Abstract
Phosphate ions (Pi) enter intracellular Ca2+ stores and precipitate Ca2+. Since transport pathways for Pi across the membrane of intracellular calcium stores have not been identified and anion channels could provide such a pathway, we have examined the Pi conductance of single anion channels from the sarcoplasmic reticulum (SR) of rabbit skeletal muscle using the lipid bilayer technique.
Two anion channels in skeletal muscle SR, the small conductance (SCl) and big conductance (BCl) chloride channels, were both found to have a Pi conductance of 10 pS in 50 mm Pi. The SCl channel is a divalent anion channel which can pass HPO42− as well as SO42− (60 pS in 100 mm free SO42−). The BCl channel is primarily a monovalent anion channel. The SCl and BCl channels are permeable to a number of small monovalent anions, showing minor selectivity between Cl−, I− and Br− (Cl− > I− > Br−) and relative impermeability to cations and large polyatomic anions (Cs+, Na+, choline+, Tris+, Hepes− and CH3O3S−).
The Pi conductance of SCl and BCl channels suggests that both channel types could sustain the observed Pi fluxes across the SR membrane. Comparison of the blocking effects of the phosphonocarboxylic acids, ATP and DIDS, on the anion channels with their effects on Pi transport suggests that the SCl channel is the more likely candidate for the SR Pi transport mechanism.
The SCl channel, with previously unknown function, provides a regulated pathway for Pi across the SR membrane which would promote Pi entry and thereby changes in the rapidly releasable Ca2+ store during onset and recovery from muscle fatigue. Anion channels may provide a pathway for Pi movement into and out of Ca2+ stores in general.
Phosphate ions (Pi, i.e. HPO42− and H2PO4−, see Methods) regulate the size of the rapidly releasable intracellular calcium pool, and hence physiological function, in a wide variety of cell types (Fulceri et al. 1993; Fryer et al. 1995; Guse et al. 1996; Mezna & Michelangeli, 1998). Pi is produced in the cytoplasm as a by-product of ATP metabolism. In resting muscle cytoplasmic [Pi] is in the range 1-5 mm, but during muscle fatigue [Pi] can rise to 20-40 mm (Godt & Nosek, 1989). At these levels Pi causes a marked reduction in Ca2+ release and peak muscle force (Fryer et al. 1995; Kabbara & Allen, 1999). Three mechanisms for this have been proposed. Firstly, depletion of the ryanodine-sensitive Ca2+ stores by activation of the calcium release channels. Bilayer studies indicate that cytoplasmic Pi can activate the calcium release channels in the sarcoplasmic reticulum (SR) (Fruen et al. 1994). Secondly, depletion of Ca2+ by reversal of the Ca2+-ATPase. High cytosolic [Pi], in conjunction with low creatine phosphate levels, is believed to reverse the SR Ca2+-ATPase in skinned muscle fibres (Duke & Steele, 2000). Finally, reduction of the free [Ca2+] in stores when Pi enters the lumen of InsP3- and ryanodine-sensitive Ca2+ stores and forms a variety of insoluble Ca2+-Pi precipitates (Fulceri et al. 1993; Fryer et al. 1995; Fryer et al. 1997). Although these effects of Pi transport across the SR can be dramatic, Pi pathways between cytoplasm and lumen have not been identified. Stefanova et al. (1991a) observed ATP-dependent transport of Pi across the membrane of SR vesicles. The fact that ATP enhanced this transport suggested an active mechanism. However, recent studies by Posterino & Fryer (1998) in skinned muscle fibres have suggested that Pi entry and exit from the SR primarily occur through a passive pathway that is not driven by ATP. In fact, these authors found that Pi entry into the SR was facilitated when ATP was omitted from the myoplasmic solution, a result consistent with Pi entry through an ATP-inhibited anion channel. However, there are no reports of Pi-permeable channels in the SR membrane.
Two SR anion channels have been identified in rabbit skeletal muscle. The BCl channel (250 pS in 250/50 mm Cl−; cis/trans) is apparently unregulated by cytoplasmic ligands and is constitutively open in lipid bilayers (Tanifuji et al. 1987; Kourie et al. 1996b). The smaller conductance SCl channel (75 pS in 250/50 mm Cl−) is highly regulated, and partially inhibited by cytoplasmic acidification (< pH7), millimolar concentrations of adenine nucleotides (Ahern & Laver, 1998; Kourie, 1999) and inositol phosphates (Kourie et al. 1997). It is voltage dependent (open at membrane potentials, Vm, between 0 and -80 mV with respect to the luminal side, Kourie et al. 1996b), activated and inhibited by oxidation and reduction, respectively (Kourie, 1997a) and activated by ≈1 μm cytoplasmic Ca2+ (Kourie et al. 1996a).
In muscle there appears to be an excess of SR Cl− channels even though Cl− is not an important counterion during Ca2+ release from SR (Coonan & Lamb, 1998). The presence of the anion channels could be understood if intracellular Cl− channels also conducted physiologically important anions other than Cl−. Thus we have measured the conductance of SCl and BCl channels to inorganic phosphate to determine whether the channels could play a role in passing these functionally important anions.
METHODS
Isolation and reconstitution SR anion channels
SR vesicles were prepared from the back and leg muscle of New Zealand White rabbits as described by Saito et al. (1984). Rabbits (≈4 kg) were killed by captive bolt before muscle was removed for biochemical processing. The procedure was carried out by the holder of a current licence granted under ACT State legislation by the Australian National University Ethics Committee. SR vesicles were isolated from muscle using techniques based on Chu et al. (1988) as previously described in Laver et al. (1995) and Kourie et al. (1996b). Briefly, cubes of muscle were homogenized in a Waring blender in homogenizing buffer (20 mm imidazole, 300 mm sucrose, pH 7.1 with HCl), centrifuged (11 000 g, 15 min) and the pellet resuspended, rehomogenized and centrifuged as above. The supernatant was filtered through cotton gauze and pelleted by centrifugation (110 000 g for 60 min) to yield a crude microsomal fraction, which was fractionated by loading onto a discontinuous sucrose gradient. Heavy SR vesicles were collected from the 35-45 % (w/v) interface, snap frozen and stored at -70 °C. SR vesicles were incorporated into artificial bilayers separating cis and trans baths. Details of the method are given elsewhere (Kourie et al. 1996a,b). Vesicles were added to the cis bath and the cytoplasmic side of incorporated channels faced the cis solution. The luminal side faced the trans solution.
Chemicals and solutions
Phosphoric acid was obtained from BDH and CsOH from ICN Biomedicals. 4′4’-Diisothiocyanatostilbene-2′2’-di-sulfonic acid (DIDS) was obtained from ICN Biomedicals. Phosphonoformic acid (PFA) and phenylphosphonic acid (PhPA) were obtained from Sigma. Lipids were obtained from Avanti Polar Lipids (Alabaster, AL, USA). Lipid bilayers were formed from phosphatidylethanolamine and phosphatidylcholine (8:2 w/w) in n-decane, across an aperture of 150-250 μm diameter in a Delrin cup.
Unless otherwise stated, solutions were buffered to pH 7.4 using 10 mm TES and also contained 0 (no added Ca2+) to 1 mm CaCl2 (Kourie et al. 1996b). The current through SCl and BCl channels was investigated in the presence of Pi solutions with Na+, Cs+ or Tris+ as the cation. NaxPi-containing solutions were made by mixing Na2HPO4 and NaH2PO4 solutions in a ratio of 81:19, titrating one with the other to give a final pH of 7.4. TrisxPi and CsxPi solutions were produced by titrating phosphoric acid with Tris-base and CsOH, respectively, to the desired pH. TES was not required to buffer the pH of Pi solutions. The relative permeability of Pi was mainly determined with 50-200 mm Pi on one side of the membrane and either 250 mm CsCl or choline-Cl on the other. Concentrations given for Pi solutions refer to Pi, not the cation.
Sulphate-containing solutions were either MgSO4 or Cs2SO4. Sulphate permeability was measured with 50-250 mm SO42− on one side of the membrane and 250 mm of either CsCl or choline-Cl on the other. The free [SO42−] was estimated using published association constants (Marks & Maxfield, 1991) and the program ‘Bound and Determined’ (Brooks and Storey, 1992). Measurement of channel permeability to Br− and I− (Y) relative to Cl− was made using 250 mm CsCl or choline-Cl on one side of the bilayer and 250 mm CsY (occasionally 50 mm CsY) on the other.
Individual ion channels were usually studied under several different ionic conditions during each experiment. Channels were initially identified as BCl or SCl by their conductance and gating properties in solutions containing 250/50 mm Cl− (cis/trans). Ionic composition of the baths was then altered using three methods. (1) Bath solution exchange by perfusion with 10 volumes of solution using a back-to-back syringe system. (2) Aliquot addition of concentrated stock solutions to the baths. (3) Rapid local perfusion achieved by flowing solutions from a tube (polyethylene, 0.5 mm diameter, 50 μm from bilayer) directly onto the bilayer surface. At a flow rate of ≈1 μl s−1, the solution at the bilayer surface could be completely replaced with the perfusing solution in less than 1 s and the changed environment could be maintained for many minutes. Details of this technique are given elsewhere (Laver & Curtis, 1996).
Calculation of liquid junction and membrane potentials
Current was measured via agar-bridge electrodes (250 mm CsCl in 1 % agar) in each solution. Bilayer potential is given with respect to the trans (luminal) bath and positive current signifies movement of positive charge from cis to trans solutions. Voltage was controlled and current recorded with an Axopatch 200B amplifier (Axon Instruments). The bilayer potential was calculated from the amplifier command potential and the liquid junction potentials at the agar electrode-bath interfaces, the cis-trans bath interface and the interface between locally perfused solutions and bath solution. In the case of solutions mainly containing monovalent ions, the liquid junction potentials can be calculated using the Henderson equation (eqn (1), e.g. Barry & Lynch, 1991).
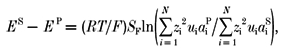 |
(1) |
where
where EP and ES are the electric potentials in solutions P and S respectively and zi, ui and ai are the valency, mobility and activity of ion species i, respectively. The Henderson equation does not accurately predict liquid junction potentials for solutions containing divalent ions. Liquid junction potentials were measured at the interface of dissimilar solutions in the cis and trans baths of the bilayer apparatus. The potential difference was measured relative to that obtained when cis and trans baths contained identical solutions (i.e. when the liquid junction potential equals zero). The potential difference between the two baths, at zero current, was measured using agar-bridge electrodes containing 4 m KCl while stirring the baths. We found that for electrolytes containing the monovalent ions used in this study, the Henderson equation predicted junction potentials within ± 2 mV of those found experimentally, but this was not always the case for solutions containing divalent ions. Hence, we frequently used the Henderson equation to predict liquid junction potentials in the absence of divalent ions. We also measured changes in the liquid junction potentials at the agar electrode-bath interface that would occur during bath perfusion and we found that they were within 2 mV of those measured in free solutions. This was done by measuring the potential difference, at zero current, between an agar electrode containing 250 mm CsCl and one containing 4 m KCl both before and after the bath solution was changed.
Recording and analysis of single channel data
During the experiments the bilayer current and potential were recorded at a bandwidth of 5 kHz, sampled at 50 kHz and simultaneously stored on computer disk using a data interface (Data Translation DT301) with in-house software written in Visual Basic. Unitary current and time-averaged currents were extracted using Channel2 software (Professor P. W. Gage & Mr M. Smith at the Australian National University).
SCl channel unitary current was measured from the difference between the channel open and baseline (closed) current levels obtained at 23 ± 2 °C. Since the BCl channels rarely showed complete closures (see Results) it was difficult to directly measure the baseline current. For BCl channels the baseline current was determined in one of two ways, (1) from the mean current at the reversal potential of the BCl channel (Vr was found by adjusting the voltage to minimise the current variance) or (2) from occasional apparent closures of the channel. Channels in ‘leaky’ bilayers, in which significant baseline conductance was present (> 100 pS), were excluded from subsequent analysis. The conductance (G) of channels was determined from the slope of their current-voltage dependence. Relative ion permeability (Pj) was derived from the reversal potentials (Vr, where the current, I, is zero) for each set of ionic conditions by numerically solving the constant field equation (eqn (2)) (Goldman 1943):
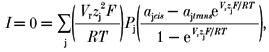 |
(2) |
where j are the ion species present, each with activity αj and valency, zj and V, F, R and T have their usual meanings. In calculating ionic permeabilities from the current reversal potentials it was assumed that cations and large anions (i.e. Cs+, Na+, choline+, Tris+, Hepes− and CH3O3S−) had zero permeability. This assumption was supported by other measurements of Vr (shown for Cs+ and choline+ in Fig. 1), which indicated that the permeability of cations and large anions, relative to Cl− was less than 0.05 in SCl and BCl channels. We found that the calculated permeability ratios for the smaller anions were insensitive to the assumed relative permeability values for cations and large anions over the range 0-0.05.
Figure 1. The conductance of anion channels from rabbit SR incorporated into lipid bilayers.
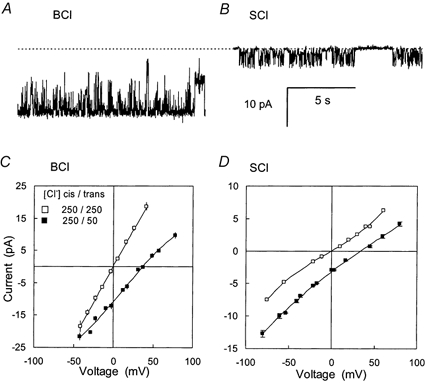
A, recording of a single BCl channel in 250/50 mm (cis/trans) choline-Cl solutions at -40 mV (cis potential) with a slope conductance of 250 pS. The dashed line shows zero current. Scale bars apply to both A and B. B, a single SCl channel with a slope conductance of 80 pS recorded under conditions described in A. C and D, mean current-voltage (I-V) data for BCl and SCl channels separating different Cl− solutions (using Cs+ or choline+ as the cation. Error bars indicate standard error of the mean voltage and current for each data point. Error bars are frequently obscured by the symbols. C, ▪, BCl channels in 250/50 mm Cl− (cis/trans, n = 6). The conductance, G, and reversal potentials, Vr, are 260 pS and 37 mV. □, BCl channels in 250/250 mm Cl− (cis/trans, n = 5), G = 445 pS and Vr = 0 mV. D, ▪, SCl channels in 250/50 mm Cl− (cis/trans, n = 7), G = 106 pS and Vr = 33 mV. □, SCl channels in 250/250 mm Cl− (cis/trans, n = 3), G = 90 pS and Vr = 0 mV.
Ion activity coefficients, γj, for each species were calculated from the total ionic strength, μ, using the Guy-Chapman ‘Limiting Law’ (eqn (3)) which provides a good approximation to the experimentally observed mean activity coefficients at ionic strengths below 1 m (Margolis, 1966):
| (3) |
The Michaelis-Menten constants were determined by fitting eqn (4) (by the method of least squares) to the substrate concentration dependence of channel conductance. Fitting was carried out using results from individual experiments rather than the averaged data presented here:
| (4) |
where Gmax is the upper limit of channel conductance at high substrate concentrations and Km is the concentration at half-maximal conductance.
The degree of channel inhibition was determined from the ratio of the time-averaged current through the channels measured under control conditions (I) to that in the presence of inhibitor (Io). Individual data were fitted with eqn (5) to determine the concentration of half-inhibition (Ki) and the Hill coefficient (H):
| (5) |
determination of association constant for cshpo4− and TrisHPO4−
Inorganic phosphate (Pi) ions associate with monovalent cations in solutions giving rise to the three predominant forms of Pi in the pH range 4-10, namely HPO42−, H2PO4− and XHPO4− where X+ represents a monovalent cation. The concentrations of these species depend on the pH, the total [X] and [Pi], and the association constants for XHPO4− and of H2PO4−. The association constants for NaHPO4− (3.5 m−1) and KHPO4− (3.1 m−1) have been determined previously (Smith & Alberty, 1956). The association constant for CsHPO4− (1.3 m−1) was measured here using the same method.
The association constant, K, was determined by measuring the midpoint of the titration of CsxPi (1 mm) with CsOH carried out in solutions containing 200 mm CsCl. Thus the titrations were carried out at near constant ionic strength. Equation (6) relates K to the difference between the pH at the titration midpoint (pHt) and the pKa for Pi, which is 6.92:
| (6) |
The titration midpoint and the values of association constants are shown in Table 1. We also carried out titrations using Na+ and K+ instead of Cs+ and obtained values of K for Na+ and K+ that were in agreement with those of Smith & Alberty (1956).
Table 1.
Determination of association constants for phosphate ion species
| Cation | ΔpH | K(M−1) |
|---|---|---|
| Na+ | 0.23 | 3.5(3.5) |
| K+ | 0.20 | 3.0(3.1) |
| Cs+ | 0.10 | 1.3 |
| Na+ | 0.21 | 3.5 |
| Cs+ | 0.12 | 1.6 |
| Tris+ | −0.06 | 0 |
The association constant, K, was determined as described in Methods. K describes the degree of binding between the cation and the divalent form of Pi (see eqn (9)). In the first three rows ΔpH is the difference between the pKa for Pi (6.92) and the midpoint of a titration in the presence of 200 mM halide salts (pKa– pHt) and is related to K by eqn (6). The values in parentheses were determined by Smith & Alberty (1956). In the last three rows ΔpH is the fall in pH upon addition of 200 mM cations to 20 mM Pi solutions. These latter measurements of K are not as reliable as those carried out at constant ionic strength because the change in ionic strength alone is likely to produce some change in pH when cations are added to the bath.
It was not possible to use the method of Smith & Alberty (1956) to find the value of K for TrisHPO4− because the titration midpoint is obscured by the effects of Tris formation, which has a pKa of 8.8. We estimated K by another method in which ionic strength was not held constant. K was calculated from the drop in pH upon the addition of 200 mm TrisCl to a solution containing 20 mm TrisxPi by solving eqns (7)-(12) (see below). These results are shown in Table 1. Association constants for K+ and Cs+ using this method are consistent with those determined at constant ionic strength and the association constant for Tris was approximately zero, which indicates negligible binding between Tris+ and HPO42−. This is consistent with the general trend that weakly charged or large anions show no significant binding with HPO42− (Smith & Alberty, 1956).
Concentrations of phosphate species in the pH range 4-10
The relative concentrations of these species can be determined by solving eqns (7)-(12). In the pH range 4-10, the concentrations of H3O+, OH− and PO43− are negligibly small. X may exist in either charged or uncharged forms depending on the pH. Thus:
| (7) |
where pKX = 8.8 for Tris. Equations (8) and (9) relate the concentrations of the Pi species:
| (8) |
| (9) |
where pKP = 6.92 and K is the association constant for cation binding to HPO42−. The total ion concentrations in the solution ([X]T and [Pi]T) are:
| (10) |
| (11) |
Electro neutrality in the solution is described by:
| (12) |
where [A−] is the concentration of additional anions.
Rationale for the choice of cations
We chose choline+, Cs+, Na+ and Tris+ as the cations to be used in these experiments. Choline + and Tris+ were used because they do not permeate the SR K+ channel, thus obviating the problem of current signals from the SR K+ channels confusing the anion channel recordings (Coronado & Miller, 1982). In addition, Tris+ was of interest because it does not bind with Pi to form significant concentrations of TrisHPO4− (see Table 1 for association constants). Hence channel conductance in the presence of Pi solutions with Tris+ as the cation could be interpreted in terms of fewer ion species than with Cs+, Na+ or K+. Cs+ was used to test for a possible XHPO4− component to Pi transport. Cs+ was more convenient choice of cation than Na+ and K+ because Cs+ has 10-20 times lower conductance in the SR K+ channels than Na+ and K+ (Cukierman et al. 1985). Thus any Cs+ current through the SR K+ channels in these experiments would have been relatively small (< 0.5 pA). Although the calcium release channels in the SR have a high conductance to Cs+ they did not present a problem in this study because (1) anion channels were frequently incorporated into the bilayer without calcium release channels, (2) in the presence of 1 mm Ca2+ in the cytoplasmic (cis) bath the calcium release channels in skeletal muscle are strongly inhibited, and (3) cation channel signals were easily distinguished from anion channels by their conductance and reversal potential. Na+ was also used as a cation because it is present in muscle and therefore the permeabilities of NaxPi complexes are important to Pi transport in vivo. However, because Na+ passes through the SR K+ channels we had to use Cs+ on the opposite side of the membrane to the Pi solution to block these channels.
RESULTS
Chloride conductance and selectivity of SCl and BCl channels
As their name suggests the SCl and BCl channels have previously been characterised in terms of their ability to pass Cl−. The characteristic Cl− conductance and gating behaviour of BCl and SCl channels are shown in Fig. 1A and B and their current-voltage (I-V) characteristics are shown in Fig. 1C and D. The BCl channels frequently underwent transitions between fully open and subconductance states while complete channel closures were uncommon. The I-V characteristics of the BCl and SCl channels where measured using Cs+ or choline+ as the cation and were found to be independent of the cation species. In symmetric 250 mm Cl− the I-V curve for the BCl channel was linear with a conductance of 445 pS. In 250 mm/50 mm Cl− (cis/trans) the I-V curve for the BCl channel has a conductance is 260 pS with a Vr of 37 mV giving permeability ratios Pcholine/PCl and PCs/PCl of ≈0. The SCl channels showed clear burst activity separated by closed periods lasting several seconds. Within bursts the channel opened to a range of subconductance states with the highest conductance states being most commonly observed. In symmetric 250 mm Cl− the I-V curve for the SCl channel was slightly non-linear with an average conductance of 90 pS (n = 5) between -40 and +40 mV. In 250 mm/50 mm Cl− (cis/trans, n = 6) the SCl channel had a conductance of 106 pS and a reversal potential (Vr) of 33 mV, giving permeability ratios for Pcholine/PCl and PCs/PCl of less than 0.04.
Phosphate conductance of SCl and BCl channels
We investigated the possibility that the Pi flow through the SCl and BCl channels was sufficiently large to produce a detectable current flow. This was done for the SCl channels by subjecting them to fast exchanges between cytoplasmic solutions containing either Cl− or Pi anions, using local perfusion (Laver & Curtis, 1996). Since continuous changes in the conductance of a single channel were observed (e.g. Fig. 2A) it was clear that the same SCl channel had a significant conductance to both Cl− and Pi. The Pi conductance of BCl channels was demonstrated in records like the one shown in Fig. 2B, which was obtained from a bilayer containing 10 BCl channels. The number of channels was determined at the beginning and end of the experiments from their total conductance in the presence of 250 mm choline-Cl (cis) and 100 mm Tris-Hepes (trans) (not shown). During the experiment the cis solution was replaced by 100 mm Tris-Hepes using local perfusion (see above). Addition of 50 mm TrisxPi to the trans bath produced a 5 pA current.
Figure 2. Pi permeates SCl and BCl channels.
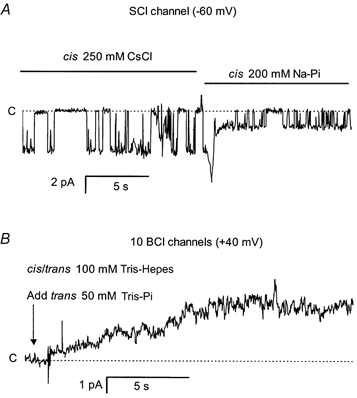
A, SCl channel openings are downward steps from each baseline (dashed lines labelled C). The SCl channel was initially recorded in CsCl solutions (250/50 mmcis/trans, left) at -60 mV (net Cl− flow cis to trans). A 200 mm NaxPi solution was continuously squirted onto the bilayer after 12 s, which replaced Cl− near the bilayer within ≈1 s. The current transient at the time of the solution exchange is an artifact of the exchange process. The current on the right is due to Pi flow from cis to trans. Clearly the same SCl channel passed both Pi and Cl− currents. B, Pi current through 10 BCl channels in a bilayer. The channels were initially detected with 250 mm choline-Cl (cis) and 100 mm Tris Hepes (trans) at +40 mV; openings are upward steps from the baseline. The cis solution was replaced by 100 mm Tris-Hepes using local perfusion, thus removing all permeant ions in the vicinity of the channels. At the beginning the trace (arrow) 50 mm TrisxPi was added to the trans bath, which induced a 5 pA current over several seconds as the Pi aliquot mixed with the bath.
The mean data for Pi conductance in the SCl and BCl channels over a range of membrane potentials and concentrations is shown in Fig. 3A and B. The I-V characteristic of the SCl channels in symmetric 200 mm NaxPi (Fig. 3A) exhibits a Pi conductance of 16 pS at 0 mV. The channel conductance showed a saturating dependence on [Pi] (Fig. 3B). Least squares fits of eqn (4) to the data showed a half-maximum at 64 ± 19 mm Pi and a maximum conductance of 22 ± 2 pS. Saturation was less pronounced for BCl channels. The fit of eqn (4) indicated a half-maximum at 450 ± 280 mm Pi and a maximum conductance of 160 ± 70 pS.
Figure 3. The conductance of SCl and BCl channels to Pi.
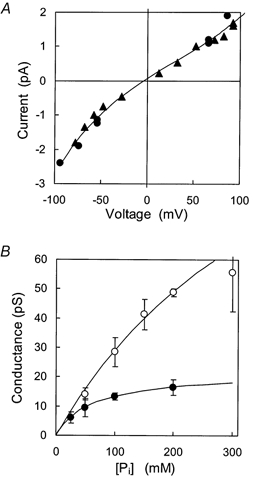
A, •, ▴, current-voltage data from two SCl channels, each shown with different symbols, with cis and trans 200 NaxPi. B, •, dependence of SCl channel conductance at -60 mV on cis[TrisxPi] with trans 25 mm TrisxPi (n = 8).○, dependence of BCl channel conductance at + 40 mV on trans[TrisxPi] with cis 250 mm choline-Cl (n = 7). Error bars show standard error of the mean. Continuous lines show fits of the Michaelis-Menten equation (eqn (4)) to the individual data comprising the mean; SCl data, Gmax = 22 ± 2 pS, Km = 64 ± 19 mm; BCl data, Gmax = 160 ± 70 pS, Km = 450 ± 280 mm.
the permeant pi species
Since Pi can exist in several different forms, it was of interest to determine which form passes predominantly through the channels. Phosphate ions in these experiments and in muscle are predominantly a mixture of three species, namely HPO42−, H2PO4− and XHPO4− where X+ represents a monovalent cation. The relative concentrations of these species depend on the pH and monovalent cation concentration. In muscle, the mole ratio concentrations of these species at rest and during fatigue are [HPO42−]= 0.8 and 14 mm, [H2PO4−]= 0.2 and 18 mm and [(Na+ K)HPO4−]= 0.4 and 8 mm (at rest total [Pi]= 1.4 mm, [Na+]+[K+]= 150 mm, pH = 7.18 and during fatigue [Pi]= 40 mm, pH ≈6.5; Godt & Maughan 1988; Godt & Nosek 1989).
We investigated the permeability of SCl and BCl channels to HPO42−, H2PO4−and XHPO4− where X represents Cs+, Na+ and Tris+. In order to obtain the permeabilities of the three Pi species it was necessary to measure reversal potentials of the anion channels at different pH and/or [X+]: conditions in which the relative concentrations of the Pi species were different (see Methods). The concentrations of each ion species (Tables 2 and 3) were calculated using eqns (7)-(12) (see Methods) and association constants given in Table 1. By applying the constant field equation to each of these situations it was possible to calculate the ion permeabilities for the individual Pi species. The effects of altering the relative concentrations of Pi species on the I-V characteristics of SCl channels are shown in Fig. 4. In Fig. 4A, the addition of CsCH3O3S to the trans chamber reduced [HPO42−] and increased [CsHPO4−] (see Tables 1 and 2). The I-V characteristic of the SCl channel was significantly affected by the addition of cations to the trans bath with a shift in the reversal potential from 19 to 36 mV (Table 2, rows 1 and 2). Solving the constant field equation for the permeability ratios of each ion species gives the selectivity sequence HPO42− > Cl− > H2PO4− and CsHPO4− (Table 4). The same selectivity sequence was independently obtained at ≈200 mm Pi by comparing the I-V characteristics obtained at pH 7.4 and 6.5 (Fig. 4B). Decreasing the pH reduced the relative concentration of HPO42− yet did not significantly change Vr (Table 2, rows 3-5). Although the permeability selectivity sequence with ≈200 mm Pi or 50 mm Pi was the same, the degree of specificity for the different ions was less at higher concentrations (Table 4). Thus the SCl channels are permeable mainly to the divalent HPO42− and the specificity is more pronounced at lower [Pi].
Table 2.
Current reversal potentials for SCl channels
| cis ion concentration(mM) | trans ion concentration(mM) | Permeant ion species Concentration, activity (mM) | Vr(mV) | |||
|---|---|---|---|---|---|---|
| 1 | 250 CsCl | 50 CsxPi pH 7.24 | [HPO42−] = 34, 10; | [H2PO4−] = 12, 9; | [CsHPO4−] = 4, 3 | 19 ± 3(4) |
| 2 | 250 CsCl | 50 CsxPi+ 200 CsCH3O3S pH 7.24 | [HPO42−] = 28, 5; | [H2PO4−] = 12, 8; | [CsHPO4−] = 10, 7 | 36 ± 3(3) |
| 3 | 250 Cl−* | 200 TrisxPi pH 7.4 | [HPO42−] = 160, 22; | [H2PO4−] = 40, 24 | 23 ± 3(2) | |
| 4 | 250 choline-Cl | 250 TrisxPi pH 6.5 | [HPO42−] = 84, 13; | [H2PO4−] = 166, 104 | 24 ± 3(2) | |
| 5 | 250 Cl−* | 200 NaxPi pH 7.4 | [HPO42−] = 88, 15; | [H2PO4−] = 22, 14; | [NaHPO4−] = 90, 57 | 21 ± 3(2) |
| 6 | 250 Cl−* | 250 MgSO4 | [SO42−] = 100,16; | [Cl−] = 250, 167 | 11 ± 2(8) | |
| 7 | 250 choline-Cl | 250 CsBr | [Br−] = 250, 167 | 5 ± 3(5) | ||
| 8 | 250 choline-Cl | 250 CsI | [I−] = 250, 167 | 6 ± 3(2) | ||
The concentrations of each Pi species were calculated using the equations and association constants given in Methods. Ion activities were calculated from ionic concentrations using eqn (3). Data are given as mean ± s.e.m.
Cations were either Cs+ or choline+ and the Cl− activity is 167 mM. Values of n are given in parentheses.
Table 3.
Current reversal potentials for BCl channels
| cis ion concentration(mM) | trans ion concentration(mM) | Permeant ion species Concentration, activity (mM) | Vr(mV) | |||
|---|---|---|---|---|---|---|
| 1 | 250 CsCl | 50 CsxPi; pH 7.24 | [HPO42−] = 34, 10; | [H2PO4−] = 12, 9; | [CsHPO4−] = 4, 3 | 52 ± 4(4) |
| 2 | 250 CsCl | 50 CsxPi+ 200 CsCH3O3S pH 7.24 | [HPO42-] = 28, 5; | [H2PO4−] = 12, 8; | [CsHPO4−] = 10, 7 | 54 ± 4(4) |
| 3 | 250 CsCl | 50 CsxPi; pH 6.5 | [HPO42−] = 15, 5; | [H2PO4−] = 30, 23; | [CsHPO4−] = 5, | 4 58 ± 4(4) |
| 4 | 250 choline-Cl | 200 TrisxPi; pH7.4 | [HPO42−] = 160, 22; | [H2PO4−] = 40, 24 | 50 ± 4(7) | |
| 5 | 250 choline-Cl | 250 TrisxPi; pH6.5 | [HPO42−] = 84, 13; | [H2PO4−] = 166, 104 | 50 ± 4(4) | |
| 6 | 200 NaxPi; pH 7.4 | 250 choline-Cl | [HPO42−] = 88, 15; | [H2PO4−] = 22, 14; | [NaHPO4−] = 90, 57 | −36 ± 3(4) |
| 7 | 250 Cl− | 250 MgSO4 | [SO42−] = 100, 16; | [Cl−] = 250, 167 | 55 ± 4(8) | |
| 8 | 250 choline-Cl | 250 CsBr | [Br−] = 250, 167 | 6 ± 3(5) | ||
| 9 | 250 choline-Cl | 250 CsI | [I−] = 250, 167 | 7 ± 3(4) | ||
Ion concentrations and activity were calculated as described in the caption to Table 2.
Figure 4. The selectivity of SCl channels to Pi solutions at different pH and in the presence of different cations.
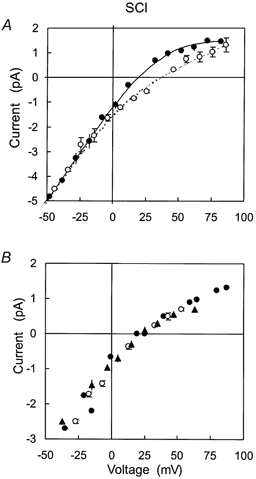
A, the cis bath contains 250 mm CsCl with trans solutions containing: •, 50 mm CsxPi, pH 7.24, Vr = 19 mV (n = 4); ○, 50 mm CsxPi (pH 7.24) + 200 mm CsCH3O3S, Vr = 36 mV (n = 3). The concentrations of each Pi species are given in Table 2. B, I-V data for SCl channels joining solutions containing 250 mm choline-Cl (cis) and trans solutions containing: ▴, 200 mm TrisxPi, pH 7.4, Vr = 23 mV (n = 2); ○, 250 mm TrisxPi, pH 6.5, Vr = 24 mV (n = 2); •, or 200 mm NaxPi, pH 7.4, Vr = 21 mV (n = 2).
Table 4.
Anion permeabilities in SCl and BCl channels
| Anion (Y) | SCl channel PY/PCl | BCl channel PY/PCl |
|---|---|---|
| 50 mM Pi | ||
| HPO42− | 3.0 ± 0.4a | 0.4 ± 0.2g |
| H2PO4− | 0a | 0.1 ± 0.1g |
| CsHPO4− | 0a | 1.7 ± 0.2g |
| ∼200 mM Pi | ||
| HPO42− | 0.9 ± 0.2b | 0.3 ± 0.2h |
| H2PO4− | 0.3 ± 0.2b | 0.1 ± 0.1h |
| NaHPO4− | 0.4 ± 0.2c | 0.5 ± 0.2i |
| SO42− | 3.0 ± 0.2d | < 0.3j |
| Br− | 0.7 ± 0.2e | 0.8 ± 0.1k |
| I− | 0.8 ± 0.1f | 0.8 ± 0.1l |
Table 4. Permeability (relative to Cl−) was calculated from Vr and known activities of ions in the bathing solutions (see Tables 2 and 3) using the constant field equation (eqn (2)). Assumed values of cation permeability over the range 0 to 0.05 had no significant effect on the predicted anion permeability.
Calculated from Table 2 (rows 1 and 2) assuming permeabilities for Cs+ and CH3O3S− equal zero.
Calculated from Table 2 (rows 3 and 4) assuming Tris+ permeability equals zero.
Calculated from Table 2 (rows 3–5) assuming Na+ permeability equals zero.
Calculated from Table 2 (row 6) assuming permeabilities for Mg2+ and choline+ equal zero.
Calculated from Table 2 (row 7) assuming permeabilities for choline+ and Cs+ equal zero.
Calculated from Table 2 (row 8) assuming permeabilities for choline+ and Cs+ equal zero.
Calculated from Table 3 (rows 1–3) assuming permeabilities for Cs+ and CH3O3S− equal zero.
Calculated from Table 3 (rows 4 and 5) assuming Tris+ permeability equals zero.
Calculated from Table 3 (rows 4–6) assuming Na+ permeability equals zero.
Calculated from Table 3 (row 7) assuming permeabilities for choline+ and Cs+ equal zero.
Calculated from Table 3 (row 8) assuming permeabilities for choline+ and Cs+ equal zero.
Calculated from Table 3 (row 9) assuming permeabilities for choline+ and Cs+ equal zero.
The effects of manipulating the relative concentrations of Pi species on BCl channels were also investigated. The effects of changing pH and [Cs+] in the Pi solutions on the BCl channel I-V characteristic are shown in Fig. 5. In contrast to results above for the SCl channel, the I-V characteristic of the BCl channel was little affected by the addition of CsCH3O3S to the trans bath, which shifted Vr from 52 to 54 mV (Table 3, rows 1-3). According to the constant field equation this indicates that the BCl channel is less selective for HPO42− than the SCl channel. Decreasing the pH of the CsxPi solution in the trans bath from 7.24 and 6.5 shifted Vr from 52 to 58 mV (Fig. 5). The constant field equation gives the selectivity sequence NaHPO4− > Cl− > HPO42− > H2PO4− (Table 4). A slightly different selectivity sequence (Cl− > NaHPO4− > HPO42− > H2PO4−) was obtained at higher Pi, ≈200 mm, by comparing the I-V characteristics obtained at pH 7.4 and 6.5 (Table 3, rows 4-6). In either case HPO42− is not the main permeant form of Pi in the BCl channel.
Figure 5. The selectivity of BCl channels to Pi solutions at different pH and in the presence of different Cs+ concentrations.
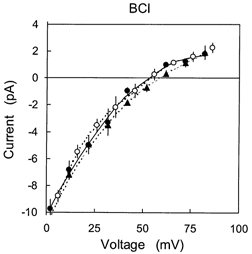
The concentrations of each Pi species are given in Table 3. The cis bath contains 250 mm CsCl with trans solutions containing: •, 50 mm CsxPi, pH 7.24, Vr = 52 mV (n = 4); ○, 50 mm CsxPi+ 200 mm CsCH3O3S, pH 7.24, Vr = 54 mV (n = 4); ▴, 50 mm CsxPi, pH 6.5, Vr = 58 mV (n = 4).
The valence selectivity of these channels was also investigated in another way by measuring the channel permeability to I− and Br− and SO42− in the SCl and BCl channels. These ions form fewer ion species than Pi. Figure 6 shows the I-V characteristics of SCl and BCl channels with solutions containing Cl− (cis) and either I− or Br− (trans) and with either Cs+ or choline+ as the cation. Current reversal potentials were found to be in the range 0-10 mV (Table 2, rows 7 and 8 and Table 3, rows 8 and 9). The conductance and relative permeabilities for both channels show little specificity between the monovalent anions, Cl− > I− > Br− (see Table 4). In contrast to the monovalent anions, SCl and BCl channels differed in their ability to conduct divalent SO42− (Fig. 7, cf. Table 2, row 6 and Table 3, row 7). For the SCl channel with 250 mm MgSO4(cis) and 250 mm Cl− (trans), Vr was 11 mV, indicating a 3-fold selectivity for SO42− over Cl−. The SCl channel conductance in 250 mm MgSO4 (cis/trans) was 60 pS (Fig. 7, filled symbols). In marked contrast, the Vr for the BCl channel (Fig. 7) under similar conditions was ≈55 mV, which was closer to the equilibrium potential for Cl− (i.e. > 100 mV) indicating selectivity for Cl−. The gating pattern associated with the BCl channel passing Cl− (e.g. Fig. 1A) was never resolved above the background noise when SO42− was the permeant ion indicating that the SO42− conductance was very small (< 10 pS). Consequently a precise determination of Vr, and hence PSO4/PCl, was not possible for the BCl channel. However we were able to obtain an upper estimate for PSO4/PCl of 0.3. Thus the BCl channel is a monovalent anion channel whereas the SCl is selective for divalent ions over monovalent ions.
Figure 6. The mean I-V characteristics of SCl and BCl channels separating equimolar ionic solutions.
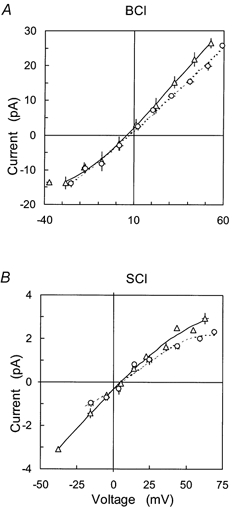
A, BCl channels in cis solutions containing 250 mm choline-Cl and with trans solutions containing: ○, 250 mm CsI, G = 440 pS and Vr = 7 mV (n = 4); ▵, 250 mm CsBr, G = 580 pS and Vr = 6 mV (n = 5).B, SCl channels in cis solutions containing 250 mm choline-Cl and with trans solutions containing: ○, 250 mm CsI, G = 43 pS and Vr = 6 mV (n = 2); ▵, 250 mm CsBr, G = 54 pS and Vr = 5 mV (n = 4).
Figure 7. Mean I-V characteristics of SCl and BCl channels in the presence of SO42−.
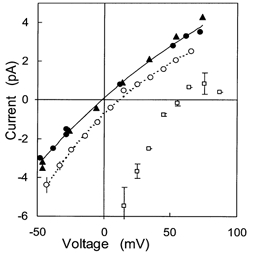
□, BCl channels with 250 mm CsCl (cis) and 250 mm MgSO4 (trans) Vr = 55 mV (n = 2).▴, •, two SCl channels in cis and trans 200 mm NaxPi (each shown with different symbols) G = 60 pS. ○, SCl channels with 250 mm MgSO4cis and 250 mm choline-Cl trans, Vr = 11 mV (n = 8).
In summary, the data give an overall picture of Pi permeation in which the SCl channel is primarily permeable to the divalent HPO42− and not the monovalent H2PO4− or XHPO4− while, in contrast, the BCl channel is impermeable to HPO42−. The mechanism for Pi permeation in the BCl channel is not clear. Reversal potential measurements in Fig. 5 suggest that CsHPO4− is the main permeant form of Pi in the BCl channels. However, this is difficult to reconcile with the fact that BCl channels also conduct Pi in the presence of TrisxPi (e.g. see Fig. 2 and Fig. 3). In these solutions the TrisHPO4− concentration would be very low and in any case TrisHPO4− is likely to be too large to fit through the pore (the BCl channel was found to be impermeable to large anions such as CH3O3S− and Hepes−). This suggests that the constant field equation does not adequately describe ion permeation in these channels under all conditions.
Anion channel inhibition by phosphonocarboxylic acids
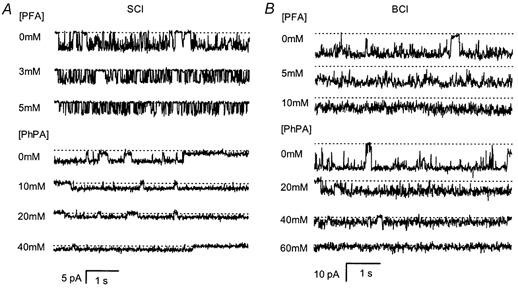
The SR Pi flux is inhibited by the phosphonocarboxylic acids (Stefanova et al. 1991b; Posterino & Fryer, 1998). Two acids from this family with significantly different inhibiting potencies were used here, namely phosphonoformic acid (PFA) and phenylphosphonic acid (PhPA). PFA is 4-fold more potent in inhibiting SR Pi flux than PhPA. Both phosphonocarboxylic acids reduced the Cl− current through SCl and BCl channels (Fig. 8). The phosphonocarboxylic acids acted predominantly by reducing the open channel conductance. PFA also slightly decreased the open probability of the SCl channel. Both PFA and PhPA increased the root mean square noise on channel openings relative to the open channel current indicating a ‘flicker’-block of the channel by these acids. The concentration dependence of inhibition under a variety of conditions is shown in Fig. 9A and B. PhPA inhibition of BCl channels was voltage dependent (being greater at negative potentials) and alleviated by increasing cis[Cl−], while PFA block was voltage-independent (Table 5). PFA was a stronger blocker of SCl channels than BCl channels (i.e. PFA blocked SCl channels with higher affinity than PhPA, Table 5). The errors on the Hill coefficient estimates were typically 0.2-0.3 and in all cases the Hill coefficients were not significantly different from unity (not shown).
Figure 8. Inhibition of BCl and SCl channels by phosphonoformic acid (PFA) and phenylphosphonic acid (PhPA).
A, two separate experiments in which different SCl channels were inhibited by PFA (top three traces) and PhPA (bottom four traces). PFA inhibition was measured in the presence of 250/50 mm CsCl (cis/trans) at -60 mV and PhPA inhibition was measured in 250 mm choline-Cl (cis) and 50 mm TrisxPi+ 100 mm Tris-Hepes (trans) at -40 mV. The channel conductance is reduced in the presence of either acid. B, two experiments in which different BCl channels were inhibited by PFA (top three traces) and PhPA (bottom four traces). PFA inhibition was measured in the presence of 250 mm choline-Cl (cis) and 50 mm TrisxPi+ 200 mm Tris-Hepes (trans) at -40 mV and PhPA inhibition was measured in 250 mm choline-Cl (cis) and 200 Tris-Hepes (trans) at -40 mV.
Figure 9. Concentration dependence of cytoplasmic phosphonocarboxylic acid inhibition of BCl and SCl channels.
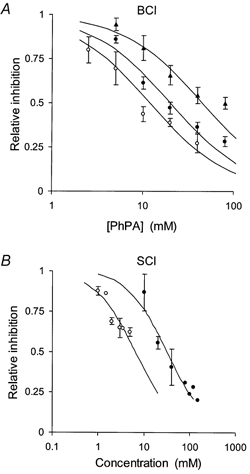
Inhibition was measured from the ratio of the time-averaged current in the presence of acid, and under control conditions. A, BCl channel inhibition by cis PhPA in the presence of trans 100 mm Tris-Hepes and under the following conditions. ▴, cis 250 mm choline-Cl, 0 mV (n = 9); •, 250 mm choline-Cl, -40 mV (n = 11); ○, 125 mm choline-Cl, -40 mV (n = 3). The curves show Hill fits to the data (Hill coefficient = 1). Half-inhibiting concentrations from these fits are given in Table 3. B, SCl inhibition by cis phosphonocarboxylic acids in the presence of trans 100 mm Tris-Hepes and cis 250 mm choline-Cl at -40 mV. ○, PFA (n = 3); •, PhPA (n = 3).
Table 5.
Block of BCl and SCl channels by phosphonocarboxylic acids
| BCl | SCl | |||
|---|---|---|---|---|
| Acid | cis[Cl−](mM) | Ki(−40 mV) | Ki(0 mV) | Ki(−40 mV) |
| PhPA | 250 | 20 ± 2(11) | 60 ± 11(9) | 30 ± 4(3) |
| PhPA | 125 | 10 ± 1.2(3) | — | — |
| PhPA | 63 | 5 ± 2(3) | — | — |
| PFA | 250 | 23 ± 3(6) | 21 ± 2(6) | 6.5 ± 1.1(3) |
Block of BCl and SCl channels by phosphonocarboxylic acids from bilayer experiments showing the concentration of acid in the cis bath that produced a 50% reduction in Cl− currents (concentrations in mM). Numbers of observations are given in parentheses.
Anion channel inhibition by DIDS
Both BCl and SCl channels frequently incorporated into bilayers in the same fusion event. These bilayers could not be used to study either channel type using Cl− based solutions. However, when SO42− was the major anion, such bilayers could be used to examine SCl channel activity, because no detectable current flowed through the BCl channels. Therefore the effect of DIDS on SCl channel activity was examined in MgSO4 solutions. The presence of Mg2+ had the added advantage of inhibiting ryanodine receptors so they did not interfere with SCl channel recordings.
DIDS is a potent inhibitor of anion channels and the Pi efflux from SR vesicles (Campbell & MacLennan, 1980). We found that addition of 20-60 μm DIDS to the cis (cytoplasmic) bath caused total inhibition of SCl channel conductance for SO42− within 30s of application (n = 5, e.g. Fig. 10A) whereas DIDS (cis 0.1 to 1 mm) produced only 14 ± 6 % inhibition of the BCl channel conductance for Cl− after several minutes of exposure (n = 4, e.g. Fig. 10B).
Figure 10. The effect of DIDS in the cytoplasmic bath on the SCl and BCl channels.
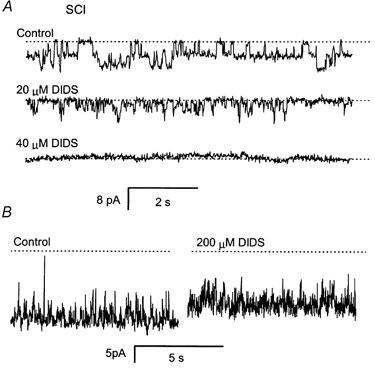
A, one of five experiments showing two SCl channels in cis 250 mm MgSO4 and trans 200 mm TrisxPi at -60 mV (top trace). The current is carried by SO42− flowing from cis to trans. The channels became substantially inhibited within 20 s of the addition of 20 μm DIDS to the cis bath (second trace). Further addition of DIDS totally inhibited the channels within 10 s (bottom trace). B, one of four experiments showing a BCl channel in cis 250 mm choline-Cl and trans 100 mm Tris-Hepes at -40 mV (left trace). The channel showed 20 % inhibition after 200 μm DIDS was added to the cis bath (right trace).
DISCUSSION
In this study we have shown that the BCl and SCl channels pass inorganic phosphate ions and thus identified the first candidates for the Pi transport mechanism in the SR. Previous studies have determined that the BCl and SCl channels are differently regulated by membrane potential and the cytoplasmic milieu (see Introduction). We find that BCl and SCl channels have distinct ion selectivities, the SCl being primarily a divalent anion channel whereas the BCl channel is primarily a monovalent anion channel. Pi-conducting ion channels have not previously been reported in muscle although a phosphate-permeable anion channel has recently been reported in the Golgi bodies of liver cells (Nordeen et al. 2000). The conductance and selectivity of the BCl channel for anions other than Pi was consistent with a previous report (Tanifuji et al. 1987).
Our key finding is that Pi flows through at least one of the anion channels of the SR. The evidence for this comes from a direct measurement of single channel current in the presence of Pi solutions. We have also inferred relative anion permeability from measurements of reversal potential. However, some caution should be exercised in interpreting these values. First, while our results provide reliable data, the available theories to interpret reversal potentials are not exact. The assumptions inherent in the constant field equation, while valid for whole cell currents, are not strictly applicable to individual channels where ion flows may not be independent. Therefore ion permeability values merely provide a condensed description of the reversal potential data. Secondly, for relative ion permeabilities a high specificity for Pi over other anions might not be an important prerequisite for a Pi transporter. Readily diffusible anions in the resting muscle cytoplasm consisted mainly of adenine nucleotides (≈6-8 mm), chloride (2-5 mm), phosphocreatine (48 mm), lactate (1.5 mm), amino acids (1 mm) glycolytic intermediates (< 4 mm), bicarbonate (< 8 mm) and inorganic phosphates (1-5 mm) (Godt & Maughan, 1988). During prolonged exercise the cytoplasmic Pi rises to 20-40 mm at the expense of phosphocreatine so that Pi becomes the dominant diffusible anion in the cytoplasm. Thus ion channels with only moderate specificity for Pi could pass substantial Pi fluxes under these conditions.
contributions of scl and bcl channels to sr pi transport
If the BCl and SCl channels are viable candidates for the physiological Pi transport mechanism then they must be sufficiently permeable to Pi to account for the magnitude of the fluxes seen in muscle. Passive Pi uptake by SR in rat muscle has been estimated as 50 μm s−1 (relative to whole fibre volume) with 20 mm cytoplasmic Pi (Fryer et al. 1997). An upper estimate of SCl and BCl channel contributions to Pi transport can be made from their Pi conductance and population density. (Channel density in muscle, D, was calculated from the number per SR vesicle, N = 0.69 (Smith et al. 1986), the radius of each vesicle, r = 0.14μm (Tanifuji et al. 1987) and the area of SR membrane per unit volume of muscle fibre A = 1μm2/μm3 (Eisenberg, 1982) using the equation: D = AN/4πr2). The Constant Field equation predicts that at Vm = 0 and 20 mm Pi (where BCl and SCl Pi conductance is similar) a unidirectional Pi current of ≈0.05 pA per fully open channel gives a SR transport rate of ≈700 μm s−1, more than enough to carry the observed Pi fluxes.
The contributions of SCl and BCl channels to physiological Pi fluxes across the SR membrane may be assessed by comparing activation and inhibition of these channels with activation and inhibition of Pi fluxes. The SR Pi flux is inhibited by phosphonocarboxylic acids; phosphonoformic acid (PFA) being approximately fourfold more potent than phenylphosphonic acid (PhPA) (Stefanova et al. 1991b). Phosphonocarboxylic acids also reduced Cl− current through the SCl and BCl channels (Fig. 8 and Fig. 9). The dependence of inhibition by the acids on voltage and cis[Cl−] indicates that the acids compete with permeant anions for a blocking site within the conduction pathway. The ratio of Ki for the two phosphonocarboxylic acids Ki (PhPa)/Ki (PFA) is in the range 1-2 for the BCl channel and 4-5 for the SCl channel (Table 5). This ratio for the SCl channel is more consistent with the relative effects of the acids on SR vesicles. Although the relative Ki values for the phosphonocarboxylic acids on the anion channels were consistent with those for Pi uptake by SR vesicles, their absolute potency for blocking BCl or SCl channels (Ki = 5-50 mm) was lower than that for Pi uptake by the SR (Ki≈1 mm). This discrepancy is most likely to be due to the high [Cl−] necessary to detect channels in bilayers, since lowering cis[Cl−] toward physiological levels of ≈5 mm lowered Ki (Cl− and phosphonocarboxylic acids act competitively, see above).
Disulfonic stilbenes are potent anion channel inhibitors but unfortunately there are conflicting interpretations of the DIDS effect on SR Pi transport. Direct measurement of Pi efflux from SR vesicles showed that DIDS produced complete inhibition at 50 μm with a half-inhibiting concentration (Ki) of 3 μm (Campbell & MacLennan, 1980). However, Pi release from the SR of skinned fibres, inferred from the Pi-induced decrease in Ca2+ release, showed that 100 μm DIDS had no significant effect on Pi transport (Posterino & Fryer, 1998). The reason for the disparate findings may lie in the promiscuous nature of DIDS, which affects a variety of membrane transporters. DIDS is known to strongly activate the Ca2+ release channel in the SR (Oba et al. 1996; Sitsapesan, 1999) so that indirect estimates of Pi transport from SR Ca2+ handling properties might not be valid. Anion channel inhibition by DIDS shown here is consistent with our previous report (Kourie et al. 1996b) that 8 μm DIDS inhibited the Cl− conductance of SCl channels but 80 μm DIDS caused only partial inhibition of BCl channels. Thus we find that the effect of DIDS on the SCl channel parallels its effect on Pi transport determined from direct measurements of efflux from SR vesicles.
Cytoplasmic ATP has been shown to inhibit Pi influx but not Pi efflux from the SR (Posterino & Fryer, 1998). BCl channels are not sensitive to ATP (< 20 mm, not shown), but SCl channels are sensitive to ATP. The Ki for ATP is 1 mm when 250 mm Cl− flows through SCl channels from cytoplasm to lumen (influx), whereas 10 mm ATP has no effect when Cl− flows in the opposite direction (efflux) (Ahern & Laver, 1998). Thus the inhibiting effects of ATP on the SCl channels, not the BCl channels, closely parallels its effects on Pi transport in muscle.
In summary, the Pi conductance and density of SCl and BCl channels suggest that both channel types could sustain the observed Pi fluxes across the SR membrane. However, the pharmacological evidence suggests that the SCl channel is the more likely candidate for the Pi transport mechanism in the SR.
Physiological roles for the SCl and BCl channels
The role of anion channels in the SR is unknown. It had been suggested that their role might be to contribute a Cl− flux to the counter current during Ca2+ release and uptake (Kourie et al. 1996a,b). However, the failure to demonstrate changes in SR [Cl−] during muscle tetanus (Somlyo et al. 1981) and the lack of an effect of Cl− removal on voltage sensor-activated contraction (Coonan & Lamb, 1998) suggest that Cl− is not an essential contributor to counter currents. It is more likely that the role of the anion channels is associated with the transport of other relatively permeant anions (e.g. SO42− and Pi). This is the first report of a high-conductance, SO42−-selective ion channel in mammalian membranes (the SCl channel). A SO42-conducting channel has recently been reported in plants (Frachisse et al. 1999). The physiological significance of the strikingly high SO42− permeability of the SCl channel is at present unclear.
The pharmacological evidence weighs against the BCl channel having a significant role in Pi transport across the SR. However, the BCl channel may provide a non-specific anion leak pathway. A physiological role for the SCl channel, and to a small extent the BCl channel, might be to allow Pi to cross the SR membrane and either associate with, or dissociate Ca2+ in, the SR lumen. The co-precipitation of Ca2+ and Pi in the SR is believed to be a major contributor to failure of Ca2+ release and muscle contraction during fatigue from prolonged activity (Fryer et al. 1995; Kabbara & Allen, 1999; Dahlstedt et al. 2000). The strongest evidence for this hypothesis comes from recent experiments on creatine kinase knock-out mice where cytoplasmic Pi does not increase during intense exercise (Dahlstedt et al. 2000). Muscle fibres from these mice were considerably more resistant to fatigue than those from normal mice. Though it is clear that Pi can cross the SR membrane, the main transport mechanism is unknown. The SCl channel is a good candidate for such a transport mechanism since (1) pharmacological agents that alter SR Pi transport have similar effects on the SCl channel and (2) they can sustain the observed Pi fluxes across the SR membrane during normal muscle function (see above) and during fatigue and recovery from fatigue where intracellular pH falls.
The high degree of regulation of the SCl channel by cytoplasmic ligands can be understood if the SR anion channels do play an essential role in regulating Pi transport. Results, here and elsewhere (Kourie et al. 1996a; Kourie, 1997a,b; Ahern & Laver, 1998), suggest that SCl channels would be further activated in muscle fatigue by raised cytoplasmic [Ca2+], luminal [Pi] (D. R. Laver, unpublished observations), reduced [ATP] and by increased concentrations of oxidants. Thus the SCl channel would increase the overall permeability of the SR membrane to Pi under conditions of muscle fatigue and ischaemia. This could serve either (1) to reduce the available Ca2+ pool under conditions of stress (fatigue or ischaemia) and so have a protective effect in conserving Ca2+ stores and reducing muscle output when energy reserves are low or (2) to aid in recovery from fatigue by allowing rapid Pi exit from the SR.
Physiological (0.5-10 mm) [Pi] has been shown to both increase or decrease (depending on the concentration) the size of intracellular Ca2+ stores that are rapidly releasable by either ryanodine (Fryer et al. 1995, 1997; Posterino & Fryer, 1998; Kabbara & Allen, 1999), InsP3 (Fulceri et al. 1993; Mezna & Michelangeli, 1998) or cADP-ribose (Guse et al. 1996), in tissues as diverse as hepatic cells, lymphocytes, platelets, brain and haematoma cells. The presence of an anion channel pathway for Pi into the SR of muscle raises the possibility that this is a general mechanism for Pi movement into and out of Ca2+ stores in many cell types.
Acknowledgments
Our thanks to Suzy Pace and Joan Stivala for preparing SR vesicles and to Drs Martin Fryer and Graham Lamb for their helpful suggestions. Dr Derek Laver was supported by a grant from the National Health & Medical Research Council of Australia (no. 9836486) and Gerlinde Lenz was supported by a grant from the Australian Research Council (ARC small grant).
References
- Ahern GP, Laver DR. ATP inhibition and rectification of a Ca2+-activated anion channel in sarcoplasmic reticulum of skeletal muscle. Biophysical Journal. 1998;74:2335–2351. doi: 10.1016/S0006-3495(98)77943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. Journal of Membrane Biology. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Brooks SP, Storey KB. Bound and determined: a computer program for making buffers of defined ion concentrations. Annals of Biochemistry. 1992;201:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- Campbell KP, MacLennan DH. DIDS inhibition of sarcoplasmic reticulum anion and calcium transport. Annals of the New York Academy of Sciences. 1980;358:328–331. doi: 10.1111/j.1749-6632.1980.tb15406.x. [DOI] [PubMed] [Google Scholar]
- Chu A, Dixon MC, Saito A, Seiler S, Fleischer S. Isolation of sarcoplasmic reticulum fractions referable to longitudinal tubules and junctional terminal cisternae from rabbit skeletal muscle. Methods in Enzymology. 1988;157:36–50. doi: 10.1016/0076-6879(88)57066-0. [DOI] [PubMed] [Google Scholar]
- Coonan JR, Lamb GD. Effect of chloride on Ca2+ release from the sarcoplasmic reticulum of mechanically skinned skeletal muscle fibres. Pflügers Archiv. 1998;435:720–730. doi: 10.1007/s004240050574. [DOI] [PubMed] [Google Scholar]
- Coronado R, Miller C. Conduction and block by organic cations in a K+ selective channel from sarcoplasmic reticulum incorporated into planar phospholipid bilayers. Journal of General Physiology. 1982;79:529–547. doi: 10.1085/jgp.79.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman S, Yellen G, Miller C. The K+ channel of sarcoplasmic reticulum. A new look at Cs+ block. Biophysical Journal. 1985;48:477–484. doi: 10.1016/S0006-3495(85)83803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Wieringa B, Westerblad H. Is creatine kinase responsible for fatigue? Studies of isolated skeletal muscle deficient in creatine kinase. FASEB Journal. 2000;14:982–990. doi: 10.1096/fasebj.14.7.982. [DOI] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Characteristics of phosphate-induced Ca2+ efflux from the SR in mechanically skinned skeletal muscle fibres. American Journal of Physiology. 2000;278:C126–135. doi: 10.1152/ajpcell.2000.278.1.C126. [DOI] [PubMed] [Google Scholar]
- Eisenberg BR. Quantitative ultrastructure of mammalian skeletal muscle. In: Peachey LD, Adrian R, editors. Handbook of Physiology, section 10, Skeletal Muscle. Bethesda, MD, USA: American Physiological Society; 1982. pp. 73–112. [Google Scholar]
- Frachisse JM, Thomine S, Colcombet J, Guern J, Barbier-Brygoo H. Sulfate is both a substrate and an activator of the voltage-dependent anion channel of arabidopsis hypocotyl cells. Plant Physiology. 1999;121:253–261. doi: 10.1104/pp.121.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruen BR, Mickelson JR, Shomer NH, Rogher TJ, Louis CF. Regulation of the sarcoplasmic reticulum ryanodine receptor by inorganic phosphate. Journal of Biochemistry. 1994;269:192–198. [PubMed] [Google Scholar]
- Fryer MW, Owen VJ, Lamb GD, Stephenson DG. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. Journal of Physiology. 1995;482:123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, West JM, Stephenson DG. Phosphate transport into the sarcoplasmic reticulum of skinned fibres from rat skeletal muscle. Journal of Muscle Research and Cell Motility. 1997;18:161–167. doi: 10.1023/a:1018605605757. [DOI] [PubMed] [Google Scholar]
- Fulceri R, Bellomo G, Gamberucci A, Romani A, Benedetti A. Physiological concentrations of inorganic phosphate affect MgATP-dependent Ca2+ storage and inositol trisphosphate-induced Ca2+ efflux in microsomal vesicles from non-hepatic cells. Biochemical Journal. 1993;289:299–306. doi: 10.1042/bj2890299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt RE, Maughan DW. On the composition of the cytosol of relaxed skeletal muscle of the frog. American Journal of Physiology. 1988;23:C591–604. doi: 10.1152/ajpcell.1988.254.5.C591. [DOI] [PubMed] [Google Scholar]
- Godt RE, Nosek TM. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. Journal of Physiology. 1989;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DE. Potential, impedance and rectification in membranes. Journal of General Physiology. 1943;27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse AH, Silva CP, Weber K, Ashamu GA, Potter BV, Mayr GW. Regulation of cADP-ribose-induced Ca2+ release by Mg2+ and inorganic phosphate. Journal of Biological Chemistry. 1996;271:23946–23953. doi: 10.1074/jbc.271.39.23946. [DOI] [PubMed] [Google Scholar]
- Kabbara AA, Allen DG. The role of calcium stores in fatigue of isolated single muscle fibres from the cane toad. Journal of Physiology. 1999;519:169–176. doi: 10.1111/j.1469-7793.1999.0169o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourie JI. A redox O2 sensor modulates the SR Ca2+ countercurrent through voltage- and Ca2+-dependent Cl− channels. American Journal of Physiology. 1997a;272:C324–332. doi: 10.1152/ajpcell.1997.272.1.C324. [DOI] [PubMed] [Google Scholar]
- Kourie JI. ATP-sensitive voltage- and calcium-dependent chloride channels in sarcoplasmic reticulum vesicles from rabbit skeletal muscle. Journal of Membrane Biology. 1997b;157:39–51. doi: 10.1007/s002329900214. [DOI] [PubMed] [Google Scholar]
- Kourie JI. pH-modulation of chloride channels from the sarcoplasmic reticulum of skeletal muscle. Journal of Membrane Biology. 1999;167:73–83. doi: 10.1007/s002329900473. [DOI] [PubMed] [Google Scholar]
- Kourie JI, Foster PS, Dulhunty AF. Inositol polyphosphates modify the kinetics of a small chloride channel in skeletal muscle sarcoplasmic reticulum. Journal of Membrane Biology. 1997;157:147–158. doi: 10.1007/s002329900224. [DOI] [PubMed] [Google Scholar]
- Kourie JI, Laver DR, Ahern GP, Dulhunty AF. A calcium-activated chloride channel in sarcoplasmic reticulum vesicles from rabbit skeletal muscle. American Journal of Physiology. 1996a;270:C1675–1686. doi: 10.1152/ajpcell.1996.270.6.C1675. [DOI] [PubMed] [Google Scholar]
- Kourie JI, Laver DR, Junankar PR, Gage PW, Dulhunty AF. Characteristics of two types of chloride channel in sarcoplasmic reticulum vesicles from rabbit skeletal muscle. Biophysical Journal. 1996b;70:202–221. doi: 10.1016/S0006-3495(96)79564-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR, Curtis BA. Surface potentials measure ion concentrations near lipid bilayers during rapid solution changes. Biophysical Journal. 1996;71:722–731. doi: 10.1016/S0006-3495(96)79271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR, Roden LD, Ahern GP, Eager KR, Junankar PR, Dulhunty AF. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. Journal of Membrane Biology. 1995;147:7–22. doi: 10.1007/BF00235394. [DOI] [PubMed] [Google Scholar]
- Margolis MJ. Chemical Principles in Calculation of Ionic Equilibria. New York: Macmillan; 1966. [Google Scholar]
- Marks PW, Maxfield FR. Preparation of solutions with free calcium concentration in the nanomolar range using 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid. Annals of Biochemistry. 1991;193:61–71. doi: 10.1016/0003-2697(91)90044-t. [DOI] [PubMed] [Google Scholar]
- Mezna M, Michelangeli F. The role of inorganic phosphate in regulating the kinetics of inositol 1,4,5-trisphosphate-induced Ca2+ release: a putative role for endoplasmic reticulum phosphate transporters. Biochimica et Biophysica Acta. 1998;1373:270–276. doi: 10.1016/s0005-2736(98)00115-1. [DOI] [PubMed] [Google Scholar]
- Nordeen MH, Jones SM, Howell KE, Caldwell JH. GOLAC: An endogenous anion channel of the golgi species. Biophysical Journal. 2000;78:2918–2928. doi: 10.1016/S0006-3495(00)76832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba T, Koshita M, Van Helden D F. Modulation of frog skeletal muscle ryanodine receptor/Ca2+ release channel gating by anion channel blockers. American Journal of Physiology. 1996;271:C819–824. doi: 10.1152/ajpcell.1996.271.3.C819. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Fryer MW. Mechanisms underlying phosphate-induced failure of Ca2+ release in single skinned skeletal muscle fibres of the rat. Journal of Physiology. 1998;512:97–108. doi: 10.1111/j.1469-7793.1998.097bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Seiler S, Chu A, Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. Journal of Cellular Biology. 1984;99:875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R. Similarities in the effects of DIDS, DBDS and suramin on cardiac ryanodine receptor function. Journal of Membrane Biology. 1999;168:159–168. doi: 10.1007/s002329900506. [DOI] [PubMed] [Google Scholar]
- Smith JS, Coronado R, Meissner G. Single-channel calcium and barium currents of large and small conductance from sarcoplasmic reticulum. Biophysical Journal. 1986;50:921–928. doi: 10.1016/S0006-3495(86)83533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Alberty RA. The apparent stability constants of ionic specieses of various adenosine phosphates with monovalent cations. Journal of Physics and Chemistry. 1956;60:180–184. [Google Scholar]
- Somlyo AV, Gonzalez-Serratos H, Shuman H, MacLellan G, Somlyo AP. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: An electron-probe study. Journal of Cell Physiology. 1981;90:577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova HI, Jane SD, East JM, Lee AG. Effects of Mg2+ and ATP on the phosphate transporter of sarcoplasmic reticulum. Biochimica et Biophysica Acta. 1991a;1064:329–34. doi: 10.1016/0005-2736(91)90319-4. [DOI] [PubMed] [Google Scholar]
- Stefanova HI, East JM, Lee AG. Covalent and non-covalent inhibitors of the phosphate transporter of sarcoplasmic reticulum. Biochimica et Biophysica Acta. 1991b;1064:321–328. doi: 10.1016/0005-2736(91)90318-3. [DOI] [PubMed] [Google Scholar]
- Tanifuji M, Sokabe M, Kasai M. An anion channel of sarcoplasmic reticulum incorporated into planar lipid bilayers: single-channel behaviour and conductance properties. Journal of Membrane Biology. 1987;99:103–111. doi: 10.1007/BF01871230. [DOI] [PubMed] [Google Scholar]


