Abstract
Whole-cell current responses to brief flashes were obtained from voltage-clamped ‘on’ bipolar cells in dark-adapted dogfish retinal slices. When internal Ca2+ was buffered to low levels, the current–voltage (I–V) relation of their flash responses was linear, with a reversal potential near 0 mV.
On elevating internal Ca2+ the light-dependent I–V relation showed outward rectification, such that the current response to a flash decreased e-fold for a hyperpolarization of 22 mV.
Inclusion of a CaMKII inhibitory peptide in the patch-pipette solution removed the rectification even in the presence of 50 μm Ca2+.
These results are consistent with CaMKII phosphorylation of cGMP-activated channels leading to a voltage-dependent reduction in conductance (outward rectification) and a reduced light response. The voltage-dependent property suggests that phosphorylation creates an energy barrier near the outer part of the channel, reducing the flow principally of monovalent cations.
This is the first reported instance of CaMKII phosphorylation acting to change the electrical characteristics of a membrane channel from linear to rectifying.
Ca2+-dependent desensitization by background light and channel rectification may underlie the change in centre–surround organization of the visual system with light adaptation.
Retinal ‘on’ bipolar cells possess a metabotropic glutamate receptor (mGluR6) linked via a cGMP cascade to the control of cGMP-activated channels (Shiells & Falk, 1990; Nawy & Jahr, 1990; Nakajima et al. 1993; de la Villa et al. 1995) which functions to generate high synaptic amplification of rod signals under dark-adapted conditions (Shiells & Falk, 1994). A major component of light adaptation of the rod visual system results from a reduction in gain in synaptic transmission from rods to ‘on’ bipolar cells, initiated by Ca2+ entry through their cGMP-activated channels which open with light (Shiells & Falk, 1999a). This action of Ca2+ is mediated by the activation of Ca2+-calmodulin kinase II (CaMKII). Evidence was presented, under conditions which bypassed the cGMP cascade, that the locus of phosphorylation was the cGMP-activated channel (Shiells & Falk, 2000). The present work confirms and extends this conclusion by showing that phosphorylation induces a voltage-dependent decrease in the conductance of the cGMP-activated channel (outward rectification). This was observed previously with elevated internal Ca2+, but the mechanism was unknown (Shiells & Falk, 1999a). This is the first reported instance of CaMKII phosphorylation acting to change the electrical characteristics of a membrane channel from linear to rectifying. This property may provide the basis for the change in centre-surround organization of the visual system with dark and light adaptation.
METHODS
Patch-clamp recording from retinal slices
Whole-cell voltage-clamp recordings were obtained from bipolar cells on, or just below, the surface of dark-adapted retinal slices prepared from the retina of the dogfish, Scyliorhinus canicula, as described previously (Shiells & Falk, 1990). Animals were killed humanely by stunning, decapitation and then pithing before removal of the eyes. The slices were continuously superfused with oxygenated Ringer solution at 16-18 °C, and were viewed under infrared illumination. The Ringer solution contained (mm): NaCl 260, KCl 3, CaCl2 4, NaHCO3 20, MgSO4 0.5, urea 350, d-glucose 10, Hepes 5, buffered to pH 7.7 when bubbled with 95 % O2-5 % CO2. Patch pipettes were coated with a heated mixture of parafilm, mineral oil and wax to improve gigaseal formation, and when filled had resistances of 2-3 MΩ. The patch-pipette solution contained (mm): CsCl 260, TEA 20, MgSO4 5, Hepes 10, urea 350, buffered to pH 7.3, to which was added 1 mm ATP and 1 mm GTP just before the experiment. The Ca2+ chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) or BAPTA at 5 mm was added to the patch-pipette solution for buffering free Ca2+ concentrations. A computer program (MaxChelator) was used to calculate the amount of CaCl2 to add to yield the free Ca2+ concentrations (1.05 mm CaCl2 with TPEN; 5.03 mm with BAPTA for 50 μm CaCl2). Fifty micromolar free Ca2+ was effective in activating CaMKII and desensitizing ‘on’ bipolar cell light responses, whilst buffering to 1 μm prevented such desensitization (Shiells & Falk, 1999a, 2000). All chemicals were obtained from Sigma unless otherwise indicated. The specific CaMKII inhibitor peptide (Ala9)-autocamtide-2 (AIP) (Ishida et al. 1995) (Calbiochem) was applied via the patch-pipette solution. High concentrations (> 100 times the concentration required for half-maximal inhibition) were used to ensure rapid and complete CaMKII inhibition. Following gigaseal formation and subsequent rupture of the membrane patch to establish the whole-cell mode, the dark membrane potential was measured in current clamp. Cells were then voltage clamped to their dark potentials (which was corrected for the tip potential) and responses to light were obtained before there was any change in the intracellular medium, as well as after full equilibration with the patch-pipette solution, usually 15 min after establishing the whole-cell configuration (Shiells & Falk, 2000). Similar equilibration times have been reported in salamander ‘on’ bipolar cells (Lasansky, 1992). Previous recordings from these cells have demonstrated that as long as ATP and GTP are included in the patch-pipette solution, the dark current and input conductance remain stable, as do the flash responses, which decline only by about 10-15 % over periods of 30-45 min (Shiells & Falk, 1995). The addition of low concentrations (20 μm) of cGMP to the patch-pipette solution was found to increase ‘on’ bipolar cell light responses in a similar manner to stimulation of soluble guanylate cyclase by NO (Shiells & Falk, 1992b). Endogenous synthesis of cGMP from GTP therefore seems to be sufficient to maintain light responses over long periods. Input conductances were measured by applying 1-5 mV voltage command pulses during the recordings and data were rejected if there were any large variations in series resistance (usually 5-10 MΩ). Current-voltage (I-V) relations of their light responses were obtained by shifting the voltage command potential to different levels about the dark potential, and membrane potentials were calculated by compensating for the voltage drop across the series resistance of the patch electrodes.
Light stimulation
The light absorbed by the rods (rhodopsin molecules isomerized per rod (Rh**)) was estimated from previous ‘on’ bipolar cell measurements in the eyecup (Ashmore & Falk, 1980), because of self-screening by the rods and the variable thickness of the slices (150-250 μm). Half-maximal ‘on’ bipolar cell flash responses were taken to correspond to one Rh**, the half-saturation intensity of the voltage response and the b-wave in eyecup measurements (Shiells & Falk, 1999b). Brief flashes (0.2 ms) were applied using a green light-emitting diode (LED) (peak emission wavelength 530 nm) mounted below the preparation. The light was calibrated with a photodiode to increase by factors of 2 over a range of 10 light intensities.
RESULTS
Figure 1 shows whole-cell voltage-clamped current responses to light flashes recorded from ‘on’ bipolar cells in dark-adapted dogfish retinal slices. Figure 1A shows a series of flash responses obtained from an ‘on’ bipolar cell on equilibration with the Ca2+ buffer 5 mm BAPTA, with no added Ca2+ in the patch-pipette solution. The flash responses showed an increase in amplitude with hyperpolarization and there was a linear relationship between flash response amplitude and membrane potential, with a reversal potential of -2 mV (Fig. 1B). This confirms previous results recorded with intracellular electrodes (Ashmore & Falk, 1980), or with patch pipettes using EGTA as an intracellular Ca2+ buffer (Shiells & Falk, 1992a). Light responses of ‘on’ bipolar cells had a linear I-V relation, with a reversal potential near 0 mV, in the dark-adapted condition with low internal Ca2+. In contrast, when ‘on’ bipolar cells were equilibrated with patch pipette solutions containing Ca2+ buffered to 50 μm, there was little or no increase in their flash response amplitudes with hyperpolarizations more negative than -35 mV (Fig. 1A). The I-V relation with 50 μm Ca2+ showed pronounced outward rectification (Fig. 1B). The voltage dependence of the light-evoked current, i(V), conformed to the following relation:
| (1) |
where i0 is the limiting current response at large hyperpolarization, Vr is the reversal potential and K is a constant. The constant K was determined from the slope of a semi-log plot of (i0 - i)/i0 against membrane potential (Fig. 1C), yielding 21.6 mV for an e-fold change in current.
Figure 1. Elevating intracellular Ca2+ to 50 μm induces outward rectification.
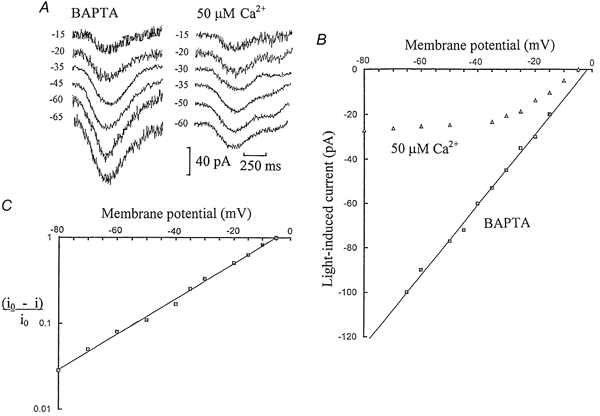
Half-maximal responses were elicited by flashes isomerizing 1 rhodopsin molecule per rod (1 Rh**) with the cells voltage clamped to their initial dark potentials. Their membrane potentials were then shifted to more positive or negative levels about their dark potentials to construct current-voltage (I-V) relations of their light responses. A, ‘on’ bipolar cell responses to 1 Rh** flashes voltage clamped to different membrane potentials, on equilibration with 5 mm BAPTA or 50 μm free Ca2+ (with 5 mm BAPTA) in the patch-pipette solution. The dark potentials were -35 and -30 mV, respectively. B, averaged I-V relations derived from current responses to 1 Rh** flashes from 5 ‘on’ bipolar cells equilibrated with 50 μm Ca2+ (with BAPTA) and from 3 cells with 5 mm BAPTA. The largest standard errors of the mean (s.e.m.) currents were ± 5 and ± 14 pA, respectively. C, semi-log plot of (i0 - i)/i0 against membrane potential for responses obtained with 50 μm free Ca2+, where i is the light-induced current and i0 is the limiting current response at large hyperpolarization. From the slope of the graph, the current response decreased e-fold for a hyperpolarization of 21.6 mV.
We have recently shown that ‘on’ bipolar cells are desensitized by background light, which results from an influx of Ca2+ (Shiells & Falk, 1999a, 2000). Elevation of Ca2+ activates CaMKII and desensitization does not occur if CaMKII is blocked by specific CaMKII peptide inhibitors. To determine whether phosphorylation of the ‘on’ bipolar cell cGMP-activated channels by CaMKII mediated the voltage-dependent change in their electrical properties, whole-cell recordings were obtained with the selective peptide inhibitor (Ala9)-autocamtide-2 (AIP) included in the patch-pipette solution with elevated free Ca2+. Figure 2A shows a series of dim (0.5 Rh**) flash responses obtained from an ‘on’ bipolar cell on equilibration with 10 μm AIP and 50 μm free Ca2+, voltage clamped to a range of membrane potentials. The flash responses show a clear increase in amplitude with hyperpolarization, similar to those obtained with BAPTA (Fig. 1A). There was a linear relationship between flash response and membrane potential when CaMKII was inhibited, even in the presence of elevated Ca2+ (Fig. 2B). This suggests that inhibition of CaMKII by AIP completely blocks the outward rectification induced by 50 μm Ca2+ and maintains linear characteristics as observed when Ca2+ was buffered to low levels.
Figure 2. Inhibition of CaMKII by AIP blocks the outward rectification induced by 50 μm Ca2+.
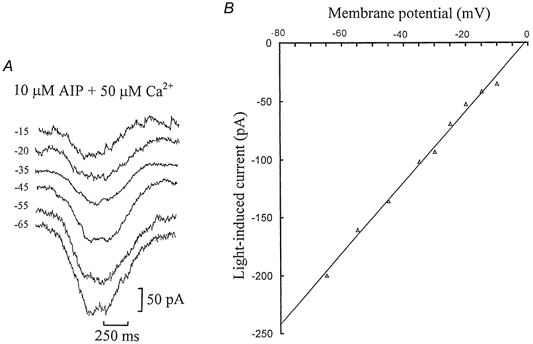
A, ‘on’ bipolar cell responses to 0.5 Rh** flashes voltage clamped to different membrane potentials, on equilibration with patch-pipette solutions containing 10 μm AIP and 50 μm free Ca2+ buffered with 5 mm TPEN. The dark potential was -35 mV. B, averaged I-V relation derived from four sets of measurements made on 2 similar ‘on’ bipolar cell recordings with AIP and 50 μm Ca2+. The largest standard error of the mean currents (s.e.m.) was ± 19 pA.
DISCUSSION
The results provide strong evidence that the outward rectification is a consequence of Ca2+ stimulating CaMKII to phosphorylate the ‘on’ bipolar cell cGMP-activated channels. In the dark-adapted condition, or with Ca2+ buffered to low levels, the I-V relations for the ‘on’ bipolar cell flash responses were linear. Exposure to dim background light induces a rise in intracellular [Ca2+], which enters via the Ca2+-permeable cGMP-activated channels (Shiells & Falk, 1999a; Nawy, 2000). This activates CaMKII resulting in ‘on’ bipolar cell desensitization, which is the initial stage of adaptation to light (Shiells & Falk, 2000). Light-adapted salamander ‘on’ bipolar cell responses to glutamate showed the same outward rectification with elevated Ca2+ (Nawy, 2000). Nawy proposed that there was a direct effect of Ca2+ binding to some site on the internal side of the channel, resulting in its down-regulation, and constituting a negative-feedback pathway. Since inhibition of CaMKII by AIP abolished the outward rectification in the presence of 50 μm Ca2+, we can conclude from the present results that the effect of Ca2+ on the channel is not direct, by binding to some regulatory site, but rather is mediated by CaMKII phosphorylation (Shiells & Falk, 2000). A major difference of ‘on’ bipolar cell cGMP-activated channels from those expressed in rods is that their conductance is not reduced by external divalent cations (Shiells & Falk, 1992a). Phosphorylation of rod cGMP-activated channels has been shown to reduce their sensitivity to cGMP, but no change in their electrical properties due to phosphorylation was reported (Gordon et al. 1992; Molokanova et al. 1999, 2000). It must be emphasized that there are no reliable measurements of single-channel characteristics, nor is there direct evidence for channel gating by cGMP. The molecular structure of the presumptive channel is unknown as the channel has thus far eluded cloning. It bears a number of similarities to the rod channel: selectivity for cGMP over cAMP, open-channel block by l-cis-diltiazem (although the locus of the blocking site differs from the rod channel; Shiells & Falk, 1992b) and the affinity for cGMP is regulated by tyrosine kinase/phosphatase (R. A. Shiells & G. Falk, unpublished observations). These features and knowledge of the threonine/serine phosphorylation site(s) deduced from light responses may contribute to the eventual elucidation of channel structures.
There are a number of models of membrane rectification (see Jack et al. 1975), among which are open-channel block (by ions driven by diffusion and the electric field) and the single energy barrier model of rectification. Since the effect of Ca2+ is mediated by phosphorylation, open-channel block would not appear to be an appropriate model. On the other hand, phosphorylation of serine/ threonine groups by CaMKII might constitute an energy barrier or steric hindrance to the passage of ions. The observed voltage dependence of the rectification, 21.6 mV for an e-fold change in current, can be analysed in terms of the relationship derived by Jack et al. (1975):
| (2) |
where i is the voltage-dependent current, a and b are constants, F is Faraday's constant, R is the gas constant, T is the absolute temperature, z is the valence and γ is the fractional distance of the barrier from the external surface of the membrane. For a single barrier at the external surface, eqn (2) reduces to:
| (3) |
At large hyperpolarization i(V) = -b =i0 and a can be evaluated from the reversal potential, a = -i0exp(-zFVr/RT) to yield eqn (1). The observed voltage dependence suggests that the position of the energy barrier would be towards the external side of the channel, i.e. with γ close to zero. When γ = 0.5 (midway) the degree of non-linearity of the I-V relation would be smaller, whilst when γ = 1 (inside) the I-V relation would show inward rectification. With a barrier near the external surface of the channel (γ = 0) and with monovalent cations constituting the principal current carriers (Ashmore & Falk, 1980), the expected voltage dependence would be RT/zF = 25 mV. However, a similar value would apply if the barrier was located at the middle of the channel and the current carriers were divalent. This possibility can be discounted because there was a high level of free Mg2+ internally in the patch-pipette solution and a similar concentration of divalent cations externally in the Ringer solution. If the permeabilities of Ca2+ and Mg2+ are the same, the constants a and b of eqn (2) would be nearly equal so that the rectification would be inward at large hyperpolarization without a limiting current. The observed value for an e-fold change of 21.6 mV, rather than 25 mV, as would apply for monovalent cations, suggests that 27 % of the current is carried by divalent cations on the assumption that the barrier for monovalent and divalent cations is at the same site near the surface. It should be noted that multiple barrier site models do not generally lead to the simple exponential relationship between current and voltage observed experimentally.
One of the characteristics of the visual system in generating high contrast sensitivity is the organisation into centre/antagonistic-surround receptive fields (Kuffler, 1953). The change of organization in receptive fields of the ‘on’ pathway from no antagonistic surround in the dark-adapted condition to a highly developed receptive field organization with light adaptation (Barlow et al. 1957; Enroth-Cugell & Shapley, 1973) can now be accounted for without needing to invoke a change in connectivity. In the dark-adapted state, the gain in synaptic transmission from rods to ‘on’ bipolar cells is at least an order of magnitude higher than that of the rod-horizontal cell synapse (Ashmore & Falk, 1980; Shiells & Falk, 1994), so the centre response dominates. In the presence of background light, the gain of the centre response is selectively reduced by desensitization mediated by CaMKII phosphorylation (Shiells & Falk, 2000), resulting in similar synaptic voltage gains in both pathways. The action of the antagonistic surround in hyperpolarizing the ‘on’ bipolar cell would shift the membrane potential to a region where the channels mediating the centre response would be in an even lower conductance state, due to their outward rectification, resulting in a further diminished centre voltage response. These control mechanisms would allow the effect of the inhibitory surround to become prominent under light adaptation. This change in receptive field organization was not observed in the ‘off’ pathway (Barlow et al. 1957), consistent with the absence of desensitization in ‘off’ bipolar cells, and their lower light sensitivity which is comparable to horizontal cells (Ashmore & Falk, 1980; Shiells & Falk, 1999a).
The conductance of AMPA receptor channels has been shown to be modulated by phosphorylation at multiple sites, with PKA dephosphorylation inducing long-term depression and CAMKII dephosphorylation inducing long-term potentiation in hippocampal neurons at distinct GluR1 Ser residues (Lee et al. 2000). In cerebellar neurons, outward rectification of AMPA receptor channels appears to be induced by the insertion of different subunits into the channels (Liu & Cull-Candy, 2000). It seems possible that bi-directional synaptic plasticity in ‘on’ bipolar cells, increasing or reducing synaptic voltage gain in the dark-adapted and light-adapted conditions, respectively, may be accounted for by dephosphorylation-CaMKII phosphorylation of their cGMP-activated channels. It remains to be determined whether more complex biochemical processes underly longer term changes in sensitivity at this first visual synapse.
Acknowledgments
We would like to thank the Wellcome Trust for financial support.
References
- Ashmore JF, Falk G. Responses of rod bipolar cells in the dark-adapted retina of the dogfish, Scyliorhinus canicula. Journal of Physiology. 1980;300:115–150. doi: 10.1113/jphysiol.1980.sp013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Fitzhugh R, Kuffler SW. Change of organization in the receptive fields of the cat's retina during dark adaptation. Journal of Physiology. 1957;137:338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Villa P, Kurahashi T, Kaneko A. L-glutamate-induced responses and cGMP-activated channels in three subtypes of retinal bipolar cells dissociated from the cat. Journal of Neuroscience. 1995;15:3571–3582. doi: 10.1523/JNEUROSCI.15-05-03571.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C, Shapley RM. Adaptation and dynamics of cat retinal ganglion cells. Journal of Physiology. 1973;233:271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SE, Brautigan DL, Zimmerman AL. Protein phosphatases modulate the apparent agonist affinity of the light-regulated ion channel in retinal rods. Neuron. 1992;9:739–748. doi: 10.1016/0896-6273(92)90036-d. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochemical and Biophysical Research Communications. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- Jack JJB, Noble D, Tsien RW. Electric Current Flow in Excitable Cells. Oxford University Press; 1975. pp. 229–234. [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. Journal of Neurophysiology. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Properties of depolarizing bipolar cell responses to central illumination in salamander retinal slices. Brain Research. 1992;576:181–196. doi: 10.1016/0006-8993(92)90679-4. [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Liu S-Q J, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–457. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- Molokanova E, Maddox F, Luetje CW, Kramer RH. Activity-dependent modulation of rod photoreceptor cyclic nucleotide-gated channels mediated by phosphorylation of a specific tyrosine residue. Journal of Neuroscience. 1999;19:4786–4795. doi: 10.1523/JNEUROSCI.19-12-04786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E, Savchenko A, Kramer RH. Interactions of cyclic nucleotide-gated channel subunits and protein tyrosine kinase probed with genistein. Journal of General Physiology. 2000;115:685–696. doi: 10.1085/jgp.115.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. Journal of Biological Chemistry. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Nawy S. Regulation of the on bipolar cell mGluR6 pathway by Ca2+ Journal of Neuroscience. 2000;20:4471–4477. doi: 10.1523/JNEUROSCI.20-12-04471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;346:269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proceedings of the Royal Society. 1990;B 242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Properties of the cGMP-activated channel of retinal on-bipolar cells. Proceedings of the Royal Society B. 1992a;247:21–25. doi: 10.1098/rspb.1992.0004. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Retinal on-bipolar cells contain a nitric oxide-sensitive guanylate cyclase. NeuroReport. 1992b;3:845–848. doi: 10.1097/00001756-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Responses of rod bipolar cells isolated from dogfish retinal slices to concentration-jumps of glutamate. Visual Neuroscience. 1994;11:1175–1183. doi: 10.1017/s0952523800006970. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Signal transduction in retinal bipolar cells. Progress in Retinal and Eye Research. 1995;14:223–247. [Google Scholar]
- Shiells RA, Falk G. A rise in intracellular Ca2+ underlies light adaptation in dogfish retinal ‘on’ bipolar cells. Journal of Physiology. 1999a;514:343–350. doi: 10.1111/j.1469-7793.1999.343ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Contribution of rod, on-bipolar, and horizontal cell light responses to the ERG of dogfish retina. Visual Neuroscience. 1999b;16:503–511. doi: 10.1017/s0952523899163119. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Activation of Ca2+-calmodulin kinase II induces desensitization by background light in dogfish retinal ‘on’ bipolar cells. Journal of Physiology. 2000;528:327–338. doi: 10.1111/j.1469-7793.2000.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


