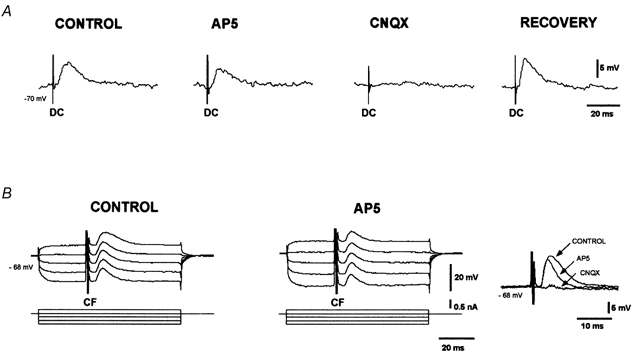
A, dorsal column EPSPs (DC) were unaffected by AP5 (50 μm), but were blocked by CNQX (10 μm). The dorsal column EPSP recovered after a 20 min washout. B, corticofugal EPSPs (CF) evoked during depolarising and hyperpolarising current pulses (current protocol is shown in the lower traces). The late voltage-dependent component was abolished by AP5 (50 μm). Right-hand traces show the block of the late and the reduction of the early component of the corticofugal EPSP by 50 μm AP5. Adding 10 μm CNQX also blocked the early component of the corticofugal EPSP.
Synaptic interactions in cells of the dorsal column nuclei
Synaptic interactions were analysed using paired stimuli (paired-pulse stimulation) delivered to one synaptic input (either the corticofugal or dorsal column, termed homosynaptic stimulation) or the two inputs in close succession (heterosynaptic stimulation). In the latter case the analysis was performed by stimulating the corresponding fibres with two electrodes as shown in Fig. 1. Subthreshold stimulation intensities were used and picrotoxin (50 μm) was added to block GABAA-dependent IPSPs (n = 69).
Paired-pulse stimulation of dorsal column fibres elicited a depression of the second EPSP at stimulation intervals of less than 50 ms in all cell tested (n = 12; Fig. 4A). A statistically significant decrease in the second EPSP was observed at intervals between 10 and 20 ms (F5,55= 49.83; P < 0.05; n = 12) and a subsequent recovery at longer intervals (Fig. 4C).
In contrast, homosynaptic paired-pulse stimulation of corticofugal fibres evoked a modest facilitation of the second EPSP at stimulation intervals of 10 ms in 6 out of 10 neurones or 60 % (F5,25= 63.31; P < 0.05; n = 6; Fig. 4C). Facilitation consisted of a selective increase of the late slow component of corticofugal EPSPs while peak amplitude was not affected (Fig. 4B).
Interaction between dorsal column and corticofugal inputs was analysed using heterosynaptic paired-pulse stimulation at different stimulus intervals. When the corticofugal stimulation preceded the dorsal column stimulation, facilitation of the dorsal column EPSP was observed at pulse intervals under 100 ms in 14 out of 22 neurones or 64 % (Fig. 5A). The change in dorsal column EPSP area relative to the area in the absence of corticofugal stimulation revealed a statistically significant facilitation of the dorsal column EPSP by the preceding corticofugal stimulation at intervals between 10 and 50 ms (F5,65= 32.21; P < 0.05; n = 14; Fig. 5B). At these stimulus intervals there was summation of the late component of the corticofugal EPSP with the subsequent dorsal column EPSP. Facilitation of the dorsal column EPSP was reduced or even totally blocked at hyperpolarised potentials below -65 mV and by superfusion with 50 μm AP5 (Fig. 5C). The effect of AP5 was not due to the decrease in the amplitude of corticofugal EPSPs because a further increase in the corticofugal stimulus intensity did not re-establish facilitation.
Figure 5. Heterosynaptic paired-pulse effects.
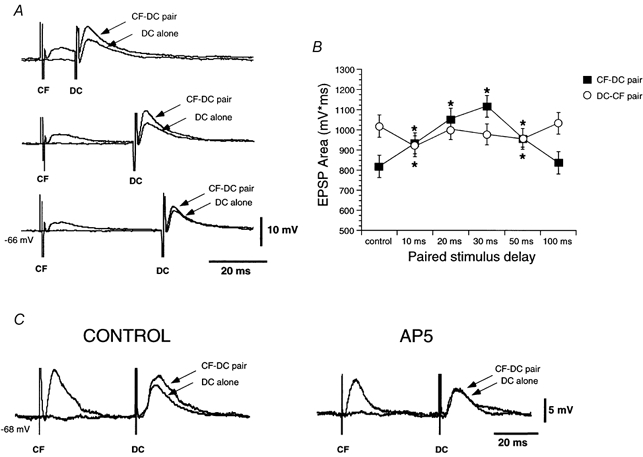
A, corticofugal (CF) stimulation facilitates dorsal column EPSPs (DC) at stimulation intervals < 50 ms (averages n = 6). B, graph of mean EPSP areas of the second EPSP as a function of the paired-pulse interval. C, heterosynaptic facilitation evoked by corticofugal stimuli (CONTROL) is abolish by addition of 50 μm AP5.
When the dorsal column EPSP preceded the corticofugal EPSP, facilitation was absent and instead a depression of the corticofugal EPSP was observed that was statistically significant at intervals of 10 and 50 ms (F5,65= 49.83; P < 0.05; n = 14; Fig. 5B).
EPSPs evoked by stimulus barrages
Barrages of stimuli were applied to examine the frequency-dependent characteristics of dorsal column and corticofugal EPSPs. Stimulation at a frequency > 10 Hz evoked a decrease in the peak amplitude of dorsal column EPSPs that started at the second or third EPSP (i.e. 20 or 30 ms) and that progressed thereafter (F5,45= 36.42; P < 0.05; n = 10; Fig. 6A and C; cf. Nuñez & Buño, 1999). This gradual depression was not modified by changes in membrane potential (Fig. 6A) but was augmented with increasing stimulation frequency (Fig. 6C). In addition, there was no temporal summation of dorsal column EPSPs with stimulation rates of up to 100 Hz (not shown).
Figure 6. Effects of stimulation barrages on dorsal column and corticofugal fibres.
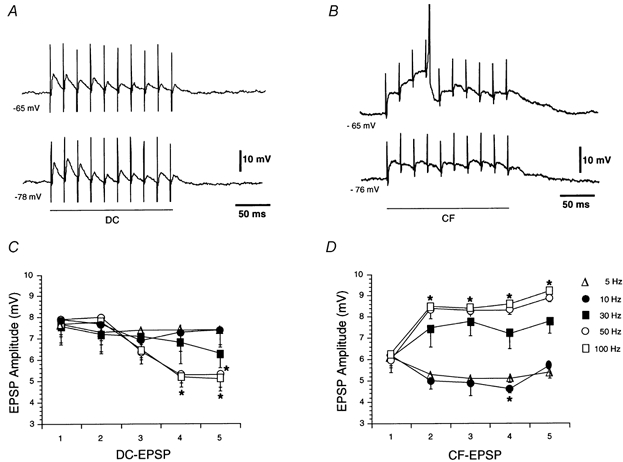
A, a progressive depression of dorsal column EPSPs (DC) was evoked by high frequency stimulation of the dorsal column (50 Hz). The effect was not voltage dependent, and there was no summation. B, progressive facilitation and temporal summation of corticofugal EPSPs (CF) were evoked by corticofugal stimulation (50 Hz). The effects were abolished by hyperpolarisation. C and D, graphs showing the peak amplitude of dorsal column and corticofugal EPSPs (at -60 mV) for successive EPSPs (1st to 5th) in the barrage at different frequencies. Note differences in behaviour between dorsal column and corticofugal EPSPs at high frequencies. Spikes are truncated.
In contrast, corticofugal EPSPs showed facilitation and temporal summation in response to stimulation barrages at frequencies > 30 Hz. These effects were obvious at depolarised membrane potentials that evoked a gradual and prolonged membrane depolarisation which could reach AP threshold (upper trace in Fig. 6B). Facilitation and temporal summation were absent at hyperpolarised membrane potentials < -65 mV (lower trace in Fig. 6B) and were inhibited by superfusion with 50 μm AP5 (n = 3; data not shown). The analysis of the frequency dependence of the effects in cells held at -60 mV revealed that facilitation and temporal summation of corticofugal EPSPs was absent at stimulation frequencies below 10 Hz, where a small depression instead of facilitation was observed. However, facilitation and temporal summation increased gradually with frequency above 30 Hz (F5,45= 40.75; P < 0.05; n = 10; Fig. 6D).
Effects of stimulus barrage on EPSPs
Barrages of stimuli applied to the corticofugal input elicited a short-lasting facilitation of both corticofugal and dorsal column EPSPs (homosynaptic and heterosynaptic facilitation, respectively). The homo- and heterosynaptic facilitation induced by corticofugal stimulation consisted of an increase in the EPSP amplitude and the efficiency with which the EPSPs elicited APs (Fig. 7Aa-b and Ba-b) that lasted up to 2 min at a stimulation frequency of 50 Hz (stimulus duration, 1 s). Averages of subthreshold dorsal column and corticofugal EPSPs also show a clear facilitation 1 min after the corticofugal stimulation barrage (Fig. 7Ac and Bc). Facilitation of both the dorsal column and corticofugal EPSP (heterosynaptic and homosynaptic, respectively) were blocked by superfusion with 50 μm AP5. An example of the blocking effect of AP5 on the heterosynaptic facilitation is shown in Fig. 7C. No changes in the membrane potential were observed during this facilitation.
Figure 7. Short-term facilitation evoked by corticofugal barrages.
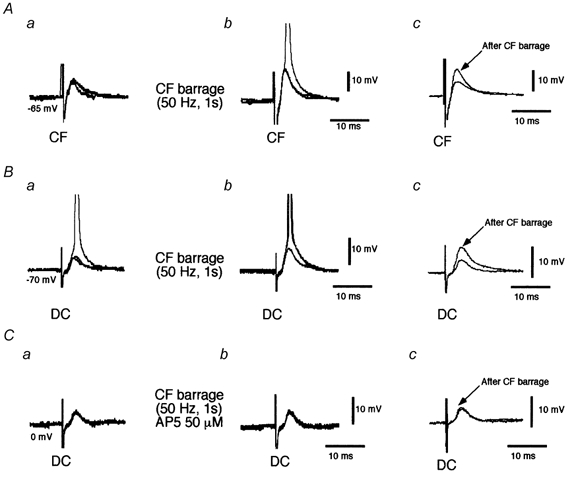
A, superimposed traces (n = 3) of corticofugal EPSPs (CF) before (a) and 1 min after (b) corticofugal stimulation (frequency, 50 Hz; duration, 1 s). The corticofugal EPSP was facilitated and elicited APs. Traces in c show average subthreshold corticofugal EPSPs (n = 8) before and after corticofugal stimulation. Note the homosynaptic facilitation. B, superimposed traces (n = 3) of dorsal column EPSPs (DC) before (a) and 1 min after (b) corticofugal stimulation (frequency, 50 Hz; duration, 1 s). The barrage of corticofugal stimulation facilitated the dorsal column EPSP. Traces in c show average dorsal column EPSPs (n = 8) before and after corticofugal stimulation. Note the heterosynaptic facilitation. C, as in B but during application of 50 μm AP5 blocked the heterosynaptic facilitation. Spikes are truncated.
It is interesting to note that the time elapsing between the high frequency stimulation of the corticofugal fibres and the onset of homosynaptic facilitation (i.e. corticofugal stimulation, corticofugal EPSP) was only a few seconds, whereas the latency of onset for the heterosynaptic facilitation was much longer and close to 1 min. (i.e. corticofugal stimulation, dorsal column EPSP). In addition, homosynaptic facilitation developed gradually, but heterosynaptic facilitation appeared rapidly with a latency of 1 min (Fig. 8A and B). The analysis of the time course of both heterosynaptic and homosynaptic facilitation clearly shows the important differences in their onset latency (Fig. 8C) suggesting that they are mediated via different cellular mechanisms. There was a statistically significant increase in the corticofugal EPSP area up to 2 min after corticofugal stimulation (F5,55= 36.42; P < 0.05; n = 12). However, the area of the dorsal column EPSP first decreased 10 s after stimulation and then increased 1 min later (F5,45= 40.75; P < 0.05; n = 10).
Figure 8. Time course of homosynaptic and heterosynaptic facilitation.
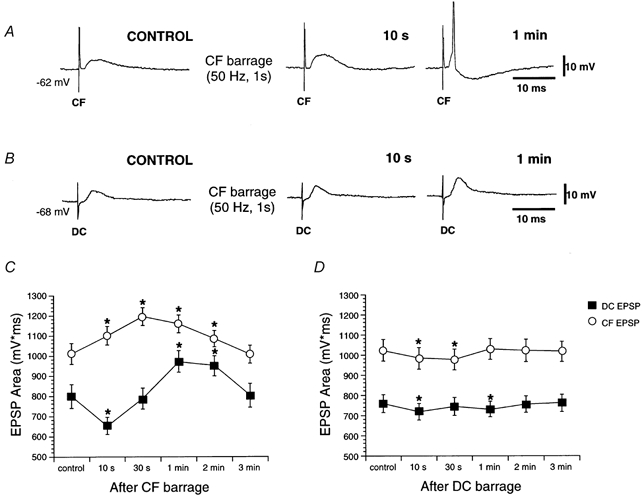
A, corticofugal EPSPs (CF) before (CONTROL) and after (10 s and 1 min) of corticofugal stimulation (frequency, 50 Hz; duration, 1 s). The corticofugal EPSP was facilitated immediately after (10 s) corticofugal stimulation, and evoked APs later. B, the dorsal column EPSP (DC) was facilitated later (1 min after corticofugal stimulation; frequency, 50 Hz; duration, 1 s). C, graph showing EPSP areas as a function of time after corticofugal stimulation. Homosynaptic and heterosynaptic facilitation appeared with different latencies. D, graph showing EPSP areas as a function of time after dorsal column stimulation.
When similar stimulation barrages were applied to the dorsal column input a depression of both dorsal column and corticofugal EPSP areas was observed (F5,40= 24.02 and 20.17, respectively; P < 0.05; n = 9; Fig. 8D).
Depolarising pulses also facilitate dorsal column EPSPs
The activation of NMDA receptors and the large depolarisation that is evoked when barrages of stimuli are applied to corticofugal fibres may activate the cellular mechanisms that underlie the facilitation described above (also see Discussion). Indeed corticofugal EPSPs show facilitation and temporal summation, thus inducing large membrane depolarisations of the neurones of the dorsal column nuclei in response to stimulation barrages. The depolarisation could relieve the voltage-dependent block of NMDA channels, increasing the NMDA current and the inflow of Ca2+. The resulting rise in intracellular Ca2+ concentration could be the signal that activates the intracellular machinery that underlies facilitation.
To test this possibility neurones of the dorsal column nuclei were depolarised by current pulses (intensity, 1-2 nA; duration, 1-2 s) that evoked responses that roughly mimicked the profile of the response elicited by corticofugal stimulation. Depolarising current pulses induced a facilitation of the dorsal column EPSP in 8 out of 10 neurones tested (F5,35= 26.12; P < 0.05; n = 8; Fig. 9A andC). It is interesting to note that the increase in amplitude of the dorsal column EPSP which characterises this facilitation appeared about 1 min after the current pulse and lasted 2-3 min. The similarity between this long latency of onset and the facilitation of dorsal column EPSPs evoked by high frequency stimulation of the corticofugal fibres suggests that similar cellular mechanisms underlie both facilitations. No changes in the membrane potential were observed during this pulse-induced facilitation of dorsal column EPSPs.
Figure 9. Facilitation of the dorsal column EPSP by depolarising pulses.
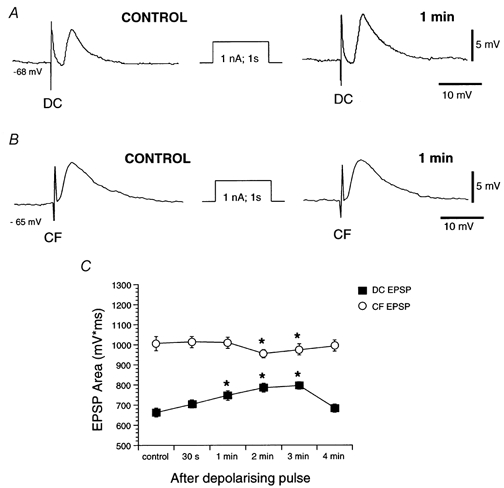
A, average (n = 6) dorsal column EPSPs (DC) before (CONTROL) and 1 min after a depolarising current pulse (amplitude, 1 nA; duration, 1 s). No changes in amplitude and duration were detected. B, average (n = 6) corticofugal EPSPs (CF) before (CONTROL) and 1 min after a depolarising current pulse (amplitude, 1 nA; duration, 1 s). Dorsal column EPSPs were facilitated 1 min after the pulse. C, graph showing EPSP areas as a function of time after a depolarising current pulse. Only dorsal column EPSPs were facilitated by depolarisation.
Similar depolarising pulses did not modify corticofugal EPSPs or decrease EPSP area at latencies of 2 and 3 min (F5,45 = 36.42; P < 0.05; n = 8; Fig. 9B and C), again suggesting that the homosynaptic and heterosynaptic facilitations induced by high frequency stimulation of the corticofugal fibres were initiated via the activation of different cellular mechanisms. These results provide evidence suggesting that the heterosynaptic facilitation of dorsal column EPSPs requires Ca2+ inflow through voltage-gated channels, whereas Ca2+ inflow through Ca2+ channels per se is probably not involved in the homosynaptic facilitation of corticofugal EPSPs.
DISCUSSION
We show that the interactions between excitatory synaptic responses evoked by activation of the two major inputs to cells of the dorsal column nuclei are far more complex than previously believed. This synaptic complexity may be of key functional importance because it could underlie the regulation of the flow of sensory information by descending cortical inputs in the dorsal column nuclei. The main feature of this interaction of excitatory inputs is the homosynaptic and heterosynaptic facilitation induced by activation of descending corticofugal inputs which could regulate both incoming sensory signals and descending inputs at the cellular level. The present results show that NMDA receptors are activated by the glutamate released from corticofugal terminals and suggest that Ca2+ inflow through voltage-gated channels at neurones of the dorsal column nuclei is an important factor in triggering homo- and heterosynaptic facilitation.
The cellular mechanisms that mediate the facilitatory action of corticofugal fibres remain uncertain. The different characteristics of the homosynaptic and heterosynaptic facilitation by corticofugal stimulation suggest different underlying cellular mechanisms for the two types of facilitation.
Characteristics of synaptic inputs
Single stimuli applied to dorsal column and corticofugal fibres always evoked EPSPs in neurones of the dorsal column nuclei and in a few cases also elicited a late IPSP. Similar sequences of EPSPs and IPSPs have been recorded intracellularly in anaesthetised animals (Andersen et al. 1964a, b; Schwartzkroin et al. 1974; Canedo et al. 2000). According to immunohistochemical studies, both dorsal column and corticofugal inputs to neurones of the dorsal column nuclei are glutamatergic (Rustioni & Cuénod, 1982; Rustioni & Weinberg, 1989; Broman, 1994; DeBiasi et al. 1994).
The EPSP evoked by stimulation of the dorsal column is mediated via the activation of non-NMDA α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA) or kainate receptors (see also Nuñez & Buño, 1999), whereas the corticofugal EPSPs also display a late NMDA-dependent component. These results are consistent with the immunohistochemical description of AMPA and NMDA receptors in neurones of the dorsal column nuclei in rat (Watanabe et al. 1994; Kus et al. 1995; Popratiloff et al. 1997).
Recently, intracellular recordings in neurones of dorsal column nuclei in a brainstem-spinal cord preparation demonstrated two types of neurones according to the responses evoked by hyperpolarising current pulses (Deuchars et al. 2000). These findings agree with our previous results (Nuñez & Buño, 1999). Deuchars et al. (2000) showed that dorsal root stimulation evoked EPSPs mediated by the activation of both non-NMDA and, to a lesser extent, NMDA receptors. The presence of an NMDA-dependent component in the dorsal column EPSP observed in that study could be due to a transient expression of NMDA receptors in the young rats used (2-5 days old) since locomotion still improves greatly and synaptic maturation probably continues with further development. An alternative explanation could be that fibres of the reticular formation, which we have not investigated and which may display an NMDA-dependent component, could have been simultaneously activated by the dorsal root volley in the immature animals used in their study.
For a number of reasons, the presence of an AP5-sensitive NMDA-dependent component in the corticofugal EPSP, but not in the dorsal column EPSP, may be responsible for the functional differences between the two types of EPSP. Firstly, the NMDA-dependent component increases the amplitude and duration of the corticofugal EPSPs evoked at depolarised membrane potentials, thus enhancing the possibility of synaptic interactions by temporal summation between successive EPSPs. Secondly, the larger amplitude and duration of the EPSPs facilitate passive electronic transfer to the soma increasing the firing probability of neurones of the dorsal column nuclei during a single corticofugal EPSP and thus the possibility of triggering more than one AP. Thirdly, the larger sustained depolarisation evoked by the NMDA-induced EPSP may activate voltage-gated Ca2+ channels, thus increasing Ca2+ inflow. Finally, and probably most importantly, Ca2+ flowing through NMDA channels may trigger different forms of synaptic plasticity as has been shown in other systems (see Edmonds et al. 1990; Malenka & Nicoll, 1993; Bliss & Collingridge, 1993; Clark & Collingridge, 1996; Thomson, 2000). Indeed, the present results show that the NMDA-dependent component of corticofugal EPSPs underlies the AP5-sensitive short-term homosynaptic and heterosynaptic facilitation that increases the synaptic efficacy of ascending and descending excitatory inputs. Moreover, the heterosynaptic facilitation of dorsal column EPSPs by high frequency stimulation of the corticofugal fibres was reproduced with depolarising pulses, indicating that Ca2+ inflow through voltage-gated channels was sufficient to trigger the underlying cellular mechanisms.
Depression and facilitation
Homosynaptic paired-pulse stimulation discloses two different types of behaviour of dorsal column and corticofugal EPSPs. While paired-pulse stimulation of dorsal column fibres induces a depression of the conditioned EPSP lasting < 50 ms, stimulation of corticofugal fibres generates a modest facilitation of the second EPSP lasting < 30 ms.
The corticofugal EPSPs exhibit paired-pulse facilitation, a phenomenon that has been traditionally attributed to a presynaptic mechanism due to residual Ca2+ in the presynaptic terminal which outlasts the first stimulation and which causes an enhanced probability of release in response to the second stimulation (e.g. Volgushev et al. 1997; Zucker, 1989). However, the facilitation of corticofugal EPSPs is small and more evident in the late NMDA-dependent component, which is absent in dorsal column EPSPs, suggesting that a postsynaptic mechanism also contributes. Indeed, this facilitation was probably mainly due to a voltage-dependent increase in the NMDA-dependent component of the corticofugal EPSP due to temporal summation with the preceding EPSP. In addition, a larger voltage-dependent AP5-sensitive homosynaptic facilitation is evoked by high frequency corticofugal stimulation (see below), again suggesting a postsynaptic mechanism involving NMDA receptor activation and possibly a transient rise in intracellular Ca2+.
The homosynaptic paired-pulse depression of dorsal column EPSPs may be due to a presynaptic mechanism caused by loss of AP conduction in the axons of the dorsal column (e.g. Newberry & Simmonds, 1984; Nuñez & Buño, 1999) or to the synaptic release and diffusion of other substances such as nitric oxide or arachidonic acid, as has been proposed to occur in the cerebellum (Reynolds & Hartell, 2000). In addition, most of neurones of the dorsal column nuclei contain the GluR2 subunit of the AMPA receptor (Popratiloff et al. 1997) which is impermeable to Ca2+. Therefore, Ca2+ inflow during the activation of the AMPA-mediated dorsal column EPSPs, that lack an NMDA-mediated component, should be negligible (Geiger et al. 1995).
Heterosynaptic paired-pulse facilitation is observed when corticofugal EPSPs precede dorsal column EPSPs at stimulation intervals of < 100 ms and only at depolarised membrane potentials. Moreover, the facilitation is blocked by AP5, implying that activation of NMDA receptors is involved (see Bliss & Collingridge, 1993).
Facilitation of dorsal column EPSPs is also induced by long-lasting depolarising current pulses, which may also lead to a substantial increase in the intracellular Ca2+ concentration, as has been demonstrated with Ca2+ imaging techniques in other cell types (Miyakawa et al. 1992; Volgushev et al. 1995). Therefore, Ca2+ inflow through NMDA and also possibly through voltage gated Ca2+ channels is probably the key factor in triggering the intracellular mechanisms that generate this type of facilitation (Bliss & Collingridge, 1993). Although in vivo the Ca2+ inflow through NMDA channels induces facilitation, in fact the mechanism is independent of NMDA because membrane depolarisation per se can induce the facilitation. Conversely, membrane depolarisation does not facilitate corticofugal EPSPs, implying that presynaptic activity and NMDA receptor activation is required for homosynaptic facilitation. Another possibility is that the corticofugal input terminates at more distal dendritic sites than dorsal column inputs (Rustioni & Sotelo, 1974) where the imposed somatic current injection may not evoke the necessary depolarisation to trigger the facilitation, whereas the localised synaptic depolarisation is sufficient to induce facilitation.
There are important differences in the temporal profiles of both types of facilitation, suggesting that they are mediated via different cellular mechanisms. Indeed, the latency of homosynaptic facilitation (i.e. corticofugal stimulation, corticofugal EPSP) is only a few seconds, whereas it is close to 1 min for the heterosynaptic facilitation (i.e. corticofugal stimulation, dorsal column EPSP). In addition, the homosynaptic facilitation developed gradually, but the heterosynaptic facilitation appeared suddenly after a latency of 1 min (Fig. 8A and B).
Although Ca2+ inflow through NMDA channels is needed for certain forms of activity-dependent synaptic plasticity as shown in the present results (see also, for example, Bliss & Collingridge, 1993), a form of NMDA-independent, Ca2+-dependent facilitation has been demonstrated in hippocampal mossy fibre-CA3 pyramidal neuron EPSPs. The mossy fibre facilitation is not affected by NMDA receptor block (Harris & Cotman, 1986), but is prevented by intracellular injection of Ca2+ chelators (e.g. Williams & Johnston, 1989), and is thought to rely on the release of Ca2+ from internal stores (Yeckel et al. 1999). It is interesting to note that cAMP-dependent protein kinase inhibitors block mossy fibre facilitation, indicating that cAMP-dependent protein kinase is required for its induction (Yeckel et al. 1999). A similar facilitation could explain the long-term effects of membrane depolarisation on dorsal column EPSPs, which do not display an NMDA-dependent component, but not the facilitation of corticofugal EPSPs, which is briefer and would require Ca2+ inflow through NMDA channels. Moreover, the delayed onset of the longer lasting facilitation of dorsal column EPSPs, as compared with corticofugal EPSPs, suggests that it could also involve the participation of cAMP-dependent protein kinases and probably phosphorylation of AMPA receptors as occurs in other systems (Lee et al. 2000).
Functional considerations
It has been recently proposed that the sensorimotor cortex exerts a selective control of somatosensory transmission in the dorsal column nuclei whereby activation of corticofugal fibres facilitates sensory responses of neurones of the dorsal column nuclei with overlapping receptive fields and inhibits those with different receptive fields (Malmierca & Nuñez, 1998). This functional arrangement probably provides a synaptic mechanism for the enhancement of contrast between sensory signals whereby the corticofugal pathway would selectively facilitate the flow of somatosensory information coming from common inputs. The present results provide a cellular mechanism by which the corticofugal inputs facilitate the synaptic responses to dorsal column inputs in cells of the dorsal column nuclei. Indeed, the facilitation evoked by activation of corticofugal inputs together with the short-term increases in synaptic efficiency may represent the cellular mechanisms whereby signals representing different receptive fields are specifically controlled in the dorsal column nuclei. This complex, activity-dependent excitatory control of the flow of peripheral information may be a fundamental step in the maintenance and plasticity of somatosensory representation in the dorsal column nuclei.
Acknowledgments
This work was supported by DGICYT-CICYT/MEC/Spain (PM98-0113 and SAF2000-0034) and NATO (CRG970212) grants. We thank Fidela de la Calzada and Francisco Peláez for their technical assistance.
References
- Andersen P, Eccles JC, Oshima T, Schmidt RF. Mechanisms of synaptic transmission in the cuneate nucleus. Journal of Neurophysiology. 1964a;27:1096–1116. doi: 10.1152/jn.1964.27.6.1096. [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Schmidt RF, Yokota T. Identification of relay cells and interneurons in the cuneate nucleus. Journal of Neurophysiology. 1964b;27:1080–1095. doi: 10.1152/jn.1964.27.6.1080. [DOI] [PubMed] [Google Scholar]
- Banna NR, Jabbur SJ. Neurochemical transmission in the dorsal column nuclei. Somatosensory and Motor Research. 1989;6:237–251. doi: 10.3109/08990228909144675. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Broman J. Neurotransmitters in subcortical somatosensory pathways. Anatomy and Embryology. 1994;189:181–214. doi: 10.1007/BF00239008. [DOI] [PubMed] [Google Scholar]
- Canedo A, Mariño J, Aguilar J. Lemniscal recurrent and transcortical influences on cuneate neurons. Neuroscience. 2000;97:317–334. doi: 10.1016/s0306-4522(00)00063-4. [DOI] [PubMed] [Google Scholar]
- Clark KA, Collingridge GL. Evidence that heterosynaptic depolarization underlies associativity of long-term potentiation in rat hippocampus. Journal of Physiology. 1996;490:455–462. doi: 10.1113/jphysiol.1996.sp021157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JD, Gordon G. Corticofugal actions on lemniscal neurons of the cuneate, gracile and lateral cervical nuclei of the cat. Experimental Brain Research. 1992;90:384–392. doi: 10.1007/BF00227252. [DOI] [PubMed] [Google Scholar]
- Debiasi S, Vitellaro-Zuccarello L, Bernardi P, Valtschanoff JG, Weinberg R. Ultrastructural and immunocytochemical characterization of terminals of postsynaptic ascending dorsal column fibres in the rat cuneate nucleus. Journal of Comparative Neurology. 1994;353:109–118. doi: 10.1002/cne.903530110. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Trippenbach T, Spyer KM. Dorsal column nuclei recorded in a brain stem-spinal cord preparation: Characteristics and their responses to dorsal root stimulation. Journal of Neurophysiology. 2000;84:1361–1368. doi: 10.1152/jn.2000.84.3.1361. [DOI] [PubMed] [Google Scholar]
- Dykes RW, Rasmusson DD, Sretavan D, Rehman NB. Submodality segregation and receptive-field sequences in cuneate, gracile and external cuneate nuclei of the cat. Journal of Neurophysiology. 1982;47:389–416. doi: 10.1152/jn.1982.47.3.389. [DOI] [PubMed] [Google Scholar]
- Edmonds B, Klein M, Dale N, Kandel ER. Contributions of two types of calcium channels to synaptic transmission and plasticity. Science. 1990;250:1142–1147. doi: 10.1126/science.2174573. [DOI] [PubMed] [Google Scholar]
- Geiger JRP, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determine gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Gordon G, Jukes GM. Dual organization of the exteroceptive components of the cat's gracile nucleus. Journal of Physiology. 1964a;173:263–290. doi: 10.1113/jphysiol.1964.sp007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon G, Jukes GM. Descending influences on the exteroceptive organization of the cat's gracile nucleus. Journal of Physiology. 1964b;173:291–319. doi: 10.1113/jphysiol.1964.sp007457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon G, Paine CH. Functional organization in nucleus gracilis of the cat. Journal of Physiology. 1960;153:331–349. doi: 10.1113/jphysiol.1960.sp006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EW, Cotman CW. Long-term potentiation of guinea pig mossy fibre responses is not blocked by N-methyl D-aspartate antagonists. Neuroscience Letters. 1986;70:132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- Jabbur SJ, Towe AL. Cortical excitation of neurons in dorsal column nuclei of cat, including an analysis of pathways. Journal of Neurophysiology. 1961;24:499–509. doi: 10.1152/jn.1961.24.5.499. [DOI] [PubMed] [Google Scholar]
- Kus L, Saxon D, Beitz AJ. NMDA R1 mRNA distribution in motor and thalamic projecting sensory neurons in the rat spinal cord and brain stem. Neuroscience Letters. 1995;25:201–204. doi: 10.1016/0304-3940(95)11878-z. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM, Tuerk JD. The distribution of the cortical fibres within the nuclei cuneatus and gracilis in the cat. Journal of Anatomy. 1964;98:143–162. [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lue JH, Jiang SY, Shieh JY, Wen CY. The synaptic interrelationships between primary afferent terminals, cuneothalamic relay neurons and GABA-immunoreactive boutons in the rat cuneate nucleus. Journal of Neuroscience Research. 1996;24:363–371. doi: 10.1016/0168-0102(95)01014-9. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends in Neuroscience. 1993;16:521–526. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- Malmierca E, Nuñez A. Corticofugal action on somatosensory response properties of rat nucleus gracilis cells. Brain Research. 1998;810:172–180. doi: 10.1016/s0006-8993(98)00920-2. [DOI] [PubMed] [Google Scholar]
- Miyakawa H, Lev Ram V, Lasser-oss N, Ross WN. Calcium transients evoked by climbing fibre and parallel fibre synaptic inputs in guinea pig cerebellar Purkinje neurons. Journal of Neurophysiology. 1992;68:1178–1189. doi: 10.1152/jn.1992.68.4.1178. [DOI] [PubMed] [Google Scholar]
- Newberry NR, Simmonds MA. The rat gracile nucleus in vitro: III. Unitary spike potentials and their conditioned inhibition. Brain Research. 1984;303:59–65. doi: 10.1016/0006-8993(84)90210-5. [DOI] [PubMed] [Google Scholar]
- Nuñez A, Buño W. In vitro electrophysiological properties of rat dorsal column nuclei neurons. European Journal of Neuroscience. 1999;11:1865–1876. doi: 10.1046/j.1460-9568.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Popratiloff A, Rustioni A, Weinberg RJ. Heterogeneity of AMPA receptors in the dorsal column nuclei of the rat. Brain Research. 1997;754:333–339. doi: 10.1016/s0006-8993(97)00177-7. [DOI] [PubMed] [Google Scholar]
- Reynolds T, Hartell NA. An evaluation of the synapse specificity of long-term depression induced in rat cerebellar slices. Journal of Physiology. 2000;527:563–577. doi: 10.1111/j.1469-7793.2000.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustioni A, Cuénod M. Selective retrograde transport of D-aspartate in spinal interneurons and cortical neurons of rats. Brain Research. 1982;236:143–155. doi: 10.1016/0006-8993(82)90041-5. [DOI] [PubMed] [Google Scholar]
- Rustioni A, Sotelo C. Synaptic organization of the nucleus gracilis of the cat. Experimental identification of dorsal root fibres and cortical afferents. Journal of Comparative Neurology. 1974;155:441–468. doi: 10.1002/cne.901550406. [DOI] [PubMed] [Google Scholar]
- Rustioni A, Weinberg RJ. The somatosensory system. In: Björklund A, Hökfelt T, Swanson LW, editors. Handbook of Chemical Neuroanatomy. Amsterdam: Elsevier; 1989. pp. 219–321. [Google Scholar]
- Schwartzkroin PA, Duijn Hv, Prince DA. Effects of projected cortical epileptiform discharges on unit activity in the cat cuneate nucleus. Experimental Neurology. 1974;43:106–123. doi: 10.1016/0014-4886(74)90136-8. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Facilitation, augmentation and potentiation at central synapses. Trends in Neuroscience. 2000;23:305–312. doi: 10.1016/s0166-2236(00)01580-0. [DOI] [PubMed] [Google Scholar]
- Towe AL, Jabbur SJ. Cortical inhibition of neurons in dorsal column nuclei of cat. Journal of Neurophysiology. 1961;24:488–498. doi: 10.1152/jn.1961.24.5.488. [DOI] [PubMed] [Google Scholar]
- Valverde F. The pyramidal tract in rodents. Zeitschrift für Zellforschung. 1966;71:297–363. A study of its relations with the posterior column nuclei, dorsolateral reticular formation of the medulla, and cervical spinal cord (Golgi and E.M. observations) [PubMed] [Google Scholar]
- Volgushev M, Voronin L, Chistiakova M, Artola A, Singer W. Long-term modifications of synaptic transmission in rat visual cortex induced by intracellular tetanization. Society Neuroscience Abstracts. 1995;21:1742. [Google Scholar]
- Volgushev M, Voronin LL, Chistiakova M, Singer W. Relations between long-term synaptic modifications and paired-pulse interactions in the rat neocortex. European Journal of Neuroscience. 1997;9:1656–1665. doi: 10.1111/j.1460-9568.1997.tb01523.x. [DOI] [PubMed] [Google Scholar]
- Walberg F. The fine structure of the cuneate nucleus in normal cats and following interruption of afferent fibres. An electron microscopical study with particular reference to findings made in Glees and Nauta sections. Experimental Brain Research. 1966;2:107–128. doi: 10.1007/BF00240401. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Mishina M, Inoue Y. Distinct distributions of five NMDA receptor channel subunit mRNAs in the brainstem. Journal of Comparative Neurology. 1994;343:520–531. doi: 10.1002/cne.903430403. [DOI] [PubMed] [Google Scholar]
- Weisberg JA, Rustioni A. Cortical cells projecting to the dorsal column nuclei of cats. An anatomical study with the horseradish peroxidase technique. Journal of Comparative Neurology. 1976;168:425–438. doi: 10.1002/cne.901680307. [DOI] [PubMed] [Google Scholar]
- Williams S, Johnston D. Long-term potentiation of hippocampal mossy fibre synapses is blocked by postsynaptic injection of calcium chelators. Neuron. 1989;3:583–588. doi: 10.1016/0896-6273(89)90268-7. [DOI] [PubMed] [Google Scholar]
- Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nature Neuroscience. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annual Review of Neuroscience. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]


