Abstract
In human subjects, a high-voltage electrical pulse between electrodes fixed over the mastoid processes activates descending tract axons at the level of the cervico-medullary junction to produce motor responses (cevicomedullary evoked responses; CMEPs) in the biceps brachii and brachioradialis muscles.
During isometric maximal voluntary contractions (MVCs) of the elbow flexors, CMEPs in the biceps brachii and brachioradialis muscles are sometimes followed by a second compound muscle action potential. This response can be observed in single trials (amplitude of up to 60 % of the maximal M wave) and follows the CMEP by about 16 ms in both muscles. The response only occurs during very strong voluntary contractions.
The second response following transmastoid stimulation appears with stimulation intensities that are at the threshold for evoking a CMEP in the contracting muscles. The response grows with increasing stimulus intensity, but then decreases in amplitude and finally disappears at high stimulation intensities.
A single stimulus to the brachial plexus during MVCs can also elicit a second response (following the M wave) in the biceps brachii and brachioradialis muscles. The latency of this response is 3–4 ms longer than that of the second response observed following transmastoid stimulation. This difference in latency is consistent with a reflex response to stimulation of large-diameter afferents.
The amplitude of the second response to transmastoid stimulation can be reduced by appropriately timed subthreshold transcranial magnetic stimuli. This result is consistent with intracortical inhibition of the response.
We suggest that transmastoid stimulation can elicit a large transcortical reflex response in the biceps brachii and brachioradialis muscles. The response travels via the motor cortex but is only apparent during near-maximal voluntary efforts.
In awake human subjects, the descending tracts of the spinal cord can be stimulated at the level of the cervicomedullary junction by passing a high-voltage electrical pulse between the mastoids or by magnetic stimulation over the back of the head (Ugawa et al. 1991, 1994). Based on collision experiments in which it has been shown that such stimuli can occlude responses to transcranial electrical or magnetic stimulation (Ugawa et al. 1991; Gandevia et al. 1999), it is believed that transmastoid stimuli activate corticospinal neurones, which in turn recruit motoneurones and evoke muscle responses that can be recorded as compound muscle action potentials (CMAPs). Stimulation may also activate other structures. In patients with cortical reflex myoclonus, a reflex response in muscle has been attributed to magnetic stimulation of ascending tracts at the foramen magnum (Ugawa et al. 1997). This reflex response could be evoked at lower intensities of stimulation than the motor response elicited through the descending tracts, and could also be evoked at appropriate latencies by the stimulation of peripheral nerves. Thus this reflex is likely to be due to the stimulation of afferent pathways. No such reflex responses were found in subjects who were neurologically normal. However, some subjects who receive electrical or magnetic descending tract stimulation report paraesthesia in the arms or legs. This indicates that in normal subjects afferent neurones can be activated by cervicomedullary stimulation.
Here we report for the first time a second motor response that can be elicited in the elbow flexor muscles of normal subjects when electrical or magnetic stimulation of the descending tracts is administered during a strong voluntary contraction. This large, synchronised CMAP appears to be a reflex response to the stimulation of afferent neurones in the spinal cord or medulla.
METHODS
Six neurologically normal subjects (age 23–46 years; three females) took part in the main studies, although not all of the subjects participated in every experiment. The study was approved by the local ethics committee and complied with the Declaration of Helsinki. All subjects gave their informed consent to participate. During the experiments, subjects sat with their right arm held in a myograph, which measured torque about the elbow. The elbow was flexed to an angle of 90 deg with the forearm supinated and held upright. Subjects received feedback about elbow flexion torque on an oscilloscope. EMGs were recorded from the biceps brachii and brachioradialis muscles through surface electrodes (9 mm diameter, Ag+/AgCl, belly-tendon configuration). EMG signals were filtered (1.6 Hz to 1 kHz) and amplified, and were recorded to computer disk via a laboratory interface using commercial software (sampling frequency 5 kHz, CED 1401 Signal; Cambridge Electronic Design). Signals were recorded in sweeps that included 50 ms before and 150 ms after the delivery of a stimulus.
In each trial, subjects performed a brief (2–3 s) voluntary elbow flexion during which a stimulus was delivered. In some trials, high-voltage electrical stimuli were delivered via electrodes fixed over the mastoid processes (100 μs pulse, 225–600 V; D180 stimulator; Digitimer). Such stimuli activate axons in the descending tracts at the level of the cervicomedullary junction (Ugawa et al. 1991; Gandevia et al. 1999). The latency of responses was monitored carefully to ensure that higher stimulation intensities did not activate the motor axons at or near the ventral roots. A jump in latency of around 2 ms is seen with stimulus spread that shifts the site of stimulation from the descending tracts to the ventral roots. In other trials, peripheral axons in the brachial plexus were stimulated via a cathode in the supraclavicular fossa and an anode on the acromion (1 ms pulse; Grass constant-current stimulator). In a separate experiment, a subthreshold transcranial magnetic stimulus was paired with a transmastoid stimulus. A transcranial magnetic stimulus can reduce the size of the response to a subsequent suprathreshold magnetic stimulus. This reduction is believed to occur through the activation of inhibitory circuits within the cortex by the subthreshold stimulus (Kujirai et al. 1993; Nakamura et al. 1997).
Intensity of transmastoid stimulation
Subjects (n = 6) received different intensities of transmastoid stimulation during brief maximal voluntary contractions (MVCs) of the elbow flexors. Subjects performed between 21 and 33 trials, which were separated by 1 min rest intervals. The stimulus intensity was initially set below the threshold for evoking a short-latency CMAP in the biceps brachii or brachioradialis muscles, and was then increased in steps of 5 % of stimulator output (100 % = 750 V) until a clear silent period could be seen after the initial CMAP. Stimulus intensity was usually increased from 25–35 % to 60–80 % of stimulator output. In each subject, the series of stimuli was repeated so that three trials were collected for each stimulation intensity.
Contraction strength
Subjects (n = 6) received transmastoid stimuli at a single intensity (40–50 % of stimulator output) during brief voluntary contractions of different target strengths. Subjects initially performed a MVC. This was followed by four trials using visual feedback to contract at 90 % MVC. A 100 % MVC then preceded four trials of 80 % MVC, and then four trials of 60 % MVC, with a final 100 % MVC trial.
Brachial plexus stimulation
Subjects (n = 4) performed brief MVCs during which stimuli were delivered to the brachial plexus. Stimulus intensities were supramaximal for eliciting maximal M waves in both the biceps brachii and brachioradialis muscles.
Effect of subthreshold transcranial magnetic stimuli
Subjects (n = 4) initially performed 20 brief 20 % MVC elbow flexions (one every 5 s) during which either a single transcranial magnetic stimulus or a pair of magnetic stimuli was delivered. Magnetic stimuli were delivered via a figure-of-eight magnetic coil (90 cm; Magstim 200 through a Bistim module, Magstim, Dyfed, UK) that was positioned over the left hemisphere at the optimal site to elicit motor evoked potentials (MEPs) in the biceps brachii and brachioradialis muscles. Threshold stimulus intensity (active threshold) was determined during a voluntary elbow flexion (20 % MVC). A stimulus intensity of 120–150 % active threshold (1.2–1.5 T) was used for the single magnetic stimulus. For the pair of magnetic stimuli, the suprathreshold stimulus was preceded by 1 or 2 ms by a subthreshold (0.7–0.8 T) stimulus. Intensities for the subthreshold stimulus ranged from 25 to 34 % of the stimulator output. Under these conditions, the subthreshold stimulus inhibited the MEPs elicited in the biceps brachii and brachioradialis muscles by the suprathreshold stimulus. The subthreshold magnetic stimulus, which was administered at the stimulus intensity and timing that had been demonstrated to result in intracortical inhibition of the MEP in each subject, was then paired with the transmastoid stimulus.
Subjects performed 30 brief maximal elbow flexions (1 min−1) during which either a transmastoid stimulus alone or a transmastoid stimulus plus a subthreshold (0.7–0.8 T) transcranial magnetic stimulus were given. The intensity of transmastoid stimulation was set at around the threshold for evoking a direct motor response during a maximal elbow flexion. Two different interstimulus intervals (ISIs; 10 trials each) were used for the pair of stimuli (transmastoid stimulus 5–8 ms or 9–12 ms before the magnetic stimulus) and the order of the three conditions was randomised. For each subject, ISIs were calculated such that any intracortical inhibitory circuits that were activated by the magnetic stimulus might affect the second response to transmastoid stimulation if that response were to travel via the motor cortex.
Additional observations
In seven subjects who made brief MVCs of the elbow flexors for other studies, magnetic stimulation over the back of the head replaced transmastoid electrical stimulation. A double cone coil was positioned with its centre 1–2 cm below the inion and up to 2 cm laterally to optimise direct motor responses in the elbow flexors of the right arm. Current flow was downwards in the junction of the coil (Ugawa et al. 1994, 1997; Taylor et al. 2000). The stimulation intensity was 80–100 % of stimulator output (Magstim 200; Magstim) and produced direct motor responses of 60–70 % of the maximal M wave during brief MVCs.
Data analysis
The peak-to-peak amplitude of each response in each trial was measured automatically between cursors set for each set of trials for each subject. Responses latencies were measured by cursor in individual trials and were averaged for each subject. All data are presented as mean ± s.d.. ANOVA (three-way) with a post hoc test to identify differences between means (Student-Newman-Keuls test) was used to compare the amplitude of responses with and without conditioning transcranial magnetic stimulation.
RESULTS
During strong voluntary contractions of the elbow flexors, transmastoid stimulation sometimes elicited two consecutive CMAPs in the biceps brachii and brachioradialis muscles (Fig. 1). The first CMAP in each muscle had a latency consistent with a response to stimulation of the descending tracts at the level of the cervicomedullary junction (cervicomedullary motor evoked potential, CMEP). In the biceps brachii the latency was 7.8 ± 0.5 ms and in the brachioradialis the latency was 9.9 ± 0.6 ms. The second response, which could sometimes be as large as the direct response, had a latency of 23.8 ± 1.1 ms and 26.4 ± 1.9 ms in the biceps brachii and brachioradialis, respectively. That is, in both muscles it occurred about 16 ms after the CMEP. The occurrence of the second response depended upon both the stimulus intensity and the contraction strength.
Figure 1. Two consecutive compound muscle action potentials (CMAPs) are evoked by transmastoid stimulation.
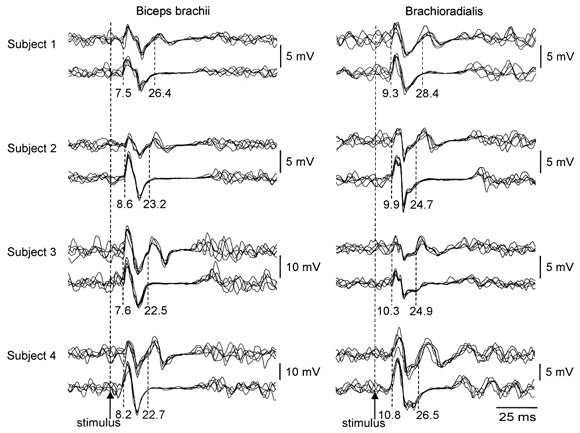
EMG traces recorded simultaneously from the biceps brachii (left) and brachioradialis (right) during brief maximal isometric elbow flexions are shown for four subjects. Two sets of five superimposed traces are shown for each muscle for each subject. In each case, both sets of traces show the initial response to transmastoid stimulation evoked by activation of descending tracts (latency of 7.5–8.6 ms for the biceps brachii and 9.3–10.8 ms for the brachioradialis). However, whereas in the lower traces for each muscle for each subject the initial response is followed by a period of EMG silence, in the upper traces it is followed by a second CMAP (latency 22.5–26.4 ms for the biceps brachii and 24.7–28.4 ms for the brachioradialis). For each subject, the intensity of the transmastoid stimulus was less for the upper traces, in which the second response is apparent, than for the lower traces.
Intensity of transmastoid stimulation
In both the biceps brachii and brachioradialis muscles, the second response was occasionally observed at low stimulus intensities when no CMEP was apparent (see Fig. 2; responses at 25–35 % of stimulator output). However, as both responses depend upon the level of voluntary contraction, there is considerable trial-to-trial variability. Averaging of the responses evoked at each intensity showed that the CMEP and the second response had similar threshold intensities of stimulation. As the CMEP grew with increasing stimulus intensity, the second response also grew. On average in six subjects, it reached 31 ± 13 % and 39 ± 5 % of the amplitude of the maximal M wave in the biceps brachii and brachioradialis muscles, respectively, while the amplitude of the CMEP was 55 ± 23 % and 51 ± 22 % of the maximal M wave, respectively. However, in individual trials the second response could reach 60 % of the maximal M wave amplitude (Fig. 2). With further increases in stimulus intensity, the size of the second response decreased while the CMEP continued to grow. At high stimulation intensities the second response disappeared completely, leaving EMG silence. When the preceding CMEP was small, at low stimulation intensities, the latency of the second response tended to be shorter than during higher-intensity stimulation (Fig. 2). The average change in latency between the two lowest and the two highest stimulus intensities that evoked responses in each subject was 2.4 ± 1.4 ms in the biceps brachii and 2.4 ± 1.6 ms in the brachioradialis. However, this latency change may be more apparent than real because the tail of the CMEP may obscure the onset of the second response.
Figure 2. Responses following transmastoid stimuli of different intensities.
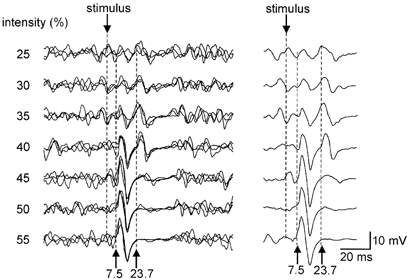
EMG traces recorded from the biceps brachii in one subject are shown. Traces show responses to transmastoid stimulation during brief maximum voluntary contractions (MVCs). The stimulus intensity was increased from 25 % to 55 % of the stimulator output and the responses to each stimulus intensity are overlaid in the left panel and averaged in the right. At the lowest stimulus intensity, responses are only apparent in the averaged display. As stimulus intensity increases, two consecutive CMAPs with latencies of 7.5–8 ms and 22–23 ms appear. With intermediate-strength stimulation intensities, both CMAPs are clear. With higher stimulus intensities, the second response disappears and only the initial response is seen.
Contraction strength
The second response only occurred during strong voluntary contractions. During MVCs, the chosen stimulus intensity elicited an average CMEP of 55 ± 25 % and a second response of 27 ± 10 % of the amplitude of the maximal M wave in the biceps brachii, and a CMEP of 54 ± 23 % and second response of 37 ± 9 % in the brachioradialis. In one subject the second response was seen with contractions of 60 % MVC or greater. In three of the six subjects it was seen during contractions of 80 % MVC or greater, but in one subject it occurred only during 100 % MVCs. Figure 3 shows responses in the biceps brachii and brachioradialis muscles from one subject. Small responses are seen after the CMEP in some of the trials for which the target force was 90 % MVC. These are more evident in the brachioradialis than in the biceps brachii. No such responses were seen in the trials performed at 80 % MVC.
Figure 3. Effect of contraction strength on the second response following transmastoid stimulation.
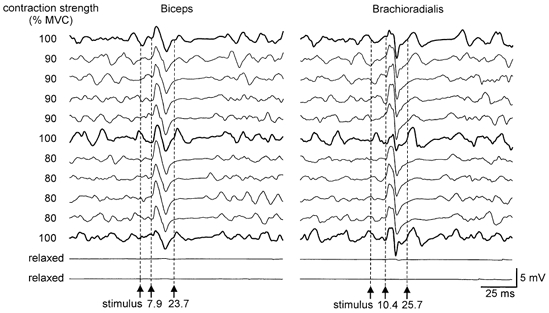
EMG traces recorded simultaneously from the biceps brachii (left) and brachioradialis (right) in one subject. Traces are shown in the order in which they were recorded and stimulus intensity was the same throughout. The top traces show responses following transmastoid stimulation during a maximal isometric elbow flexion (100 % MVC; thick traces). After the initial CMAP, a second response is seen in traces recorded from both the biceps brachii and brachioradialis. In the next four sets of traces, the subject aimed to make a 90 % maximal contraction. The second response can be seen in some traces, but is more obvious in the brachioradialis than in the biceps brachii. The next set of traces was again recorded during a MVC and again shows the second response. In the four sets of traces recorded during 80 % MVCs, no second response was elicited, but during a final 100 % MVC it became apparent once again in both muscles. The final two sets of traces were recorded from relaxed muscles and show that the stimulus was almost below threshold for any response under these conditions.
Brachial plexus stimulation
In three out of four subjects a second response was apparent following the M waves evoked by supramaximal stimulation in both the biceps brachii and brachioradialis. The latency of the M wave was 5.0 ± 0.4 ms in the biceps brachii and 7.1 ± 0.4 ms in the brachioradialis, while that of the subsequent (second) response was 27.0 ± 0.7 ms and 29.0 ± 1.1 ms, respectively. That is, responses occurred about 22 ms apart in both muscles. Figure 4 shows, for the biceps brachii in one subject, responses following brachial plexus stimulation and responses following transmastoid stimulation. When contraction strength was submaximal the second response elicited following brachial plexus stimulation disappeared in a similar way to that following transmastoid stimulation (Fig. 4, lower traces).
Figure 4. Consecutive CMAPs evoked by stimulation of the brachial plexus.
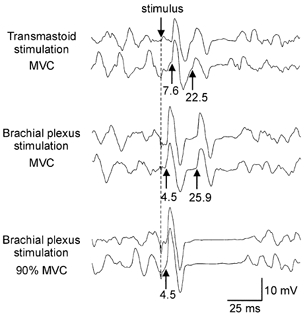
EMG traces recorded from the biceps brachii in one subject. The top pair of traces show examples of the initial and second CMAPs elicited following transmastoid stimulation during a maximal elbow flexion. The middle pair of traces show the responses elicited following supramaximal stimulation of the brachial plexus during a MVC in the same subject. The initial response here (elicited through the stimulation of peripheral motor axons) has a latency that is about 3 ms less than the response to transmastoid stimulation. The latency of the second response (3.5 ms longer than the second response to transmastoid stimulation) is consistent with a reflex response to the activation of large-diameter afferents. The bottom pair of traces shows responses to brachial plexus stimulation when the subject performed a weaker contraction (90 % MVC). There is no second response to the stimulus.
Effect of a subthreshold transcranial magnetic stimulus
In each of the four subjects studied, MEPs in the biceps brachii and brachioradialis were inhibited by a subthreshold magnetic stimulus that preceded the suprathreshold magnetic stimulus by 1 or 2 ms. MEPs recorded during contractions at 20 % MVC were reduced in amplitude to 78 ± 20 % in the biceps brachii and 67 ± 8 % in the brachioradialis. The mean latency of MEPs in the biceps brachii was 12.1 ± 0.5 ms, and in the brachioradialis it was 14.2 ± 1.0 ms. Appropriate timing of the subthreshold magnetic stimulus relative to a transmastoid stimulus was then calculated such that the magnetic stimulus was delivered over the cortex 1 or 2 ms before output cells in the motor cortex would need to fire to produce the second response to the transmastoid stimulation. The assumed conduction time from the motor cortex to the muscle was based on the latency of MEPs elicited during voluntary contraction. Thus, timing was calculated by measuring the latency of the second response to transmastoid stimulation and subtracting the latency of the MEP and then subtracting 1 or 2 ms, depending upon which interval had proved effective in causing inhibition of the MEP in the paired-pulse trials. This calculation resulted in ISIs of between 9 and 12 ms (transmastoid stimulus before magnet) in the four subjects. A second ISI (5–8 ms) was also used in each subject, in which the magnetic stimulus was delivered 4 ms earlier.
The amplitude of the second response to transmastoid stimulation could be reduced by a subthreshold transcranial magnetic stimulus. Figure 5A shows averaged EMG traces from the biceps brachii in one subject. Note that the CMEP is small in these traces so that the recording is dominated by the second response to transmastoid stimulation. With the paired stimuli at an ISI of 9 ms, the second response is reduced to 74 % of its control amplitude, whereas with an ISI of 5 ms the response is not reduced. This trend was also apparent overall (Fig. 5B). When data from the biceps brachii and brachioradialis were analysed together, the second response to transmastoid stimulation was significantly reduced by the weak magnetic cortical stimulation at the longer ISI but not the shorter ISI (P < 0.05; three-way ANOVA and post hoc Student-Newman-Keuls test). On average, the amplitude of the second response in the biceps brachii was decreased to 75 ± 5 % at the longer ISI and to 87 ± 11 % at the shorter ISI. In the brachioradialis the effects were smaller but still apparent (90 ± 6 % at the longer ISI and 96 ± 15 % at the shorter ISI).
Figure 5. Inhibition of second response to transmastoid stimulation by subthreshold transcranial magnetic stimulation.
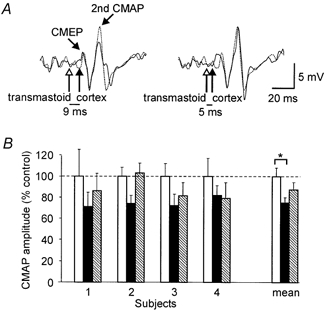
A, EMG traces recorded from the biceps brachii in one subject. Each trace is an average of 10 trials and shows a small cervicomedullary motor evoked potential (CMEP) followed by the second response to transmastoid stimulation. In each pair of traces, the dotted trace shows the responses elicited following transmastoid stimulation (open arrow) delivered alone, whereas the continuous trace shows responses elicited when a weak transcranial magnetic stimulus (filled arrow) was paired with the transmastoid stimulus. With an interstimulus interval (ISI) of 9 ms (on the left), the second response to transmastoid stimulation was reduced to 74 % of its control amplitude. With an ISI of 5 ms (on the right), the second response was not reduced. B, amplitude of the second response to transmastoid stimulation when the transmastoid stimulus was given alone (open bars) and when it was followed by a subthreshold transcranial magnetic stimulus (ISI 9–12 ms, filled bars; ISI 5–8 ms, hatched bars). Data shown are from responses elicited in the biceps brachii. For each subject, the amplitude of the response is normalised to its mean amplitude in the control condition (transmastoid stimulus alone). Each bar shows mean ±s.e.m.. Bars on the far right of the graph show mean data for the group. The second response to transmastoid stimulation is significantly reduced (P < 0.05) by a weak transcranial magnetic stimulus delivered 9 ms after the transmastoid stimulus.
Additional observations
When magnetic stimulation close to the inion replaces transmastoid electrical stimulation (Taylor et al. 2000; see Methods), a second EMG response can also occur in biceps brachii and brachioradialis. Such responses have a similar latency to those evoked by electrical stimulation. We have observed these responses during brief MVCs in all seven of the subjects who performed such trials in experiments undertaken for other purposes.
DISCUSSION
The main finding reported here is the occurrence of a second CMAP that can be observed in the biceps brachii and brachioradialis muscles after the initial motor response to transmastoid stimulation. Whereas the initial response is evoked through stimulation of the descending tracts (Gandevia et al. 1999), our observations suggest that the second large synchronised volley is a reflex response to stimulation of afferents in the spinal cord or medulla and probably travels through a supraspinal pathway that involves the motor cortex.
In the biceps brachii, the response has a latency of around 24 ms, while in the brachioradialis the latency is 2 ms longer. With lower stimulus intensities the latencies in both muscles decreased by about 2 ms. This may indicate that at higher stimulation intensities the onset of the second response is masked by the tail of the larger CMEP. The difference in latency between the second responses in the two muscles is consistent with the difference in conduction time from the site of descending tract stimulation to the muscles. The latency of the CMEP in the brachioradialis is 9.9 ms, compared to 7.8 ms in the biceps brachii. The time between the CMEP and the subsequent response is around 16 ms in both muscles (or 14 ms with a weaker stimulus). This suggests that the responses in the two muscles travel along pathways of similar length. Thus, it is unlikely that the second response is evoked as a consequence of the actions of the CMEP in the periphery.
This leaves a number of ways in which the second response could occur. These include double firing of the motoneurones in response to a single descending volley, although this is unlikely (Berardelli et al. 1991), antidromic activation of descending tracts to produce direct or recurrent excitation of corticospinal neurones, activation of slow or polysynaptic descending pathways, and activation of afferent axons in the spinal cord or medulla as one limb of a long-latency reflex response. However, demonstration of a similar response at an appropriate latency after brachial plexus stimulation strongly supports the last possibility. In both muscles, the latency of the response after brachial plexus stimulation was around 3.5 ms longer than the response after transmastoid stimulation. This difference is consistent with the longer afferent pathway and, together with the occurrence of the response with relatively low-intensity transmastoid stimulation, suggests that large-diameter afferents are involved. While the exact site of afferent stimulation is uncertain, comparison with the site of activation of the descending tracts suggests that the afferent pathways are activated at the level of the cuneate nucleus or slightly above in the medial lemniscus.
Although reflex responses to transmastoid stimulation have not been reported previously in normal subjects, responses with a latency of around 27 ms were seen during weak contractions in hand muscles in patients with cortical reflex myoclonus (Ugawa et al. 1997). These patients also show giant somatosensory evoked potentials in response to median nerve stimulation, and enhanced long-loop reflexes (Shibasaki, 2000 for review). Ugawa et al. (1997) suggest that in these patients the reflex response to transmastoid stimulation occurred through stimulation of group Ia afferents and travelled a transcortical route. Although the latency between the CMEP and the second response in the patients (10–11 ms) was short compared to the latency we observed in normal subjects (14–18 ms), the reflex responses in patients were more variable and less synchronous than the responses reported here.
Our findings suggest that the second response observed following transmastoid stimulation in normal subjects also follows a reflex transcortical route. The site of stimulation at the cervicomedullary junction suggests a supraspinal pathway, and the reduction in size of the response effected by subthreshold transcranial magnetic stimuli is consistent with passage through the motor cortex. Transcranial magnetic stimulation at low intensities (70–80 % of that required to elicit an EMG response during voluntary contraction) is believed to activate inhibitory interneurones within the cortex and can inhibit the response to a subsequent magnetic cortical stimulus. This period of intracortical inhibition is effective from around 1–5 ms after the subthreshold stimulus (Kujirai et al. 1993; Ridding et al. 1995; Abbruzzese et al. 1999). When a subthreshold magnetic stimulus was delivered over the motor cortex 1 or 2 ms before the time at which motor cortical output cells would need to fire in order to produce the second response to transmastoid stimulation, the response was inhibited. Note that this timing occurred with the longer ISI, in which the stimulus to the motor cortex was delivered 9–12 ms after the transmastoid stimulus, and thus about 13 ms before the second response occurred in the biceps brachii. When the magnetic stimulus was delivered earlier (around 6 ms before the estimated activation of cortical output cells) the response was not inhibited. This behaviour is consistent with intracortical inhibition of a reflex response to transmastoid stimulation. A second possibility is that suppression of the second response to transmastoid stimulation occurred through collision or occlusion with the subthreshold response to magnetic stimulation within the cortex. However, suppression through either mechanism would suggest a transcortical route for the response. Furthermore, as the estimated conduction time for the efferent pathway was based on the latency of the MEP, it is likely that the second response to transmastoid stimulation, like the MEP, travels via corticospinal neurones.
The second response to transmastoid stimulation has some distinctive features. First, it only occurs during very strong voluntary contractions. This suggests that some segment of the pathway needs to be strongly excited before it can respond. It seems unlikely that it is the motoneurones that must be strongly activated, as the immediate history of the motoneurones is determined by their response to the initial descending volley. Similar CMEPs evoked during strong and weaker contractions should leave the motoneurones in similar states. Secondly, when the response does occur it is large, synchronous and is easily seen in single trials. Thirdly, the second response to transmastoid stimulation appears when the CMEP is small; it grows larger with increased stimulus intensity but then becomes smaller and disappears when stimulus intensity increases further. It is as if stimulating more corticospinal axons to generate a large CMEP somehow occludes the later response. In contrast, when the second CMAP is elicited by stimulating the brachial plexus, it can follow a stimulus that produces a maximal M wave. The antidromic invasion of motoneurones after brachial plexus stimulation (with limited synaptic activation through Ia afferents) may not leave them in the same state as synaptic activation through a descending volley. Thus, one explanation for the decrease in the second response with the increase in the CMEP could be that firing of the motoneurones is followed by a hyperpolarisation or inhibition that prevents them from being activated a second time, so that only motoneurones that did not participate in the CMEP might fire in the second response. Alternatively, the response might be inhibited at the motor cortex; increasing intensities of transmastoid stimulation could lead to cortical inhibition via collaterals of the antidromically activated corticospinal axons (Krnjevic et al. 1966; Ghosh & Porter, 1988).
The relationship between the second response after transmastoid stimulation and the long-latency reflex pathway activated by muscle stretch is unclear. With a conduction time of 2.5–3 ms between the motor cortex and the site of transmastoid stimulation, and a similar conduction time to the sensory cortex, the central delay of 10–11 ms is consistent with previous studies on long-latency reflexes in hand muscles (Deuschl et al. 1989; Kurusu & Kitamura, 1999). In the biceps brachii, small long-latency responses to muscle stretch were seen during weak contractions and were revealed in averaged trials. If the afferent limb of the stretch response travels via group Ia axons, its latency of around 45 ms would give a similar central time to that for the second response following transmastoid stimulation. However, whereas for distal muscles the long-latency response to muscle stretch almost certainly occurs via a transcortical pathway (Matthews et al. 1990; Day et al. 1991; Deuschl et al. 1991; Taylor et al. 1995), studies in patients do not suggest this route for the biceps brachii (Thilmannn et al. 1991; Fellows et al. 1996).
In summary, this study has defined a new putative reflex response to the stimulation of large-diameter afferent axons. This large, synchronised response has a central delay of around 10 ms, requires strong voluntary contraction and may be ‘occluded’ at high stimulus intensities of corticospinal stimulation. Most likely, the reflex is transcortical with an efferent limb via corticospinal axons.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia.
References
- Abbruzzese G, Assini A, Buccolieri A, Schieppati M, Trompetto C. Comparison of intracortical inhibition and facilitation in distal and proximal arm muscles in humans. Journal of Physiology. 1999;514:895–903. doi: 10.1111/j.1469-7793.1999.895ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Inghilleri M, Rothwell JC, Cruccu G, Manfredi M. Multiple firing of motoneurones is produced by cortical stimulation but not by direct activation of descending motor tracts. Electroencephalography and Clinical Neurophysiology. 1991;81:240–242. doi: 10.1016/0168-5597(91)90078-c. [DOI] [PubMed] [Google Scholar]
- Day BL, Riescher H, Struppler A, Rothwell JC, Marsden CD. Changes in the response to magnetic and electrical stimulation of the motor cortex following muscle stretch in man. Journal of Physiology. 1991;433:41–57. doi: 10.1113/jphysiol.1991.sp018413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, Ludolph A, Schenck E, Lücking CH. The relations between long-latency reflexes in hand muscles, somatosensory evoked potentials and transcranial stimulation of motor tracts. Electroencephalography and Clinical Neurophysiology. 1989;74:425–430. doi: 10.1016/0168-5597(89)90031-2. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Michels R, Berardelli A, Schenk E, Inghilleri M, Lücking CH. Effects of electric and magnetic transcranial stimulation on long latency reflexes. Experimental Brain Research. 1991;83:403–410. doi: 10.1007/BF00231165. [DOI] [PubMed] [Google Scholar]
- Fellows SJ, Töpper R, Schwarz M, Thilmann AF, Noth J. Stretch reflexes of the proximal arm in a patient with mirror movements: absence of bilateral long-latency components. Electroencephalography and Clinical Neurophysiology. 1996;101:79–83. doi: 10.1016/0924-980x(95)00247-i. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE, Taylor JL. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. Journal of Physiology. 1999;521:749–759. doi: 10.1111/j.1469-7793.1999.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Porter R. Morphology of pyramidal neurones in monkey motor cortex and the synaptic actions of their intracortical axon collaterals. Journal of Physiology. 1988;400:593–615. doi: 10.1113/jphysiol.1988.sp017138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K, Randic M, Straughan DW. An inhibitory process in the cerebral cortex. Journal of Physiology. 1966;184:16–48. doi: 10.1113/jphysiol.1966.sp007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu K, Kitamura J. Long-latency reflexes in contracted hand and foot muscles and their relations to somatosensory evoked potentials and transcranial magnetic stimulation of the motor cortex. Clinical Neurophysiology. 1999;110:2014–2019. doi: 10.1016/s1388-2457(99)00166-2. [DOI] [PubMed] [Google Scholar]
- Matthews PBC, Farmer SF, Ingram DA. On the localisation of the stretch reflex of intrinsic hand muscles in a patient with mirror movements. Journal of Physiology. 1990;428:561–577. doi: 10.1113/jphysiol.1990.sp018228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. Journal of Physiology. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. Journal of Physiology. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki H. Electrophysiological studies of myoclonus. Muscle and Nerve. 2000;23:321–335. doi: 10.1002/(sici)1097-4598(200003)23:3<321::aid-mus3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Gandevia SC. Magnetic stimulation of the descending tracts in human subjects. Proceedings of the Australian Neuroscience Society. 2000;11:48. [Google Scholar]
- Taylor JL, Fogel W, Day BL, Rothwell JC. Ipsilateral cortical stimulation inhibits the long latency response to stretch in the long finger flexors in humans. Journal of Physiology. 1995;488:821–831. doi: 10.1113/jphysiol.1995.sp021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilmann AF, Schwarz M, Töpper R, Fellows SJ, Noth J. Different mechanisms underlie the long-latency stretch reflex response of active human muscle at different joints. Journal of Physiology. 1991;444:631–643. doi: 10.1113/jphysiol.1991.sp018898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Annals of Neurology. 1991;29:418–427. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation of corticospinal pathways at the foramen magnum level in humans. Annals of Neurology. 1994;36:618–624. doi: 10.1002/ana.410360410. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation of the descending and ascending tracts at the foramen magnum level. Electroencephalography and Clinical Neurophysiology. 1997;105:128–131. doi: 10.1016/s0924-980x(97)96141-5. [DOI] [PubMed] [Google Scholar]


