Abstract
The relative roles of sympathetic nerve activity and circulating catecholamines for adipose tissue lipolysis during exercise are not known.
Seven paraplegic spinal cord injured (SCI, injury level T3-T5) and seven healthy control subjects were studied by microdialysis and 133xenon washout in clavicular (Cl) and in umbilical (Um) (sympathetically decentralized in SCI) subcutaneous adipose tissue during 1 h of arm cycling exercise at ∼60 % of the peak rate of oxygen uptake.
During exercise, adipose tissue blood flow (ATBF) and interstitial glycerol, lactate and noradrenaline concentrations increased significantly in both groups. Plasma catecholamine levels increased significantly less with exercise in SCI than in healthy subjects. The exercise-induced increase in interstitial glycerol concentration in subcutaneous adipose tissue was significantly lower in SCI compared with healthy subjects (SCI: 25 ± 12 % (Cl), 36 ± 20 % (Um); healthy: 60 ± 17 % (Cl), 147 ± 45 % (Um)) and the increase in ATBF was significantly lower (Cl) or similar (Um) in SCI compared with healthy subjects (SCI: 1.2 ± 0.3 ml (100 g)−1 min−1 (Cl), 1.0 ± 0.3 ml (100 g)−1 min−1 (Um); healthy: 2.8 ± 0.7 ml (100 g)−1 min−1 (Cl), 0.6 ± 0.3 ml (100 g)−1 min−1 (Um)). Accordingly, in both adipose tissues lipolysis increased less in SCI compared with healthy subjects, indicating that circulating catecholamines are important for the exercise-induced increase in subcutaneous adipose tissue lipolysis. In SCI subjects, the exercise-induced increase in subcutaneous adipose tissue lipolysis was not lower in decentralized than in sympathetically innervated adipose tissue. During exercise the interstitial noradrenaline and adrenaline concentrations were lower in SCI compared with healthy subjects (P < 0.05) and always lower than arterial plasma catecholamine concentrations (P < 0.05).
It is concluded that circulating catecholamines are important for the exercise-induced increase in subcutaneous adipose tissue lipolysis while sympathetic nerve activity is not.
Adipose tissue lipolysis increases during exercise (Arner et al. 1990; Wennlund et al. 1994; Hellström et al. 1996), and both an increase in sympathoadrenergic activity and a decrease in plasma insulin concentration are believed to enhance adipose tissue lipolysis (Arner, 1995). Systemic β-blockade diminishes free fatty acid (FFA) turnover in running dogs (Issekutz, 1978) and plasma concentrations of both FFAs and glycerol in exercising humans (Bülow, 1981; Macdonald et al. 1984). Also, local administration of a β-adrenergic blocker into adipose tissue via a microdialysis probe during exercise produces a pronounced reduction in local glycerol concentrations, which is presumed to reflect lipolysis (Arner et al. 1990; Hellström et al. 1996). Finally, experiments using selective β-adrenergic blockade have shown that the β1- rather than the β2-adrenoceptor mediates lipolysis during exercise (Lundborg et al. 1981; van Baak, 1988). On the basis of these experiments it is evident that sympathoadrenergic activity plays a role in adipose tissue lipolysis during exercise, but the relative importance of local sympathetic nerve activity and circulating catecholamines remains to be clarified.
Spinal cord injured (SCI) individuals with a cervical or a high thoracic lesion experience diminished sympathetic nerve traffic below the level of injury (Normell, 1974), including reduced leg sympathetic nerve activity recorded by microneurography (Wallin & Stjernberg, 1984) and a lower whole-body noradrenaline spillover (Krum et al. 1990), as well as impaired release of adrenaline from the adrenal medulla (Schmid et al. 1998). From this, it is to be presumed not only that these individuals experience a smaller increase in overall sympathoadrenergic activity during arm exercise, but also that sympathetic activity to subcutaneous adipose tissue will be less affected in areas proximal (e.g. the clavicular region) than in areas distal (e.g. the umbilical region) to the injury. Lower plasma catecholamine responses to arm exercise have in fact been found in SCI compared with healthy subjects (Kjær et al. 1996). In accordance with a differentiated autonomic response, hand heating elicits perspiration above but not below the segmental level of interrupted sympathetic output (Karlsson et al. 1997).
In the present investigation we studied SCI subjects with a high thoracic lesion (paraplegic subjects) to clarify the role of local sympathetic nerve activity relative to circulating catecholamines during the increase in adipose tissue lipolysis in response to voluntary arm exercise. The rate of lipolysis was determined from interstitial glycerol concentrations and local blood flow and compared between clavicular and umbilical subcutaneous adipose tissues. Because an influence of sympathetic nerve activity might escape attention if the responsiveness to hormonal stimulation is higher in umbilical compared with clavicular adipose tissue, healthy subjects were also studied. This also enabled the evaluation of the role of circulating catecholamines per se. Using this design any enhancing role of circulating catecholamines on adipose tissue lipolysis would be revealed by an impaired exercise-induced increase in lipolysis in clavicular adipose tissue in SCI compared with healthy subjects. An enhancing role of local sympathetic nerves would be revealed by the impaired exercise-induced increase in lipolysis in umbilical adipose tissue in SCI subjects and identical responses in clavicular adipose tissue in SCI compared with healthy subjects. Finally, an enhancing influence of both circulating catecholamines and regional sympathetic nerve activity would be revealed by a relatively higher impairment of exercise-induced lipolysis in umbilical than in clavicular adipose tissue in SCI compared with healthy subjects.
METHODS
Subjects
Seven SCI and seven healthy subjects gave their written informed consent according to the Declaration of Helsinki II to participate in the study, which was approved by the Ethics Committee for Medical Research in Copenhagen (KF 01–167/96). The SCI subjects had complete spinal cord lesions (Frankel class A, Frankel et al. 1969) at T3–T5 (range) for at least 2 years (range 2–15 years) before the start of the study. Anthropometric data for the two groups are shown in Table 1.
Table 1.
Anthropometric data
| SCI | Healthy | |
|---|---|---|
| Sex (male/female) | 5/2 | 5/2 |
| Age (years) | 39 ± 4 | 38 ± 4 |
| Weight (kg) | 67 ± 6 | 73 ± 6 |
| Height (cm) | 175 ± 4 | 176 ± 3 |
| Body mass index (kg m−2) | 21.8 ± 1.4 | 23.4 ± 1.2 |
| Body fat (%) | 31 ± 4 | 20 ± 4 |
| Abdominal skinfold (mm) | 15 ± 3 | 12 ± 2 |
| V̇O2,peak for arm exercise (ml (kg LBM)−1 min−1) | 26 ± 2 | 31 ± 3 |
| V̇O2 during arm exercise (ml (kg LBM)−1 min−1) | 15.4 ± 0.8 | 17.3 ± 1.5 |
| V̇O2 during exercise (% of V̇O2,peak) | 61 ± 3 | 57 ± 4 |
| RER during exercise | 0.88 ± 0.03 | 0.85 ± 0.02 |
| Heart rate at rest (beats min−1) | 69 ± 3 | 63 ± 4 |
| Heart rate during exercise (beats min−1) | 122 ± 9 | 116 ± 7 |
| Rate of perceived exertion (Borg scale) | 13 ± 1 | 14 ± 1 |
Values are means ±s.e.m. for 7 SCI and 7 healthy subjects. LBM, lean body mass; RER, respiratory exchange ratio.
Protocol
At least 3 days before the experimental day subjects performed an arm cycling test to exhaustion to determine the peak rate of oxygen uptake (V̇O2,peak) on a Monarch (Stockholm, Sweden) ergometer. The sympathetic denervation level of the skin was determined by applying iodized starch to the chest prior to the test to visualize perspiration (Guttmann, 1976). Darker areas on the skin after exercise indicated the presence of intact sympathetic innervation of sweat glands. The level of sympathetic disruption was within plus or minus one level of sensory disruption. On the day before the experiment subjects avoided strenuous physical activity. After an overnight fast and abstinence from coffee, tea and tobacco, the subjects were brought to the laboratory at 08.00 h where they were DEXA-scanned to determine body composition (Lunar DPX-IQ, software version 4.6c, fast mode and extended research analysis; Lunar Corporation, Madison, WI, USA) and had abdominal skinfold thickness measured. During the experiments subjects wore light clothes and the room temperature was ∼24 °C. After insertion of microdialysis and arterial catheters microdialysis perfusion was started and after a 60 min equilibration period the experiment was initiated. The experiment consisted of a 60 min basal and a 60 min exercise period in which subjects arm cycled aiming at 60 %V̇O2,peak. During these periods subjects were in a semi-sitting position (60 deg to the couch). Four times during the exercise period rates of O2 consumption (V̇O2) and CO2 production (V̇CO2) were measured by an open circuit ventilated hood system (Oxycon Champion; Jaeger, Würzburg, Germany). The electrocardiogram and heart rate were measured continuously via ECG electrodes.
Microdialysis
Microdialysis was performed in principle as described previously (Stallknecht et al. 1997). After local anaesthesia (0.05 ml lidocaine (lignocaine), 10 mg ml−1) of the skin at the site of perforation four microdialysis catheters (CMA 60; CMA/Microdialysis AB, Stockholm, Sweden) were placed in the clavicular subcutaneous adipose tissue transversely and in parallel ∼1.5 cm apart with the most distal catheter ∼5 cm above the mammilla. Another four catheters (CMA 60) were placed in the umbilical subcutaneous adipose tissue transversely and in parallel ∼1.5 cm apart. Two of the catheters in each area were used for the determination of interstitial glycerol and lactate, and dialysate glucose concentrations and the remaining catheters were used for the determination of interstitial noradrenaline and adrenaline concentrations. All probes were perfused at a rate of 1 μl min−1 using a high-precision syringe pump (CMA 100; CMA/Microdialysis AB). The in vivo relative recovery (RR) was determined by the internal reference calibration technique (Scheller & Kolb, 1991). In catheters used for determination of metabolites perfusate consisted of Ringer acetate solution with 100 μm glycerol, 1 mm lactate, 4 mm glucose, 1.3 μm[3H]glycerol (specific activity, 7.4 GBq mmol−1; NEN, Du Pont, Belgium) and 2.0 μm[14C]lactate (specific activity, 5.7 GBq mmol−1; Amersham, Bucks, UK). In catheters used for determination of catecholamines perfusate consisted of Ringer acetate solution with 100 μm glycerol, 1 mm lactate and 4 mm glucose, and in addition 4 mm reduced glutathione was added to prevent degradation of catecholamines during sampling. After the experiment 7.6 nm[3H]noradrenaline (specific activity, 485 GBq mmol−1; NEN) was added to the perfusate in catheters used for the analysis of catecholamines in order to determine the RR of catecholamines. RR of adrenaline (MW, 183) was assumed to equal RR of noradrenaline (MW, 169). [3H]Noradrenaline was not added during the experiment due to the potential local effect of the relatively high noradrenaline concentration. After addition of [3H]noradrenaline catheters were perfused for 15 min in order to create a steady state and then the dialysate was sampled for three periods of 10 min.
Catheterization
Under local anaesthesia (5 ml lidocaine, 10 mg ml−1) a catheter was introduced percutaneously into one of the femoral arteries for blood sampling and continuous measuring of blood pressure. The catheter was kept patent with isotonic saline delivered by an automatic flushing device (MX9504; Medex Medical, Lancashire, UK). In one SCI subject we were not able to catheterize an artery, and, hence, we have no blood sample data from this subject.
Blood flow
Subcutaneous adipose tissue blood flow (ATBF) was measured by the local 133xenon-washout method (Stallknecht et al. 1995) in the clavicular and in the umbilical regions contralateral to the regions in which microdialysis was performed. The tissue-to-blood partition coefficient (λ) for 133Xe was calculated according to the equation derived for abdominal subcutaneous adipose tissue by Bülow et al. (1987) as 0.22SFT + 2.99 (ml g−1), where SFT is abdominal skinfold thickness (in mm) measured using Harpenden skinfold calipers. λ was assumed not to vary between clavicular and abdominal adipose tissue as no (Bülow et al. 1987) or minor (Jansson & Lönnroth, 1995) variation in λ has been found between other subcutaneous sites.
Radiation
The total dose of radiation subjects were exposed to in this experiment was ∼0.03 mSv.
Sampling and analyses
Dialysate was collected in 200 μl capped microvials at 30 and 15 min intervals in the basal state and during exercise, respectively, for analysis of metabolites and at 60 min intervals for analysis of catecholamines. Dialysate sampling was delayed by 3 min relative to the rest of the experimental protocol to compensate for the transit time in the outlet tubing. Blood for determination of metabolites and hormones was collected in iced tubes from the femoral artery and immediately centrifuged. Blood was sampled (7.5 ml) 15 and 45 min into the basal period and 7, 22, 37 and 52 min into the exercise period. Blood for the determination of glycerol, lactate, glucose, β-hydroxybutyrate and triglyceride (TG) was stabilized with 20 i.u. heparin; blood for determination of free fatty acids (FFA), insulin, cortisol and growth hormone (GH) was stabilized with 500 kallikrein inhibitory units of aprotinin (Trasylol) and 1.5 mg EDTA, and blood for determination of catecholamines was stabilized with 4 μmol reduced glutathione and 20 i.u.heparin (ml blood)−1.
For plasma samples each value represents the average of duplicate determinations and for microdialysate samples each value represents a single determination. Plasma glucose and lactate concentrations were determined at once using a YSI 2300 glucose/lactate analyser (YSI Incorporated, Yellow Springs, OH, USA). All other plasma and microdialysate samples were kept at −20 °C until analysis, except samples for FFA and catecholamines, which were kept at −80 °C. Microdialysate glycerol, lactate and glucose concentrations were determined using a CMA 600 microdialysis analyser (CMA/Microdialysis AB). After precipitation of plasma proteins with perchloric acid plasma glycerol concentrations were determined by fluorometry (Frayn et al. 1989) adapted to a Monarch centrifugal analyser (Instrumentation Laboratory, Warrington, Cheshire, UK). Plasma FFA concentrations were determined using a commercial enzymatic kit (Wako) adapted to the Monarch centrifugal analyser, plasma TG concentrations were determined by spectrophotometry (Humphreys et al. 1990) adapted to the Monarch centrifugal analyser, and plasma d-β-hydroxybutyrate concentrations were determined by fluorometry (Galbo et al. 1979). Plasma insulin concentrations were determined using a commercial ELISA kit (DAKO Diagnostics, Cambs, UK). Plasma cortisol and growth hormone concentrations were determined by commercial RIA kits (CIS bio international, Gif-Sur-Yvette, France). Plasma and microdialysate catecholamine concentrations were determined by a single-isotope radioenzymatic assay (Ben-Jonathan & Porter, 1976). Briefly, 0.05 ml of plasma or dialysate was diluted 1:1 with 0.6 n perchloric acid containing 0.1 % ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid and incubated with [3H]S-adenosyl-l-methione (Amersham, Bucks, UK) and catechol-O-methyl transferase purified from rat liver. The O-methylated tritiated derivatives, [3H]normetadrenaline and [3H]metadrenaline, were extracted with toluene:isoamyl alcohol (3:2) and separated into two discrete spots using two-dimensional thin-layer chromatography. After drying the spots were cut out, extracted in 0.1 n acetic acid and the tritium activity was determined by liquid scintillation counting (2200CA, Packard Instrument Company, USA). The quantitative evaluation of samples was based upon standard curves. The limit of sensitivity for the assay was 3 and 2 fmol, respectively, for noradrenaline and adrenaline. The intra-assay coefficients of variation were 21 and 11 %, respectively, for noradrenaline and adrenaline concentrations of 0.1 nm, 7 and 10 %, respectively, for 1 nm, and 7 and 9 %, respectively, for 6 nm.
Calculations
The RR was calculated as (Dpmp–Dpmd)/Dpmp, where Dpmp is disintegrations per minute in 5 μl perfusate and Dpmd is disintegrations per minute in 5 μl dialysate. Interstitial concentrations were calculated as (Cd–Cp)/RR +Cp, where Cd is dialysate concentration and Cp is perfusate concentration.
Concentrations in venous plasma water (Cv,calc) were calculated by the principle described in Intaglietta & Johnson (1978). The calculation is based on Fick's law of diffusion for a thin membrane: J =–PS (Cc–Ci), where J is the substrate flux, P is the membrane permeability of the substrate, S the membrane surface area, Cc the concentration of substrate in the capillary and Ci is the substrate concentration in interstitial water. If this equation is integrated over the entire length of a capillary, the following expression is obtained: (Cv,calc–Ci)/(Ca–Ci) = e−PS/Q giving Cv,calc= (Ca–Ci)e−PS/Q+Ci for the tissue uptake situation or Cv,calc= ((Ci–Ca)(1–e−PS/Q)) +Ca for the tissue output situation. Ca is concentration in arterial plasma water, and Q is the blood water flow in which the metabolite is distributed. The PS product was set to 3 ml (100 g)−1 min−1 for lactate and glycerol, since values in this range have been found for molecules of similar sizes (Linde et al. 1974). The PS product was assumed to be constant within the range of blood flow variations registered.
Adipose tissue metabolite uptake or output was calculated as the product of the blood water flow in which the metabolite is distributed and the difference between Cv,calc and Ca.
Net adipose tissue glycerol output is equivalent to glycerol release and, accordingly, adipose tissue lipolysis, as glycerol utilization in adipose tissue is negligible (Dominguez & Herrera, 1976) and because one glycerol molecule is released for each triglyceride molecule hydrolysed.
Statistics
The computer program SigmaStat for Windows version 1.02 (Jandel Scientific Software, San Rafael, CA, USA) was used for statistical analysis. All data are presented as means ±s.e.m.. A two-way repeated measures analysis of variance (RM ANOVA) with site (clavicular/umbilical/plasma) and time as factors was used to test whether data measured at multiple sites changed with time for SCI or healthy subjects. If data were not normally distributed, a natural log (ln) transformation was performed before data analysis. Another two-way RM ANOVA with site and group (SCI/healthy) as factors was used to test whether data differed between sites or groups in the basal state or during exercise. If values differed between sites a Student-Newman-Keuls test was used post hoc to locate the difference. For substances measured only in plasma a two-way RM ANOVA with group and time as factors was used to test whether concentrations differed between groups or changed with time. Student's t test for unpaired data (data normally distributed) or a Mann-Whitney rank sum test (data not normally distributed) was used to test whether anthropometric data and exercise-induced changes differed between SCI and healthy subjects. A significance level of 0.05 in two-tailed testing was chosen a priori.
RESULTS
Anthropometric data
The SCI and healthy subjects did not differ significantly with respect to sex, age, body weight and height, abdominal skinfold thickness, whole body fat percentage and arm exercise V̇O2,peak (P > 0.05, Table 1). During exercise relative and absolute V̇O2, respiratory exchange ratio (RER), heart rate and rate of perceived exertion did not differ between SCI and healthy subjects (P > 0.05, Table 1).
Subcutaneous adipose tissue blood flow
In the basal state blood flow did not differ between SCI and healthy subjects (P > 0.05), and in both groups blood flow was higher in clavicular than in umbilical adipose tissue (P < 0.05, Fig. 1). In response to exercise adipose tissue blood flow increased in both SCI and healthy subjects. The exercise-induced increase in blood flow was considerably higher in clavicular adipose tissue of healthy subjects (P = 0.05) than in umbilical adipose tissue of these subjects and in both adipose tissues of SCI subjects (Table 2).
Figure 1.
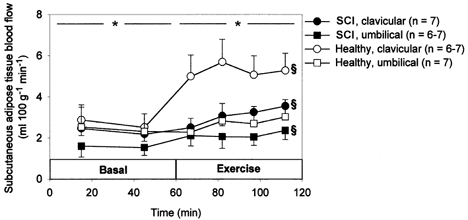
Blood flow in clavicular and umbilical subcutaneous adipose tissue measured by local 133xenon washout technique in 7 SCI and 7 healthy subjects in the basal state and during 60 min arm cycling at ∼60 %V̇O2,peak. Values are means ±s.e.m..*P < 0.05 between clavicular and umbilical adipose tissue. § Significant change with time.
Table 2.
Summary of major exercise-induced changes
| SCI | Healthy | ||||||
|---|---|---|---|---|---|---|---|
| Cl | Pl | Um | Cl | Pl | Um | 2-way RM ANOVA | |
| ATBF | ↑ | ↑ | ↑↑ | ↑ | Healthy-Cl > all others | ||
| Interstitial glycerol | ↑ | ↑↑ | ↑↑ | ↑↑↑ | SCI < Healthy, Cl < Um | ||
| Plasma glycerol | ↑ | ↑ | |||||
| Glycerol output | ↑ | ↑↑ | ↑↑ | ↑↑↑ | |||
| Interstitial lactate | ↑ | ↑ | ↑↑ | ↑↑ | SCI < Healthy | ||
| Plasma lactate | ↑ | ↑↑ | SCI < Healthy | ||||
| Plasma catecholamines | ↑ | ↑↑ | SCI < Healthy | ||||
No. of arrows indicates size of increase. Cl, clavicular adipose tissue; Pl, plasma; Um, umbilical adipose tissue; 2-way RM ANOVA, two-way repeated measures analysis of variance; ATBF, adipose tissue blood flow.
Relative recovery of glycerol, lactate and noradrenaline
The relative recovery (RR) of glycerol and lactate did not differ between SCI and healthy subjects (P > 0.05) or between clavicular (Cl) and umbilical (Um) adipose tissue (P > 0.05). RR of glycerol and lactate increased from rest to exercise (P < 0.05) (glycerol: SCI, 0.67 ± 0.07 to 0.75 ± 0.06 (Cl), 0.61 ± 0.07 to 0.69 ± 0.08 (Um); healthy, 0.74 ± 0.04 to 0.84 ± 0.03 (Cl), 0.66 ± 0.08 to 0.72 ± 0.07 (Um); lactate: SCI, 0.62 ± 0.08 to 0.71 ± 0.07 (Cl), 0.57 ± 0.07 to 0.65 ± 0.08 (Um); healthy, 0.73 ± 0.05 to 0.82 ± 0.04 (Cl), 0.65 ± 0.09 to 0.70 ± 0.10 (Um)). RR of noradrenaline also did not differ between SCI and healthy subjects (P > 0.05) or between clavicular and umbilical adipose tissue (P > 0.05) (SCI, 0.69 ± 0.04 (Cl), 0.63 ± 0.03 (Um); healthy, 0.71 ± 0.04 (Cl), 0.71 ± 0.05 (Um)).
Glycerol concentrations and output
In the basal state plasma and interstitial glycerol concentrations did not differ between SCI and healthy subjects (P > 0.05), and in both groups interstitial glycerol concentration was higher in umbilical than in clavicular adipose tissue (P < 0.05, Fig. 2). In response to exercise interstitial glycerol concentrations always increased (P < 0.05). The percentage increase in interstitial glycerol concentration during exercise was lower (P < 0.05) and the absolute increase in interstitial glycerol tended to be lower (P = 0.06) in SCI compared with healthy subjects and in clavicular compared with umbilical adipose tissue (percentage increase, P = 0.05; absolute increase, P = 0.1; Table 2). Arterial plasma glycerol concentrations increased in response to exercise in both SCI and healthy subjects (P < 0.05) with no significant difference between groups. Plasma glycerol concentrations were lower than interstitial glycerol concentrations (P < 0.05) both in the basal state and during exercise. In the basal state calculated glycerol output was higher in SCI compared with healthy subjects, but the difference was not significant (Fig. 2). Basal glycerol output did not differ significantly between clavicular and umbilical adipose tissue in either SCI or healthy subjects (P > 0.05). The percentage increase in glycerol output during exercise was lower in SCI compared with healthy subjects in both clavicular (38 vs. 85 %) and umbilical (89 vs. 286 %) adipose tissue although differences did not prove significant (Table 2).
Figure 2.
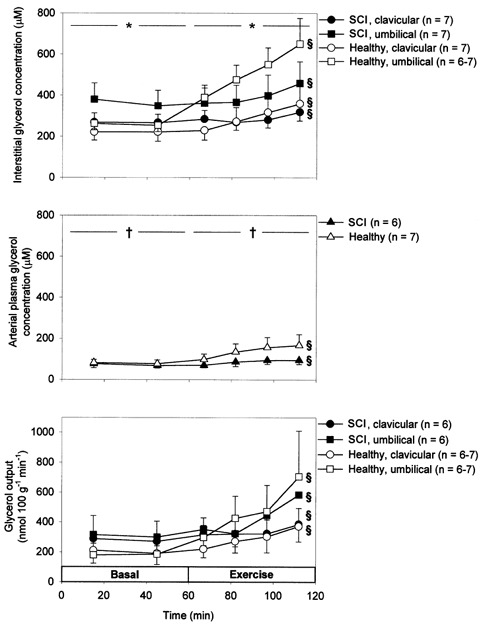
Arterial plasma (middle) as well as interstitial (top) glycerol concentrations and glycerol output (bottom) from clavicular and umbilical subcutaneous adipose tissue in 7 SCI and 7 healthy subjects in the basal state and during 60 min arm cycling at ∼60 %V̇O2,peak. Values are means ±s.e.m..*P < 0.05 between clavicular and umbilical adipose tissue. § Significant change with time. †P < 0.05 between interstitial and plasma concentration.
Lactate concentrations and exchange
In the basal state arterial plasma lactate concentrations were lower than interstitial concentrations (P < 0.05, Fig. 3). These concentrations, as well as calculated lactate output, did not differ between SCI and healthy subjects in the basal state (P > 0.05, Fig. 3). However, interstitial concentrations as well as lactate output in the basal state were higher in clavicular than in umbilical adipose tissue (P < 0.05). In response to exercise the increase in plasma lactate was accompanied by a transient lactate uptake in the studied adipose tissues. Plasma lactate concentrations increased less in SCI compared with healthy subjects (P < 0.05) as did interstitial lactate concentrations (P < 0.05, Table 2).
Figure 3.
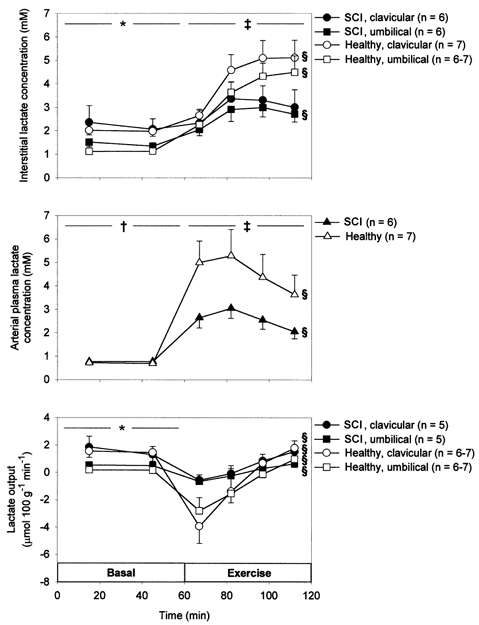
Arterial plasma (middle) as well as interstitial (top) lactate concentrations and lactate output (bottom) from clavicular and umbilical subcutaneous adipose tissue in 7 SCI and 7 healthy subjects in the basal state and during 60 min arm cycling at ∼60 %V̇O2,peak. Values are means ±s.e.m..*P < 0.05 between clavicular and umbilical adipose tissue. ‡P < 0.1 between SCI and healthy subjects. § Significant change with time. †P < 0.05 between interstitial and plasma concentration.
Glucose concentrations
Arterial plasma glucose concentration did not differ between SCI and healthy subjects (P > 0.05), and in both groups arterial concentration decreased in response to exercise (P < 0.05, Table 3). Dialysate glucose concentration tended to be lower in SCI compared with healthy subjects both in the basal state (P = 0.09) (SCI, 4.16 ± 0.13 (Cl), 4.06 ± 0.09 (Um); healthy, 4.51 ± 0.28 (Cl), 4.41 ± 0.25 (Um)) and during exercise (P = 0.08) (SCI, 4.13 ± 0.18 (Cl), 4.16 ± 0.08 (Um); healthy, 4.44 ± 0.18 (Cl), 4.97 ± 0.37 (Um)).
Table 3.
Arterial plasma hormone and metabolite concentrations in the basal state and during 60 min arm cycling at ∼60 %V̇O2,peak
| Basal | Exercise | ||||||
|---|---|---|---|---|---|---|---|
| 15 min | 45 min | 7 min | 22 min | 37 min | 52 min | ||
| Insulin (pM) *§ | SCI | 45 ± 6 | 48 ± 7 | 49 ± 6 | 43 ± 8 | 34 ± 8 | 30 ± 6 |
| Healthy | 26 ± 3 | 26 ± 4 | 31 ± 4 | 29 ± 2 | 28 ± 2 | 22 ± 3 | |
| Cortisol (nm)§ | SCI | 152 ± 28 | 199 ± 46 | 170 ± 38 | 249 ± 48 | 287 ± 52 | 321 ± 51 |
| Healthy | 263 ± 53 | 227 ± 52 | 268 ± 63 | 349 ± 72 | 334 ± 76 | 323 ± 75 | |
| GH (ng ml−1)*§ | SCI | 0.09 ± 0.02 | 0.22 ± 0.12 | 0.94 ± 0.77 | 4.14 ± 1.65 | 7.11 ± 1.69 | 8.33 ± 1.95 |
| Healthy | 1.41 ± 0.78 | 1.98 ± 1.03 | 2.19 ± 0.94 | 10.4 ± 3.9 | 17.3 ± 6.2 | 15.9 ± 5.6 | |
| Glucose (mm)§ | SCI | 5.27 ± 0.14 | 5.25 ± 0.13 | 5.26 ± 0.12 | 4.94 ± 0.20 | 4.75 ± 0.24 | 4.69 ± 0.24 |
| Healthy | 5.51 ± 0.13 | 5.48 ± 0.14 | 5.45 ± 0.16 | 5.34 ± 0.18 | 5.25 ± 0.20 | 5.09 ± 0.17 | |
| FFA (μm)§ | SCI | 652 ± 93 | 620 ± 80 | 454 ± 84 | 424 ± 111 | 409 ± 102 | 459 ± 74 |
| Healthy | 712 ± 113 | 707 ± 135 | 552 ± 143 | 688 ± 223 | 815 ± 274 | 920 ± 297‖ | |
| TG (μm) | SCI | 1259 ± 264 | 1296 ± 287 | 1332 ± 307 | 1316 ± 305 | 1280 ± 303 | 1245 ± 299 |
| Healthy | 1098 ± 164 | 1147 ± 161 | 1177 ± 175 | 1148 ± 179 | 1341 ± 183 | 1127 ± 180 | |
| d-β-Hydroxy-butyrate (μm)§ | SCI | 157 ± 37 | 155 ± 36 | 96 ± 21 | 77 ± 17 | 73 ± 14 | 63 ± 12 |
| Healthy | 380 ± 203 | 350 ± 187 | 229 ± 129 | 181 ± 101 | 161 ± 73 | 150 ± 61 | |
Values are means ±s.e.m. for 6 SCI and 7 healthy subjects.
P < 0.05 between groups.
Significant change with time.
P < 0.05vs. exercise time 7 min.
GH, growth hormone; FFA, free fatty acids; TG, triglyceride.
Catecholamine concentrations
In the basal state arterial noradrenaline and adrenaline concentrations did not differ significantly between SCI and healthy subjects (P > 0.05, Fig. 4). The concentrations were higher than interstitial catecholamine concentrations, which also did not differ significantly between groups (P > 0.05) or between clavicular and umbilical adipose tissue (P > 0.05, Fig. 4). In response to exercise, plasma catecholamine concentrations increased less markedly in SCI compared with healthy subjects (P < 0.05, Table 2). This was true also for interstitial concentrations (P < 0.05), which remained lower than plasma concentrations (P < 0.05) indicating the net uptake of both noradrenaline and adrenaline in adipose tissue. Ratios between interstitial and arterial plasma catecholamine concentrations were always higher for noradrenaline than for adrenaline (all: P < 0.05) (basal: SCI, 0.56 ± 0.10 (noradrenaline) vs. 0.30 ± 0.09 (adrenaline) (Cl), 0.50 ± 0.18 vs. 0.29 ± 0.06 (Um); healthy, 0.63 ± 0.12 vs. 0.34 ± 0.04 (Cl), 0.46 ± 0.04 vs. 0.29 ± 0.05 (Um); exercise: SCI, 0.46 ± 0.09 vs. 0.29 ± 0.05 (Cl), 0.38 ± 0.10 vs. 0.25 ± 0.06 (Um); healthy, 0.56 ± 0.10 vs. 0.29 ± 0.06 (Cl), 0.63 ± 0.11 vs. 0.26 ± 0.04 (Um)). Furthermore, ratios between interstitial and arterial plasma noradrenaline concentrations tended to be lower in umbilical adipose tissue in SCI compared with healthy subjects (0.38 ± 0.10 (n = 5) vs. 0.63 ± 0.11 (n = 6), P > 0.05) and in umbilical compared with clavicular adipose tissue in SCI subjects (0.38 ± 0.10 (n = 5) vs. 0.46 ± 0.09 (n = 6), P > 0.05). Corresponding ratios between interstitial and arterial plasma adrenaline concentrations did not differ significantly (0.25 ± 0.06 (n = 5) vs. 0.26 ± 0.04 (n = 6), P > 0.05, and 0.25 ± 0.06 (n = 5) vs. 0.29 ± 0.05 (n = 6), P > 0.05).
Figure 4.
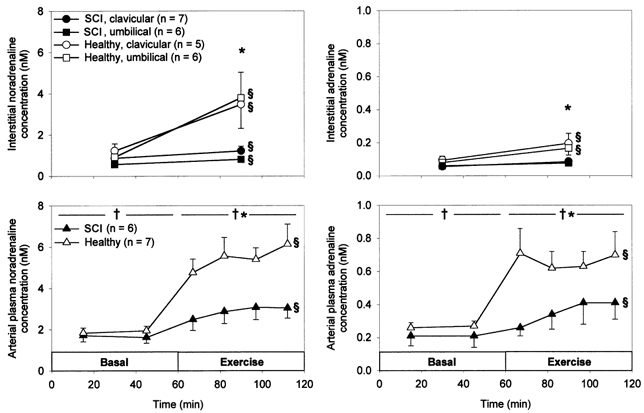
Arterial plasma noradrenaline (left, bottom) and adrenaline (right, bottom) concentrations as well as interstitial noradrenaline (left, top) and adrenaline (right, top) concentrations in clavicular and umbilical subcutaneous adipose tissue in 7 SCI and 7 healthy subjects in the basal state and during 60 min arm cycling at ∼60 %V̇O2,peak. Values are means ±s.e.m..*P < 0.05 between SCI and healthy subjects. § Significant change with time. †P < 0.05 between interstitial and plasma concentration.
Plasma insulin, cortisol, GH, FFA, TG and d-β-hydroxybutyrate concentrations
Plasma insulin concentration was higher in SCI than in healthy subjects both in the basal state and during exercise (P < 0.05, Table 3). In response to exercise plasma insulin concentration decreased in both groups (P < 0.05). The percentage decrease in plasma insulin concentration did not differ significantly between groups (P > 0.05), but the absolute decrease tended to be larger (P = 0.07) in SCI compared with healthy subjects. Plasma cortisol concentration did not differ between groups at rest (P > 0.05) and always increased in response to exercise (P < 0.05). Plasma GH concentration was lower in SCI compared with healthy subjects at rest (P < 0.05) and increased markedly in response to exercise in both groups (P < 0.05). Neither the absolute nor the percentage exercise-induced increases of plasma cortisol and GH differed significantly between SCI and healthy subjects. Plasma FFA and d-β-hydroxybutyrate concentrations did not differ between SCI and healthy subjects (P > 0.05) and decreased in both groups at the onset of exercise (P < 0.05, Table 3). During the exercise period FFA concentrations increased again in healthy subjects (P < 0.05). Plasma TG concentrations did not differ between SCI and healthy subjects (P > 0.05) and did not change with time (P > 0.05, Table 3).
DISCUSSION
In the present study the exercise-induced increase in interstitial glycerol concentration in subcutaneous adipose tissue was significantly lower in SCI compared with healthy subjects (Fig. 2, Table 2). Furthermore, in SCI subjects the exercise-induced increase in subcutaneous adipose tissue blood flow was significantly lower (clavicular region) or equal to (umbilical region) the increase in healthy subjects (Fig. 1, Table 2). As the increase in arterial plasma glycerol did not differ significantly between groups it follows that the exercise-induced increase in lipolysis was lower in SCI compared with healthy subjects. This interpretation of data is substantiated by the calculation of a lower exercise-induced increase in subcutaneous adipose tissue glycerol output, i.e. lipolysis, in SCI compared with healthy subjects, although the difference between groups, probably reflecting the composite nature of the variable and the inherent high variation (Stallknecht et al. 1999), did not prove significant (Fig. 2, Table 2). Because a lower exercise-induced increase in lipolysis in SCI compared with healthy subjects was found in both sympathetically innervated and sympathetically decentralized adipose tissue and because plasma adrenaline and noradrenaline concentrations were reduced in SCI subjects, the results indicate that circulating catecholamines account for the exercise-induced increase in lipolysis (Fig. 4).
The exercise-induced increase in interstitial glycerol concentration was significantly higher in umbilical compared with clavicular adipose tissue in both SCI and healthy subjects (Fig. 2, Table 2). On the other hand, the exercise-induced increase in blood flow was significantly higher in clavicular adipose tissue of healthy subjects than in umbilical adipose tissue of these subjects as well as in both adipose tissues of SCI subjects (Fig. 1, Table 2). The percentage increase with exercise of calculated adipose tissue glycerol output was higher in umbilical compared with clavicular adipose tissue in both SCI and healthy subjects (Fig. 2, Table 2), but the difference did not prove significant, a fact probably reflecting the high variation inherent in a composite variable (Stallknecht et al. 1999). The fact that the exercise-induced increase in lipolysis was not lower in umbilical relative to clavicular adipose tissue in SCI compared with healthy subjects indicates that sympathetic nerves are not important for the exercise-induced increase in lipolysis.
From the present experiment we cannot conclude whether the sensitivity of lipolysis to catecholamines or other hormones differs between SCI and healthy subjects or between different adipose tissue depots, but this could be the case. Chronic compensatory mechanisms may have developed in response to the primary defect in SCI subjects, which makes it difficult to compare SCI and healthy subjects. Previously, we found no indication of hypersensitivity to adrenaline in adrenalectomized subjects (Howlett et al. 1999). In contrast, hypersensitivity to adrenaline has been demonstrated in diabetics with autonomic neuropathy and normal basal plasma adrenaline concentrations (Hilsted et al. 1987) indicating that loss of sympathetic nerve terminals is essential for the development of adrenergic hypersensitivity. The existence of such hypersensitivity would, in our study, tend to blur the influence of sympathetic decentralization in umbilical adipose tissue. Thus, even though it is unlikely that adrenergic hypersensitivity fully compensated for the loss of neuronal activity, we cannot completely rule out that sympathetic nerve activity plays a role in exercise-induced lipolysis. Additional experiments in which adrenaline was infused during exercise in SCI subjects to mimic levels seen in healthy subjects would have been helpful but were not acceptable to the patients.
As insulin is known to inhibit adipose tissue lipolysis (Hales et al. 1978), differences in insulin concentrations might influence our results. In fact, plasma insulin concentrations were higher in SCI than in healthy subjects (Table 3), which can be ascribed either to decreased insulin sensitivity in SCI subjects (Karlsson, 1999) due to a more inactive lifestyle or to a loss of tonic inhibitory sympathetic control of insulin secretion in SCI subjects. However, plasma insulin concentration decreased similarly in both SCI and healthy subjects in response to exercise and, accordingly, the influence of plasma insulin on adipose tissue lipolysis was probably the same in the two groups. It has previously been shown that cortisol and GH play a role in lipolysis at rest (Frayn, 1998). In the present study basal plasma cortisol concentrations did not differ between groups, but basal plasma GH concentrations were lower in SCI compared with healthy subjects (Table 3). However, neither the exercise-induced increase in plasma cortisol nor the exercise-induced increase in plasma GH differed significantly between SCI and healthy subjects. Accordingly, it is unlikely that differences between groups in exercise-induced lipolysis were due to differences in GH and cortisol responses to exercise. This view is supported by the fact that the effects of increases in plasma concentrations of these hormones on lipolysis would not be expected to occur within the relatively short duration of the exercise (Frayn, 1998).
The lower exercise-induced increase in lipolysis in SCI compared with healthy subjects was not accompanied by a lower rate of fat oxidation during exercise as judged from RER measurements. This may reflect the fact that the supply of FFA from plasma is not limiting FFA oxidation during exercise or that oxidation of intramuscular TG is compensatorily increased when FFA availability is reduced.
Previous microdialysis studies in which microdialysate glycerol concentration increased during exercise in umbilical (Arner et al. 1990; Wennlund et al. 1994; Hellström et al. 1996) and gluteal (Arner et al. 1990) adipose tissue have suggested that exercise stimulates lipolysis in subcutaneous adipose tissue. An increased dialysate glycerol concentration can, however, reflect an increased relative recovery of glycerol as well as an increased interstitial glycerol concentration. The present study adds to previous studies by showing that both the interstitial glycerol concentration (Fig. 2) and relative recovery of glycerol increase during exercise in both umbilical and clavicular adipose tissue.
One previous study has addressed subcutaneous adipose tissue lipolysis in exercising SCI and healthy control subjects, and in that study 3–6 min handgrip exercise was used as intervention (Karlsson et al. 1997). This mild workload, however, in healthy subjects only stimulated lipolysis in the umbilical region (Karlsson et al. 1997), a fact that made it impossible to evaluate the relative role of impaired sympathetic nerve activity and impaired plasma catecholamine response, respectively, for the demonstrated reduction in exercise-induced lipolysis in the umbilical region of SCI subjects (Karlsson et al. 1997). In our study we detected an increase in lipolysis both at the clavicular and at the umbilical level in both SCI and healthy subjects most likely because of the more prolonged and strenuous dynamic exercise protocol that we applied. Also, in the previous study no attempt was undertaken to determine interstitial concentrations of noradrenaline in the tissues studied (Karlsson et al. 1997).
In the present study, plasma noradrenaline and adrenaline concentrations were lower during exercise in SCI compared with healthy subjects (Fig. 4). Usually, the plasma catecholamine concentration depends on the percentage of maximal oxygen uptake (Kjær et al. 1988) and in the present study SCI and healthy subjects exercised at similar percentages of peak oxygen uptake (Table 1). Correspondingly, heart rate and rate of perceived exertion did not differ between groups (Table 1) and hence the degree of sympathetic stimulation from the autonomic centres in the brain was probably the same in the two groups. The lower plasma noradrenaline concentration during exercise in SCI compared with healthy subjects probably reflects a lower release of noradrenaline from the decentralized nerves in the lower part of the body in the SCI subjects. That release of noradrenaline during exercise is in fact lower in the decentralized adipose tissue is indicated by the ratios between interstitial and arterial plasma noradrenaline concentrations which tended to be lower in umbilical adipose tissue in SCI compared with healthy subjects and in umbilical compared with clavicular adipose tissue in SCI subjects.
While the ratio between interstitial and arterial plasma noradrenaline concentration may be an indicator of local sympathetic nerve activity, the interstitial noradrenaline concentration per se is apparently a poor indicator of local sympathetic activity in adipose tissue. Thus, the interstitial concentrations in both SCI and healthy subjects were lower than plasma concentrations and, furthermore, in SCI subjects identical in sympathetically innervated and decentralized regions, respectively (Fig. 4). The finding that for adrenaline a concentration gradient from plasma to adipose tissue interstitial fluid was seen is in accordance with the fact that adrenaline is primarily secreted in the adrenal medulla. Noradrenaline, however, is primarily released from sympathetic nerves in tissues, and we expected a concentration gradient from interstitial fluid to plasma (Goldstein et al. 1983). Nevertheless, in a recent study Coppack et al. (1998) found noradrenaline concentrations in the basal state to be slightly lower in a vein draining subcutaneous, abdominal adipose tissue than in arterial plasma (lean subjects, 0.80 ± 0.08 vs. 0.88 ± 0.08 nm; obese subjects, 0.73 ± 0.07 vs. 0.93 ± 0.09 nm) indicating net uptake of noradrenaline in adipose tissue. It has been estimated that lung contributes 30 % and kidney and skeletal muscle each contribute 20 % to whole body noradrenaline spillover to plasma at rest (Esler et al. 1984). Probably, sympathetic outflow is higher to other organs than to subcutaneous adipose tissue making the contribution from subcutaneous adipose tissue to whole body noradrenaline spillover small and promoting a net uptake of noradrenaline in this tissue. Our study indicated that noradrenaline is, in fact, released in adipose tissue, whereas adrenaline is not, since there were higher concentration ratios between interstitial fluid and plasma for noradrenaline than for adrenaline both at rest and during exercise (Fig. 4).
Interstitial catecholamine concentrations determined in the present study are minimum concentrations, as we cannot completely exclude the possibility that catecholamines were degraded during the sampling of dialysate. However, in a previous study we infused adrenaline intravenously in healthy subjects (Stallknecht et al. 1997). It is reassuring that ratios between interstitial and plasma adrenaline concentrations in that study (0.31–0.47) were similar to ratios in the present study even though dialysate collection time in the present study was 60 min compared with 15 min in the previous study (Stallknecht et al. 1997).
In the present study lactate was released from adipose tissue in the basal state (Fig. 3) in accordance with previous findings (Stallknecht et al. 1997; Ellmerer et al. 1998). We found interstitial lactate concentrations to be higher in clavicular than in umbilical adipose tissue in both SCI and healthy subjects (Fig. 3), which is in contrast to a study by Karlsson et al. (1995) in which interstitial lactate concentration did not differ between tissues. We can think of no reason for this discrepancy. In the present study, during exercise arterial lactate concentrations increased prior to the increase in interstitial lactate concentrations indicating lactate uptake in adipose tissue at the onset of exercise (Fig. 3). Using the open-flow microperfusion technique, uptake of lactate in adipose tissue during exercise has previously been demonstrated (Ellmerer et al. 1998). Also using the arterio-venous catheterization technique lactate uptake in subcutaneous adipose tissue during exercise has been found (Mulla et al. 2000). In the present study lactate output/uptake did not differ significantly between SCI and healthy subjects (Fig. 3), which is in agreement with a previous study in which dialysate lactate concentration from subcutaneous, abdominal adipose tissue in healthy subjects was not influenced by either α- or β-adrenoceptor blockade during exercise (Hellström et al. 1996). In the present study plasma lactate concentrations increased less in SCI compared with healthy subjects during exercise (Fig. 3, Table 2), which can probably be ascribed to the lower plasma adrenaline response in the former subjects. In line with this view, adrenaline may enhance muscle glycogenolysis during exercise and in exercising adrenalectomized subjects adrenaline infusion has been shown to increase plasma lactate concentrations (Howlett et al. 1999).
In the present study blood flow was always higher in clavicular than in umbilical adipose tissue both in the basal state and during exercise (Fig. 1). In the study by Karlsson et al. (1997) there was no significant difference in blood flow between these tissues in SCI subjects, whereas in healthy subjects blood flow was 75 % higher in clavicular than in umbilical tissue. In the present study adipose tissue blood flow did not differ significantly between SCI and healthy subjects in the basal state and generally increased in response to exercise (Fig. 1). A normal subcutaneous adipose tissue blood flow has previously been found in SCI subjects (Sørensen et al. 1994), and sympathetic blockade by epidural anaesthesia in healthy volunteers has been shown not to influence subcutaneous blood flow (Henriksen & Alsner, 1975). Also, in agreement with our findings, in dogs neither basal nor exercise-induced increase in adipose tissue blood flow was influenced by acute denervation of the adipose tissue (Bülow & Madsen, 1986). In a previous study it was suggested that adipose tissue vasodilatation during exercise is secondary to metabolic events associated with lipolysis (Bülow, 1981). However, this mechanism cannot explain the higher clavicular relative to umbilical adipose tissue blood flow during exercise seen in the present study because adipose tissue lipolysis was lower in clavicular compared with umbilical tissue (Fig. 2).
In conclusion, subcutaneous adipose tissue lipolysis is stimulated less in SCI compared with healthy control subjects during submaximal exercise at comparable relative workloads. This difference probably reflects impaired increase in plasma catecholamine concentrations rather than impaired local sympathetic nerve activity during exercise in SCI subjects. Adipose tissue blood flow generally increases during exercise and is not significantly lower in sympathetically decentralized than in innervated adipose tissue and is not intimately coupled to local lipolysis. As blood flow differs between clavicular and umbilical adipose tissue the interstitial glycerol concentration per se cannot be interpreted as an accurate marker of lipolysis. Lactate metabolism in adipose tissue is not influenced by the presence of sympathetic nerves. Finally, the interstitial noradrenaline concentration per se is a poor marker of local sympathetic activity in subcutaneous adipose tissue.
Acknowledgments
We thank Inge Rasmussen, Regitze Kraunsøe, Lisbeth Kall, Vibeke Staffeldt and Connie Temdrup for skilled technical assistance. This study was supported by grants from the Novo Nordisk Foundation, Danish Diabetes Association, Weimann's foundation and Danish National Research Foundation (504–14).
References
- Arner P. Impact of exercise on adipose tissue metabolism in humans. International Journal of Obesity. 1995;19(suppl. 4):S18–21. [PubMed] [Google Scholar]
- Arner P, Kriegholm E, Engfeldt P, Bolinder J. Adrenergic regulation of lipolysis in situ at rest and during exercise. Journal of Clinical Investigation. 1990;85:893–898. doi: 10.1172/JCI114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N, Porter JC. A sensitive radioenzymatic assay for dopamine, norepinephrine, and epinephrine in plasma and tissue. Endocrinology. 1976;98:1497–1507. doi: 10.1210/endo-98-6-1497. [DOI] [PubMed] [Google Scholar]
- Bülow J. Human adipose tissue blood flow during prolonged exercise, III. Effect of beta-adrenergic blockade, nicotinic acid and glucose infusion. Scandinavian Journal of Clinical and Laboratory Investigation. 1981;41:415–424. doi: 10.3109/00365518109092065. [DOI] [PubMed] [Google Scholar]
- Bülow J, Jelnes R, Astrup A, Madsen J, Vilmann P. Tissue/blood partition coefficients for xenon in various adipose tissue depots in man. Scandinavian Journal of Clinical and Laboratory Investigation. 1987;47:1–3. doi: 10.1080/00365518709168861. [DOI] [PubMed] [Google Scholar]
- Bülow J, Madsen J. Exercise-induced increase in dog adipose tissue blood flow before and after denervation. Acta Physiologica Scandinavica. 1986;128:471–474. doi: 10.1111/j.1748-1716.1986.tb08001.x. [DOI] [PubMed] [Google Scholar]
- Coppack SW, Horowitz JF, Paramore DS, Cryer PE, Royal HD, Klein S. Whole body, adipose tissue, and forearm norepinephrine kinetics in lean and obese women. American Journal of Physiology. 1998;275:E830–834. doi: 10.1152/ajpendo.1998.275.5.E830. [DOI] [PubMed] [Google Scholar]
- Dominguez MC, Herrera E. The effect of glucose, insulin and adrenaline on glycerol metabolism in vitro in rat adipose tissue. Biochemical Journal. 1976;158:183–190. doi: 10.1042/bj1580183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellmerer M, Schaupp L, Trajanoski Z, Jobst G, Moser I, Urban G, Skrabal F, Wach P. Continuous measurement of subcutaneous lactate concentration during exercise by combining open-flow microperfusion and thin-film lactate sensors. Biosensors and Bioelectronics. 1998;13:1007–1013. doi: 10.1016/s0956-5663(98)00002-5. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Leonard P, Sacharias N, Burke F, Johns J, Blombery P. Contribution of individual organs to total noradrenaline release in humans. Acta Physiologica Scandinavica. 1984;(suppl. 527):11–16. [PubMed] [Google Scholar]
- Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, Vernon JD, Walsh JJ. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- Frayn KN. Regulation of fatty acid delivery in vivo. Advances in Experimental Medicine and Biology. 1998;441:171–179. doi: 10.1007/978-1-4899-1928-1_16. [DOI] [PubMed] [Google Scholar]
- Frayn KN, Coppack SW, Humphreys SM, Whyte PL. Metabolic characteristics of human adipose tissue in vivo. Clinical Science. 1989;76:509–516. doi: 10.1042/cs0760509. [DOI] [PubMed] [Google Scholar]
- Galbo H, Holst JJ, Christensen NJ. The effect of different diets and of insulin on the hormonal response to prolonged exercise. Acta Physiologica Scandinavica. 1979;107:19–32. doi: 10.1111/j.1748-1716.1979.tb06438.x. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, McCarty R, Polinsky RJ, Kopin IJ. Relationship between plasma norepinephrine and sympathetic neural activity. Hypertension. 1983;5:552–559. doi: 10.1161/01.hyp.5.4.552. [DOI] [PubMed] [Google Scholar]
- Guttmann L. Spinal Cord Injuries. Comprehensive Management and Research. Oxford: Blackwell Scientific; 1976. [Google Scholar]
- Hales CN, Luzio JP, Siddle K. Hormonal control of adipose-tissue lipolysis. Biochemical Society Symposia. 1978;43:97–135. [PubMed] [Google Scholar]
- Hellström L, Blaak E, Hagström-Toft E. Gender differences in adrenergic regulation of lipid mobilization during exercise. International Journal of Sports Medicine. 1996;17:439–447. doi: 10.1055/s-2007-972875. [DOI] [PubMed] [Google Scholar]
- Henriksen O, Alsner T. Effect of spinal sympathetic blockade upon local regulation of blood flow in subcutaneous tissue. Acta Physiologica Scandinavica. 1975;95:83–88. doi: 10.1111/j.1748-1716.1975.tb10028.x. [DOI] [PubMed] [Google Scholar]
- Hilsted J, Richter E, Madsbad S, Tronier B, Christensen NJ, Hildebrandt P, Damkjaer M, Galbo H. Metabolic and cardiovascular responses to epinephrine in diabetic autonomic neuropathy. New England Journal of Medicine. 1987;317:421–426. doi: 10.1056/NEJM198708133170705. [DOI] [PubMed] [Google Scholar]
- Howlett K, Galbo H, Lorentsen J, Bergeron R, Zimmerman-Belsing T, Bülow J, Feldt-Rasmussen U, Kjær M. Effect of adrenaline on glucose kinetics during exercise in adrenalectomised humans. Journal of Physiology. 1999;519:911–921. doi: 10.1111/j.1469-7793.1999.0911n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys SM, Fisher RM, Frayn KN. Micro-method for measurement of sub-nanomole amounts of triacylglycerol. Annals of Clinical Biochemistry. 1990;27:597–598. doi: 10.1177/000456329002700613. [DOI] [PubMed] [Google Scholar]
- Intaglietta M, Johnson PC. Principles of capillary exchange. In: Johnson PC, editor. Peripheral Circulation. New York: Wiley; 1978. pp. 141–166. [Google Scholar]
- Issekutz B., Jr Role of beta-adrenergic receptors in mobilization of energy sources in exercising dogs. Journal of Applied Physiology. 1978;44:869–876. doi: 10.1152/jappl.1978.44.6.869. [DOI] [PubMed] [Google Scholar]
- Jansson PA, Lönnroth P. Comparison of two methods to assess the tissue/blood partition coefficient for xenon in subcutaneous adipose tissue in man. Clinical Physiology. 1995;15:47–55. doi: 10.1111/j.1475-097x.1995.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Karlsson AK. Insulin resistance and sympathetic function in high spinal cord injury. Spinal Cord. 1999;37:494–500. doi: 10.1038/sj.sc.3100844. [DOI] [PubMed] [Google Scholar]
- Karlsson AK, Attvall S, Jansson PA, Sullivan L, Lönnroth P. Influence of the sympathetic nervous system on insulin sensitivity and adipose tissue metabolism: A study in spinal cord-injured subjects. Metabolism: Clinical and Experimental. 1995;44:52–58. doi: 10.1016/0026-0495(95)90289-9. [DOI] [PubMed] [Google Scholar]
- Karlsson AK, Elam M, Friberg P, Biering-Sørensen F, Sullivan L, Lönnroth P. Regulation of lipolysis by the sympathetic nervous system: a microdialysis study in normal and spinal cord-injured subjects. Metabolism: Clinical and Experimental. 1997;46:388–394. doi: 10.1016/s0026-0495(97)90053-6. [DOI] [PubMed] [Google Scholar]
- Kjær M, Bangsbo J, Lortie G, Galbo H. Hormonal response to exercise in humans: influence of hypoxia and physical training. American Journal of Physiology. 1988;254:R197–203. doi: 10.1152/ajpregu.1988.254.2.R197. [DOI] [PubMed] [Google Scholar]
- Kjær M, Pollack SF, Mohr T, Weiss H, Gleim GW, Bach FW, Nicolaisen T, Galbo H, Ragnarsson KT. Regulation of glucose turnover and hormonal responses during electrical cycling in tetraplegic humans. American Journal of Physiology. 1996;271:R191–199. doi: 10.1152/ajpregu.1996.271.1.R191. [DOI] [PubMed] [Google Scholar]
- Krum H, Brown DJ, Rowe PR, Louis WJ, Howes LG. Steady state plasma [3H]-noradrenaline kinetics in quadriplegic chronic spinal cord injury patients. Journal of Autonomic Pharmacology. 1990;10:221–226. doi: 10.1111/j.1474-8673.1990.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Linde B, Chisolm G, Rosell S. The influence of sympathetic activity and histamine on the blood- tissue exchange of solutes in canine adipose tissue. Acta Physiologica Scandinavica. 1974;92:145–155. doi: 10.1111/j.1748-1716.1974.tb05731.x. [DOI] [PubMed] [Google Scholar]
- Lundborg P, Aström H, Bengtsson C, Fellenius E, Von Schenck H, Svensson L, Smith U. Effect of beta-adrenoceptor blockade on exercise performance and metabolism. Clinical Science. 1981;61:299–305. doi: 10.1042/cs0610299. [DOI] [PubMed] [Google Scholar]
- Macdonald IA, Bennett T, Brown AM, Wilcox RG, Skene AM. The effects of acute or chronic ingestion of propranolol or metoprolol on the metabolic and hormonal responses to prolonged, submaximal exercise in hypertensive men. British Journal of Clinical Pharmacology. 1984;17:283–293. doi: 10.1111/j.1365-2125.1984.tb02343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulla NA, Simonsen L, Bülow J. Post-exercise adipose tissue and skeletal muscle lipid metabolism in humans: the effects of exercise intensity. Journal of Physiology. 2000;524:919–928. doi: 10.1111/j.1469-7793.2000.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normell LA. Distribution of impaired cutaneous vasomotor and sudomotor function in paraplegic man. Scandinavian Journal of Clinical and Laboratory Investigation. 1974;138:25–41. [PubMed] [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. Journal of Neuroscience Methods. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Schmid A, Huonker M, Stahl F, Barturen JM, König D, Heim H, Lehmann M, Keul J. Free plasma catecholamines in spinal cord injured persons with different injury levels at rest and during exercise. Journal of the Autonomic Nervous System. 1998;68:96–100. doi: 10.1016/s0165-1838(97)00127-6. [DOI] [PubMed] [Google Scholar]
- Sørensen JL, Hauge EN, Wroblewski H, Biering-Sørensen F. Cutaneous and subcutaneous blood flow rates in paraplegic humans investigated by 133xenon wash-out. Methodological considerations. Clinical Physiology. 1994;14:281–289. doi: 10.1111/j.1475-097x.1994.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Stallknecht B, Bülow J, Frandsen E, Galbo H. Desensitization of human adipose tissue to adrenaline stimulation studied by microdialysis. Journal of Physiology. 1997;500:271–282. doi: 10.1113/jphysiol.1997.sp022017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallknecht B, Madsen J, Galbo H, Bülow J. Evaluation of the microdialysis technique in the dog fat pad. American Journal of Physiology. 1999;276:E588–595. doi: 10.1152/ajpendo.1999.276.3.E588. [DOI] [PubMed] [Google Scholar]
- Stallknecht B, Simonsen L, Bülow J, Vinten J, Galbo H. Effect of training on epinephrine-stimulated lipolysis determined by microdialysis in human adipose tissue. American Journal of Physiology. 1995;269:E1059–1066. doi: 10.1152/ajpendo.1995.269.6.E1059. [DOI] [PubMed] [Google Scholar]
- Van Baak MA. Beta-adrenoceptor blockade and exercise. An update. Sports Medicine. 1988;5:209–225. doi: 10.2165/00007256-198805040-00002. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Stjernberg L. Sympathetic activity in man after spinal cord injury. Outflow to skin below the lesion. Brain. 1984;107:183–198. doi: 10.1093/brain/107.1.183. [DOI] [PubMed] [Google Scholar]
- Wennlund A, Wahrenberg H, Hagström-Toft E, Bolinder J, Arner P. Lipolytic and cardiac responses to various forms of stress in humans. International Journal of Sports Medicine. 1994;15:408–413. doi: 10.1055/s-2007-1021079. [DOI] [PubMed] [Google Scholar]


