Abstract
During strenuous exercise lactic acid accumulates producing a reduction in muscle pH. In addition, exercise causes a loss of muscle K+ leading to an increased concentration of extracellular K+ ([K+]o). Individually, reduced pH and increased [K+]o have both been suggested to contribute to muscle fatigue.
To study the combined effect of these changes on muscle function, isolated rat soleus muscles were incubated at a [K+]o of 11 mm, which reduced tetanic force by 75 %. Subsequent addition of 20 mm lactic acid led, however, to an almost complete force recovery. A similar recovery was observed if pH was reduced by adding propionic acid or increasing the CO2 tension.
The recovery of force was associated with a recovery of muscle excitability as assessed from compound action potentials. In contrast, acidification had no effect on the membrane potential or the Ca2+ handling of the muscles.
It is concluded that acidification counteracts the depressing effects of elevated [K+]o on muscle excitability and force. Since intense exercise is associated with increased [K+]o, this indicates that, in contrast to the often suggested role for acidosis as a cause of muscle fatigue, acidosis may protect against fatigue. Moreover, it suggests that elevated [K+]o is of less importance for fatigue than indicated by previous studies on isolated muscles.
Contracting muscles lose K+ and plasma [K+] may reach 10 mm during strenuous exercise (Sréter, 1963; Hermansen et al. 1984; Hallen et al. 1994; Sejersted & Sjogaard, 2000). Close to the muscle fibres, in the interstitium, the increase in K+ may be even larger (Hnik et al. 1976; Juel et al. 2000; Green et al. 2000). Based on experiments on isolated, resting muscles, a [K+]o of 10 mm or more leads to a loss of force (see e.g. Bouclin et al. 1995; Cairns et al. 1997) and the work-induced increase in interstitial [K+] has, therefore, been suggested to contribute to muscle fatigue (for review, see Sejersted & Sjogaard, 2000). Simultaneous with the increase in [K+]o, blood lactate may increase to 20 mm or more during fatiguing work (Fitts, 1994). Since both the lactate ion (Hogan et al. 1995) and reduced pH can impair force production in isolated muscle, exercise-induced accumulation of lactic acid and ensuing reduction in muscle pH are also suggested to contribute to fatigue (for review, see Fitts, 1994), and although the role of reduced muscle pH has been questioned (see e.g. Lamb & Stephenson, 1994; Pate et al. 1995; Westerblad et al. 1997; Posterino & Fryer, 2000) it is still by many considered to be important in muscle fatigue during intensive exercise (Fitts, 1994).
In this study the combined effect of elevated [K+]o and reduced pH on muscle function was examined on isolated rat soleus muscles. In contrast to the often suggested role for acidosis as a cause of muscle fatigue, it is shown that in muscles where force was depressed by high [K+]o, acidification by lactic acid produced a pronounced recovery of force. Since intense exercise is associated with increased [K+]o, this indicates that acidosis may protect against fatigue rather than being a cause of fatigue.
METHODS
Animal handling and muscle preparation
All handling and use of animals complied with Danish animal welfare regulations. All experiments were carried out using soleus muscles from 4-week-old Wistar rats weighing 60-70 g (own breed). The rats were fed ad libitum and were maintained at constant temperature (21 °C) and day length (12 h). Animals were killed by decapitation, and muscles were isolated with the proximal end attached to the bone and the distal end with an intact tendon, as previously described (Overgaard & Nielsen, 2001). For experiments with stimulation via the motor nerve, approximately 10 mm of the nerve was left attached to the muscle. The standard incubation medium was Krebs-Ringer bicarbonate buffer containing (mm): 122 NaCl, 25 NaHCO3, 2.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.3 CaCl2 and 5.0 d-glucose. If not otherwise stated the buffer was maintained at 30 °C and equilibrated with a mixture of 95 % O2-5 % CO2 throughout the experiment (pH 7.4). In K+-enriched buffers an equivalent amount of Na+ was omitted to maintain isoosmolarity. To avoid large excursions in pH, buffers containing l-lactic or propionic acid were equilibrated for 20 min with 95 % O2-5 % CO2 before use. In all experiments, the muscles were mounted isometrically at optimal length in the standard incubation medium at least 30 min before starting the experiment. Other mixtures of O2 and CO2 were made using a gas mixing pump (type M201, Wösthoff oHG, Germany).
Isometric force measurement and electrical stimulation
Before starting the experiment, muscles were adjusted to optimal length for force production. Isometric force development was measured using a force displacement transducer as previously described (Overgaard & Nielsen, 2001), and recorded with a chart recorder and/or digitally on a computer. In general, contractions were evoked via field stimulation using constant voltage pulses (10 V) applied through two platinum wire electrodes passing current over the central part of the muscle. If not otherwise noted pulses of 1 ms duration were used. In experiments where M-waves were measured, contractions were evoked via nerve stimulation using fixed current pulses (5 mA, 0.2 ms duration) applied through a glass suction electrode (Overgaard & Nielsen, 2001). The pulses were found to be supramaximal for stimulation of the motor nerve without producing any direct stimulation of muscle fibres. If not otherwise stated, contractions were elicited every 10 min throughout the experiments using 30 Hz pulse trains of 1.5 or 2 s duration which assured full development of tetanic force also in the modified buffers.
Recordings of membrane potential and muscle compound action potentials (M-waves)
The method used for measuring M-waves was as previously described (Overgaard & Nielsen, 2001). In brief, muscles were stimulated via the motor nerve through a stimulus isolator (ISU 165, Cibertec, Spain). Unipolar M-wave signals were recorded from a circular silver electrode with a recording area of 0.79 mm2 placed in close contact with the muscle between the innervation zone and the tendon. The diameter of the electrode was approximately half the width of the muscle, allowing a relatively large number of fibres to contribute to the M-wave recordings. The resting membrane potential of surface muscle fibres was measured by standard electrophysiological technique using glass microelectrodes (Overgaard & Nielsen, 2001).
Ca2+ influx, Ca2+ content and Na+-K+ pump activity
Ca2+ influx and Ca2+ content were measured as previously described (Everts & Clausen, 1986). The activity of the Na+-K+ pump was expressed as the ouabain-sensitive K+ influx calculated from measurements of the ouabain-sensitive unidirectional 86Rb+ influx as previously described (Andersen & Clausen, 1993). In short, muscles receiving no electrical stimulation were incubated for 10 min in buffer with or without 10−3m ouabain, then 10 min in buffer containing 0.1 μCi 86Rb+ ml−1, and finally washed four times for 15 min at 0 °C in Na+-free non-radioactive Tris-sucrose buffer (pH 7.4).
Intracellular pH
Muscles were mounted on a myograph for use on a microscope (Leica DM-IRB) and were loaded with 20 μm 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF AM) for 30 min and then washed. To measure pHi, the preparation was excited alternately with wavelengths of 495 and 450 nm from a 75 W xenon lamp that led into a monochromator (Zeiss, M4, GII). The emission from the muscle was collected by the microscope, led through a bandpass filter (530-585 nm), and picked up by a photomultiplier system (PTI). Data were stored on a computer, and the ratio of emission at the two excitation wavelengths (495/440 nm) was calculated after subtracting the background fluorescence. The emission ratio was calibrated to pHi by the high K+-nigericin technique (Thomas et al. 1979) and the data were fitted by linear regression (r2 > 0.94 for all calibrations). To reduce dye bleaching, measurements of pHi were made for only 1 s every 30 s during experiments. The presented values for muscle pH in the various buffers were recorded after at least 30 min incubation in the given buffer.
Statistics
All data are expressed as means ±s.e.m. The statistical significance of difference between two groups was ascertained using Student's two-tailed t test for non-paired observations.
RESULTS
To explore the combined effect of elevated [K+]o and lactic acid acidosis on contractile function, buffer containing 20 mm lactic acid was added to isolated muscles in which force had been depressed by exposure to elevated [K+]o. Figure 1 shows that incubation at a [K+]o of 11 mm for 90 min reduced tetanic force to 25 % of the control force determined at 4 mm K+. Subsequent addition of 20 mm lactic acid led to an almost complete recovery of force that was maintained for at least 50 min. Moreover, adding lactic acid at the same time as [K+]o was increased completely prevented the reduction in force. Similar effects of lactic acid were observed on twitch force (data not shown).
Figure 1. Effect of 20 mm lactic acid on tetanic force in rat soleus muscles exposed to a [K+]o of 11 mm.
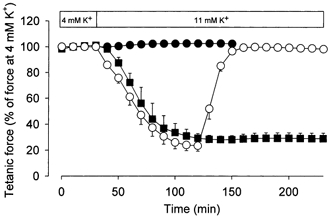
[K+]o was increased to 11 mm at time 30 min. ▪, controls, n = 2; •, lactic acid added together with the increase in [K+]o, n = 6; ○, lactic acid added after 90 min at 11 mm K+, n = 6. Muscles were stimulated tetanically every 10 min using 30 Hz pulse trains of 1.5 s duration and a pulse duration of 0.2 ms. Data are means and s.e.m.
In muscles excited directly with field stimulation after complete blockage of the neuro-muscular junction by 10−5m tubocurarine (Overgaard & Nielsen, 2001), the force recovery induced by the addition of lactic acid (from 32 ± 5 to 85 ± 9 % of control force, n = 4) was similar to the recovery seen in control muscles (Fig. 2). This shows that the recovery of force was related to an effect of lactic acid on the muscle fibres themselves rather than to an effect on the motor nerve.
Figure 2. Effect on force and intracellular pH of 20 mm lactic acid, 20 mm propionic acid or an increase in CO2 from 5 to 23 %.
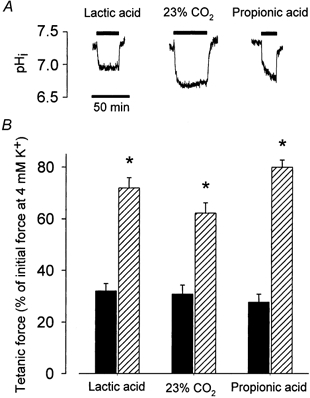
A, representative traces showing changes in pHi in muscles exposed to lactic acid, propionic acid or 23 % CO2 as indicated by the bars. B, tetanic force in muscles incubated at 12.5 mm K+ for 70 min (▪) and after a further 50 min incubation in buffer containing both 12.5 mm K+ and the indicated acids ( ). Muscles were stimulated tetanically every 10 min using 30 Hz pulse trains of 2 s duration and a pulse duration of 1 ms. Data show means and s.e.m. from 6-12 muscles. * Force before and after indicated addition is significantly different (P < 0.001).
). Muscles were stimulated tetanically every 10 min using 30 Hz pulse trains of 2 s duration and a pulse duration of 1 ms. Data show means and s.e.m. from 6-12 muscles. * Force before and after indicated addition is significantly different (P < 0.001).
The presence of 20 mm lactic acid reduced buffer pH by 0.64 ± 0.03 units (from 7.44 ± 0.02 to 6.80 ± 0.02, n = 8). Figure 2A shows that lactic acid also produced a reduction in intracellular pH (pHi) that was maintained as long as the acid was present. In 20 muscles the reduction in pHi was 0.39 ± 0.04 units (from 7.28 ± 0.05 to 6.89 ± 0.06). To distinguish whether the recovery of force at high [K+]o was related to the reductions in pH or, perhaps, to a metabolic effect of lactic acid (Van Hall, 2000), two series of experiments were made. In one series, muscles at standard pH were exposed to 12.5 mm K+ for 70 min whereafter the concentration of glucose in the buffer was increase from the standard 5 mm to 20 mm. In contrast to lactic acid, increased glucose concentrations had no effect on muscle force (n = 4, data not shown). In the other series of experiments, the effect of lactic acid on force and pH was compared to the effect of 20 mm propionic acid or an increase in CO2 from 5 to 23 %. Both acids led to maintained reductions in pHi (Fig. 2A) and in buffer pH that were of similar size as with 20 mm lactic acid (the reduction in pHi and buffer pH, respectively, were 0.32 ± 0.08 and 0.66 ± 0.04 (n = 4) with proprionic acid, and 0.39 ± 0.04 and 0.56 ± 0.03 (n = 4) with CO2). Figure 2B shows that also the force recovery produced by the two acids was of the same magnitude as with lactic acid.
Intracellular acidification of mammalian muscle has been shown to reduce the Ca2+ sensitivity of the contractile filaments, producing a reduction in tetanic force (Fitts, 1994). The effect is, however, sensitive to temperature and almost non-existent at 37 °C (Pate et al. 1995; Westerblad et al. 1997; Bruton et al. 1998). In our experiments, addition of 20 mm lactic acid to muscles incubated at 4 mm K+ and at 30 °C did not inhibit force but actually produced a minor albeit significant increase in tetanic force (by 8 ± 1 %, n = 4, P < 0.05). Other experiments with muscles at 11 mm K+ showed that at 37 °C the addition of lactic acid also produced a recovery of tetanic force (from 40 ± 8 to 81 ± 7 % of the control force, n = 4). Experiments where the calcium handling of resting muscles was examined showed that 30 min incubation with 20 mm lactic acid had no effect on the unidirectional Ca2+ influx or on total muscle Ca2+ content (Ca2+ influx was 2.3 ± 0.2 and 2.7 ± 0.2 nmol (g wet wt)−1 min−1 with and without lactic acid, respectively, whereas Ca2+ content was 0.71 ± 0.03 and 0.64 ± 0.03 μmol (g wet wt)−1, respectively, n = 6, P > 0.1).
There is substantial evidence that in muscles depressed by elevated [K+]o, stimulation of the electrogenic Na+-K+ pump by, for example, hormones can cause considerable recovery of force (Nielsen et al. 1998; Overgaard et al. 1999). To examine a possible role for the Na+-K+ pump in the force recovery produced by low pH, we determined the effect of 20 mm lactic acid on the ouabain-sensitive uptake of K+. In control muscles the uptake was 241 ± 41 nmol (g wet wt)−1 min−1, which was not significantly different from the uptake in muscles exposed to 20 mm lactic acid (196 ± 34 nmol (g wet wt)−1 min−1, 6 vs. 6 muscles, P > 0.1). Moreover, even after 30 min incubation with 10−3m ouabain, addition of 20 mm lactic acid produced an around 2-fold increase in tetanic force in muscles incubated at 10 mm K+ (n = 4). Thus, the recovery of force by lactic acid could not be related to stimulation of the Na+-K+ pump.
The force-depressing effect of elevated [K+]o is most likely to be related to depolarisation of the muscle fibres leading to a reduction in the amplitude of action potentials and, eventually, to conduction failure (Cairns et al. 1995, 1997; Overgaard et al. 1999; Sejersted & Sjogaard, 2000). This loss of excitability can be seen in Fig. 3, where the area of the compound action potential (M-wave) is shown before and after elevation of [K+]o to 10 mm. In agreement with other studies (Overgaard et al. 1999; Overgaard & Nielsen, 2001) the reduction in force was well correlated to a concomitant reduction in M-wave area that was associated with an increased width but reduced amplitude of the M-wave. To investigate whether the recovery of force induced by lactic acid was related to improved excitability of the muscle, we examined the effect of lactic acid on M-waves in muscles exposed to high [K+]o. To minimise stimulation artifacts, the muscles were excited via the motor nerve. Compared to direct field stimulation, excitation via the nerve leads to more exacerbated effects of elevated [K+]o on force (Nielsen et al. 1998; Overgaard & Nielsen, 2001) and for that reason only 10 mm K+ was used in these experiments. As shown in Fig. 3, the recovery of force produced by lactic acid coincided with an almost complete recovery of M-wave area. In muscles incubated at 10 mm K+, addition of 20 mm lactic acid only produced a small and non-significant increase in the membrane potential (from −62 ± 3 to −64 ± 4 mV in 40 min, n = 4 muscles), making a repolarisation an unlikely cause for the recovery of excitability.
Figure 3. Effect of high [K+]o and lactic acid on M-wave and contractile properties.
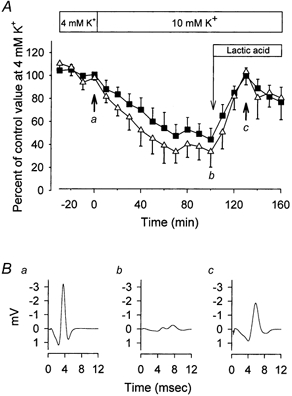
After determination of control values, [K+]o was increased to 10 mm K+ and after 100 min 20 mm lactic acid was introduced, as indicated. Muscle contractions were elicited via the motor nerve using a suction electrode. A, time course of M-wave area and tetanic force. ▪, tetanic force; ▵, M-wave area. Data show means and s.e.m. from 4 muscles. B, representative M-wave traces from a single muscle obtained at the time points indicated by a, b, and c in A.
To study further the effect of reduced pH on muscle function, the effect of various K+ concentrations on force was determined in control muscles and in muscles exposed to 20 mm lactic acid or increased CO2. Figure 4 shows that in control muscles the tetanic force starts decreasing at [K+]o values above 8 mm and at a [K+]o of around 11 mm, force is reduced by 50 %. When pH was lowered by lactic acid or CO2, [K+]o had to be increased to more that 10 mm to produce any force reduction and to 13 mm to reduce force by 50 %. Thus, low pH produced an around 2 mm right-shift in the relation between [K+]o and tetanic force, making the muscles less sensitive to high [K+]o. The effect of lactic acid on force at high [K+]o depended on the concentration of the acid. Thus, in muscles depressed by 12.5 mm K+, 10 mm lactic acid (buffer pH 7.10 ± 0.04) increased force from 34 ± 2 to 48 ± 3 % (n = 16) of control force whereas 20 mm lactic acid increased force to 83 ± 3 % (n = 12).
Figure 4. Effect of lactic acid or increased CO2 tension on the relation between steady state tetanic force and [K+]o.
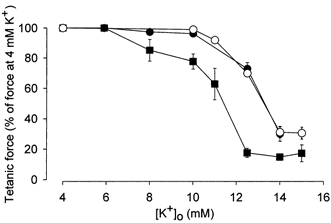
Muscles were incubated at different [K+]o values until a steady force level was obtained. ▪, control muscles, buffer pH 7.4 (n = 4–6); ○, 20 mm lactic acid added, buffer pH 6.82 (n = 8–10); •, CO2 increased to 50 %, buffer pH 6.72 (n = 8). Data show means and s.e.m. At all [K+]o values above 8 mm, the relative force of the muscles at low pH was significantly higher than in the control muscles at the corresponding [K+]o (P < 0.05).
DISCUSSION
In both humans and a large number of animals, strenuous exercise is associated with accumulation of lactic acid and an increased [K+]o (for reviews, see Fitts, 1994; Sejersted & Sjogaard, 2000). During fatiguing work, the concentration of blood lactate may reach 20 mm or more (Fitts, 1994). In conflict with several previous publications (see e.g. Fitts, 1994) but in concord with others (see e.g. Fitts, 1994; Pate et al. 1995; Westerblad et al. 1997; Bruton et al. 1998), the present study failed to demonstrate any inhibitory effect on muscle contractility of even 20 mm lactic acid.
More importantly, this study also shows that in muscles where force and excitability are depressed by high [K+]o, lactic acid produces a pronounced recovery of force. A recovery of force could also be obtained by acidifying the muscle fibres using CO2 or propionic acid. In contrast, 20 mm glucose failed to induce a recovery of force. These findings indicate that the effect of lactic acid was related to the acidification of the muscles rather than to a possible metabolic effect (Van Hall, 2000). The pH of the muscles in the presence of 20 mm lactic acid was similar to the values for muscle pH observed after intensive exercise (Sjøgaard et al. 1985). In contrast, the extracellular pH was somewhat lower than normally seen in intact organisms, and in some experiments a reversal of the pH gradient across the sarcolemma was seen. Despite this, the present data indicate that when muscles are placed in a milieu that with respect to [K+]o mimics the milieu seen by the muscle fibres during intensive exercise, lactic acid acidosis not only does not cause a reduction in force but actually increases force by counteracting the force-depressing effects of high [K+]o. The present study therefore suggests that instead of being a cause of muscle fatigue, accumulation of lactic acid during intensive work actually protects muscles against fatigue.
The force-depressing effect of elevated [K+]o is likely to be related to membrane depolarisation and ensuing slow inactivation of Na+ channels (Ruff et al. 1988; Ruff, 1997). The resulting loss of excitation-induced activation of the muscles could potentially be compensated for by an accumulation of muscle Ca2+ causing an increase in the cytosolic Ca2+ transient in response to stimulation. Lactic acid was, however, without effect on both the Ca2+ influx and the total Ca2+ content of the muscles, making an accumulation of Ca2+ an unlikely explanation for the recovery of force. In contrast, measurement of M-waves demonstrated that the effect of acidification on force in muscles at high [K+]o could be fully explained by a recovery of muscle excitability. Since the recovery of excitability was not caused by repolarisation of the sarcolemma it can be envisaged that it was related to a direct effect on the Na+ channels. It has previously been shown that lowering intracellular pH reduces fast inactivation of Na+ channels (Nonner et al. 1980). Although slow and fast inactivation are structurally distinct processes (Vedantham & Cannon, 1998) it is possible that low pH also reduces slow inactivation perhaps by shifting the steady-state voltage dependence of inactivation towards less negative potentials. Such a shift would make the excitability and, thus, the force production of muscles less prone to inhibition caused by work-induced elevations in [K+]o. Since extracellular K+ is most likely only to accumulate to inhibitory levels at exercise intensities where lactic acid acidosis also occurs, the present study not only suggests that accumulation of lactic acid protects against muscle fatigue but also that exercise-induced increases in [K+]o are of less importance for muscle fatigue than indicated by previous experiments on isolated muscles.
Acknowledgments
This study was supported by The Carlsberg Foundation. The technical assistance of Marianne Stürup-Johansen, Tove Lindahl Andersen and Vibeke Uhre is gratefully acknowledged. Also we thank Dr Torben Clausen for providing valuable comments on an earlier version of this manuscript.
References
- Andersen SL, Clausen T. Calcitonin gene-related peptide stimulates active Na+-K+ transport in rat soleus muscle. American Journal of Physiology. 1993;264:C419–429. doi: 10.1152/ajpcell.1993.264.2.C419. [DOI] [PubMed] [Google Scholar]
- Bouclin R, Charbonneau E, Renaud JM. Na+ and K+ effect on contractility of frog sartorius muscle: implication for the mechanism of fatigue. American Journal of Physiology. 1995;268:C1528–1536. doi: 10.1152/ajpcell.1995.268.6.C1528. [DOI] [PubMed] [Google Scholar]
- Bruton JD, Lannergren J, Westerblad H. Effects of CO2-induced acidification on the fatigue resistance of single mouse muscle fibers at 28 degrees C. Journal of Applied Physiology. 1998;85:478–483. doi: 10.1152/jappl.1998.85.2.478. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Flatman JA, Clausen T. Relation between extracellular [K+], membrane potential and contraction in rat soleus muscle: modulation by the Na+-K+ pump. Pflügers Archiv. 1995;430:909–915. doi: 10.1007/BF01837404. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Hing WA, Slack JR, Mills RG, Loiselle DS. Different effects of raised [K+]o on membrane potential and contraction in mouse fast- and slow-twitch muscle. American Journal of Physiology. 1997;273:C598–611. doi: 10.1152/ajpcell.1997.273.2.C598. [DOI] [PubMed] [Google Scholar]
- Everts ME, Clausen T. Effects of thyroid hormones on calcium contents and 45Ca exchange in rat skeletal muscle. American Journal of Physiology. 1986;251:E258–265. doi: 10.1152/ajpendo.1986.251.3.E258. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiological Reviews. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Green S, Langberg H, Skovgaard D, Bulow J, Kjaer M. Interstitial and arterial-venous [K+] in human calf muscle during dynamic exercise: effect of ischaemia and relation to muscle pain. Journal of Physiology. 2000;529:849–861. doi: 10.1111/j.1469-7793.2000.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallen J, Gullestad L, Sejersted OM. K+ shifts of skeletal muscle during stepwise bicycle exercise with and without β-adrenoceptor blockade. Journal of Physiology. 1994;477:149–159. doi: 10.1113/jphysiol.1994.sp020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansen L, Orheim A, Sejersted OM. Metabolic acidosis and changes in water and electrolyte balance in relation to fatigue during maximal exercise of short duration. International Journal of Sports Medicine. 1984;5:110–115. [Google Scholar]
- Hnik P, Holas M, Krekule I, Kuriz N, Mejsnar J, Smiesko V, Ujec E, Vyskocil F. Work-induced potassium changes in skeletal muscle and effluent venous blood assessed by liquid ion-exchanger microelectrodes. Pflügers Archiv. 1976;362:85–94. doi: 10.1007/BF00588685. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Gladden LB, Kurdak SS, Poole DC. Increased [lactate] in working dog muscle reduces tension development independent of pH. Medicine and Science in Sport and Exercise. 1995;27:371–377. [PubMed] [Google Scholar]
- Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. American Journal of Physiology. 2000;278:R400–406. doi: 10.1152/ajpregu.2000.278.2.R400. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. Journal of Physiology. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen OB, Hilsted L, Clausen T. Excitation-induced force recovery in potassium-inhibited rat soleus muscle. Journal of Physiology. 1998;512:819–829. doi: 10.1111/j.1469-7793.1998.819bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W, Spalding BC, Hille B. Low intracellular pH and chemical agents slow inactivation gating in sodium channels of muscle. Nature. 1980;284:360–363. doi: 10.1038/284360a0. [DOI] [PubMed] [Google Scholar]
- Overgaard K, Nielsen OB. Activity-induced recovery of excitability in K+-depressed rat soleus muscle. American Journal of Physiology. 2001;280:R48–55. doi: 10.1152/ajpregu.2001.280.1.R48. [DOI] [PubMed] [Google Scholar]
- Overgaard K, Nielsen OB, Flatman JA, Clausen T. Relations between excitability and contractility in rat soleus muscle: role of the Na+-K+ pump and Na+/K+ gradients. Journal of Physiology. 1999;518:215–225. doi: 10.1111/j.1469-7793.1999.0215r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. Journal of Physiology. 1995;486:689–694. doi: 10.1113/jphysiol.1995.sp020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino GS, Fryer MW. Effects of high myoplasmic L-lactate concentration on E-C coupling in mammalian skeletal muscle. Journal of Applied Physiology. 2000;89:517–528. doi: 10.1152/jappl.2000.89.2.517. [DOI] [PubMed] [Google Scholar]
- Ruff RL. Sodium channel regulation of skeletal muscle membrane excitability. Annals of the New York Academy of Sciences. 1997;835:64–76. doi: 10.1111/j.1749-6632.1997.tb48618.x. [DOI] [PubMed] [Google Scholar]
- Ruff RL, Simoncini L, Stuhmer W. Slow sodium channel inactivation in mammalian muscle: a possible role in regulating excitability. Muscle and Nerve. 1988;11:502–510. doi: 10.1002/mus.880110514. [DOI] [PubMed] [Google Scholar]
- Sejersted OM, Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiological Reviews. 2000;80:1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G, Adams RP, Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee exercise. American Journal of Physiology. 1985;248:R190–196. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- Sréter FA. Cell water, sodium, and potassium in stimulated red and white mammalian muscles. American Journal of Physiology. 1963;205:1295–1298. doi: 10.1152/ajplegacy.1963.205.6.1295. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Van Hall G. Lactate as a fuel for mitochondrial respiration. Acta Physiologica Scandinavica. 2000;168:643–656. doi: 10.1046/j.1365-201x.2000.00716.x. [DOI] [PubMed] [Google Scholar]
- Vedantham V, Cannon SC. Slow inactivation does not affect movement of the fast inactivation gate in voltage-gated Na+ channels. Journal of General Physiology. 1998;111:83–93. doi: 10.1085/jgp.111.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lannergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. Journal of Physiology. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]


