Abstract
Most of the liquid that fills the lung of the fetal sheep in late gestation is cleared by the end of labour. Clearance of this liquid has a beneficial effect on postnatal gas exchange and therefore represents an important adaptation for postnatal life. Despite its importance, there is disagreement about whether clearance begins prior to labour, or occurs entirely within labour.
To address this issue, we made serial determinations of lung liquid volume by indicator dilution during late gestation and labour in the fetal sheep.
Regression analysis demonstrated that lung liquid volume exhibited a plateau level in the near-term fetus before it began to decline. Two models provided a fit to the decline in volume. In one, lung liquid clearance occurred in two linear phases, the first beginning 70 h before the study was terminated when the ewe was in advanced labour, the second occupying the last 8 h of the study period. In the initial phase, average lung liquid volume fell from 38.3 to 26.4 ml kg−1 before a rapid decline in the second phase reduced the volume to 13.8 ml kg−1. An exponential decay model was also found to fit the data; this showed a gradual decline in lung liquid volume in the 2 days preceding onset of labour, followed by a much more rapid decline within labour.
The rate of lung liquid secretion also declined in two linear phases, both of which commenced earlier than the changes in lung liquid volume. An exponential decay model also gave a significant fit to the data, but the fit was significantly weaker than that achieved with the two-slope model.
We conclude that clearance of lung liquid begins well before commencement of labour in the full term fetal sheep, and then accelerates once labour is established. In our study, lung liquid volume fell even in the absence of reabsorption of liquid across the pulmonary epithelium, indicating that outflow of liquid through the trachea must have occurred at a rate in excess of the secretion rate.
The fetal lung secretes a liquid which distends the future airspaces and plays a crucial role in promoting lung growth. Although essential for normal fetal development, both experimental (Berger et al. 1996) and clinical (Hales et al. 1993; Morrison et al. 1995) evidence supports the view that prenatal clearance of lung liquid before birth is critical for the establishment of normal respiratory function immediately after delivery. It is now clear that the vast bulk of this liquid leaves the lung before birth (Berger et al. 1998) and that at least a portion of the clearance occurs during labour (Brown et al. 1983) when rising concentrations of circulating adrenaline trigger liquid absorption by stimulating an active reabsorptive process mediated by transepithelial Na+ transport. However, evidence is conflicting as to whether there are other mechanisms that bring about a decline in lung liquid volume before the onset of labour, thereby contributing to liquid clearance before birth. An early study reported evidence that lung liquid volume starts to decline several days before labour (Dickson et al. 1986), whereas data presented in a number of recent reports suggest that lung liquid volume continues to rise until the day before labour, reaching an average value as high as 50 ml (kg body wt)−1 (Hooper & Harding, 1995; Harding & Hooper, 1996; Lines et al. 1997).
Both sources of evidence are open to criticism. The study that reported a pre-labour decline in lung liquid volume did not monitor uterine muscle activity (Dickson et al. 1986), leading to the suggestion that the animals may have been in early labour at the time a decline in lung liquid volume was observed (Lines et al. 1997). In the later series of studies (Hooper & Harding, 1995; Harding & Hooper, 1996; Lines et al. 1997), Blue Dextran was used as the impermeant tracer for lung liquid volume determinations. It has now been shown that a substantial proportion of this agent binds to the pulmonary epithelium of the near-term fetal sheep (Pfister et al. 1999). Accordingly, use of this tracer may lead to a substantial overestimate of lung liquid volume, potentially giving rise to an artefactual rise in lung liquid volume in late gestation.
The experiments reported here were undertaken to determine conclusively whether lung liquid volume declines over the last days of gestation before the onset of labour, or whether the decline is restricted to labour itself. As the impermeant tracer we used radio-iodinated human serum albumin (RISA), because we have shown that it does not bind to any significant extent to the pulmonary epithelium and that loss of tracer across the epithelium is minimal (Pfister et al. 1999).
METHODS
Surgery
All surgical and experimental procedures conformed with the guidelines established by the National Health and Medical Research Council of Australia and had the approval of the Standing Committee in Ethics in Animal Experimentation of Monash University.
Operations were performed on twelve fetuses of pregnant Border-Leicester ewes between 131 and 133 days gestation (G131 to G133; term at G147). Anaesthesia was induced with an intravenous injection of propofol (5 mg kg−1, Zeneca Ltd, Macclesfield, UK) and maintained with a mixture of 1-2 % halothane, 66 % N2O and 32-33 % O2. The maternal abdomen was opened in the midline and the fetal head was delivered through a uterine incision. After a midline ventral opening was made in the fetal neck, the carotid artery was catheterised non-occlusively using a teflon cannula-tygon tubing assembly. Two wide-bore silastic catheters (i.d. 3 mm, o.d. 6 mm; Sil-Med Corporation, Taunton, MA, USA) were introduced through a single tracheostomy, with one catheter directed rostrally so that its tip lay 2 cm or more from the larynx, while the other catheter was directed caudally towards the lung. The two free catheter ends were connected to form a liquid-filled loop. The skin incision was sutured and a liquid-filled catheter open to the amniotic space was secured to the skin nearby. The thorax was opened at the level of the 9th intercostal space to expose the diaphragm and bipolar harpoon EMG electrodes (Cooke et al. 1990) were inserted into the diaphragm for recording electromyogram activity (EMGd). The intercostal space was then closed with a silk ligature. Harpoon electrodes were also inserted into the external oblique muscle of the abdomen and into the external intercostal muscle of the 6th interspace after exposing each muscle through small skin incisions.
The fetus was returned to the uterus which was carefully closed to prevent leakage of amniotic fluid. Finally a bipolar harpoon EMG electrode was pushed into the uterine muscle (EMGu) and its lead was sutured to the uterus. The abdominal wall of the ewe was sutured and all catheters and wires were tunnelled subcutaneously to the flank where they exited through a small incision and were stored in a bag secured under a stocking encircling the abdomen. All animals received post-operative analgesia (50 mg Finadyne, Schering-Plough, Australia) and daily intramuscular antibiotic treatment (500 mg procaine penicillin, 500 mg dihydrostreptomycin). Animals were allowed to recover for 7 to 9 days before observations commenced.
Estimation of fetal body weight
All fetuses were studied on a number of days. Fetal body weight was calculated for each gestational age studied, using published fetal sheep growth curves (Lumbers et al. 1985; Davis et al. 1992), corrected for each fetus with its post-mortem weight according to the following formula:
where FBW is the desired weight for a particular fetus and gestation, a value that is calculated from FBWave, the mean weight of a sheep fetus at that gestation taken from sheep growth curves, multiplied by the ratio of FBWPM (actual weight of the fetus at post-mortem) divided by FBWavePM (mean fetal weight from sheep growth curves at the gestational age at termination of the experiment). The mean weights used for each gestational age were derived from the mean of the two published formulae (Lumbers et al. 1985; Davis et al. 1992) which provided comparable values with a difference between two estimates of 2.8 ± 0.5 % (mean ±s.e.m.).
Experimental protocol
On the day of each experiment, fetal carotid and amniotic fluid catheters were connected to pressure transducers (Hewlett-Packard 1280) and amplifiers (Hewlett-Packard 8805B). The diaphragm, external oblique and external intercostal EMG electrodes were connected to differential amplifiers (Neomedix NT114) and band-pass filtered (40 Hz to 2 kHz). All signals were displayed continuously on a chart recorder (Neotrace 800Z, Neomedix), digitized using a Maclab/16S system (ADInstruments, Castle Hill, NSW, Australia) and stored on a personal computer.
Measurement of pressure in the future airspace of the lung
During post-mortem examination of near-term fetuses in which a tracheal loop had been implanted, we have occasionally found a thick mucus plug near one or both cannula tips, suggesting that the loop could become obstructed in some fetuses. We reasoned that an obstruction anywhere in the tracheal loop would dam liquid in the lung and raise hydrostatic pressure within the lung. At the start of each experimental day we therefore recorded pressure in the lung end of the tracheal loop until we obtained a period of constant pressure and there was no fetal activity evident in the available EMG signals; this value was corrected by subtracting amniotic fluid pressure (PAF) measured at the same time.
Determination of lung liquid volume and secretion rate
Under sterile conditions, the lung end of the tracheal loop was connected to a temperature-controlled (40 °C) glass burette that wa sealed at its top with a rubber stopper. An 18-gauge needle was passed through the stopper and a Millipore filter was attached to the needle. With this arrangement, atmospheric pressure and sterility were maintained in the burette. We drained liquid from the lung into the burette and reinstilled it into the lung for a period of 30 min before taking a sample (0.5 ml) to measure its background radioactivity; this step was omitted for the first experiment in each fetus, since there was no radioactivity present in the lung at the start of this experiment. A volume of 50-100 ml was then collected in a container, weighed and a known quantity of radio-iodinated human serum albumin (RISA), prepared as described previously (Pfister et al. 1999) was thoroughly mixed into it (3-8 μC for measurements separated by 1 day or more; 10-20 μC when a second measurement was made on the day of labour). In addition, 0.5-1 ml of fetal plasma, obtained just before the start of the experiment, was added as a source of ‘cold’ albumin. A small sample was then taken from the container to determine its level of radioactivity. The remaining liquid was weighed accurately (Mettler AE 166 delta range), returned to the burette and reinstilled into the lung. The tracer was mixed with lung liquid by repeated cycles of drainage and reinstillation over at least 30 min, using a maximum hydrostatic pressure of ±15 cmH2O. After 30 min of mixing, 12-15 samples of 0.5 ml were taken at intervals of 5-10 min whilst cycling was continued. In one animal, due to concern that labour was progressing rapidly, only two samples were taken in advanced labour, allowing us to estimate lung liquid volume (VL), but not secretion rate (JV).
Concentration of RISA in each sample was measured on a gamma counter (1282 Compugamma, LKB, Wallac, Finland; window set between 20 and 100 keV). Lung liquid volume and secretion rate were calculated according to established and verified techniques (Brown et al. 1983; Pfister et al. 1999). Jv, based on the slope of the relationship between lung liquid volume and time, was calculated using the least squares method. VL at the time of interruption of the tracheal loop (VL, when t = 0) was obtained by extrapolation.
During the course of each experiment, several samples of carotid arterial blood were taken to analyse fetal blood gas and acid-base status at an assumed body temperature of 40 °C (Radiometer ABL500, Radiometer, Copenhagen) and to measure haemoglobin concentration and O2 saturation (OSM2 Hemoximeter, Radiometer).
Lung liquid secretion rate and lung liquid volume were determined longitudinally in each animal, with the first experiment performed at G140 (in one fetus the first study was performed at G141) and every 1-2 days thereafter until the detection of labour. Continuous monitoring of uterine activity (EMGu), PAF and fetal breathing activity (EMGd) was instituted at G142. This allowed recognition of the early signs of labour (see later) and an immediate determination of VL and Jv was performed. Another determination was made 7-24 h later unless the appearance of fetal parts and vigorous maternal pushing suggested there was insufficient time to complete the experiment. After the final labour determination was made, the ewe and fetus were killed with an overdose of anaesthetic administered via the jugular vein of the ewe (150 mg kg−1; Lethabarb, Virbac, Peakhurst, NSW, Australia). A post-mortem examination was performed, checking for signs of fetal infection by inspecting the appearance and smell of the fetus, placenta and amniotic fluid, as well as its internal organs and fluids. The fetus was weighed and the lung was dissected before placing it in an oven at 95 °C for approximately 2 weeks until it reached a constant dry weight.
Definition of labour
As recognition of labour was crucial to the study, animals were monitored continuously from G142. Ewes were observed at regular intervals for clinical signs of impending labour, chiefly a reddening of the vulva and the appearance of mucus at the introitus. Behavioural changes were also carefully monitored, including decreased feeding and increased restlessness, as evinced by repeated change of posture from sitting to standing, and a frequent scraping of the forefeet on the floor of the pen. As the stages of labour are usually based on clinical findings and are ill-defined (Crawford, 1983, 1985) we based our definitions on EMGu and PAF recordings. Typically, in late gestation, but before labour was clear-cut, we observed infrequent and irregular short bursts of EMGu activity with little or no increase in PAF (Fig. 1, left panel) similar to the contractions manually palpated and described in the human by John Braxton Hicks (Hicks, 1871). As the ewe entered labour, we observed increasingly regular bursts of uterine activity separated by inactive periods some 12-24 h before fetal parts became apparent at the introitus. Early labour (approximating Stage I in the human) was diagnosed when synchronous amniotic fluid pressure waves of 5-15 cmH2O accompanied EMGu activity on a regular basis (Fig. 1, middle panel). Advanced labour (approximating Stage II in the human) was diagnosed when the ewe started active and repeated pushing, giving rise to amniotic fluid pressure peaks that reached >20 cmH2O (Fig. 1, right panel).
Figure 1. Uterine activity and amniotic fluid pressure before and during labour.
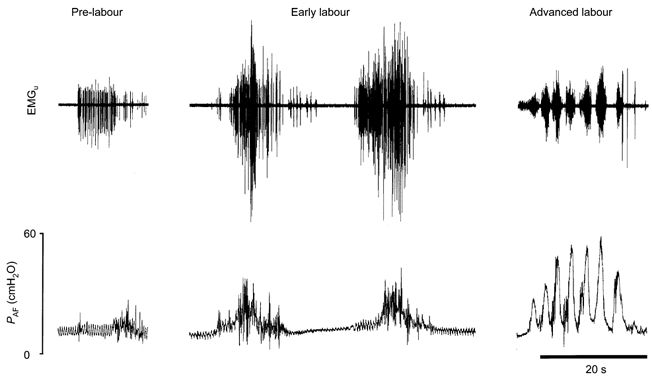
Uterine electromyogram (EMGu) and amniotic fluid pressure (PAF) from a fetus taken before labour (pre-labour), during early labour and in advanced labour. Before the establishment of labour, infrequent uterine contractions gave rise to a small increase in amniotic fluid pressure. In early labour, uterine contractions were more frequent and powerful, while in advanced labour maternal pushing was evident and it was sometimes in phase with the EMGu as shown in this record.
Exclusion criteria
The study was designed to be longitudinal, so it was decided at the outset that fetuses would be excluded unless lung liquid volume and secretion rates were determined at least once before and once during labour. In addition, since it was our intention to study only fetuses that had experienced a normal gestation and delivery, we decided to exclude fetuses if they entered advanced labour earlier than 141 days gestation (G141). This lower limit was chosen because lambs that deliver vaginally at this age or later manifest a normal rapid increase in arterial oxygen levels (Berger et al. 1990), suggesting all had experienced normal adaptations for birth.
Data analysis
The time at which a lung liquid volume and secretion rate determination was made during each study in a fetus was referenced to time zero, which was either the time the last determination began in advanced labour (8 of the 9 animals studied), or, in the fetus in which we did not perform an experiment in advanced labour, we took the time that the ewe was killed. After establishing that the data were not skewed, we subjected the raw data to a least squares regression analysis (SPSS, SPSS Inc., Chicago, IL, USA). The variation attributable to animals was removed before examining the effect of time on lung liquid volume and secretion rate, the two dependent variables. We tested four models on the entire data sets: first, a model in which each variable had a plateau level before a single slope decline occurred until time zero; second, a model in which a plateau level was followed by a two-slope decline in each variable; third, a model in which there was an increase in each variable before a decline to time zero, and fourth, an exponential decay model in which each variable declined in a continually accelerating manner throughout the study period. The first and second models were designed to test the claim that lung liquid volume declines before the onset of labour (Dickson et al. 1986), while the third model tested the claim that it continues to increase until the start of labour (Hooper & Harding, 1995; Harding & Hooper, 1996; Lines et al. 1997). The fourth model tested whether the decline in each variable is best fitted via a continuously augmenting process. The data were also analysed after the early and advanced labour values were removed; however, in this analysis, we tested only the first and third models. In the first three models tested, the pivot points between the two or three segments of the relationship between each variable and time were shifted progressively along the time axis, and the positions giving rise to the minimum residual variation were accepted as providing the best fit to the data.
Volumes, secretion rates and lung weights were expressed per kilogram of body weight. Blood gas, haemoglobin and pH values were compared by one-way ANOVA after binning the data. When a fetus had more than one value in a bin we used the mean value. For a number of comparisons we used the Student's unpaired or paired t test as appropriate. Values are given as means ±s.e.m. Differences were considered significant when P < 0.05.
RESULTS
Of the 12 chronically instrumented fetal sheep that entered the study, labour occurred prematurely in two and one had an obstructed tracheal loop at G142, as confirmed at post-mortem by the presence of a kink in the tracheal tubing close to the fetal neck. Blood gas, acid-base and haemoglobin levels over the final days of gestation and labour indicate that fetuses were healthy throughout the study period, and no significant change was found in any of the variables over the period of study (Table 1). Fetuses reached advanced labour at a mean gestational age of 145.4 ± 0.8 days when the study was terminated and fetal weight was taken (5.1 ± 0.2 kg). Post-mortem examination revealed no abnormality in any of the fetuses. Dry lung weight (3.3 ± 0.1 g (kg body weight)−1) was similar to that reported in an earlier study (Berger et al. 1998).
Table 1.
Respiratory variables during the course of the study, showing data binned according to the timing of each study relative to advanced labour
| Pre-labour > 6 days (n = 3) | Pre-labour 3–6 days (n = 7) | Pre-labour 1–3 days (n = 8) | Early labour (n = 8) | Advanced labour (n = 8) | |
|---|---|---|---|---|---|
| [Hb] (g dl−1) | 11.3 ± 0.3 | 11.3 ± 0.3 | 11.9 ± 0.4 | 12.1 ± 0.6 | 11.7 ± 0.4 |
| pHa | 7.35 ± 0.00 | 7.34 ± 0.01 | 7.35 ± 0.00 | 7.34 ± 0.01 | 7.33 ± 0.01 |
| Sa,O2 (%) | 53.5 ± 4.3 | 55.8 ± 2.1 | 52.6 ± 2.6 | 47.2 ± 2.3 | 43.7 ± 4.9 |
| Pa,O2 (mmHg) | 23.9 ± 2.6 | 24.8 ± 1.3 | 23.9 ± 1.4 | 22.8 ± 1.1 | 21.7 ± 1.3 |
| Pa,CO2 (mmHg) | 53.9 ± 0.8 | 53.9 ± 0.8 | 53.2 ± 0.8 | 55.1 ± 1.0 | 54.6 ± 1.3 |
Values are means ± S.E.M. Note that n signifies the number of fetuses contributing data to each bin. [Hb], haemoglobin concentration; pHa, arterial pH; Sa,O2, arterial saturation; Pa,O2, arterial partial pressure of O2; Pa,CO2, arterial partial pressure CO2.
Early and advanced labour were recognised, according to the definitions outlined in Methods, in all nine animals. However, due to rapid progress, complete experiments in advanced labour were possible in only seven of the nine fetuses studied. In one of the remaining two fetuses, only two lung liquid samples were obtained before the experiment was terminated because of concern that delivery might occur; in this animal we could therefore estimate lung liquid volume (VL) in advanced labour, but not secretion rate (Jv). In the second fetus, no determination of lung liquid volume or Jv was possible during advanced labour. The determination made in early labour preceded that in advanced labour by 15.4 ± 2.7 h.
Lung liquid volume
Between three and eight determinations of VL were made in the nine fetuses studied. These values spanned a period of approximately 8 days before termination of the experiment in advanced labour. The raw data for each fetus are shown in Fig. 2, with values pertaining to each fetus connected by dashed lines. Variation attributable to animals was removed before fitting one of the four models tested. The first model incorporated a plateau level for VL followed by a single slope regression. As can be seen from Table 2, this model accounted for a considerable proportion of the variation in the data, and the pivot point (the position on the time axis at which VL began to decline) providing the best fit to the data was at 37 h before time zero. A significant improvement in the data fit was achieved when a second slope was added to the model, as shown in Fig. 2. The two pivot points giving the best fit to the data were at 70 h and 8 h before time zero. Based upon the regression, the average initial VL was 38.3 ml kg−1 before it declined to 26.4 ml kg−1 in early labour and to 13.8 ml kg−1 in advanced labour. The initial rate of decline of VL at 70 h was 0.19 ml kg−1 h−1, increasing to 1.58 ml kg−1 h−1 over the last 8 h of labour studied. When we tested a model in which VL increased initially before declining to time zero, analysis showed that the slope of the initial rise in VL was not significantly different from zero and this model did not account for more of the variation in the data than the plateau plus two-slope model. When we fitted an exponential model to the data, we found a significant relationship of the form VL = 37.8 - 23.4exp(0.0560)h, where h is the number of hours (with a negative value) before time zero. This model, accounted for less of the variation in the data than the two-slope model, although the difference was not significant (F1,30 = 2.5, n.s.).
Figure 2. Change in lung liquid volume in the last days of gestation and labour.
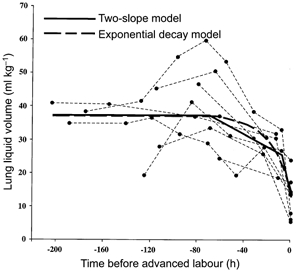
Data points for each animal are joined by dashed lines. The continuous line shows the mean lung liquid volume for all the fetuses, as derived from the regression analysis examining the goodness-of-fit of a two-slope model. The thick dashed line represents the fit achieved with the exponential decay model.
Table 2.
Analysis of variance for lung liquid volume (VL) data in Fig. 2
| Source of variation in VL | Degrees of freedom | Sum of squares | Mean square | F |
|---|---|---|---|---|
| Animals | 8 | 1981 | 248 | 4.5 |
| Time | ||||
| Plateau plus one-slope regression | 2 | 2934 | 1467 | 26.7 |
| Incorporation of second slope | 1 | 306 | 306 | 5.6 |
| Residual | 30 | 1651 | 55 | — |
| Total | 41 | 6872 | — | — |
The plateau plus one-slope regression is significant at the 0.1% level. A significantly improved fit to the data was achieved by the incorporation of a second slope to the model (P < 0.025).
When only the pre-labour data were included in the analysis, we found that the best fit was achieved with a plateau and a one-slope model, with the pivot point 56 h before time zero.
Lung liquid secretion rate
Secretion rates are plotted in Fig. 3 and again the values for individual fetuses are connected by dashed lines. After removal of animal variation, the Jv data were tested with the same four models used to fit the VL data. As seen in Table 3, the plateau plus one-slope model accounted for a considerable proportion of the variation in the data; the pivot point between the plateau and the single slope at which the residual error was minimised was at 33 h before time zero. Addition of a second slope to the model gave a further significant improvement in fit, and the pivot points leaving the minimum residual error were at 180 and 12 h before time zero (see Fig. 3). In the first phase of its decline, mean secretion rate fell from 5.3 to 3.1 ml kg−1 h−1, before decreasing in the second phase to 1.8 ml kg−1 h−1. As was the case for VL, there was no evidence of an increase in Jv before it began to decline. Fitting an exponential model to the data led to the relationship Jv = 4.74 - 3.07 exp(0.0267)h, where h is the number of hours before time zero. This model, however, accounted for significantly less of the variation in the data than the two-slope model (F1,29 = 4.36, P < 0.05).
Figure 3. Change in lung liquid secretion in the last days of gestation and labour.
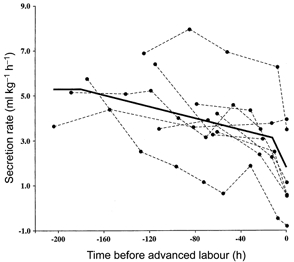
Values for each animal are joined by dashed lines. The continuous line shows the mean for the study group derived from regression analysis.
Table 3.
Analysis of variance for lung liquid secretion (JV) data in Fig. 3
| Source of variation in JV | Degrees of freedom | Sum of squares | Mean square | F |
|---|---|---|---|---|
| Animals | 8 | 90.0 | 11.25 | 10.9 |
| Time | ||||
| Plateau plus one-slope regression | 2 | 40.9 | 20.45 | 19.9 |
| Incorporation of second slope | 1 | 6.75 | 6.75 | 6.6 |
| Residual | 29 | 29.9 | 1.03 | — |
| Total | 40 | 167.6 | — | — |
The plateau plus one-slope regression is significant at the 0.1% level. A significantly improved fit to the data was achieved by the incorporation of a second slope to the model (P < 0.025).
When the secretion rates in early and advanced labour data were removed from the data set, the best fit to the data was produced with a plateau and a one-slope decline to time zero. The pivot point in this reduced data set occurred at 180 h before time zero. It was noteworthy that Jv measured in early labour (2.9 ± 0.8 ml kg−1 h−1, n = 8) was significantly greater than zero (P < 0.05) and that Jv in advanced labour (1.4 ± 0.7 ml kg−1 h−1, n = 7) was not significantly different from zero; these values were not significantly different from one another. Only a single animal manifested reabsorption of lung liquid in our study group, and this animal reabsorbed liquid both in early (-0.4 ml kg−1 h−1) and advanced labour (-0.8 ml kg−1 h−1).
Intra-pulmonary pressure and lung liquid volume
The relationship between lung liquid volume and intra-pulmonary pressure, measured immediately on opening the tracheal loop at the start of each experiment, is shown in Fig. 4. Using all values, the calculated regression line (y = 2.1x+ 32.2; r = 0.76) predicted a lung volume of 32.2 ml kg−1 at zero pressure. We also performed a regression analysis using only those data points with a zero or positive intra-pulmonary pressure; that is, we excluded negative values since these appear to fall on the lower limb of what looks like the normal sigmoid pressure-volume curve of the lung, except that the upper plateau is absent (Fig. 4). In this case, shown by the line in Fig. 4, the calculated regression (y = 4.1x+ 28.8; r = 0.64, P < 0.001) predicted a lung liquid volume of 28.8 ml kg−1 at zero intra-pulmonary pressure, rising by 4.1 ml kg−1 (cmH2O)−1. In all advanced labour studies where we determined intra-pulmonary pressure (n = 6), we measured values of zero and below. In three of the seven measurements made in early labour, the intra-pulmonary pressure was negative. One negative intra-pulmonary pressure value was obtained in a fetus 61 h before time zero. When the values during labour were excluded, the mean intra-pulmonary pressure in the fetuses studied was 2.2 ± 0.5 cmH2O.
Figure 4. Relationship between lung liquid volume and intra-pulmonary pressure.
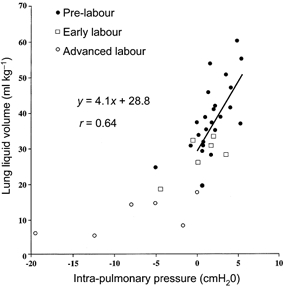
The regression line shown was calculated using lung liquid volumes associated with intra-pulmonary pressures of zero and above.
DISCUSSION
Our key findings are that lung liquid volume and secretion rate do not increase over the last days of gestation, but instead both begin to decline well before the onset of labour. Our analysis shows that for the decline in both lung liquid volume and secretion rate, a plateau plus two-segment model gives a closer fit to the data than an exponential decay model. The difference between the linear and exponential models was not significant for lung liquid volume, but it was significant for secretion rate. Our results indicate that secretion rate begins its decline before lung liquid volume, and the accelerated decline in both variables near the end of our study period also occurs first in secretion rate.
We can now consider whether the mathematical models that fit our data provide any insight into the mechanisms that control lung liquid volume and secretion near term. For lung liquid volume, the (statistical) equivalence of the two-segment and exponential decay models indicates that clearance of liquid from the lung could involve two discrete events, the first of which is switched on 3 days before labour and the second within labour, or it could involve a single continuously accelerating process. Although the two-segment model offers the advantage that it points to discrete times at which clearance processes begin and accelerate, further work is required before a choice can be made between the two models that fit our data. The better fit to the secretion rate data by the plateau plus two-segment model suggests that a process is set in train well before the onset of labour that either gradually reduces secretion rate, or activates an absorptive mechanism. Published data support the idea that transport of Na+ ions across the pulmonary epithelium begins in late gestation (Olver et al. 1986), since amiloride treatment of the near-term fetus leads to an increased rate of secretion. Our findings are consistent with the level of Na+ transport increasing as term approaches so that the absorptive and secretory processes are almost in balance once the fetus enters advanced labour. Such a possibility is supported by evidence that Na+ channels on the apical (luminal) side of the pulmonary epithelium increase in number with the approach of delivery (see Baines et al. 2000).
We found that reabsorption of lung liquid occurred in only one of our fetuses, whereas an earlier study showed that reabsorption of liquid occurs during the last 1-2 h before delivery (Brown et al. 1983). In the current series of experiments, however, it is likely that observations were terminated before circulating adrenaline levels had risen sufficiently to initiate Na+ transport across the pulmonary epithelium (Olver et al. 1986). Nevertheless, the important insight generated by our results is that even before active reabsorption commences a very large decline in lung liquid volume has taken place. On average, we found that lung liquid volume falls by approximately 25 ml kg−1 between G140 and advanced labour, a volume that represents the major part of the 75 % decline in lung liquid volume shown to occur before birth in previous work (Berger et al. 1998).
This and earlier studies (Brown et al. 1983; Berger et al. 1998) enable us to define the time course of changes in lung liquid volume in the days immediately before birth. Initially, volume declines relatively slowly but the process occupies a period of more than 2 days leading up to early labour, resulting in the removal of a large volume of liquid. During this initial phase, the two-slope model indicates that lung liquid volume falls by approximately 30 %, from 38.3 ml kg−1 several days before birth to 26.4 ml kg−1 in early labour; the exponential decay model predicts a fall from 37.8 to 22.8 ml kg−1 over the same period. A more rapid fall between early and advanced labour then takes lung liquid volume to 13.8 ml kg−1 (two-slope model), or to 14.4 ml kg−1 (exponential decay model). Although we terminated the study at this point, it is highly likely that liquid clearance would have continued had we not done so. Indeed, Berger et al. (1998) showed that by the time of birth, the mean volume of liquid in the fetal lung was only 6.8 ml kg−1, just half that at termination of the current study. We suggest that the final step in the clearance of lung liquid involves active reabsorption, a process that has been shown to be stimulated by the catecholamine surge which occurs just before the end of labour (Brown et al. 1983). Reabsorption of liquid from the lung is driven by active Na+ transport which then continues to play a dominant role in keeping the airspace dry throughout postnatal life (Ramsden et al. 1992).
Comparison with earlier work
Our results confirm an earlier demonstration that the volume of liquid in the fetal lung starts to decline before the onset of labour (Dickson et al. 1986). This report has been criticised because labour was not monitored with a uterine electromyogram, leading to the suggestion that fetuses may already have been in labour when the decline in lung liquid volume was first observed (Lines et al. 1997). No such criticism can be made of the current study, since uterine EMG and amniotic pressure were monitored continuously from G142. Even acknowledging the difficulty of precisely identifying the onset of labour, it is unlikely that labour had begun when we first observed a decline in lung liquid volume, since this would require labour to have begun up to 70 h before we terminated the study in advanced labour.
Our results run counter to the conclusion, based on a compilation of results from several sets of experiments (Hooper et al. 1988; Hooper & Harding, 1989, 1990, 1995; Wallace et al. 1990, 1995, 1996a, b; Miller et al. 1993), that lung liquid volume continues to rise until the day labour begins. By this time, the volume of liquid in the lung reportedly reaches a mean level as high as 50 ml kg−1 (Hooper & Harding, 1995; Harding & Hooper, 1996; Lines et al. 1997). We suggest that two methodological problems could account for the disparate results. The first problem arises from the use of Blue Dextran as the impermeant tracer whose dilution is used to calculate volume in studies reporting a continuous increase in lung liquid volume until labour. This molecule has a high affinity for proteins (Dean & Watson, 1979) and binds to the pulmonary epithelium in substantial quantities (Pfister et al. 1999). Such binding, by reducing the quantity of free Blue Dextran in lung liquid, is likely to result in an overestimation of lung liquid volume. We have also shown that the magnitude of this overestimation may increase with gestation, as considerably more Blue Dextran binds at G140 than at G125 (Pfister et al. 1999).
A second potential source of error is one that is common to virtually all studies of lung liquid volume in the chronically catheterised fetal sheep. An exteriorised loop of tubing is implanted in the trachea to allow lung liquid to be sampled and its volume determined (Adamson et al. 1975). This tubing must be long and of narrow bore, its diameter being set by that of the trachea and a thick wall being needed to avoid kinking. Unavoidably, therefore, the tubing increases resistance to the outflow of liquid through the trachea, and may increase the distending pressure and volume of liquid in the lung. The presence of highly viscous mucous in the loop would accentuate this effect, and mucous masses are commonly seen flowing in the tubing when liquid is drained from the lung at the start of experiments. The potential for the tracheal loop to increase lung liquid volume substantially is evident from Fig. 4, which shows that in the positive pressure range the compliance of the lung is 4.1 ml kg−1 (cmH2O)−1, a value close to that reported earlier (Dickson & Harding, 1991). Consistent with our view that the tracheal loop leads to over-inflation of the lung, the mean lung liquid volume in the present study at G140 (35.1 ± 3.1 ml kg−1) shows a trend to higher values (P = 0.07) than reported in 10 fetuses of the same age (28.2 ± 1.8 ml kg−1) in an earlier study which did not employ a tracheal loop (Berger et al. 1998).
Lung liquid secretion and reabsorption
Lung liquid secretion rate began to decline well in advance of labour, thereby confirming the conclusions of two earlier studies (Kitterman et al. 1979; Dickson et al. 1986). Relatively low rates of liquid secretion were reported in another study in the late gestation sheep and, as outlined earlier, addition of amiloride to the lung lumen resulted in a significant increase in liquid secretion (Olver et al. 1986). Thus secretion may decline near term as a result of a progressive increase in active Na+ transport competing with the Cl− secretory mechanism.
Negative intra-pulmonary pressure in labour
For the first time we demonstrate a large negative intra-pulmonary pressure in advanced labour, a finding that demonstrates lung volume is less than functional residual capacity, and also that liquid must not be able to enter the lung from the amniotic cavity; the mechanism by which amniotic fluid is excluded from the lung was demonstrated almost 20 years ago, when it was shown that the larynx acts as a one-way valve allowing only liquid outflow under normal circumstances (Brown et al. 1983). The finding of a negative intra-pulmonary pressure near the end of labour may in part explain reports that the first inspiration in vaginally delivered human infants does not require diaphragmatic contraction (Geubelle et al. 1959; Karlberg, 1960; Karlberg et al. 1962a, b). Karlberg proposed that ‘vaginal squeeze’, applied to the fetal thorax during the expulsion phase of delivery, reduces lung volume below functional residual capacity. In his conception, elastic recoil of the chest wall, once it is delivered from the birth canal, causes passive inflow of air. Our data show that a negative intra-pulmonary pressure is already established in advanced labour before the thorax enters the birth canal. It is also likely that the liquid absorption seen in the last 1-2 h of labour (Brown et al. 1983) reduces intra-pulmonary pressure to levels below those seen in this study.
It is interesting to consider the extent to which the observed negative intra-pulmonary pressure in labour would affect lung liquid secretion or reabsorption. Active reabsorption of liquid, driven by Na+ transport in the last hours of labour, would progressively reduce lung volume, increase chest wall recoil, and result in an increasingly negative intra-pulmonary pressure. The increasingly negative intra-pulmonary pressure established during labour would, however, favour passive movement of liquid across the epithelium and into the lung lumen and potentially counterbalance active absorptive mechanisms. Rather than reduce the luminal space to dryness, a balance would be achieved at which active reabsorption matched passive trans-epithelial inflow of liquid, with no further net liquid movement.
Resistance in tracheal loop catheter
The reported compliance of the liquid-filled fetal lung is high, with values around 5 ml (cmH2O)−1 kg−1 (Dickson & Harding, 1991), or slightly less according to our results. Any obstruction in the tracheal loop could therefore give rise to a large volume increase in the lung while causing only a slow build-up of pressure that would act to overcome the obstruction. In an attempt to assess whether an obstruction was present in the tracheal loop, we measured intra-pulmonary pressure at the start of each experiment. Two potential sources of error should be considered in this measurement. First, all intra-pulmonary pressure values were referenced to amniotic fluid pressure measured through an open-ended catheter. If the tip of this catheter did not lie in a liquid pool that was contiguous within the amniotic sac, as could occur with low amniotic fluid levels near term, amniotic pressure might not be correctly determined. Second, the measurement of intra-pulmonary pressure was taken from the caudal part of the loop at the start of the experiment, and if an obstruction existed along this tube we might have underestimated intra-pulmonary pressure. In retrospect, we should have determined intra-pulmonary pressure after eliminating any obstructing plug by vigorous aspiration and flushing of the tubing. Even so, our results show that in some cases the tracheal loop did indeed raise pressure in the lung, with some values in the 4-5 cmH2O range, considerably above the average of 2-3 cmH2O (Vilos & Liggins, 1982; Dickson & Harding, 1991).
mechanisms underlying the decline in vL during labour
Having established that VL declines over the course of the last days of gestation, and that the process begins well before the start of labour, and in the absence of active liquid reabsorption, the key issue is what brings it about. There is considerable evidence that there is a radical alteration of fetal activity in labour, characterised by a cessation of breathing, the appearance of long-lasting contractions of the diaphragm, and activity resembling coughing (Berger et al. 1986). One possibility is that lung liquid volume declines in labour directly as a result of the loss of fetal breathing movements, since blockade of fetal breathing has been reported to lead to a 24 % decline in lung liquid volume (Miller et al. 1993). However, the reported decline in volume was measured 2 days after the institution of blockade and the relevance of this finding to labour is uncertain. During the course of the present study, we observed long-lasting and simultaneous contractions of thoracic, abdominal and diaphragm muscles during labour. As we have suggested, activity in fetal muscles could expel liquid from the fetal lung (Berger et al. 1998), so long as its net effect is to cause an increase in intrapleural pressure. What elicits these trunk muscle contractions is unknown. Levels of cortisol and many other circulating hormones are known to rise during labour, and one or more of these agents could trigger the fetus to alter its behaviour. In agreement with an earlier study (Berger et al. 1986), we found no significant hypoxia, hypercapnia or acidosis during labour at a time when fetal lung liquid volume had declined substantially, demonstrating that fetal asphyxia is not involved.
Earlier work demonstrates that Na+ reabsorption, activated by the adrenaline surge of labour, begins to clear liquid from the lung in the last 1-2 h before birth (Brown et al. 1983). The extent to which this mechanism reduces lung liquid volume cannot be decided from our study, but it could be responsible for the decline in volume from the 13.8 ml kg−1 (or 14.4 ml kg−1, according to the exponential decay model) we observed in advanced labour to the level of 6.8 ml kg−1 reported at the end of labour (Berger et al. 1998). It is difficult to envisage how active expulsive efforts by the fetus could achieve this final stage in the decline in lung liquid volume, since it occurs in a lung that is already well below functional residual capacity and in conditions of substantial negative intra-pleural pressure. Although the fall in lung liquid volume that may be attributable to reabsorption is only a small fraction of the total liquid clearance before delivery, previous work indicates that it would have a substantial beneficial effect on postnatal gas exchange (Berger et al. 1996, 2000). Once switched on in labour, the Na+ absorptive mechanism continues to play a crucial role in pulmonary function throughout postnatal life (Ramsden et al. 1992). Its importance is underlined by the respiratory distress experienced by newborn guinea-pigs in which pulmonary Na+ channels are blocked with amiloride (O'Brodovich et al. 1990). Perhaps more tellingly, when the Na+ channel is rendered defective genetically, newborn mice die of respiratory failure within 8 to 40 h (Hummler et al. 1996).
Clinical significance
Our elucidation of the timetable with which lung liquid volume and secretion decline before term delivery emphasises the importance of the last days of gestation in adapting the fetus for postnatal life. The benefits of labour to the newborn are already well illustrated by the higher incidence of respiratory compromise (‘wet lung’) in human infants delivered by Caesarean section with absent (12.4 %) or reduced (5.6 %) labour, compared with a much lower incidence (0.6 %) in infants that deliver vaginally (Hales et al. 1993). The mechanisms that adapt the lung for postnatal life can now be seen to include a prolonged and gradual clearance of lung liquid beginning well before the onset of labour, together with an acceleration of clearance once labour is established. The respiratory hazard that elective Caesarean delivery represents may, therefore, not simply be attributable to the absence of labour, but also to the newborn missing out on a process that clears liquid from the lung over a period of days leading up to labour.
Acknowledgments
This research was supported by the National Health Medical Research Council of Australia (Project Grant 990682), the Swiss National Foundation, Litta Foundation, Ciba-Geigy Jubilee, Glaxo Wellcome, Roche Research Foundation, Ritchie Centre for Baby Health Research and Monash University. We are especially grateful to Mr George Rennie for his expert statistical treatment of our data.
References
- Adamson TM, Brodecky V, Lambert TF, Maloney JE, Ritchie BC, Walker AM. Lung liquid production and composition in the ‘in utero’ foetal lamb. Australian Journal of Experimental Biology and Medical Science. 1975;53:65–75. doi: 10.1038/icb.1975.7. [DOI] [PubMed] [Google Scholar]
- Baines DL, Folkesson HG, Norlin A, Bingle CD, Yuan HT, Olver RE. The influence of mode of delivery, hormonal status and postnatal O2 environment on epithelial sodium channel (ENaC) expression in perinatal guinea-pig lung. Journal of Physiology. 2000;522:147–157. doi: 10.1111/j.1469-7793.2000.t01-2-00147.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger PJ, Horne RSC, Soust M, Walker AM, Maloney JE. Breathing at birth and the associated blood gas and pH changes in the lamb. Respiration Physiology. 1990;82:251–266. doi: 10.1016/0034-5687(90)90039-2. [DOI] [PubMed] [Google Scholar]
- Berger PJ, Kyriakides MA, Smolich JJ, Ramsden CA, Walker AM. Massive decline in lung liquid before vaginal delivery at term in the fetal lamb. American Journal of Obstetrics and Gynecology. 1998;178:223–227. doi: 10.1016/s0002-9378(98)80004-5. [DOI] [PubMed] [Google Scholar]
- Berger PJ, Kyriakides MA, Smolich JJ, Ramsden CA, Walker AM. Influence of prenatal adrenaline infusion on arterial blood gases after Caesarean delivery in the lamb. Journal of Physiology. 2000;527:377–385. doi: 10.1111/j.1469-7793.2000.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger PJ, Smolich JJ, Ramsden CA, Walker AM. Effect of lung liquid volume on respiratory performance after Caesarean delivery in the lamb. Journal of Physiology. 1996;492:905–912. doi: 10.1113/jphysiol.1996.sp021356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger PJ, Walker AM, Horne R, Brodecky V, Wilkinson MH, Wilson F, Maloney JE. Phasic respiratory activity in the fetal lamb during late gestation and labour. Respiration Physiology. 1986;65:55–68. doi: 10.1016/0034-5687(86)90006-x. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Olver RE, Ramsden CA, Strang LB, Walters DV. Effects of adrenaline and of spontaneous labour on the secretion and absorption of lung liquid in the fetal lamb. Journal of Physiology. 1983;344:137–152. doi: 10.1113/jphysiol.1983.sp014929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke IRC, Brodecky V, Berger PJ. Easily-implantable electrodes for chronic recording of electromyogram activity in small fetuses. Journal of Neuroscience Methods. 1990;33:51–54. doi: 10.1016/0165-0270(90)90081-p. [DOI] [PubMed] [Google Scholar]
- Crawford JS. The stages and phases of labour: an outworn nomenclature that invites hazard. Lancet. 1983;2:271–272. doi: 10.1016/s0140-6736(83)90246-5. [DOI] [PubMed] [Google Scholar]
- Crawford JS. The phases and stages of labour. British Journal of Hospital Medicine. 1985;34:32–36. [PubMed] [Google Scholar]
- Davis TA, Gause G, Perks AM, Cassin S. Effects of intravenous saline infusion on fetal ovine lung liquid secretion. American Journal of Physiology. 1992;262:R1117–1120. doi: 10.1152/ajpregu.1992.262.6.R1117. [DOI] [PubMed] [Google Scholar]
- Dean PD, Watson DH. Protein purification using immobilized triazine dyes. Journal of Chromatography. 1979;165:301–316. doi: 10.1016/s0021-9673(00)88187-x. [DOI] [PubMed] [Google Scholar]
- Dickson KA, Harding R. Compliances of the liquid-filled lungs and chest wall during development in the fetal sheep. Journal of Developmental Physiology. 1991;16:105–113. [PubMed] [Google Scholar]
- Dickson KA, Maloney JE, Berger PJ. Decline in lung liquid volume before labour in fetal lambs. Journal of Applied Physiology. 1986;61:2266–2272. doi: 10.1152/jappl.1986.61.6.2266. [DOI] [PubMed] [Google Scholar]
- Geubelle F, Karlberg P, Koch G, Lind J, Wallgren G, Wegelius C. L'aeration du poumon chez le nouveau-né. Biology of the Neonate. 1959;1:169–210. [PubMed] [Google Scholar]
- Hales KA, Morgan MA, Thurnau GR. Influence of labor and route of delivery on the frequency of respiratory morbidity in term neonates. International Journal of Gynaecology and Obstetrics. 1993;43:35–40. doi: 10.1016/0020-7292(93)90271-w. [DOI] [PubMed] [Google Scholar]
- Harding R, Hooper S. Regulation of lung expansion and lung growth before birth. Journal of Applied Physiology. 1996;81:209–224. doi: 10.1152/jappl.1996.81.1.209. [DOI] [PubMed] [Google Scholar]
- Hicks JB. On the contractions of the uterus throughout pregnancy: their physiological effects and their value in the diagnosis of pregnancy. Transactions of the Obstetrical Society of London. 1871;13:216–231. [Google Scholar]
- Hooper SB, Dickson KA, Harding R. Lung liquid secretion, flow and volume in response to moderate asphyxia in fetal sheep. Journal of Developmental Physiology. 1988;10:473–485. [PubMed] [Google Scholar]
- Hooper SB, Harding R. Effect of β-adrenergic blockade on lung liquid secretion during fetal asphyxia. American Journal of Physiology. 1989;257:R705–710. doi: 10.1152/ajpregu.1989.257.4.R705. [DOI] [PubMed] [Google Scholar]
- Hooper SB, Harding R. Changes in lung liquid dynamics induced by prolonged fetal hypoxemia. Journal of Applied Physiology. 1990;69:127–135. doi: 10.1152/jappl.1990.69.1.127. [DOI] [PubMed] [Google Scholar]
- Hooper SB, Harding R. Fetal lung liquid: a major determinant of the growth and functional development of the fetal lung. Clinical and Experimental Pharmacology and Physiology. 1995;22:235–247. doi: 10.1111/j.1440-1681.1995.tb01988.x. [DOI] [PubMed] [Google Scholar]
- Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in αENaC-deficient mice. Nature Genetics. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- Karlberg P. The adaptive changes in the immediate postnatal period, with particular reference to respiration. The Journal of Pediatrics. 1960;56:585–604. doi: 10.1016/s0022-3476(60)80332-0. [DOI] [PubMed] [Google Scholar]
- Karlberg P, Adams FH, Geubelle F, Wallgren G. Alteration of the infant's thorax during vaginal delivery. Acta Obstetricia et Gynecologica Scandinavica. 1962a;41:223–229. doi: 10.3109/00016346209158099. [DOI] [PubMed] [Google Scholar]
- Karlberg P, Cherry RB, Escardó FE, Koch G. Respiratory studies in Newborn Infants. II. Acta Paediatrica. 1962b;51:121–136. doi: 10.1111/j.1651-2227.1962.tb08666.x. [DOI] [PubMed] [Google Scholar]
- Kitterman JA, Ballard PL, Clements JA, Mescher JE, Tooley WH. Tracheal fluid in fetal lambs: spontaneous decrease prior to birth. Journal of Applied Physiology. 1979;47:985–989. doi: 10.1152/jappl.1979.47.5.985. [DOI] [PubMed] [Google Scholar]
- Lines A, Hooper SB, Harding R. Lung liquid production rates and volumes do not decrease before labour in healthy fetal sheep. Journal of Applied Physiology. 1997;82:927–932. doi: 10.1152/jappl.1997.82.3.927. [DOI] [PubMed] [Google Scholar]
- Lumbers ER, Smith FG, Stevens AD. Measurement of net transplacental transfer of fluid to the fetal sheep. Journal of Physiology. 1985;364:289–299. doi: 10.1113/jphysiol.1985.sp015745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AA, Hooper SB, Harding R. Role of fetal breathing movements in control of fetal lung distension. Journal of Applied Physiology. 1993;75:2711–2717. doi: 10.1152/jappl.1993.75.6.2711. [DOI] [PubMed] [Google Scholar]
- Morrison JJ, Rennie JM, Milton PJ. Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective caesarean section. British Journal of Obstetrics and Gynaecology. 1995;102:101–106. doi: 10.1111/j.1471-0528.1995.tb09060.x. [DOI] [PubMed] [Google Scholar]
- O'Brodovich H, Hannam V, Seear M, Mullen JBM. Amiloride impairs lung water clearance in newborn guinea pigs. Journal of Applied Physiology. 1990;68:1758–1762. doi: 10.1152/jappl.1990.68.4.1758. [DOI] [PubMed] [Google Scholar]
- Olver RE, Ramsden CA, Strang LB, Walters DV. The role of amiloride-blockable sodium transport in adrenaline-induced lung liquid reabsorption in the fetal lamb. Journal of Physiology. 1986;376:321–340. doi: 10.1113/jphysiol.1986.sp016156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister RE, Ramsden CA, Neil HL, Kyriakides MA, Berger PJ. Errors in estimating lung liquid volume in fetal lambs using radiolabeled serum albumin and blue dextran. Journal of Applied Physiology. 1999;87:2366–2374. doi: 10.1152/jappl.1999.87.6.2366. [DOI] [PubMed] [Google Scholar]
- Ramsden CA, Markiewicz M, Walters DV, Gabella G, Parker KA, Barker PM, Neil HL. Liquid flow across the epithelium of the artificially perfused lung of fetal and postnatal sheep. Journal of Physiology. 1992;448:579–597. doi: 10.1113/jphysiol.1992.sp019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilos GA, Liggins GC. Intrathoracic pressures in fetal sheep. Journal of Developmental Physiology. 1982;4:247–256. [PubMed] [Google Scholar]
- Wallace MJ, Hooper SB, Harding R. Regulation of lung liquid secretion by arginine vasopressin in fetal sheep. American Journal of Physiology. 1990;258:R104–111. doi: 10.1152/ajpregu.1990.258.1.R104. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Hooper SB, Harding R. Effects of elevated fetal cortisol concentrations on the volume, secretion, and reabsorption of lung liquid. American Journal of Physiology. 1995;269:R881–887. doi: 10.1152/ajpregu.1995.269.4.R881. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Hooper SB, Harding R. Role of the adrenal glands in the maturation of lung liquid secretory mechanisms in fetal sheep. American Journal of Physiology. 1996a;270:R33–40. doi: 10.1152/ajpregu.1996.270.1.R33. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Hooper SB, McCrabb GJ, Harding R. Acidaemia enhances the inhibitory effect of hypoxia on fetal lung liquid secretion in sheep. Reproduction, Fertility and Development. 1996b;8:327–333. doi: 10.1071/rd9960327. [DOI] [PubMed] [Google Scholar]


