Abstract
Group I projections from intrinsic plantar muscles to motoneurones (MNs) of human leg and thigh muscles were investigated. Changes in firing probability of single motor units (MUs) in the tibialis anterior (TA), peroneus brevis (Per brev), soleus (Sol), gastrocnemius medialis (GM), vastus lateralis (VL), semitendinosus (ST) and biceps (Bi) were studied after electrical stimuli applied to: (i) the tibial nerve (TN) at ankle level, (ii) the corresponding homonymous nerve, and (iii) the skin of the heel, to mimic the TN-induced cutaneous sensation.
Homonymous facilitation, attributable to monosynaptic Ia excitation, was found in all the sampled units. Early heteronymous excitation elicited by TN stimulation was found in many MUs. Later effects (3–5 ms central delay) were bigger and more frequently observed: excitation in most TA and Per brev MUs, and inhibition in most Sol, GM and Bi MUs and in many ST and VL MUs. The low threshold (∼0.5–0.6 × motor threshold) and the inability of a pure cutaneous stimulation to reproduce these effects (except the late excitation in TA MUs) indicate that they were due to stimulation of group I muscle afferents.
The early excitation was accepted to be monosynaptic when its central delay differed from that of the homonymous Ia excitation by less than 0.5 ms. Such a significant TN-induced monosynaptic Ia excitation was found in MUs belonging to all leg and thigh motor nuclei tested. Although its mean strength was relatively weak, it is argued that these monosynaptic connections might affect already depolarized MNs.
The late excitation found in TA and Per brev MUs is argued to be mediated through interneurones located rostral to MNs.
The late suppression, found in most Sol, GM and Bi MUs, and in many ST and VL MUs, was the dominant effect. It was accompanied by an inhibition of the Sol and quadriceps H reflexes at rest, and therefore reflects an inhibition directed to MNs. Its long latency is argued to reflect transmission by interneurones located rostral to MNs (the inhibitory counterpart of non-monosynaptic excitation).
The functional implications of these connections are discussed with respect to the requirements of the stance phase of human walking and running.
In man, large stretch responses, due to activation of homonymous Ia and group II afferents, are elicited in the flexor digitorum brevis (FDB) by perturbation of stance (Schieppati et al. 1995; Corna et al. 1995; Schieppati & Nardone, 1997). The question then arises whether muscle spindle afferent discharges from intrinsic plantar muscles are confined to homonymous pathways or have more extensive projections through heteronymous monosynaptic connections of Ia afferents and/or interneurones co-activated by group I and group II afferents.
Heteronymous monosynaptic Ia connections are indeed much more widespread in the human lower limb than in the cat (Eccles et al. 1957) or the baboon (Hongo et al. 1984) hindlimb; whereas in the latter species they mainly link motoneurones (MNs) of muscles acting synergistically at the same joint, transjoint Ia connections between ankle and knee muscles are almost the rule in humans (Meunier et al. 1993). This is probably to provide the more elaborate reflex assistance required in bipedal stance and gait. The first aim of the present study was therefore to investigate the extent to which such a broadening of transjoint monosynaptic Ia connections in humans also concerns projections from intrinsic plantar muscles.
In addition, leg and thigh muscles in man are linked by an oligosynaptic group I excitation, which also has a very diffuse pattern of distribution, since stimulation of the femoral, the posterior tibial or the common peroneal nerve is able to elicit this effect in all leg and thigh muscles tested (Chaix et al. 1997). This oligosynaptic group I excitation is mediated through interneurones presumably located rostral to MNs (Chaix et al. 1997) and controlled by inhibitory interneurones facilitated by corticospinal volleys (Marchand-Pauvert et al. 1999). The second aim of the present investigation was to investigate: (i) the extent to which group I volleys from intrinsic plantar muscles elicit oligosynaptic facilitation–or suppression–in MNs supplying proximal muscles, as already shown for tibial nerve-induced inhibition- facilitation of gastrocnemius-soleus (GS) MNs (Abbruzzese et al. 1996); and (ii) in the case of suppression, whether it reflects a direct inhibition of MNs (inhibitory postsynaptic potentials (IPSPs) in MNs) or their disfacilitation (i.e. inhibition of pre-motoneurones mediating excitation to the MNs).
METHODS
The experiments were carried out on nine healthy subjects (aged 22–65 years), all of whom gave written informed consent (obtained according to the Declaration of Helsinki) to the experimental procedure, which was approved by the institutional ethics committee. The subjects were seated in an armchair and the examined leg was loosely fixed with the hip semi-flexed (120 deg), the knee slightly flexed (160 deg) and the ankle at 110 deg plantar flexion.
Recording
The EMG was recorded by surface electrodes 1 cm apart: either 0.8 cm2 silver plates or differential electrodes DE-2.3 (Delsys Inc., Boston, USA). They were secured to the skin over the corresponding muscle belly: quadriceps (Q; vastus lateralis (VL) and vastus intermedius (VI), 25–30 and 5–10 cm above the patella, on the lateral and anterior aspect of the thigh, respectively), biceps femoris (Bi), semitendinosus (ST), soleus (Sol), gastrocnemius medialis (GM), peroneus brevis (Per brev) and tibialis anterior (TA). Motor units (MUs) in flexor digitorum brevis (FDB) and flexor hallucis brevis (FHB) were also recorded to calculate the conduction velocity (CV) in the fastest Ia afferents in the tibial nerve.
Subjects were instructed to perform a weak tonic voluntary contraction (strength below 5 % of maximal voluntary force) and to maintain it long enough (up to several hours in some experiments) to investigate changes in firing probability of the same MU with various stimulus intensities.
Conditioning stimuli
Electrical pulses of 1 ms duration were delivered through: (i) 1.5 cm diameter bipolar surface electrodes 1.5 cm apart (AG/AGCL, Niko, UK) to stimulate the tibial nerve (TN) at ankle level behind the medial malleolus, and the common peroneal nerve (CPN) at the level of the caput fibulae; (ii) half-ball electrodes 2 cm apart to stimulate the sciatic nerve at the upper part of the posterior aspect of the thigh; and (iii) unipolar electrodes to stimulate the femoral nerve (FN, active cathode in the femoral triangle, anode on the posterior aspect of the thigh) and the posterior tibial nerve (PTN, active cathode in the popliteal fossa, anode on the anterior aspect of the knee). The intensity of the nerve stimuli was expressed as multiples of the motor threshold (× MT).
Cutaneous stimuli
The cutaneous sensation (weak local and/or radiating paraesthesia) evoked by TN stimulation was mimicked by pure cutaneous stimuli to estimate the contribution of cutaneous afferents. The local sensation was reproduced by surface electrodes (AG/GCL) placed on the lateral and medial parts of the heel and the radiating paraesthesia by plate electrodes placed over the nerve projection area (big toe), allowance being made for the extra peripheral conduction time. The stimulus intensity was adjusted to imitate the sensation evoked by TN stimulation, and it was often impossible for the subject to make the distinction between the sensation elicited by TN stimulation and this pure cutaneous stimulation.
Study of single motor units
Method of post-stimulus time histogram (PSTH)
The EMG potentials of single MUs recorded with surface electrodes were converted into standard pulses by a discriminator with variable trigger levels and these were fed to a computer which subsequently triggered the stimulators about once every 0.7 s. Stimuli were delivered in relation to the MU discharge to avoid the period of after-hyperpolarisation. PSTHs of MUs from various thigh (VL, Bi, ST) and leg (Sol, GM, TA, Per brev) muscles were constructed for the 20–90 ms following a conditioning stimulation (0.5 ms bin width). Histograms of the firing probability were constructed after the conditioning stimulus (□ in the left panels of Figs 1–4) and in a control situation without stimulation (▪ in the left panels of Figs 1–4).
Figure 1. Conduction velocity in tibial nerve Ia afferents between ankle and knee levels.
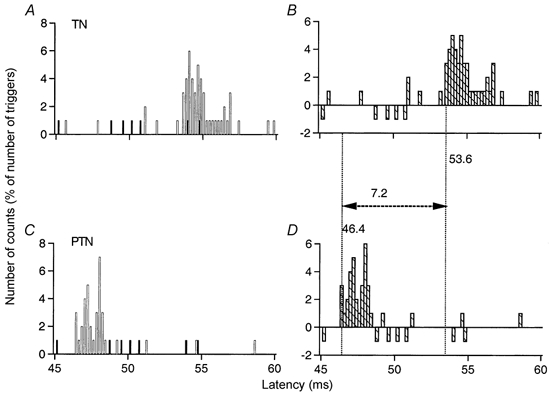
Post-stimulus time histograms (PSTHs, 0.2 ms bin width) of a flexor digitorum brevis (FDB) unit after stimulation of the tibial nerve (TN) at ankle level (1 × MT; A and B) and of the posterior tibial nerve (PTN) at knee level (0.7 × MT; C and D). The histograms in A and C show discharges of the voluntary activated MU under control conditions (▪) and after stimulation of the nerve (□). The differences between these two histograms, expressed as a percentage of the number of triggers, are plotted in B and D ( ) against the latency from the stimulation. Vertical dotted lines indicate the latency of the peaks. The difference between the latencies of the peak of excitation elicited by stimulation of the TN at ankle (B, 53.6 ms) and PTN at knee (D, 46.4 ms) levels was 7.2 ms. Distance between ankle and knee electrodes, 42 cm.
) against the latency from the stimulation. Vertical dotted lines indicate the latency of the peaks. The difference between the latencies of the peak of excitation elicited by stimulation of the TN at ankle (B, 53.6 ms) and PTN at knee (D, 46.4 ms) levels was 7.2 ms. Distance between ankle and knee electrodes, 42 cm.
Figure 4. Changes in firing probability of a soleus MU after stimulation of the homonymous and heteronymous group I and cutaneous afferents.
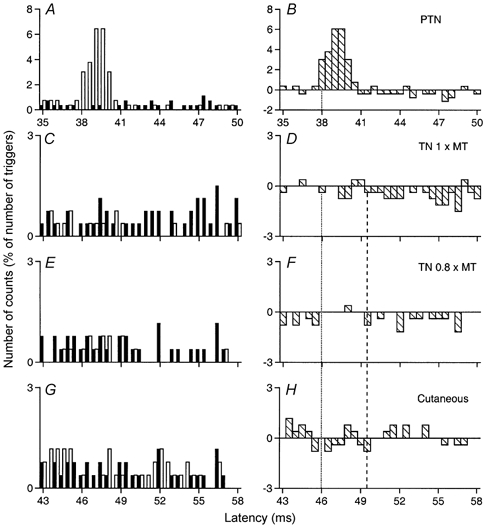
Comparison of PSTHs (0.5 ms bin width) of a soleus (Sol) MU after stimulation of the homonymous Ia afferents in the PTN at knee level (0.7 × MT; A and B), of the TN at ankle level at 1 × MT (C and D) and at 0.8 × MT (E and F), and of the skin of the heel, to mimic the cutaneous sensation elicited by the TN at 0.8 × MT (G and H). Abscissa and ordinate, and column symbols as in Fig. 1. Distance between ankle and knee stimulation sites and L2 vertebra: 97 and 56 cm, respectively. CV in the fastest Ia afferents in the PTN and TN: 66 and 58 m s−1, respectively. Vertical dotted lines indicate the latency of the homonymous peak (38 ms, P < 0.001; B) and the estimated monosynaptic heteronymous latency (46 ms, 38 + 8 {0.97/58–0.56/66}; D and F). The vertical dashed line indicates the latency of the inhibition (49.5 ms).
Organisation of the experiments
The control situation without stimulation, stimulation of the homonymous nerve (FN, sciatic, PTN or CPN), of the TN at ankle level and cutaneous stimulation mimicking the cutaneous sensation evoked by TN stimulation were randomly alternated in the same sequence so that the TN-induced changes in firing probability were always compared to those elicited by stimulation of the homonymous nerve and by stimulation of cutaneous afferents recorded under the same conditions. To clarify the differences between the results obtained in control and conditioned situations the control value in each bin was subtracted from that observed after conditioning stimulation ( in Figs 1–6, in which the number of counts in each bin is expressed as a percentage of the total number of corresponding stimuli delivered during the sequence). The exceptional sequences in which a change in the control sequence significantly contributed to the differences seen between the two situations were not retained for further analysis.
in Figs 1–6, in which the number of counts in each bin is expressed as a percentage of the total number of corresponding stimuli delivered during the sequence). The exceptional sequences in which a change in the control sequence significantly contributed to the differences seen between the two situations were not retained for further analysis.
Figure 6. Changes in firing probability of a vastus lateralis MU after stimulation of the homonymous and heteronymous group I and cutaneous afferents.
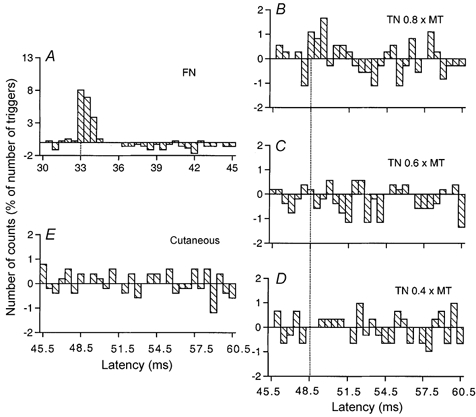
Comparison of PSTHs (0.5 ms bin width) of a vastus lateralis (VL) MU after stimulation of the homonymous Ia afferents in the femoral nerve (FN) at hip level (0.7 × MT, A), of the TN at ankle level at 0.8 × MT (B), 0.6 × MT (C) and 0.4 × MT (D), and of the skin of the heel, to mimic the cutaneous sensation elicited by the TN at 0.8 × MT (E). Abscissa and ordinate as in Fig. 1. Only the differences between conditioned and control histograms are shown ( ). Vertical dotted lines indicate the latency of the peaks: homonymous, 33 ms (P < 0.001; A); heteronymous, 48.5 ms (P < 0.05; B). Distance between TN and FN stimulation sites and L2 vertebra: 112 and 26 cm, respectively. CV in the fastest Ia afferents in the FN and TN: 60 and 58 m s−1, respectively. Same subject as in Fig. 1. The difference in latencies of heteronymous and homonymous peaks (48.5–33 = 15.5 ms) differed by only 0.5 ms from the difference in afferent conduction times (1.12/0.58–0.26/0.60 = 15 ms).
). Vertical dotted lines indicate the latency of the peaks: homonymous, 33 ms (P < 0.001; A); heteronymous, 48.5 ms (P < 0.05; B). Distance between TN and FN stimulation sites and L2 vertebra: 112 and 26 cm, respectively. CV in the fastest Ia afferents in the FN and TN: 60 and 58 m s−1, respectively. Same subject as in Fig. 1. The difference in latencies of heteronymous and homonymous peaks (48.5–33 = 15.5 ms) differed by only 0.5 ms from the difference in afferent conduction times (1.12/0.58–0.26/0.60 = 15 ms).
Statistical analysis
The statistical analysis of changes in firing probability was confined to a window that (i) started with the time of arrival at MN level of the TN (or homonymous) fastest Ia volley (estimated from the CV in Ia afferents and the distance from the stimulation site to L2 vertebra, see below), and (ii) lasted for 12 ms to avoid contamination by the long-latency M2 response (see Marsden et al. 1983). Within this window of analysis, each group of consecutive bins exhibiting an increase (or a decrease) in firing probability was grouped together and tested with a χ2 test to determine the extent to which the distribution of firing probability after stimulation within this group differed from that in the control situation. A peak of excitation (or a trough of suppression) was accepted if there was a significant (at least P < 0.05) increase (or decrease) in firing probability in a group of adjacent bins (a single bin was admitted for the peak of monosynaptic Ia excitation, as in Fig. 5B). The latency of the first bin of the increased (or decreased) firing probability was taken to be the latency of the excitation (or inhibition) provided that the probability was significantly changed in this bin or in the first group of two or three adjacent bins. Although the relation between the amplitude of a peak (or a trough) in the PSTH and that of the underlying excitatory postsynaptic potential (EPSP) (or IPSP) is complex (see Gustafsson & McCrea, 1984), the larger the EPSP the higher the peak (and the more profound the IPSP the larger the trough). Thus, the size of the peak (or the trough) was estimated as the sum of the differences (conditioned–control counts) in the different consecutive bins with increased (or decreased) firing probability contributing to a given peak or trough, e.g. there was a peak of 10.5 % between 51 and 53.5 ms in Fig. 2D, or a trough of 10 % between 49.5 and 56.5 ms in Fig. 4D.
Figure 5. Changes in firing probability of a gastrocnemius medialis MU after stimulation of the homonymous and heteronymous group I and cutaneous afferents.
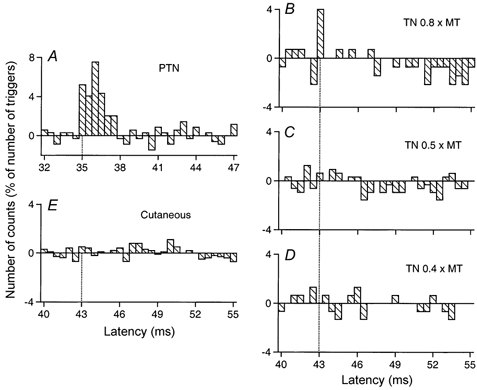
Comparison of PSTHs (0.5 ms bin width) of a gastrocnemius medialis (GM) MU after stimulation of the homonymous Ia afferents in the PTN at knee level (0.7 × MT; A), of the TN at ankle level at 0.8 × MT (B), 0.5 × MT (C) and 0.4 × MT (D), and of the skin of the heel, to mimic the cutaneous sensation elicited by the TN at 0.8 × MT (E). Abscissa and ordinate as in Fig. 1. Only the differences between conditioned and control histograms are shown ( ). Vertical dotted lines indicate the latency of the peaks: homonymous, 35 ms (P < 0.001; A); heteronymous, 43 ms (P < 0.01; B). Distance between ankle and knee stimulation sites and L2 vertebra: 97 and 57 cm, respectively. CV in the fastest Ia afferents in the PTN and TN: 65 and 58 m s−1, respectively. Same subject as in Fig. 4. The difference in latencies of heteronymous and homonymous peaks (43–35 = 8 ms) can be entirely explained by the difference in afferent conduction times (0.97/58–0.57/65 = 8 ms).
). Vertical dotted lines indicate the latency of the peaks: homonymous, 35 ms (P < 0.001; A); heteronymous, 43 ms (P < 0.01; B). Distance between ankle and knee stimulation sites and L2 vertebra: 97 and 57 cm, respectively. CV in the fastest Ia afferents in the PTN and TN: 65 and 58 m s−1, respectively. Same subject as in Fig. 4. The difference in latencies of heteronymous and homonymous peaks (43–35 = 8 ms) can be entirely explained by the difference in afferent conduction times (0.97/58–0.57/65 = 8 ms).
Figure 2. Changes in firing probability of a tibialis anterior MU after stimulation of the homonymous and heteronymous group I and cutaneous afferents.
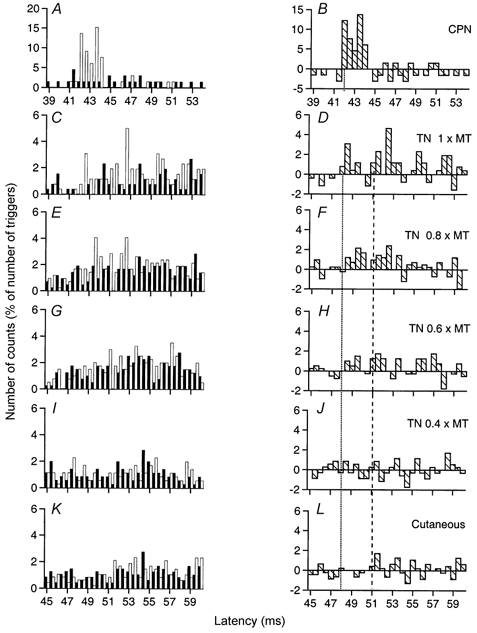
Comparison of PSTHs (0.5 ms bin width) of a tibialis anterior (TA) MU after stimulation of the homonymous Ia afferents in the common peroneal nerve (CPN) at knee level (0.8 × MT; A and B), of the TN at ankle level at 1 × MT (C and D), 0.8 × MT (E and F), 0.6 × MT (G and H) and 0.4 × MT (I and J), and of the skin of the heel to mimic the cutaneous sensation elicited by the TN at 0.8 × MT (K and L). Abscissa and ordinate, and column symbols as in Fig. 1. Vertical dotted lines indicate the latency of the peaks: homonymous, 42 ms (P < 0.001; B); heteronymous, 48 ms (P < 0.001; D). Distance between ankle and knee stimulation sites and L2 vertebra: 97 and 70 cm, respectively. CV in the fastest Ia afferents in the CPN and TN: 68 and 60 m s−1, respectively. The vertical dashed line indicates the latency of the ‘late’ excitation (51 ms; D, F and H).
Homonymous Ia facilitation
Changes in firing probability were always studied after stimulation of the nerve containing the afferents from the muscle investigated (referred to as the ‘homonymous’ nerve): FN for VL, sciatic nerve for ST and Bi, PTN for Sol and GM, and CPN for TA and Per brev. Stimulation of the ‘homonymous’ nerve at 1 × MT, or usually below in order not to elicit a compound H reflex, always evoked an early increase in firing probability. After correction for the trigger delay (delay between the real onset of the MU potential and the onset of the triggered pulse), the actual latency of this early peak was identical to that of the H reflex in the corresponding muscle, obtained at rest (Sol, VL) or during voluntary contraction. This early peak can therefore be attributed to the monosynaptic Ia EPSP (Mao et al. 1984).
Procedure used to define coupling (mono- or oligosynaptic) of the heteronymous effects
The same procedure was used as in previous studies (Meunier et al. 1990, 1993). Latencies of the early facilitation evoked in the same MU by stimulation of the homonymous nerve and of the TN were compared. Since the efferent conduction times were identical (same MU), the difference between the two latencies must reflect the difference in the afferent conduction times and/or in the central (synaptic) delay of the Ia effects evoked by homonymous and heteronymous stimulation. If, like homonymous, heteronymous excitation is mediated through a monosynaptic pathway, the difference in latency between heteronymous and homonymous excitations should be entirely explained by the difference in afferent conduction times.
Latency of non-monosynaptic events
This was measured from the heteronymous monosynaptic Ia latency. In MUs in which there was no heteronymous monosynaptic Ia peak, heteronymous latency was estimated as the following sum: homonymous monosynaptic Ia latency + the difference in afferent conduction times between TN and homonymous Ia volleys from stimulation sites to the spinal cord.
Afferent conduction times
Differences in afferent conduction times for the fastest Ia homonymous and TN volleys were estimated from: (i) the distance from stimulation sites of the electrodes eliciting the afferent volleys (homonymous or TN) to the L2 vertebra (entrance in the spinal cord) measured on the skin of the lower limb, and (ii) the CV in Ia afferents. The CV in Ia afferents in the TN was calculated from the latency of the monosynaptic Ia peaks measured in the PSTH of the same FDB or FHB MU after stimulation of Ia afferents at knee and ankle levels using 0.2 ms bins (see Fig. 1, and Appendix in Hultborn et al. 1987). Those in the CPN, PTN, FN and sciatic nerve had been already and similarly calculated for most subjects from the latency of the monosynaptic Ia peaks measured in the PSTH of the same MU in the corresponding muscle after stimulation of homonymous Ia afferents (Meunier et al. 1993; Simonetta-Moreau et al. 1999).
H reflex studies
Experiments using the H reflex in Sol and Q (VI) muscles were performed to investigate whether results obtained with PSTH tests could also be obtained in the absence of voluntary contraction. This was particularly important in the case of a decrease in firing probability in PSTHs to distinguish between inhibition or disfacilitation of the MNs (see Discussion). The Sol and Q H reflexes were obtained by unipolar stimulation of the PTN and FN, respectively. Because the sensitivity of H reflexes of small size varies with the amplitude of the unconditioned reflex (Crone et al. 1990), the size of the unconditioned reflex was adjusted to between 15 and 30 % of the maximum M wave. The reflex responses were measured as the peak-to-peak amplitude of muscle action potentials. In each experimental run, 20 control and 20 conditioned reflexes were randomly alternated for each conditioning-test interval. Conditioned reflexes were expressed as a percentage of control reflexes. An F test (Scheffé's test) was used to determine whether the changes evoked by the conditioning stimulation were significant.
RESULTS
Conduction velocity of Ia afferents from intrinsic plantar muscles
As explained in Methods, the evidence that heteronymous projections of Ia afferents from intrinsic plantar muscles to MNs supplying proximal muscles are monosynaptic relies on the fact that the difference between the latencies of the peaks of excitation elicited by stimulation of heteronymous Ia afferents in the TN at ankle level and of homonymous Ia afferents can be entirely explained by the difference in afferent conduction times of the two volleys. It was therefore necessary to assess the CV in Ia afferents in the TN. To that end, homonymous monosynaptic Ia projections to MNs of FDB and FHB were used. The latencies of the peaks of homonymous Ia excitation elicited in the same FDB or FHB MU by stimulation of Ia afferents in the TN at ankle level and in the PTN at knee level were therefore compared, using 0.2 ms bins. Given that the peak elicited by PTN stimulation was larger than that evoked by TN stimulation and that increasing the PTN stimulation invariably resulted in a H reflex in short flexors of the toes, it can be assumed that the PTN-induced peak was mainly due to stimulation of Ia afferents from intrinsic plantar muscles.
An example of the results obtained in a FDB MU is shown in Fig. 1. The difference between the latencies of the peaks elicited by stimulation of the TN (Fig. 1A and B) and PTN (Fig. 1C and D) was 7.2 ms, and the distance between the electrodes was 42 cm. This gave a CV of 58 m s−1 (0.42/0.0072). Similar measurements were performed in 20 MUs (8 subjects). The mean value (± s.e.m.) was 58 ± 0.8 m s−1 (range, 53–60 m s−1) for the fastest Ia afferents in the TN, thus significantly less than the mean values found by Meunier et al. (1993) for the fastest Ia afferents in the PTN (65 m s−1) or the CPN (68 m s−1). It is of interest that in the baboon lower limb the CV in Ia afferents from intrinsic plantar muscles is similarly slower (Hongo et al. 1984).
Early excitations compatible with a monosynaptic heteronymous Ia connection will be first considered.
Early heteronymous excitation from intrinsic plantar foot muscles in single units
Evidence for monosynaptic heteronymous Ia excitation of tibialis anterior MUs
The effects elicited by various stimuli in a TA MU are illustrated in Fig. 2. Stimulation of the CPN (0.8 × MT) at knee level (in a distal position close to the tibial crest to favour stimulation of TA fibres) elicited a highly significant (P < 0.001) peak of homonymous Ia excitation at a latency of 42 ms (Fig. 2A and B). After correction for the trigger delay of the MU (5 ms, see Methods), this latency exactly corresponded to that of the TA H reflex of this subject (37 ms), and can therefore be attributed to a monosynaptic Ia EPSP (Mao et al. 1984).
Heteronymous stimulation of the TN (1 × MT) at ankle level elicited a highly significant (P < 0.001) peak of excitation that occurred at a latency of 48 ms (Fig. 2C and D), i.e. 6 ms later than the peak of homonymous Ia excitation, and lasted for 2 ms. The distances from CPN and TN stimulation to the L2 vertebra were 70 and 97 cm, respectively. Given that, in this subject, the CV for the fastest Ia afferents in the CPN and TN were measured at 68 and 60 m s−1, respectively, the supplementary peripheral conduction time for the TN volley should be at least 5.87 ms (0.97/60–0.70/68). This entirely accounts for the 6 ms difference in the latency of the two peaks of excitation, and thus strongly suggests that the heteronymous and homonymous excitations had the same central delay, i.e. that the heteronymous connection also corresponds to a monosynaptic linkage. When the stimulus intensity was decreased to 0.8 × MT (Fig. 2E and F) and 0.6 × MT (Fig. 2G and H), a smaller but still significant (P < 0.01 and 0.05, respectively) TN-induced heteronymous excitation persisted within the window 48.5–50.5 ms, but disappeared at 0.4 × MT (Fig. 2I and J), attesting that the threshold of the heteronymous excitation was low (within the range found for human group I afferents; Meunier et al. 1990, 1993). Note that the decrease in the underlying EPSP, attested by the reduction of the peak, was then accompanied by an increase in the latency of the peak in the PSTH, as described previously in animal experiments (Fetz & Gustafsson, 1983; Gustafsson & McCrea, 1984). Figure 2K and L shows that a cutaneous stimulation mimicking exactly the sensation evoked by TN stimulation at 0.8 × MT (see Methods) did not modify the firing probability of the MU at that latency.
A similar significant early increase in firing probability, compatible with a monosynaptic linkage, was found in 18/42 (43 %) TA MUs (from 6/8 subjects) after TN stimulation.
Evidence for monosynaptic coupling for various muscle-nerve combinations
In MUs of the other muscles tested, the same criteria were applied to provide evidence for a heteronymous monosynaptic group I excitation: (i) difference in central delays of homonymous and heteronymous peaks of ≤ 0.5 ms when the intensity of the heteronymous stimulation was equal to 0.8–1 × MT; (ii) low threshold; and (iii) absence of effects evoked at the same latency by pure cutaneous stimuli.
Peroneus brevis
In the Per brev MU illustrated in Fig. 3, the difference (53–46.5 = 6.5 ms) between the latencies of the monosynaptic CPN-induced peak (Fig. 3A and B) and of the early peak elicited by TN stimulation at 1 × MT (Fig. 3C and D; P < 0.01) could also be entirely explained by the difference in afferent conduction times (see legend to Fig. 3). When the stimulus intensity was decreased to 0.8 × MT a smaller but still significant (P < 0.05) TN-induced heteronymous excitation persisted within the window 53.5–54 ms (Fig. 3E and F). A similar TN-induced monosynaptic excitation was only observed in 4/21 (19 %) MUs (belonging to 4 different subjects).
Figure 3. Changes in firing probability of a peroneus brevis MU after stimulation of the homonymous and heteronymous group I and cutaneous afferents.
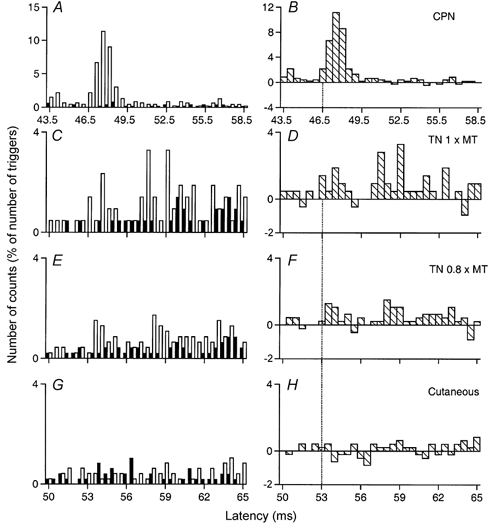
Comparison of PSTHs (0.5 ms bin width) of a peroneus brevis (Per brev) MU after stimulation of the homonymous Ia afferents in the CPN at knee level (0.8 × MT; A and B), of the TN at ankle level at 1 × MT (C and D) and at 0.8 × MT (E and F), and of the skin of the heel, to mimic the cutaneous sensation elicited by the TN at 0.8 × MT (G and H). Abscissa and ordinate, and column symbols as in Fig. 1. Vertical dotted lines indicate the latency of the peaks: homonymous, 46.5 ms (P < 0.001; B); heteronymous, 53 ms (P < 0.01; D). Distance between ankle and knee stimulation sites and L2 vertebra: 97 and 65 cm, respectively. CV in the fastest Ia afferents in the CPN and TN: 68 and 60 m s−1, respectively. Same subject as in Fig. 2. The difference in latencies of heteronymous and homonymous peaks (53–46.5 = 6.5 ms) can be entirely explained by the difference in afferent conduction times (0.97/60–0.65/68 = 6.6 ms).
Soleus
In the Sol MU illustrated in Fig. 4, there was no heteronymous excitation at monosynaptic latency (46 ms, see legend). However, evidence for a significant TN-induced monosynaptic Ia excitation was found in 10/22 (45 %) Sol MUs (from 4/5 subjects).
Gastrocnemius medialis
In the GM MU illustrated in Fig. 5, the difference between the latencies of the peaks of homonymous (A) and heteronymous (B, a single bin but significant at P < 0.01) excitations (43–35 = 8 ms) could again be entirely explained by the difference in peripheral conduction times (see legend to Fig. 5). Evidence for heteronymous monosynaptic Ia excitation was found in 5/15 MUs (33 %) tested (from 4/7 subjects).
Vastus lateralis
In Fig. 6 the difference in latencies of the homonymous and heteronymous peaks (48.5–33 = 15.5 ms) differed by only 0.5 ms from the estimated difference in afferent conduction times (see legend to Fig. 6) and was therefore compatible with a monosynaptic connection. Evidence for a heteronymous monosynaptic Ia excitation was found in 10/25 VL MUs (40 %) tested (from 4/8 subjects).
Hamstrings
A significant excitation occurring at monosynaptic latency was found in 11/26 ST MUs (42 %) (from 5/9 subjects) and 4/15 Bi MUs (27 %) (from 2/8 subjects).
Strength of the different monosynaptic heteronymous group I connections
The strength of the monosynaptic group I connections from the TN in the different muscles are compared in Table 1 Two factors were considered when estimating the strength of these connections: (i) the frequency of occurrence, with the number (numerator) of MUs where heteronymous Ia excitation reached statistical significance (at least P < 0.05), and the number (divisor) of explored MUs, and, in parentheses, the former as a percentage of the latter; (ii) the mean value (± s.e.m.) of the size of the heteronymous peak assessed at 0.8–1 × MT, as explained in Methods and expressed as a percentage of the number of triggers, calculated in those experiments with a significant heteronymous excitation. Two remarks may be made: (i) monosynaptic group I projections from intrinsic plantar muscles to MNs supplying leg and thigh muscles were found in all seven motor nuclei explored; and (ii) when they existed, the mean values of the excitation were small (∼3 % of the number of triggers), far below the size of the homonymous peak evoked with lower stimulus intensities (0.6–0.7 × MT, to be below the threshold of the compound H reflex).
Table 1.
Strength, latency and duration of monosynaptic Ia excitation, late excitation and late inhibition elicited by group I afferents in the different MN pools tested
| TA | Per brev | Sol | GM | VL | ST | Bi | |
|---|---|---|---|---|---|---|---|
| Monosynaptic excitation | |||||||
| Frequency | 18/42 (43 %) | 4/21 (19%) | 10/22 (45 %) | 5/15 (33 %) | 10/25 (40 %) | 11/26 (42 %) | 4/15 (27 %) |
| Size | 4.2 ± 0.5 | 3 ± 0.7 | 2.7 ± 0.7 | 2.9± 0.3 | 3 ± 0.7 | 2.2 ± 0.5 | 3 ± 0.4 |
| Late excitation | |||||||
| Frequency | 30/42 (71 %) | 20/21 (95%) | 1/17 (6%) | 0/15 | 4/25 (16 %) | 2/26 (13 %) | 1/15 (6 %) |
| Size | 8.5 ± 1.1 | 6.3 ± 1 | 2 | — | 3.1 ± 1 | 2.1 ± 0.3 | 6 |
| Latency | 4.1 ± 0.4 | 6.2 ± 0.5 | 3.5 | — | 4.8 ± 1.5 | 5.8 ± 0.2 | 6.6 |
| Duration | 2.8 ± 0.3 | 5.1 ± 0.5 | 4 | — | 1.1 ± 0.9 | 1.8 ± 0.3 | 0.5 |
| Late inhibition | |||||||
| Frequency | 0/42 | 0/21 | 19/22 (86 %) | 11/15 (73 %) | 10/25 (40 %) | 12/26 (46 %) | 11/15 (73 %) |
| Size | — | — | 13.3 ± 1.6 | 11.4 ± 0.9 | 5.7 ± 1.1 | 4.6 ± 0.7 | 6.2 ±0.9 |
| Latency | — | — | 3.9± 0.5 | 2.6 ± 0.2 | 4.7 ± 0.5 | 3.4 ± 0.4 | 3.5 ± 0.7 |
| Duration | — | — | 12.3 ± 1.2 | 7.8 ± 1.1 | 5 ± 1.1 | 5 ± 0.6 | 7.3 ± 1 |
TA, tibialis anterior; Per brev, peroneus brevis; Sol, soleus; GM, gastrocnemius medialis; VL, vastus lateralis; ST, semitendinosus; Bi, biceps femoris. Frequency: frequency of occurrence, expressed as a ratio (i.e. the number of MUs in which a significant effect (P < 0.05 at least) was evoked/the number of units tested (raw values)), and, in parentheses, as a percentage (i.e. the former as a percentage of the latter). Size: mean values (±s.e.m.) of the size of the peak (Monosynaptic and Late excitation) or trough (Late inhibition), assessed as explained in Methods and expressed as a percentage of the number of triggers, calculated in all experiments with a significant effect. Latency and Duration: mean values (±s.e.m.) of the latency and duration of the effect, in milliseconds.
Late effects
In many MUs there was a late effect, which occurred with a central delay of several milliseconds with respect to monosynaptic latency. This effect, which was stronger than the early excitation, had a different effect in the different motor nuclei: (i) exclusively excitatory in TA and Per brev MUs; (ii) almost exclusively inhibitory in Sol, GM and Bi MUs; and (iii) predominantly inhibitory in VL and ST MUs.
Late excitation
Tibialis anterior
Figure 2C and D shows the late excitation (P < 0.001) induced by TN stimulation (1 × MT) in a TA MU, which occurred with a central delay of 3 ms (vertical dashed line) with respect to monosynaptic latency and lasted for 3 ms. This excitation still existed (P < 0.01) at 0.8 × MT (Fig. 2E and F) and 0.6 × MT (Fig. 2G and H) but disappeared at 0.4 × MT (Fig. 2I and J). As illustrated in Fig. 2K and L, cutaneous afferents somewhat (P < 0.05) contributed to this excitation. A statistically significant late excitation was observed in 30/42 TA MUs (71 %), and most often, in this combination, the cutaneous contribution reached statistical significance.
Peroneus brevis
Figure 3C and D shows that in this Per brev MU, after the relatively weak peak of monosynaptic Ia excitation (at 53–55 ms), stimulation of the TN at 1 × MT elicited a second and bigger peak that occurred with a central delay of 4 ms between 57 and 62 ms (P < 0.001). This peak was still present and significant when the TN intensity was decreased to 0.8 × MT (Fig. 3E and F) and 0.6 × MT (not illustrated). Cutaneous stimulation mimicking the sensation elicited by TN stimulation also evoked an excitation (Fig. 3G and H) but it was much weaker (2 vs. 12 % of the number of triggers). A similar statistically significant late excitation was observed in 20/21 MUs (95 %). The cutaneous contribution to this late effect was always either nil or not statistically significant.
Other muscles
A similar late excitation evoked by stimuli below 1 × MT was very rare in MUs of thigh muscles (1/15 in Bi, 2/26 in ST, 4/25 in VL) and was never found in Sol (except 1 MU) and GM. In these muscles the usual low-threshold late effect was indeed a suppression.
Table 1 shows the frequency of occurrence and mean values (± s.e.m.) for the strength (expressed as a percentage of the number of triggers), the central delay (latency) and the duration of this late excitation in the different muscles.
Late suppression
Late suppression was the dominant effect observed in ankle extensors, Q and hamstrings. Figure 5 (a GM MU) shows that the peak of monosynaptic Ia excitation evoked by TN stimulation at 0.8 × MT was followed by a profound suppression (15 % of the number of triggers, P < 0.001; Fig. 5B), which had a long latency (3.5 ms longer than monosynaptic excitation) and a long duration (7.5 ms). This highly significant suppression had a very low threshold since it persisted when the TN stimulus intensity was reduced to 0.5 × MT (Fig. 5C), but disappeared at 0.4 × MT (Fig. 5D). The pure cutaneous stimulation mimicking the sensation elicited by TN stimulation, if anything, evoked an increase in firing probability (Fig. 5E). Figure 4 shows that a profound suppression (8 % of the number of triggers, P < 0.001) was also evoked in a Sol MU by TN stimuli at 1 × MT (Fig. 4C and D) and 0.8 × MT (Fig. 4E and F) with similar characteristics: 3.5 ms central delay with respect to the estimated monosynaptic latency (see vertical dotted and dashed lines) and long duration. Again, this suppression was still present when TN stimulation was reduced to 0.5 × MT (not illustrated) and was not evoked by cutaneous stimulation (Fig. 4G and H).
Table 1 shows the frequency of occurrence and mean values for the strength, the central delay (latency) and the duration of the late suppression in the different muscles. Whether or not preceded by a peak of monosynaptic excitation, a similar profound, low-threshold (having no equivalent evoked by the pure cutaneous stimulation), long-latency and long-lasting suppression was observed in 19/22 (86 %) Sol, 11/15 (73 %) GM, 11/15 (73 %) Bi, 12/26 (46 %) ST and 10/25 (40 %) VL MUs. Table 1 shows that the late inhibition was particularly profound in ankle extensors (Sol and GM) and was never observed in TA and Per brev.
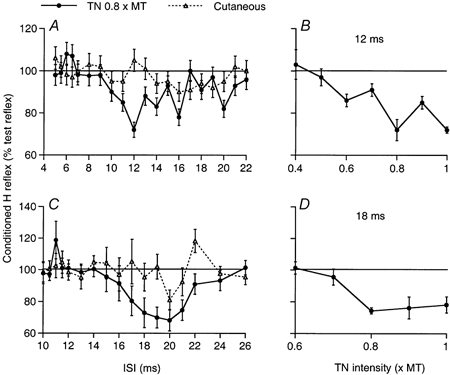
Modulation of the H reflex
Figure 7A and C shows the time courses of the changes in the Sol (A) and Q (VI, C) H reflexes when preceded by a stimulation at 0.8 × MT to the TN. In both cases there was a weak early facilitation occurring at the 6.5 and 11 ms interstimulus intervals (ISIs), respectively, corresponding (in this 163 cm tall subject) to a simultaneous arrival of conditioning and test Ia volleys at MN level, and probably reflecting the weak Ia connections from intrinsic plantar muscles to Sol and Q MNs. The very brief duration (0.5 ms) of this weak facilitation and its abrupt termination suggest that the initial conditioning monosynaptic Ia EPSP is truncated by a disynaptic IPSP, probably Ib in origin. The absence of any change in the H reflex in the following 3 ms indicates that the net result of the monosynaptic EPSP and of the disynaptic Ib IPSP is then zero. At later ISIs, TN stimulation (0.8 × MT, •) evoked an inhibition of the Sol and Q H reflex that appeared 3.5–4 ms later than the heteronymous monosynaptic Ia facilitation, and lasted for 7 ms. The cutaneous stimulation (▵), mimicking the sensation elicited by TN stimulation, elicited a much smaller depression (if any), which occurred later (after allowance for the extra peripheral conduction time). Varying the intensity of TN stimulation at the peak of inhibition, when there was no effect of the cutaneous stimulation (12 ms ISI in the Sol, 18 ms ISI in the Q), showed that the threshold of the inhibition was at 0.6 × MT in Sol (Fig. 7B) and 0.7 × MT in Q (Fig. 7D). Similar results were obtained in four subjects, with, in particular, a depression of the Q H reflex which was as profound as that of the Sol H reflex. The discrepancy between the large and constant inhibition of the Q H reflex and the weaker and less constant inhibition found in the PSTHs of single Q MUs is most probably due to the fact that these responses originated from different heads of the Q: VL for the MU PSTHs, VI for the H reflex (see Methods).
Figure 7. Modulation of the soleus and quadriceps H reflexes by a conditioning stimulation to the tibial nerve.
A and C, time courses of the variations in the Sol (A) and Q (C) H reflexes when preceded by a conditioning stimulation to the TN (0.8 × MT; •) or a cutaneous stimulation to the skin of the heel (▵; allowance being made for the extra peripheral conduction time); changes in the conditioned H reflex (expressed as a percentage of the unconditioned reflex) are plotted against the interstimulus interval (ISI). B and D, effects of varying the TN stimulus intensity on the Sol (B, 12 ms ISI) and the Q (D, 18 ms ISI) H reflexes. Each symbol represents the mean of 20 measurements. Vertical bars = 1 s.e.m. Same subject as in Figs 2 and 3.
DISCUSSION
Changes in the firing probability of voluntarily activated MUs from various leg and thigh muscles were investigated after TN stimulation. Conditioning volleys to TN were shown to elicit a statistically significant, but generally weak, early increase in the firing probability of MUs belonging to all muscles tested (TA, Per brev, Sol, GM, VL, ST, Bi), a stronger late excitation in almost all TA and Per brev MUs and a profound late suppression in most Sol, GM and Bi MUs, and in many ST and VL MUs.
Peripheral pathway
All effects had an electrical threshold between 0.5 and 0.6 × MT, i.e. as low as that of lower limb group Ia afferents (Meunier et al. 1990, 1993). The latency of the earliest excitation was so brief that, even when assuming a mediation through the most rapid central pathway (monosynaptic, see below), the CV of the responsible afferents was compatible with that of the most rapid Ia afferents in the TN measured in each subject. Except for the late excitation of TA MUs, any significant contribution from cutaneous afferents to the TN-induced effects was eliminated by control experiments (Figs 2L, 3H, 4H, 5E, 6E, ▵ in Fig. 7A and C). The low threshold of the different effects and the high CV of afferents responsible for the early excitation, and the inability of a pure cutaneous stimulation to reproduce most effects, indicate that they are due to stimulation of group I muscle afferents (except the late excitation of TA MUs for which a cutaneous contribution was often found).
It was not possible to stimulate separately afferents from the different intrinsic plantar muscles innervated by the TN (FDB, FHB, abductor hallucis, abductor digiti minimi). However, as, during gait or standing on the tip of toes, intrinsic plantar muscles have been shown to act as a group (Mann & Inman, 1964), volleys elicited by TN stimulation are probably representative of the group I volleys originating from the group of intrinsic plantar muscles under physiological conditions.
Heteronymous monosynaptic Ia projections
A comparison was drawn between the difference in the latencies of excitations evoked by homonymous and TN afferent volleys. This difference was then compared to the difference in afferent conduction times for the two volleys, calculated from: (i) CVs in the fastest Ia fibres, and (ii) the distances between stimulation sites and the entrance of the volleys in the spinal cord at the level of the L2 vertebra. Evidence for a similar central delay in homonymous and heteronymous pathways was accepted when the difference in latencies of the peaks did not differ from the estimated difference in afferent conduction times by more than 0.5 ms. Since the onset of the homonymous peak, occurring at the same latency as the H reflex, is known to be monosynaptic (Mao et al. 1984), a monosynaptic transmission was admitted in such cases. The validity of this conclusion depends on the time resolution of the method and on the reliability of latency measurements and estimates concerning peripheral afferent conduction times.
Time resolution of the method
The earliest disynaptic group I effects in humans start to manifest themselves at least 0.8 ms after the earliest monosynaptic Ia excitation (Pierrot-Deseilligny et al. 1981b; Day et al. 1984; Hultborn et al. 1987). One can therefore estimate that a disynaptic effect should have a central delay exceeding by more than 0.5 ms (the time resolution of the method) the estimated monosynaptic group I-induced central delay.
Trigger delay
Because of the trigger delay, i.e. the delay between the exact onset of the EMG potential and the moment at which the computer was triggered by the rapidly rising phase of the potential, there was some uncertainty regarding the absolute latency of the peaks. However, the trigger delay was the same for homonymous and heteronymous peaks in a given MU, since they were investigated in the same sequence, and a greater trigger delay would not alter the difference in latencies of the two peaks, the critical measurement in these experiments.
Estimate of afferent conduction times
Another source of uncertainty may result from the estimates in peripheral afferent conduction times relying on distances measured on the skin. In the case of muscles innervated by the sciatic nerve and its branches, the comparison of the distances for TN and homonymous nerves were fairly reliable, since TN and homonymous volleys run along the same nerve between knee (or thigh) level and the spinal cord.
In the case of the femoral nerve, the common ‘intra-abdominal part’ of femoral and TN nerves can only be measured approximately. However, it has been calculated that a 3 cm error in this common part would only alter by 0.1 ms the difference between heteronymous and homonymous afferent conduction times (the critical measurement in these experiments; Meunier et al. 1990).
Since MN pools supplying intrinsic plantar muscles are located in L5-S1 (Kendall et al. 1971), Ia afferents from these muscles enter the spinal cord approximately at the same level as some tested MN pools (Sol, GM, ST, Bi) or more caudal (TA, Per brev or VL). In the latter case, an intraspinal conduction time should therefore be added to the afferent conduction time of the heteronymous Ia volley. However, because this is compensated for by a longer extraspinal conduction time for the homonymous afferent volley in the lumbar plexus, this factor can be neglected.
The onset of the heteronymous excitation, like that of the homonymous excitation, therefore reflects a monosynaptic connection of group I afferents, i.e. of Ia afferents, since they are the only ones to have monosynaptic projections to MNs. It is conceded that oligosynaptic group I EPSPs (Jankowska & McCrea, 1983) can contribute to the late bins of the peak in the PSTH (Burke et al. 1984). However, peaks longer than 1 ms do not necessarily imply a contribution of oligosynaptic pathways, since a small underlying monosynaptic EPSP during its decay phase, added to the synaptic noise, can still cause the MN to fire (Fetz & Gustafsson, 1983).
In the baboon, transjoint heteronymous monosynaptic Ia projections from intrinsic plantar muscles are only significant onto GM MNs, and are totally absent onto Q, ST, TA or peroneal MNs (Hongo et al. 1984). This therefore contrasts with the very diffuse pattern described in the present investigation, which is in keeping with the extensive transjoint connections between ankle and knee muscles found in humans (Meunier et al. 1993).
Finally, it must be pointed out that these monosynaptic Ia connections, which are weak with respect to homonymous connections, have approximately the same strength in all seven MN pools tested.
Late effects
Because they had the same threshold as the monosynaptic heteronymous Ia excitation, the long latency of the late effects cannot be attributed to a longer peripheral afferent conduction time.
Late excitation
The long latency of the late excitation found in almost all TA and Per brev MUs reflects a long central delay, but the very low threshold (and sharp onset) of the effect suggests an oligosynaptic transmission. The long latency could then be explained by a mediation through interneurones located rostral to the MNs, as has been argued for the diffusely distributed non-monosynaptic group I excitation between leg and thigh muscles (Chaix et al. 1997). However, the late excitation from intrinsic plantar muscles differs from the non-monosynaptic group I excitation between leg and thigh muscles in two essential aspects: (i) in TA MUs a significant contribution from cutaneous afferents from the skin of the heel was almost constantly observed; and (ii) the pattern of distribution was not diffuse since the late excitation was only exceptionally found in ankle extensors, VL and hamstrings, where it was replaced by a suppression.
Late inhibition
It might be argued that the late trough seen in MU PSTHs of ankle extensors, VL and hamstrings is due to a disynaptic Ib inhibition, but that it appeared in the MN with a long latency because it was superimposed on the preceding monosynaptic Ia excitation. In fact, a similar significant trough in the PSTH existed in those MUs without monosynaptic excitation (e.g. Fig. 4C–F), and, in some MUs with monosynaptic excitation, the trough persisted when the excitation had disappeared at low stimulus intensities (Fig. 5C). The long latency therefore reflects a long central delay. Here again, because of the very low threshold of the effect (and of its sharp onset) it is likely to reflect an oligosynaptic transmission through interneurones located rostral to the MNs (the inhibitory counterpart of oligosynaptic excitation).
The trough observed in the PSTHs may reflect a direct inhibition of MNs (IPSP in MNs) or their disfacilitation (i.e. inhibition of pre-motoneurones mediating descending excitation to the MN maintaining the voluntary level of firing during PSTH experiments). Group I-induced oligosynaptic excitation of ankle or knee muscle MUs elicited by a given group I volley from any of these muscles is suppressed by a group I volley originating from another muscle, and this diffuse ‘heteronymous’ suppression has been argued to reflect a disfacilitation (Chaix et al. 1997). The finding that, in the present investigation, the TN-induced inhibition of Sol and Q H reflexes at rest had exactly the same characteristics (low-threshold, long latency, long duration) as the TN-induced troughs observed in the PSTHs of Sol and Q MUs indicates that group I volleys from intrinsic plantar muscles evoke a direct inhibition of the MNs.
Depressive effects elicited by group I afferents from intrinsic plantar muscles therefore differ from the diffuse ‘heteronymous’ group I-induced disfacilitation described by Chaix et al. (1997) in two essential aspects, since: (i) they are not diffuse, but are focused on MNs of extensors (Sol, GM, Q, and hamstrings which are hip extensors); and (ii) they directly inhibit the MNs. This last point is of particular interest, since this is the first evidence for inhibitory projections to MNs in this system of human interneurones co-activated by group I and group II afferents (Simonetta-Moreau et al. 1999; Marchand-Pauvert et al. 1999), assumed to be homologues of the feline intermediate zone/ventral horn midlumbar interneurones (Edgley & Jankowska, 1987).
Functional implications
It has been suggested that the particular pattern of monosynaptic heteronymous Ia connections observed in the cat and in the baboon has evolved to assist locomotion in each species (Lundberg, 1969; Hongo et al. 1984). Thus, the diffuse Ia projections from intrinsic plantar muscles found in man might be related to the more elaborate reflex assistance required in bipedal stance and gait. However, because of the weakness of these connections, they probably only affect already depolarised MNs, providing a safety factor during their firing. A possible role for monosynaptic projections from intrinsic plantar muscles must therefore be sought during postural tasks and phases of locomotion where there is a co-contraction of these intrinsic muscles and various proximal muscles.
Different synergistic combinations occur in different tasks, e.g. the co-contraction of intrinsic plantar muscles and ankle extensors is associated with a contraction of hamstrings while leaning forwards, but with a contraction of Q while hopping. The selection of the heteronymous Ia connections adequate for a given task might be controlled through different spinal pathways. (i) Presynaptic inhibition of Ia terminals has been proposed (Meunier et al. 1993). In this connection, presynaptic inhibition of Sol and Q Ia terminals is increased by group I volleys from intrinsic plantar muscle (Iles, 1996). (ii) Matched heteronymous recurrent inhibition (Barbeau et al. 2000); however, there is no recurrent inhibition from intrinsic plantar muscles, because of the probable absence (as in the cat) of recurrent collaterals from axons of distal muscles (Rossi & Mazzochio, 1991). (iii) A good candidate to oppose unwanted excitatory Ia effects could then be the profound late inhibition from intrinsic plantar muscles described above.
Intrinsic plantar muscles are especially active at the end of the stance phase of human walking (Mann & Inman, 1964) and running, where they exhibit a lengthening contraction known to evoke strong muscle spindle afferent discharges (Burke et al. 1978). The functional role of the excitatory Ia projections must be considered, whether transmission of the opposite group I inhibitory effects onto extensor MNs is assumed to be depressed or not.
During running
Under the assumption that transmission of the group I-induced inhibition is then depressed, the Ia discharge might play a twofold role: (i) to secure the extensor thrust occurring during the stance phase with the contractions of the flexors of the toes, GS, Q (and also hamstrings; Schwab et al. 1983) (weak TA and peroneal muscle contractions playing the role of lateral riggings maintaining the foot); and (ii) to favour a rapid automatic compensation to irregularities of the ground.
During walking
Transmission of group I-induced inhibition to ankle and knee extensors should, on the contrary, not be depressed. (i) During the stance phase, GS contraction resists the passive ankle dorsiflexion produced by the resultant of the extrinsic forces (kinetic force and gravity) but it is imperative that GS tension be overcome by this resultant if the body is to be brought forward; the group I inhibition from intrinsic plantar muscles, which has been shown to be facilitated by cutaneous afferents from the foot sole (Abbruzzese et al. 1996), together with other inhibitory mechanisms (see Pierrot-Deseilligny et al. 1981a), could contribute to this. (ii) At the end of the stance phase, there is ample flexion of the knee, which makes the inhibition of Q MNs by the group I discharge from intrinsic plantar muscles purposeful. Finally, the facilitation of TA MNs by cutaneous afferents from the heel might secure the TA contraction occurring at heel strike.
Acknowledgments
Our thanks are due to A. Rigaudie and M. Dodo for excellent technical assistance. This work was supported by grants from Assistance Publique-Hôpitaux de Paris, Ministère de la Recherche (UPRES EA 2393) and IRME. Véronique Marchand-Pauvert was supported by a grant from Fondation pour la Recherche Médicale and Philippe Marque by grants from Hôpitaux de Toulouse and Assistance Publique-Hôpitaux de Paris.
References
- Abbruzzese M, Rubino V, Schieppati M. Task-dependent effects evoked by foot muscle afferents on leg muscle activity in humans. Electroencephalography and Clinical Neurophysiology. 1996;101:339–348. doi: 10.1016/0924-980x(96)95682-9. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Marchand-Pauvert V, Meunier S, Nicolas G, Pierrot-Deseilligny E. Posture-related changes in heteronymous recurrent inhibition from quadriceps to ankle muscles in humans. Experimental Brain Research. 2000;130:345–361. doi: 10.1007/s002219900260. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. Journal of Neurophysiology. 1984;52:435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Löfstedt L. Muscle spindle activity in man during shortening and lengthening contractions. Journal of Physiology. 1978;277:131–142. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix Y, Marque P, Meunier S, Pierrot-Deseilligny E, Simonetta-Moreau M. Further evidence for non-monosynaptic group I excitation of motoneurones in the human lower limb. Experimental Brain Research. 1997;115:35–46. doi: 10.1007/pl00005683. [DOI] [PubMed] [Google Scholar]
- Corna S, Grasso M, Nardone A, Schieppati M. Selective depression of medium-latency leg and foot muscle responses to stretch by an α2-agonist in humans. Journal of Physiology. 1995;484:803–809. doi: 10.1113/jphysiol.1995.sp020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazières L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Experimental Brain Research. 1990;81:35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Day BL, Marsden CD, Obeso JA, Rothwell JC. Reciprocal inhibition between the muscles of the human forearm. Journal of Physiology. 1984;349:519–534. doi: 10.1113/jphysiol.1984.sp015171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents onto many different species of alpha motoneurones. Journal of Physiology. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. Journal of Physiology. 1987;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. Journal of Physiology. 1983;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. Journal of Physiology. 1984;347:431–451. doi: 10.1113/jphysiol.1984.sp015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T, Lundberg A, Phillips CG, Thompson RF. The pattern of monosynaptic Ia-connections to hindlimb motor nuclei in the baboon: a comparison with the cat. Proceedings of the Royal Society B. 1984;221:261–289. doi: 10.1098/rspb.1984.0034. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia fibres: a study in man and the cat. Journal of Physiology. 1987;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. Journal of Physiology. 1996;491:197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, McCrea D. Shared reflex pathways from Ib tendon organ afferents and Ia muscle spindle afferents in the cat. Journal of Physiology. 1983;338:99–111. doi: 10.1113/jphysiol.1983.sp014663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall HO, Kendall FP, Wadsworth GE. Muscles. Testing and Function. Baltimore, USA: The William & Wilkins Company; 1971. [Google Scholar]
- Lundberg A. The Nansen Memorial Lecture, V. Oslo: Universiteitsforlaget; 1969. Reflex control of stepping; pp. 1–42. [Google Scholar]
- Mann R, Inman V. Phasic activity of intrinsic muscles of the foot. Journal of Bone and Joint Surgery. 1964;46A:469–481. [PubMed] [Google Scholar]
- Mao CC, Ashby P, Wang M, McCrea D. Synaptic connections from large muscle afferents to the motoneurones of various leg muscles in man. Experimental Brain Research. 1984;56:341–350. doi: 10.1007/BF00236290. [DOI] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Simonetta-Moreau M, Pierrot-Deseilligny E. Cortical control of spinal pathways mediating group II excitation to human thigh motoneurones. Journal of Physiology. 1999;517:301–313. doi: 10.1111/j.1469-7793.1999.0301z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Rothwell JC, Day BL. Long-latency automatic responses to muscle stretch in man: origin and function. In: Desmedt JE, editor. Motor Control Mechanisms in Health and Disease. New York: Raven Press; 1983. pp. 509–539. [PubMed] [Google Scholar]
- Meunier S, Pénicaud A, Pierrot-Deseilligny E, Rossi A. Monosynaptic Ia excitation and recurrent inhibition from quadriceps to ankle flexors and extensors in man. Journal of Physiology. 1990;423:661–675. doi: 10.1113/jphysiol.1990.sp018046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E, Simonetta-Moreau M. Pattern of monosynaptic heteronymous Ia connections in the human lower limb. Experimental Brain Research. 1993;96:533–544. doi: 10.1007/BF00234121. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Bergego C, Katz R, Morin C. Cutaneous depression of Ib reflex pathways to motoneurones in man. Experimental Brain Research. 1981a;42:351–361. doi: 10.1007/BF00237500. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Morin C, Bergego C, Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Experimental Brain Research. 1981b;42:337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Rossi A, Mazzochio R. Presence of homonymous recurrent inhibition in motoneurones supplying different lower limb muscles in humans. Experimental Brain Research. 1991;84:367–373. doi: 10.1007/BF00231458. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Nardone A. Medium-latency stretch reflexes of foot and leg muscles analysed by cooling the lower limb in standing humans. Journal of Physiology. 1997;503:691–698. doi: 10.1111/j.1469-7793.1997.691bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieppati M, Nardone A, Siliotto R, Grasso M. Early and late stretch responses of human foot muscles induced by perturbation of stance. Experimental Brain Research. 1995;105:411–422. doi: 10.1007/BF00233041. [DOI] [PubMed] [Google Scholar]
- Schwab GH, Moynes DR, Jobe FW, Perry J. Lower extremity electromyographic analysis of running gait. Clinical Orthopedics. 1983;176:166–170. [PubMed] [Google Scholar]
- Simonetta-Moreau M, Marque P, Marchand-Pauvert V, Pierrot-Deseilligny E. The pattern of excitation of human lower limb motoneurones by probable group II muscle afferents. Journal of Physiology. 1999;517:287–300. doi: 10.1111/j.1469-7793.1999.0287z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


