Abstract
Because individuals differ in the phase angle at which their circadian rhythms are entrained to external time cues, averaging group data relative to clock time sometimes obscures abrupt changes that are characteristic of waveforms of the rhythms in individuals. Such changes may have important implications for the temporal organization of human circadian physiology.
To control for variance in phase angle of entrainment, we used dual internal reference points – onset and offset of the nocturnal period of melatonin secretion – to calculate average profiles of circadian rhythm data from five previously published studies.
Onset and/or offset of melatonin secretion were found to coincide with switch-like transitions between distinct diurnal and nocturnal periods of circadian rhythms in core body temperature, sleepiness, power in the theta band of the wake EEG, sleep propensity and rapid eye movement (REM) sleep propensity.
Transitions between diurnal and nocturnal periods of sleep–wake and cortisol circadian rhythms were found to lag the other transitions by 1–3 h.
When the duration of the daily light period was manipulated experimentally, melatonin-onset-related transitions in circadian rhythms appeared to be entrained to the light-to-dark transition, while melatonin-offset-related transitions appeared to be entrained to the dark-to-light transition.
These results suggest a model of the human circadian timing system in which two states, one diurnal and one nocturnal, alternate with one another, and in which transitions between the states are switch-like and are separately entrained to dawn and dusk.
This description of the human circadian system is similar to the Pittendrigh–Daan model of the rodent circadian system, and it suggests that core features of the system in other mammals are conserved in humans.
Sometimes, investigators who study human circadian physiology use sine functions to model circadian rhythms, as if they were inherently continuous and sinusoidal. Many investigators, however, recognize that human circadian rhythms exhibit discontinuous changes and seem to be governed by multiple processes that require more complex models and methods of analysis. In this regard, a series of observations in our laboratory led us to propose a specific model for the human circadian system in which two states, one diurnal and one nocturnal, alternate with one another, and in which transitions between the states are switch-like and are separately entrained to dawn and dusk.
Although circadian rhythms are generated within the organism and persist in the absence of external input, it is obvious that the temporal programme outlined above mirrors the contours of the external day-night cycle, in effect creating a ‘day within’ (Pittendrigh, 1988). This parallel has obvious functional implications. It seems likely that this programme enables humans to anticipate and to adapt automatically to the contrasting conditions of their daytime and night-time worlds (Rusak, 1989). This interpretation is consistent with the fact that the circadian pacemaker entrains the transitions between diurnal and nocturnal states to dawn and dusk, and the fact that it can adjust the length of the interval between these transitions to accommodate seasonal changes in day length (Pittendrigh & Daan, 1976; Illnerova & Vanecek, 1982; Wehr et al. 1993; Elliott & Tamarkin, 1994).
Conceptually, this model of the human circadian system is similar to the classic Pittendrigh-Daan model of the rodent circadian system (Pittendrigh & Daan, 1976). In rodents, the model appears to describe the behaviour of processes that take place in the circadian pacemaker itself, in addition to the rhythms that the pacemaker regulates (Sumova et al. 1995; Mrugala et al. 2000). Although these processes cannot be measured directly in humans, the observations in rodents suggest that the temporal organization of overt rhythms in humans is likely to reflect the temporal organization of processes that take place in their pacemaker, too.
METHODS
The model that we propose here for the human circadian timing system is based on new analyses of data from five previously published studies from our laboratory.
Subjects
Healthy volunteers were screened with interviews, physical examinations and routine laboratory tests and procedures. None had medical or psychiatric illnesses, and none had taken any medication for at least 3 weeks before the studies. Subjects who were living in experimental light-dark cycles (Studies 1 and 2, below) had rigidly prescribed sleep-rest schedules. Those who were living in their usual environment (Studies 3-5, below) were required to maintain regular sleep schedules with deviations of no more than 1.5 h in the timing and duration of sleep during the 10 days preceding circadian rhythm assessments. Their compliance was monitored with sleep logs. Volunteers participated in five different studies, the details of which have been published elsewhere (Wehr et al. 1993, 1995a, 2001; Wehr, 1996; Aeschbach et al. 1997, 1999). All research was consistent with the Declaration of Helsinki and was approved by the institutional review board of the Intramural Research Program of the National Institute of Mental Health (NIMH). All participants gave written informed consent for their participation.
Experimental conditions and circadian rhythm measurements
Study 1
Healthy volunteers were admitted to an inpatient research unit where they lived for one or more weeks under controlled light-dark cycles. Circadian rhythms were measured on two occasions, once after the volunteers had been exposed to a conventional schedule of 16 h of light and 8 h of darkness each day for 1 week (LD 16:8), and once after they had been exposed to 10 h of light and 14 h of darkness each day for 4 weeks (LD 10:14) (for details see Wehr et al. 1993).
During the dark phase of each of the two light-dark cycles, polysomnographic recordings of sleep were obtained, and 30 s epochs were scored visually according to conventional criteria (Rechtshaffen & Kales, 1968). From these records, nightly times of onset and offset of sleep and times of occurrence of REM sleep were determined. For times of onset and offset of sleep, mean values for the last six nights of each of the two light-dark cycles were calculated for each individual. From sleep recordings from the last six nights of the 14 h dark periods, the average percentage of REM sleep that occurred in successive 5 min bins was calculated for each individual.
Immediately after the last night of each light-dark cycle schedule, circadian rhythms in sleepiness, and in plasma levels of melatonin and cortisol secretion, were measured in a 30 h constant routine protocol in which individuals were kept constantly awake, ate small isocaloric meals every 2 h, and remained seated (except for bathroom breaks) in constant dim (< 1 lx) light. This protocol was designed to eliminate or hold constant behavioural and environmental factors that would otherwise mask the intrinsic waveform of circadian rhythms. Every 30 min during the constant routine protocol, subjects used the Stanford Sleepiness Scale (Hoddes et al. 1973) to rate their level of sleepiness, and blood samples were obtained via an in-dwelling intravenous catheter and subsequently analysed with radioimmunoassays for plasma levels of melatonin and cortisol.
Study 2
Healthy volunteers were admitted to an inpatient research unit where they lived under two different light-dark cycles, as described in Study 1. After they were exposed to each light-dark cycle, circadian rhythms in sleep propensity and plasma melatonin levels were measured (Wehr, 1996). To measure sleep propensity, subjects were deprived of sleep for one night. Beginning at 9 a.m., they were then asked to lie down in the dark and sleep during 10 min intervals that were scheduled every 30 min for 30 h (Lavie & Zvuluni, 1992). Polysomnographic recordings of sleep were obtained during these intervals and were visually scored according to conventional criteria (Rechtshaffen & Kales, 1968). For each 10 min interval, total minutes of sleep were calculated for each individual. Subjects ate small isocaloric meals every 2 h and remained in dim light (< 1 lx) throughout the period of sampling. Every 30 min, during the 20 min intervals between sleep opportunities, blood samples were obtained via an in-dwelling intravenous catheter and subsequently analysed with radioimmunoassays for plasma levels of melatonin.
Study 3
Healthy volunteers lived with their usual schedules in their usual environments at 39 deg N latitude. On two occasions, once in winter and once in summer, circadian rhythms of sleepiness (Hoddes et al. 1973), rectal temperature and plasma levels of melatonin and cortisol were measured in a constant routine protocol, as described for Study 1, during brief admissions to a research unit. On these occasions, the average times (±s.d.) of sunrise and sunset were 07.19 ± 00.10 h and 17.06 ± 00.18 h in winter and 05.51 ± 00.07 h and 20.33 ± 00.04 h in summer, respectively (for details see Wehr et al. 1995a).
Study 4
Healthy volunteers lived with their usual schedules in their usual environments at 39 deg N latitude. On two occasions, once in winter and once in summer, circadian rhythms in plasma melatonin levels were measured for 24 h beginning at 5 p.m. These measurements were obtained during brief admissions to a research unit during which subjects slept according to their habitual sleep schedules and remained in constant dim (< 1 lx) light when they were awake (for details see Wehr et al. 2001).
Study
5. Healthy volunteers lived with their usual schedules in their usual environments at 39 ° N latitude. On one occasion, at various times of the year, circadian rhythms in plasma melatonin levels and in EEG theta activity (power density in the 4.25-8.0 Hz band) of the waking EEG were measured in a constant routine protocol, as described for Study 1, during brief admissions to a research unit (for details see Aeschbach et al. 1997, 1999).
Hormone radioimmunoassays
Plasma levels of melatonin were measured in duplicate by radioimmunoassay (RIA) by StockGrand Ltd, at the Department of Biochemistry, University of Surrey, Guildford, Surrey, UK. The melatonin assay had a detection limit of 3-5 pg ml−1 for all studies (Fraser et al. 1983). Plasma levels of cortisol were measured in duplicate by RIA by Hazleton Laboratories (Vienna, VA, USA). The cortisol assay had a detection limit of 0.5 μg dl−1.
Circadian rhythm parameters
For circadian rhythms that exhibited discrete transitions between distinctive diurnal and nocturnal periods, times of onset and offset of the nocturnal periods were defined and identified as follows:
Melatonin
For 24 h profiles of plasma levels of melatonin, three independent raters who were blind to the identity of subjects and to measurement conditions visually identified the times of onset and offset of the nocturnal period of melatonin secretion in each subject in all conditions of Studies 1-5. The time of onset of secretion was defined as the time midway between the last non-detectable level and the first detectable level in the evening that was followed by a sustained elevation of levels throughout the night. The end of secretion was defined, as described by Lewy et al. (1999), as the time of the last local maximum value that remained within the range of high nocturnal levels and that was followed by a rapid decline towards non-detectable levels in the morning (Fig. 1). Data were included in the analysis if at least two of the three raters agreed on the timing of each of the two events, which was the case in 96 % of subjects (Wehr et al. 2001). Data for 81 pairs of profiles were available for analysis from 10 men and one woman from Study 1, three men and three women from Study 2, nine men from Study 3, and 21 men and 32 women from Study 4. Data for 10 profiles from seven men and three women from Study 5 were also available for analysis.
Figure 1. Onset and offset of nocturnal melatonin secretion.
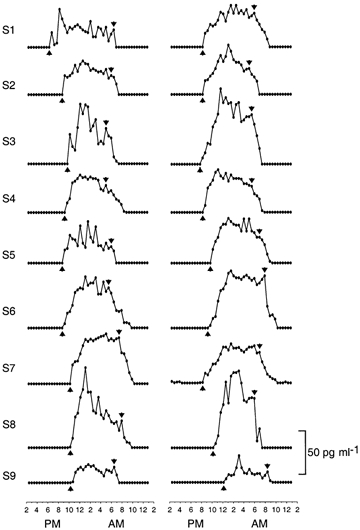
Twenty-four hour profiles of plasma melatonin levels in summer (left panel) and winter (right panel) in nine men who remained awake and at rest in constant dim light. For each profile, arrows indicate when the onset and offset of nocturnal period of melatonin secretion were judged to have occurred. Simultaneous profiles of rectal temperature appear in Fig. 2. Group-average profiles appear in Fig. 3. Data are extracted from Wehr et al. (1995a).
Rectal temperature
For 24 h profiles of rectal temperature in the constant routines in Study 3, the times of onset and offset of a nocturnal period of declining core body temperature were defined, respectively, as the time of the beginning of a rapid decline from high daytime levels and the time of the beginning of a rapid rise from low night-time levels to high daytime levels (Fig. 2). Data for nine pairs of profiles from nine men were available for analysis. Their age was 32.6 ± 8.6 (mean ±s.d.).
Figure 2. Onsets of evening decline and morning rise of core body temperature.
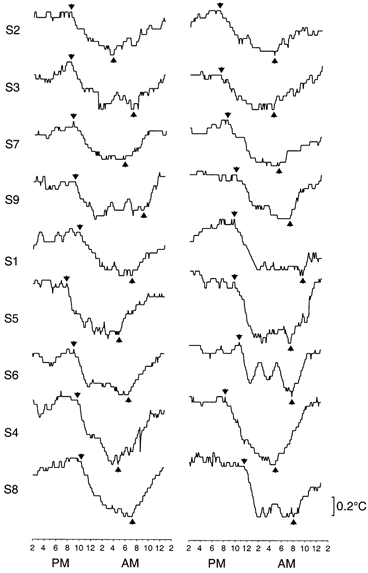
Twenty-four hour profiles of rectal temperature in summer (left panel) and winter (right panel) in nine men who remained awake and at rest in constant dim light. For each profile, arrows indicate when the temperature was judged to have begun a rapid decline from high daytime levels to low night-time levels and when it was judged to have begun a rapid rise from low night-time levels to high daytime levels. Simultaneous profiles of plasma melatonin levels appear in Fig. 1. Group-average profiles appear in Fig. 3. Data are extracted from Wehr et al. (1995a).
Sleepiness
For 24 h profiles of scores on the Stanford Sleepiness Scale in each individual, the times of onset and offset of a nocturnal period of increasing sleepiness were defined, respectively, as the last time, following the beginning of the measurement period, that the individual recorded their lowest rating on this scale and the first subsequent time that they recorded their highest rating on this scale, respectively (Wehr et al. 1993). Data for 18 pairs of profiles from 10 men and one woman in Study 1 and from nine men in Study 3 were available for analysis. In each study, subjects were assessed in constant routine measurement periods. Since the methods and conditions used for data acquisition during the constant routine measurement periods were identical in the two conditions of each study, data from the two conditions were averaged for each subject to reduce variance arising from measurement error and biological variability. Since the methods and conditions during the measurement periods were also identical across studies, the data for the subjects in both studies were combined in the subsequent analysis and display of data, for the same purpose. For two subjects who participated in both studies, data from the four conditions were averaged for each subject, so that the total number of subjects was 17 men and one woman. Their age was 33.2 ± 5.8 (mean ±s.d.).
Cortisol
For 24 h profiles of plasma levels of cortisol, the times of onset and offset of a nocturnal period of increasing plasma levels were defined as the times of the minimum and maximum values, respectively. Data for nine pairs of profiles from nine men were available for analysis from Study 1, in which subjects were assessed in constant routine measurement periods following chronic exposures to two different artificial light-dark cycles. Since the methods and conditions used for data acquisition during the constant routine measurement periods were identical in the two conditions, data from the two conditions were averaged for each subject to reduce variance arising from measurement error and biological variability. This practice acted as a smoothing procedure that tended to diminish the effects of pulsatility of secretion on the timing of maximia and minima. The data for each condition were also analysed separately to compare after-effects of the two light-dark cycles on the duration of nocturnal periods of melatonin and cortisol secretion. The subjects’ age was 29.4 ± 6.7 (mean ±s.d.).
Sleep propensity
Sleep propensity was defined as the minutes of sleep that were obtained during 10 min sleep opportunities that were scheduled every 30 min during the sampling period. For 24 h profiles of sleep propensity, the time of onset of a nocturnal period of increasing sleep propensity was considered to begin at the end of the ‘evening wakefulness maintenance zone’ (see Lavie & Zvuluni, 1993), which was defined as the time of the last local minimum value of sleep in the evening that was followed by a sustained elevation of levels throughout the night. The time of the end of this period was defined as the time of the last local maximum value that remained within the range of high nocturnal levels and that was followed by a rapid decline towards low levels in the morning. Since the methods and conditions used for the collection of data in each of the two conditions of Study 2 were identical, in the analysis and display of results the data from the two conditions were averaged for each subject to reduce variance arising from measurement error and biological variability. Data for six pairs of profiles from three men and three women were available for analysis. Their age was 33 ± 8.3 (mean ±s.d.).
EEG theta activity
For 24 h profiles of wake EEG theta activity in Study 5, the time of onset of a nocturnal period of increasing theta activity was defined as the time of the minimum value. Data for 10 profiles from seven men and three women were available for analysis. Their age was 25.4 ± 2.8 (mean ±s.d.).
REM sleep
For profiles of REM sleep from 14 h recording periods in Study 1, the time of offset of a nocturnal period of increasing REM sleep was defined as the time of the bin with the highest amount of REM sleep (expressed as a percentage of each 5 min recording bin). The recordings from the 14 h dark periods of Study 1 were selected for analysis because they encompassed a much larger portion of the night than the 8 h dark periods in that study. Data for 11 profiles from 10 men and one woman were available for analysis. Their age was 30.2 ± 6.2 (mean ±s.d.).
Sleep
Data for 70 pairs of times of sleep onset and offset were available for analysis from sleep EEG recordings for nine men in Study 1 and from sleep logs for eight men in Study 3 and 21 men and 32 women in Study 4. Their age was 37.6 ± 9.8 (mean ±s.d.).
Data analysis
Onset and offset of the nocturnal period of melatonin secretion were used as internal frames of reference for measuring the timing, respectively, of onset and offset of nocturnal periods of other circadian rhythms, as defined above. Specifically, for each individual, differences were calculated between the time of onset of the nocturnal period of melatonin secretion and the time of onset of the nocturnal period of each of the other circadian rhythms. Similarly, differences were calculated between the time of offset of the nocturnal period of melatonin secretion and the time of offset of the nocturnal period of each of the other circadian rhythms. For each of these differences, group means, standard deviations and 95 % confidence intervals were calculated. Student's t test was used to determine whether the differences in timing of the onset or offset of melatonin secretion and the onset or offset, respectively, of the nocturnal period of any other circadian rhythm was significantly different from zero.
To facilitate visual inspection of the phase relationships between the nocturnal period of melatonin secretion and the nocturnal periods of other circadian rhythms, average 24 h profiles of the latter are shown together with average 24 h profiles of the former. These plots were created by referencing data for the other circadian rhythms to the times of onset and offset of melatonin secretion, as described in the legend for Fig. 3.
Figure 3. Simultaneous evening and morning transitions in melatonin and temperature circadian rhythms.
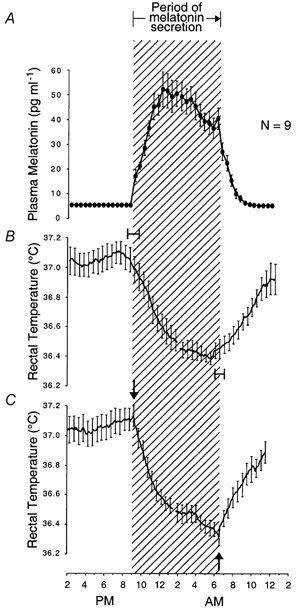
Group-average 24 h profiles (±s.e.m.) of plasma melatonin levels and rectal temperature in winter and summer in individuals who remained awake and at rest in constant dim light. Onset of melatonin secretion coincides with the beginning of a rapid, exponential decline of temperature from high daytime values to low night-time values. Offset of melatonin secretion coincides with the beginning of a rapid, exponential rise of temperature from low night-time values to high daytime values. A and B, melatonin and temperature data in the left half of each circadian rhythm profile are averaged across subjects with reference to their time of onset of melatonin secretion (shown in Fig. 1). This reference point in average profiles is positioned over the abscissa at its average time of occurrence in the group, indicated by the left margin of the cross-hatched area delineating the period of nocturnal melatonin secretion. Melatonin and temperature data in the right half of each circadian rhythm profile are averaged across subjects with reference to their time of offset of melatonin secretion (shown in Fig. 1). This reference point in average profiles is positioned over the abscissa at its average time of occurrence in the group, indicated by the right margin of the cross-hatched area delineating the period of nocturnal melatonin secretion. Ninety-five per cent confidence intervals for onset of evening decline in temperature relative to onset of melatonin secretion, and onset of morning rise in temperature relative to offset of melatonin secretion, are shown. C, temperature data in the left half of each circadian rhythm profile are averaged across subjects with reference to their time of beginning of rapid decline in temperature from high daytime levels to low night-time levels (shown in Fig. 2). Temperature data in the right half of each circadian rhythm profile are averaged across subjects with reference to their time of beginning of rapid rise in temperature from low night-time levels to high daytime levels (shown in Fig. 2). These reference points in average profiles are positioned over the abscissa at their average time of occurrence in the group, indicated by arrows. Note that average times of corresponding reference points in temperature and melatonin rhythms coincide exactly. Since data in the right half of each melatonin profile were averaged across subjects with reference to a local maximum that was identified as the end of secretion, a local maximum has been preserved in the average profile at this point. Data are from nine pairs of profiles in nine subjects extracted from Wehr et al. (1995a).
Study 1 was designed to determine whether experimental manipulations of the duration of the dark portion of the light-dark cycle (the scotoperiod) to which individuals are exposed can cause them to make corresponding adjustments in the duration of nocturnal intervals of melatonin and other circadian rhythms. To examine this question, the durations of nocturnal periods of circadian rhythms after exposure to artificial 14 h ‘nights’ and after exposure to artificial 8 h ‘nights’ were compared, and the statistical significance of differences between these conditions was assessed with paired t tests. Because we predicted on the basis of animal models that nocturnal periods of the rhythms would be longer after exposure to long nightly dark periods than after exposure to short ones, one-tailed tests were used.
In all instances in which an individual's data from two conditions of a study were combined, means of corresponding values from the two conditions were used for each individual when group-averages and tests of statistical significance were calculated. In a few instances, subjects participated in more than one study: one man and one woman in Studies 1 and 2, and two men in Studies 1 and 3. In these cases, data from all conditions of both studies were averaged for the subject, so that each contributed only one set of data to the final analysis.
RESULTS
The 24 h profiles of circadian rhythms that were averaged with reference to the onset and offset of nocturnal melatonin secretion appeared to be organized into distinct diurnal and nocturnal states with switch-like transitions between the states.
In one group of rhythms, the transitions between diurnal and nocturnal states coincided with corresponding transitions in the rhythm of melatonin secretion (Table 1 and Figs 3-6). Thus, onset of melatonin secretion coincided with transitions from diurnal periods of high core body temperature, low sleepiness, decreasing sleep propensity and decreasing wake EEG theta activity to nocturnal periods of rapidly decreasing core body temperature, increasing sleepiness, increasing sleep propensity and increasing wake EEG theta activity (Figs 3-6). Offset of melatonin secretion coincided with transitions from nocturnal periods of low core body temperature, increasing sleepiness and increasing REM sleep to diurnal periods of rapidly rising core body temperature, decreasing sleepiness and decreasing REM sleep (Figs 3, 4 and 7).
Table 1.
Phase relationship between nocturnal period of melatonin (MEL) secretion and nocturnal periods of other circadian rhythms
| Nocturnal period of circadian rhythm | |||||||
|---|---|---|---|---|---|---|---|
| Type of circadian rhythm | d.f. | Time of onset relative to MEL onset (h) x± s.d. | t value | P value | Time of offset relative to MEL offset (h) x ± s.d. | t value | P value |
| REM sleepa | 10 | — | — | — | 0.0 ± 0.9 | 0.9 | n.s. |
| Rectal temperatureb | 8 | 0.2 ± 1.3 | 0.4 | n.s. | 0.0 ± 1.2 | 0.0 | n.s. |
| Sleepinessa,b | 17 | −0.1 ± 2.3 | 0.2 | n.s. | −0.8 ± 2.7 | 1.2 | n.s. |
| Wake EEG thetad,e | 9 | 1.0 ± 1.8 | 1.8 | n.s. | — | — | — |
| Sleep propensityc | 5 | −0.2 ± 0.5 | 1.2 | n.s. | 1.7 ± 0.5 | 7.8 | 0.001 |
| Plasma cortisola | 13 | 1.5 ± 1.3 | 4.5 | 0.001 | 1.3 ± 1.3 | 3.7 | 0.003 |
| Sleep: | |||||||
| EEGa | 9 | 1.2 ± 1.0 | 2.9 | 0.02 | 1.7 ± 1.0 | 5.7 | 0.0003 |
| Diaryb,f | 61 | 2.6 ± 1.1 | 18.1 | 0.0001 | 1.5 ± 1.5 | 7.5 | 0.0001 |
Figure 6. Simultaneous evening transitions in melatonin and EEG theta activity circadian rhythms.
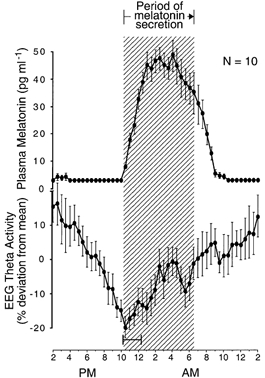
Group-average 24 h profiles (±s.e.m.) of plasma melatonin levels and power density in the theta band of wake EEG in individuals who remained awake and at rest in constant dim light. The wake-dependent component that was approximated by a saturating exponential function was removed from the theta profile. The hatched area delineates the nocturnal period of melatonin secretion. The onset of melatonin secretion coincides with the transition from the diurnal period of decreasing theta power density to the nocturnal period of increasing power density. The 24 h profiles are constructed as described for the left half of A and B in Fig. 3. Ninety-five per cent confidence intervals are shown for onset of the nocturnal period of increasing theta power, relative to onset of melatonin secretion. Data are from 10 rhythm profiles in 10 subjects extracted from Aeschbach et al. (1999).
Figure 4. Simultaneous evening and morning transitions in melatonin and sleepiness circadian rhythms.
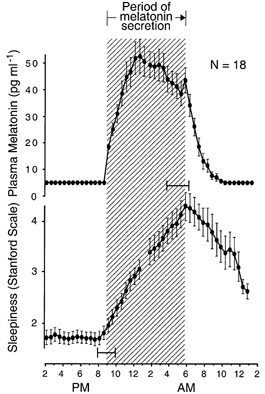
Group-average 24 h profiles (±s.e.m.) of plasma melatonin levels and self-ratings of sleepiness on the Stanford Sleepiness Scale in individuals who remained awake and at rest in constant dim light. Onset and offset of melatonin secretion coincide with onset and offset of nocturnal period of increasing sleepiness. The hatched area delineates the nocturnal period of melatonin secretion. Ninety-five per cent confidence intervals are shown for onset and offset of the nocturnal period of increasing sleepiness, relative to onset and offset, respectively, of melatonin secretion. 24 h profiles are constructed as described for A and B in Fig. 3. Data are from 18 pairs of rhythm profiles in 18 subjects extracted from Wehr et al. (1993) and Wehr et al. (1995a).
Figure 7. Simultaneous morning transitions in melatonin and REM sleep propensity circadian rhythms.
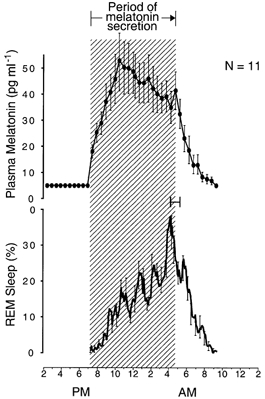
Group-average 24 h profiles (±s.e.m.) of plasma melatonin levels and amount of REM sleep in individuals who slept during a 14 h dark period from 6 p.m. to 8 a.m. for several weeks. The hatched area delineates the nocturnal period of melatonin secretion. Offset of melatonin secretion coincides with the transition from the nocturnal period of increasing frequency of REM sleep to the diurnal period of decreasing frequency of REM sleep (as a percentage of recording time). 24 h profiles are constructed as described for A and B in Fig. 3. Ninety-five per cent confidence intervals are shown for offset of the nocturnal period of increasing frequency of REM sleep, relative to offset of melatonin secretion. Data are from 10 six-night-average REM sleep profiles in 10 subjects extracted from Wehr et al. (1993).
In a second group of rhythms, the transitions between diurnal and nocturnal states lagged the onset and offset of the nocturnal period of melatonin secretion by 1-3 h (Table 1 and Fig. 8 and Fig. 9). These included transitions from diurnal periods of wakefulness and decreasing plasma cortisol levels to nocturnal periods of sleep and increasing plasma cortisol levels (Fig. 8 and Fig. 9). They also included transitions from nocturnal periods of sleep, increasing sleep propensity and increasing plasma cortisol levels to diurnal periods of wakefulness, decreasing sleep propensity and decreasing plasma cortisol levels (Figs 5, 8 and 9).
Figure 8. One to three hour lag between evening and morning transitions of cortisol and sleep-wake circadian rhythms relative to those of melatonin circadian rhythm.
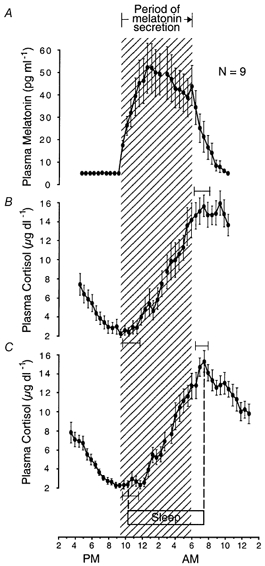
Group-average 24 h profiles (±s.e.m.) of plasma melatonin levels and plasma cortisol levels in individuals who remained awake and at rest in constant dim light. Onset and offset of the nocturnal period of increasing cortisol levels lag by 1-3 h onset and offset, respectively, of nocturnal melatonin secretion. The hatched area delineates the nocturnal period of melatonin secretion. Ninety-five per cent confidence intervals are shown for onset and offset of the nocturnal period of increasing cortisol levels, relative to onset and offset, respectively, of melatonin secretion. A and B, 24 h profiles constructed as described for A and B in Fig. 3. C, sleep and cortisol data in the left half of the graph are averaged across subjects with reference to their time of onset of sleep. Sleep and cortisol data in the right half of the graph are averaged across subjects with reference to their time of offset of sleep. These reference points in average profiles are positioned over the abscissa at their average times of occurrence in the group, indicated by dotted lines on the left and right, respectively. Ninety-five per cent confidence intervals are shown for onset and offset of the nocturnal period of increasing cortisol levels, relative to onset and offset respectively, of sleep. Data are from nine pairs of rhythm profiles in nine subjects extracted from Wehr et al. (1993).
Figure 9. Morning and evening transitions in circadian rhythms separately entrained to dawn and dusk.
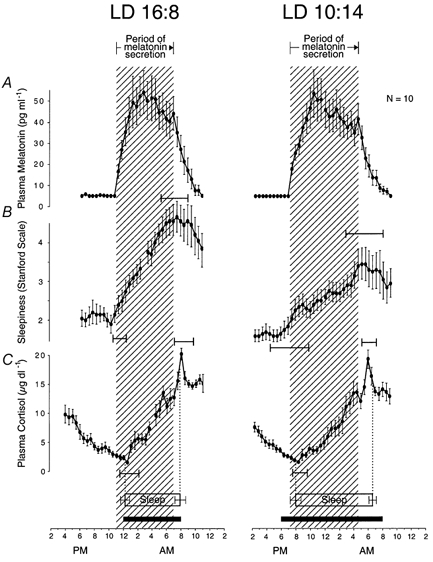
Group-average 24 h profiles (±s.e.m.) of plasma melatonin levels, sleepiness self-ratings (Stanford Scale), and plasma cortisol levels in individuals who remained awake and at rest in constant dim light after they were chronically exposed to short artificial ‘nights’ (left panel) and to long artificial ‘nights’ (right panel). Group-average profiles (±s.e.m.) of the prior sleep period are also shown. Nocturnal periods of melatonin secretion, increasing sleepiness and increasing cortisol were longer after exposure to long nights compared with short nights. The black horizontal bars indicate duration of nightly periods of darkness. The hatched areas delineate nocturnal periods of melatonin secretion. Ninety-five per cent confidence intervals are shown for onset and offset of nocturnal periods of increasing sleepiness and increasing plasma cortisol levels relative to onset and offset, respectively, of melatonin secretion. A and B, 24 h profiles of rhythms constructed as described for A and B in Fig. 3. C, 24 h profiles of rhythms constructed as described for C in Fig. 8. Data are from nine pairs of rhythm profiles in nine subjects extracted from Wehr et al. (1993).
Figure 5. Simultaneous evening transitions in melatonin and sleep propensity circadian rhythms.
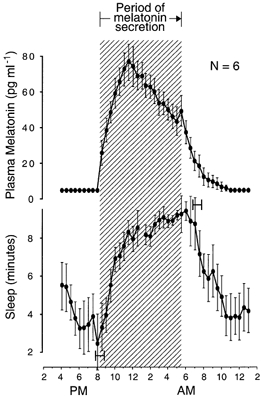
Group-average 24 h profiles (±s.e.m.) of plasma melatonin levels and sleep propensity in individuals who were given a 10 min sleep opportunity every 30 min. The hatched area delineates the nocturnal period of melatonin secretion. Onset of melatonin secretion coincides with onset of the nocturnal period of increasing sleep propensity. Ninety-five per cent confidence intervals are shown for onset and offset of the nocturnal period of increasing sleep propensity, relative to onset and offset, respectively, of melatonin secretion. 24 h profiles are constructed as described for A and B in Fig. 3. Data from six pairs of rhythm profiles in six subjects are extracted from Wehr (1996).
The times of minimum and maximum plasma cortisol levels in a constant routine were significantly later than the times of onset (P = 0.001) and offset (P = 0.001) of melatonin secretion in the constant routine (Table 1) but were not significantly different from the mean times of onset (0.2 ± 1.5 h, d.f. = 8, t = 0.5, n.s.) and offset (0.3 ± 1.1 h, d.f. = 8, t = 0.9, n.s.) of sleep during the six nights that preceded the constant routine. In addition, the transition between a nocturnal period of increasing cortisol levels to a diurnal period of decreasing cortisol levels was more sharply defined when cortisol data were averaged with reference to habitual offset of sleep than when they were averaged with reference to offset of melatonin secretion (Fig. 8).
After individuals in Study 1 were transferred from light-dark cycles with short nightly dark periods to cycles with long nightly dark periods, the interval between transitions into and out of nocturnal periods of several circadian rhythms became longer (Fig. 9). These included the nocturnal periods of melatonin secretion (7.9 ± 1.6 versus 9.4 ± 1.4 h (d.f. = 9, t = 3.9, P = 0.002)), increasing sleepiness (7.6 ± 3.0 versus 10.3 ± 5.1, d.f. = 9, t = 1.9, P = 0.05), and increasing plasma cortisol levels (7.4 ± 2.9 versus 9.6 ± 2.6, d.f. = 8, t = 1.7, P = 0.06) in constant routine protocols and average sleep during the six nights that preceded the constant routine protocols (7.6 ± 0.2 versus 10.5 ± 0.9, d.f. = 10, t = 11.5, P = 0.0001; all t tests one-tailed) (Fig. 9).
DISCUSSION
Biological day and night, dawn and dusk
Twenty-four hour profiles of circadian rhythms in arousal state, core body temperature and hormone secretion were found to exhibit distinct diurnal and nocturnal states - a biological ‘day’ and ‘night’ - and to exhibit abrupt, switch-like transitions between the states - a biological ‘dawn’ and ‘dusk’ (Fig. 11).
Figure 11. Group-average 24 h melatonin profiles referenced to external clock-time and internal circadian time.
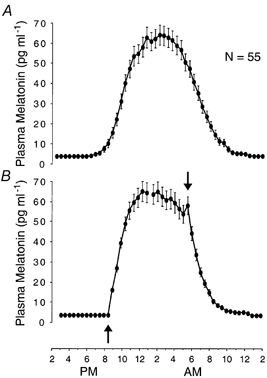
Group-average 24 h profiles (±s.e.m.) of plasma melatonin levels in constant dim light in 55 healthy individuals (23 men and 32 women) who slept according to their habitual sleep schedules and remained in constant dim (< 1 lx) light when they were awake. A,melatonin data are averaged across subjects with reference to clock-time. B, same data averaged with reference to onset (up-arrow) and offset (down-arrow) of melatonin secretion (B), as described for A and B in Fig. 3. Because individuals differ in schedules to which their circadian rhythms are entrained and in phase angles at which their circadian rhythms are entrained to these schedules, abrupt changes that are characteristic of rhythm waveforms in individuals, such as onset of melatonin secretion (see examples in Fig. 1), are obscured when data are averaged relative to external phase markers (A). These features are preserved when data are averaged relative to internal phase markers (B). Data extracted from Wehr et al. (2001).
The rhythms could be divided into two groups based on the timing of these transitions. Within each of the two groups, the times of transitions between diurnal and nocturnal states of the different rhythms appeared to coincide. Between the groups, however, the times of the transitions in one group lagged those in the other by 1-3 h. The leading group included circadian rhythms in melatonin secretion, core body temperature, sleepiness, wake EEG theta power (dusk transition), sleep propensity (dusk transition) and REM sleep propensity (dawn transition) (Table 1 and Figs 4-7 and 10). The lagging group included circadian rhythms in sleep and in plasma levels of cortisol (Table 1 and Figs 8-10).
Figure 10. Temporal organization of the human circadian timing system.
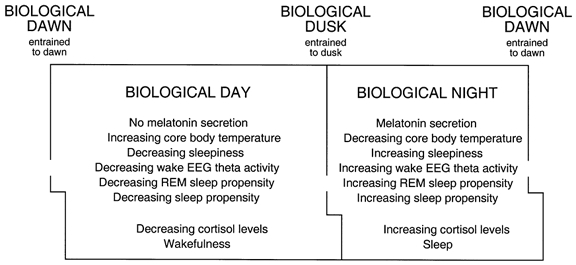
Profiles of a number of circadian rhythms in humans exhibit distinct diurnal and nocturnal states with abrupt switch-like transitions between them. These states and transitions can be conceptualized as a biological day and night and a biological dawn and dusk. They are generated within the organism and mirror and anticipate features of the solar day to which they correspond, and with which they are synchronized.
The time of offset of the nocturnal period of increasing sleep propensity is uncertain. It is possible that it coincided with offset of melatonin secretion but was masked by a ceiling effect. The design of the experiment prevented subjects from sleeping more than 10 min in naps that were scheduled every 30 min and these naps were saturated with sleep during the hours that preceded the estimated time of offset of increasing sleep propensity (see Fig. 5).
Since REM sleep can only be measured during sleep, and since sleep was restricted to 14 h nightly dark periods, we cannot be said to have completely measured its circadian rhythm. However, the existence and the timing of the transition from a nocturnal interval of increasing REM sleep to a diurnal interval of decreasing REM sleep is consistent with previously published descriptions of the circadian rhythm of REM sleep propensity (Dijk & Czeisler, 1995). Whether the transition from the diurnal period to the nocturnal period of the rhythm would be abrupt or smooth cannot be determined from the present data. A previous study, however, suggests a close temporal relationship between the onset of melatonin secretion and the onset of a nocturnal period of increasing frequency of REM sleep (Dijk et al. 1997).
internal versus external frames of reference
Often, an external frame of reference, such as clock-time, is used to calculate average profiles of human circadian rhythms. However, because individuals differ in the schedules to which their circadian rhythms are entrained and in the phase angles at which the rhythms are entrained to these schedules, this method of averaging tends to smooth out abrupt changes, like the onset of melatonin secretion, that might be characteristic of the waveform of the rhythm in an individual profile (Fig. 11). To control for inter-individual differences in the phase angle of entrainment and preserve abrupt changes in the average profiles, we used dual internal reference points - the onset and offset of melatonin secretion - as a template for these calculations.
When we used the melatonin circadian rhythm as a template for calculating group-average profiles of other rhythms, it was necessary to identify both onset and offset of nocturnal melatonin secretion. Onset is clear-cut and readily identifiable, and has been used extensively as a circadian phase marker (Lewy et al. 1999) (see examples in Fig. 1). Offset is more difficult to identify (Lewy et al. 1999). The use of offset (like the use of onset of the decline in core body temperature) is a novel element in the present analysis of previously published data. The existence of correlates of offset of melatonin secretion, namely, the almost simultaneous transitions from nocturnal periods of low core body temperature, increasing sleepiness, and increasing REM sleep to diurnal periods of rapidly increasing core body temperature, decreasing sleepiness and decreasing REM sleep (Figs 3, 4 and 7), together with the replicability of these findings (Fig. 8), tends to support the validity of the concept of a discrete offset of melatonin secretion and the reliability of its identification.
Relation to previous studies
Our results are generally consistent with those of Cajochen et al. (1999), who found a high correlation between melatonin plasma levels and subjective sleepiness, Wyatt et al. (1998), who found a close temporal relationship between the nocturnal rise in melatonin secretion and a nocturnal rise in sleep efficiency, Dijk et al. (1999), who found a close temporal relationship between the onset of melatonin secretion and the onset of the nocturnal rise in sleep propensity, Kräuchi et al. (2000), who found a close temporal relationship between the onset of melatonin secretion and the onset of the nocturnal decline in core body temperature, and Duffy et al. (1999), whose data appear to show small lags in the timing of onset and offset of habitual sleep relative to onset and offset of melatonin secretion. However, a detailed comparison of these investigators’ results with ours is not possible because most did not seek to identify onset and offset of melatonin secretion and relate these events to transitions in waveforms of circadian rhythms in other variables. On the other hand, while we found that the nocturnal rise in sleep propensity coincided with the onset of melatonin secretion, Shochat et al. (1997) reported that the rise in sleep propensity (the ‘sleep gate’) occurred 100-120 min later than the onset of melatonin secretion in their subjects. The reasons for this discrepancy are unclear. In their study, sleep-sampling periods were shorter (7 min vs. 10 min), and schedules of sleep and light exposure during the period that preceded sleep propensity measurements were less rigidly controlled.
Results of other investigators’ experiments suggest that the event that we characterize as biological dawn also coincides with the phase in the circadian pacemaker's rhythm at which phase-resetting responses to light switch from maximal phase delays to maximal phase advances. This ‘crossover point’ in the pacemaker's phase-response curve (PRC) for light coincides with the minimum of the circadian temperature rhythm, which corresponds to biological dawn in our data (Fig. 3). The crossover point is a point of singularity in the pacemaker's rhythm at which exposure to light can abolish the rhythm (Jewett et al. 1994).
A hierarchy of rhythms
Inferences about causal relationships among the transitions between diurnal and nocturnal states of circadian rhythms can be drawn from the order in which these transitions occurred and from what is already known of their interactions. For example, if the administration of melatonin facilitates the onset of sleep, as has been claimed, then the onset of its secretion might contribute to the rise in sleepiness, theta activity and sleep propensity that occurs in the evening, especially since this rise begins when melatonin begins to be secreted. Conversely, the rapid disappearance of melatonin after the cessation of its secretion in the morning might also contribute to the simultaneous decline in sleepiness at that time, though experimental evidence for this effect is lacking (Figs 4-6). Any effects of melatonin on sleepiness and sleep must be relative rather than absolute, however, because individuals who secrete no melatonin at all seem to sleep normally (authors’ unpublished observations).
The onset of melatonin secretion probably helps to initiate the rapid decline in core body temperature that begins at the same time (Fig. 3), because administration of melatonin has been shown to lower core body temperature by inducing distal vasodilatation and heat-loss (reviewed in Kräuchi et al. 2000). There is some evidence that this vasodilatation and heat-loss in turn contributes to the increase in sleepiness and sleep propensity that begins when melatonin secretion begins (Fig. 4 and Fig. 5) (Kräuchi et al. 2000). A corollary is that the offset of melatonin secretion probably helps to initiate the rapid rise in core body temperature and decline in sleepiness that begin at the same time. These thermoregulatory effects of melatonin might also account for the changes in REM sleep that we observed. One of us proposed that a function of REM sleep is to selectively warm the central nervous system during the nocturnal period in which core body temperature is down-regulated (Wehr, 1992). This hypothesis is consistent with observations in the present study of an inverse relationship between core body temperature and REM sleep and a simultaneity of transitions between nocturnal and diurnal trends in their levels (Fig. 3 and Fig. 7).
The gradual increase in sleepiness and sleep propensity that begins when melatonin secretion begins must contribute to the subsequent decision to go to bed. This decision seems to represent a threshold phenomenon, because it occurs 2-3 h after melatonin secretion and sleepiness begin to increase. All of these changes must then facilitate the onset of sleep that occurs shortly thereafter (Fig. 8). Conversely, the gradual decline in sleepiness, sleep propensity and REM sleep propensity that begins when melatonin secretion stops (Figs 1, 3 and 4) must facilitate the onset of wakefulness and the decision to get up that occur 1-2 h later (Fig. 8). Possibly consistent with this model, the interval between biological dawn and dusk is shorter in habitual short sleepers than it is in habitual long sleepers (Aeschbach et al. 2001).
Two observations indicate that the timing of the constant routine cortisol circadian rhythm may be more closely related to the timing of the sleep-wake cycle during the nights that preceded the constant routine than to the constant routine melatonin circadian rhythm. First, transitions between diurnal and nocturnal periods of the cortisol rhythm coincided with times of transitions between habitual wakefulness and sleep rather than transitions in melatonin secretion (Fig. 8). Second, the transition from the nocturnal period when cortisol levels were rising to the diurnal period when they were declining was sharper when cortisol data were averaged with reference to habitual offset of sleep than when they were referenced to offset of melatonin secretion (Fig. 8).
The chain of events that might cause the onset of the nocturnal period of rising cortisol levels to be synchronized with habitual onset of sleep, and the onset of the diurnal period of declining cortisol levels to be synchronized with habitual onset of wakefulness, is unclear. The fact that these phase relationships are preserved in a constant routine protocol from which sleep is excluded indicates that the acts of going to sleep and of waking up do not directly induce these transitions in cortisol secretion. Instead, they indicate that the transitions are governed by circadian processes that persist when sleep is suspended. These circadian processes might nevertheless be entrained to the acts of going to sleep and waking up and/or to changes in retinal exposure to light that are associated with these acts. More than three decades ago, Orth & Island (1969) provided evidence that it is the latter rather than the former events that entrain the peak of the corticosteroid circadian rhythm. Alternatively, the 1-3 h lag between transitions in the melatonin rhythm and transitions in the cortisol rhythm might reflect the lags that are known to exist in the transmission of secretory stimuli from the hypothalamus to the pituitary, and from the pituitary to the adrenal cortex. With regard to this interpretation, however, it should be noted that suprachiasmatic nucleus (SCN) regulation of adrenocortical secretion appears partly to be effected through a multisynaptic autonomic pathway from the SCN to the adrenal that alters the response of the gland to ACTH (Buijs et al. 1999).
The extent to which our observations about relationships between sleep and other variables can be generalized to unrestricted sleep schedules is unknown. Since our subjects were required to sleep only during the night-time dark periods in the various studies, we are unable to say whether any of these relationships would be preserved in other situations in which individuals also sleep: for example, during afternoon naps.
Entrainment to dawn and dusk
The hypothesis that transitions between diurnal and nocturnal periods of circadian rhythms are separately entrained to dawn and dusk was supported by a previously published analysis of data from the experiment in Study 1 (Wehr et al. 1993), which suggested that these transitions were separately entrained to lights-on and lights-off (see also Burefsova et al. 1992). This finding was confirmed in the present analysis, in which a more appropriate indicator of offset of melatonin secretion was used (Fig. 9). In this conclusion, we assume that the individuals’ responses to lights-off and lights-on in an artificial light-dark cycle are predictive of responses to dusk and dawn in a natural light-dark cycle. Although the responses to natural and artificial stimuli might be qualitatively similar, they might be quantitatively different, because the signal properties of the two types of stimuli may be different, and the light-dark cycle that is perceived in everyday life can be modified by eye closure during sleep.
The concept of entrainment does not imply that the evening and morning transitions in the circadian rhythms are phase-locked to dusk and dawn. Internal coupling between the circadian processes that control these transitions will limit the extent to which the phase angle between them can be changed in response to changes in the interval between dusk and dawn. This can be seen clearly in Fig. 9, which shows that the phase angle between the light-to-dark transition and the onset of melatonin secretion and the phase angle between the dark-to-light transition and offset of melatonin secretion both increase when the dark period is lengthened.
Conclusions
The present data highlight similarities between humans and other animals in the temporal organization of the circadian system, and they show that the classic Pittendrigh & Daan (1976) model of the rodent circadian system could be extended to the human case. In that model, and in its elaboration by Illnerova & Vanecek (1982), it is proposed that the circadian pacemaker consists of two component oscillators. One is entrained to dusk and controls an evening bout of locomotor activity and the onset of melatonin secretion in nocturnal rodents. The other is entrained to dawn and controls a morning bout of locomotor activity and the offset of melatonin secretion. Separate entrainment of the oscillators to dawn and dusk makes it possible for the pacemaker to adjust the duration of nocturnal periods of activity and melatonin secretion to conform to seasonal changes in night length. As applied to humans, the dusk- and dawn-entrained components of the complex circadian pacemaker could be considered to control evening and morning transitions in melatonin secretion, core body temperature, sleepiness, EEG theta activity, sleep propensity, REM sleep propensity, cortisol secretion and sleep-wake state, and to adjust the timing of these transitions in response to seasonal changes in day length.
In their model, Pittendrigh and Daan conceptualized its dusk- and dawn-entrained components as controlling the occurrence of two bouts of activity, one at the beginning and one at the end of the nocturnal period during which activity is expressed in nocturnal rodents. Although the nocturnal period of melatonin secretion sometimes also exhibits separate, evening and morning bouts of secretion (Wehr et al. 1995b), Illnerova and Vanecek linked the dusk- and dawn-entrained components not to these bouts, but to the transitions that mark the beginning and end of the nocturnal period of secretion. These transitions correspond to the onset of the evening activity bout and the offset of the morning activity bout in the Pittendrigh-Daan model. In our application of the model to humans, we follow Illnerova and Vanecek in focusing on transitions between diurnal and nocturnal states of circadian rhythms.
In this interpretation of our results, we use melatonin secretion as a surrogate marker for processes in the circadian pacemaker that delineate a biological night but cannot be measured directly in humans. Recently, direct neurophysiological evidence was obtained from pacemaker tissue in rodents that supports the complex pacemaker model. Abrupt, switch-like transitions between periods of low and high firing rates were detected in in vitro recordings of electrical activity, and the transitions were found to be separately entrained to light-dark and dark-light transitions of the light-dark cycle to which animals previously had been exposed (Mrugala et al. 2000). Ongoing research promises to expose molecular substrates of these processes (Sumova et al. 1995; Illnerova et al. 1999; Messager et al. 2000). In this regard, there is evidence that the various autoregulatory feedback cycles in clock gene expression that give rise to circadian rhythms may be differentially entrained to dawn and dusk and thus might differentially control the timing of evening and morning transitions in circadian rhythms (Daan et al. 2001).
Changes in the duration of melatonin secretion that are induced by changes in the duration of the photoperiod are used by other mammals to regulate seasonal behaviour (Gorman et al. 2001). The possibility that the cortisol rhythm plays a similar mediating role is raised by our finding that the photoperiod duration is encoded in its peak-to-nadir interval, and by a recent report that circadian clocks in peripheral tissues may be entrained to the circadian pacemaker's rhythm via the cortisol rhythm (Balsalobre et al. 2000). The human circadian timing system appears to be homologous to that of other mammals with regard to its temporal organization and its capacity to encode seasonal changes in day length. However, the extent to which seasonal changes in day length act through this system to modify reproduction and other functions is unclear and is a focus of ongoing investigation (Wehr, 2001).
Acknowledgments
The authors would like to thank Charles Barker for technical assistance in the analysis and graphic presentation of data.
References
- Aeschbach D, Matthews J, Postolache T, Jackson M, Giesen HA, Wehr TA. Dynamics of the human EEG during prolonged wakefulness: Evidence for frequency-specific circadian and homeostatic influences. Neuroscience Letters. 1997;239:121–124. doi: 10.1016/s0304-3940(97)00904-x. [DOI] [PubMed] [Google Scholar]
- Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Two circadian rhythms in the human electroencephalogram during wakefulness. American Journal of Physiology. 1999;277:R1771–1779. doi: 10.1152/ajpregu.1999.277.6.R1771. [DOI] [PubMed] [Google Scholar]
- Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. Sleep. 2001;24(suppl.):A6. doi: 10.1210/jc.2002-020827. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MGP, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. European Journal of Neuroscience. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Burefsova M, Dvograkova M, Zvolsky P, Illnerova H. Human circadian rhythm in serum melatonin in short winter days and in simulated artificial long days. Neuroscience Letters. 1992;136:173–176. doi: 10.1016/0304-3940(92)90042-6. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk D-J. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. American Journal of Physiology. 1999;277:R640–649. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- Daan S, Albrecht U, Van Der Horst GTJ, Illnerova H, Roenneberg T, Wehr TA, Schwartz WJ. Assembling a clock for all seasons: Are M and E oscillators in the genes? Journal of Biological Rhythms. 2001;16:105–116. doi: 10.1177/074873001129001809. [DOI] [PubMed] [Google Scholar]
- Dijk D-J, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. Journal of Physiology. 1999;516:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk D-J, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. Journal of Physiology. 1997;505:851–858. doi: 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Dijk D-J, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. Journal of Investigational Medicine. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- Elliott JA, Tamarkin L. Complex circadian regulation of pineal melatonin and wheel-running in Syrian hamsters. Journal of Comparative Physiology. 1994;174:469–484. doi: 10.1007/BF00191713. [DOI] [PubMed] [Google Scholar]
- Fraser S, Cowen P, Franklin M, Franey C, Arendt J. Direct radioimmunoassay for melatonin in plasma. Clinical Chemistry. 1983;29:396–397. [PubMed] [Google Scholar]
- Gorman MR, Goldman BD, Zucker I. Mammalian photoperiodism. In: Takahashi J, Turek FW, Moore RY, editors. Handbook of Behavioral Neurobiology: Circadian Clocks. New York: Plenum-Kluwer; 2001. pp. 481–508. [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Illnerova H, Travnickova Z, Jac M, Sumova A. Comparison of the pineal and SCN rhythmicity. Effect of photic and non-photic stimuli, photoperiod and age. Advances in Experimental Medicine and Biology. 1999;460:247–260. doi: 10.1007/0-306-46814-x_27. [DOI] [PubMed] [Google Scholar]
- Illnerova H, Vanecek J. Two-oscillator structure of the pacemaker controlling the circadian rhythm of N-acetyltransferase in the rat pineal gland. Journal of Comparative Physiology. 1982;145:539–548. [Google Scholar]
- Jewett ME, Kronauer RE, Czeisler CA. Phase-amplitude resetting of the human circadian pacemaker via bright light: a further analysis. Journal of Biological Rhythms. 1994;9:295–314. doi: 10.1177/074873049400900310. [DOI] [PubMed] [Google Scholar]
- Kräuchi K, Cajochen C, Werth E, Wirz-Justice A. Functional link between distal vasodilation and sleep-onset latency. American Journal of Physiology. 2000;278:R741–748. doi: 10.1152/ajpregu.2000.278.3.R741. [DOI] [PubMed] [Google Scholar]
- Lavie P, Zvuluni A. The 24-h sleep propensity function: Experimental bases for somnotypology. Psychophysiology. 1992;29:566–575. doi: 10.1111/j.1469-8986.1992.tb02032.x. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. Journal of Biological Rhythms. 1999;14:227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- Messager S, Haxlerigg DG, Mercer JG, Morgan PJ. Photoperiod differentially regulates the expression of Per1 and ICER in the pars tuberalis and the suprachiasmatic nucleus of the Siberian hamster. European Journal of Neuroscience. 2000;12:2865–2870. doi: 10.1046/j.1460-9568.2000.00174.x. [DOI] [PubMed] [Google Scholar]
- Mrugala M, Zlomanczuk P, Jagota A, Schwartz WJ. Rhythmic multiunit neural activity in slices of hamster suprachiasmatic nucleus reflect prior photoperiod. American Journal of Physiology. 2000;278:R987–994. doi: 10.1152/ajpregu.2000.278.4.R987. [DOI] [PubMed] [Google Scholar]
- Orth DN, Island DP. Light synchronization of the circadian rhythm in plasma cortisol (17-OHCS) concentration in man. Journal of Clinical Endocrinology and Metabolism. 1969;29:479–486. doi: 10.1210/jcem-29-4-479. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. The photoperiodic phenomena: seasonal modulation of the ‘day within’. Journal of Biological Rhythms. 1988;3:173–188. doi: 10.1177/074873048800300206. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker structure: A clock for all seasons. Journal of Comparative Physiology. 1976;A 106:333–355. [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. Bethesda, MD, USA: US Department of Health, Education and Welfare, Public Health Service; 1968. [Google Scholar]
- Rusak B. The mammalian circadian system: models and physiology. Journal of Biological Rhythms. 1989;4:121–134. [PubMed] [Google Scholar]
- Shochat T, Luboshitzky R, Lavie P. Nocturnal melatonin onset is phase locked to the primary sleep gate. American Journal of Physiology. 1997;273:R364–370. doi: 10.1152/ajpregu.1997.273.1.R364. [DOI] [PubMed] [Google Scholar]
- Sumova A, Travnickova Z, Peters R, Schwartz WJ, Illnerova H. The rat suprachiasmatic nucleus is a clock for all seasons. Proceedings of the National Academy of Sciences of the USA. 1995;92:7754–7758. doi: 10.1073/pnas.92.17.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA. A brain-warming function for REM sleep. Neuroscience and Biobehavioral Reviews. 1992;16:379–397. doi: 10.1016/s0149-7634(05)80208-8. [DOI] [PubMed] [Google Scholar]
- Wehr TA. A ‘clock for all seasons’ in the human brain. In: Buijs RM, Kalsbeek A, Romhin RH, Pennartz CMA, Mirmiran M, editors. Progress in Brain Research. Vol. 111. Amsterdam: Elsevier; 1996. pp. 319–340. [PubMed] [Google Scholar]
- Wehr TA. Photoperiodism in humans and other primates: evidence and implications. Journal of Biological Rhythms. 2001;16:348–364. doi: 10.1177/074873001129002060. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Duncan WC, Jr, Sher L, Aeschbach D, Schwartz PE, Turner EH, Postolache TT, Rosenthal NE. A circadian signal of change of season in patients with seasonal affective disorder. Archives of General Psychiatry. 2001 doi: 10.1001/archpsyc.58.12.1108. in the Press. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Giesen HA, Moul DE, Turner EH, Schwartz PJ. Suppression of human responses to seasonal changes in daylength by modern artificial lighting. American Journal of Physiology. 1995a;269:R173–178. doi: 10.1152/ajpregu.1995.269.1.R173. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Moul DE, Giesen HA, Seidel JA, Barker C, Bender C. Conservation of photoperiod-responsive mechanisms in humans. American Journal of Physiology. 1993;265:R846–857. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Schwartz PJ, Turner EH, Feldman-Naim S, Drake C, Rosenthal NE. Bimodal patterns of human melatonin secretion consistent with a two-oscillator model of regulation. Neuroscience Letters. 1995b;194:105–108. doi: 10.1016/0304-3940(95)11740-n. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk D-J. Circadian temperature and melatonin rhythms, sleep, and a neurobehavioral function in humans living on a 20-h day. American Journal of Physiology. 1998;277:R1152–1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]


