Abstract
The regulation of Maxi Cl− channels by 17β-oestradiol and non-steroidal triphenylethylene antioestrogens represents a rapid, non-classical effect of these compounds. In the present study we have investigated the signalling pathways used for the regulation of Maxi Cl− channel activity by oestrogens and antioestrogens in C1300 neuroblastoma cells.
Whole-cell Maxi Cl− currents were readily and reversibly activated by tamoxifen, toremifene and the membrane-impermeant ethyl-bromide tamoxifen, only when applied to the extracellular medium.
Pre-treatment of C1300 cells with oestrogen or cAMP prevented the antioestrogen-induced activation of Maxi Cl− channels. The inhibitory effect of 17β-oestradiol and cAMP was abolished by the kinase inhibitor staurosporine.
Current activation was unaffected by the removal of intracellular Ca2+ and Mg2+, but was completely abolished in the presence of okadaic acid. These results are consistent with the participation of an okadaic acid-sensitive serine/threonine protein phosphatase in the activation of Maxi Cl− channels. However, neither oestrogen or antioestrogen treatment modified the total activity of the two major serine/threonine phosphatases, PP1 and PP2A, in C1300 cells.
Although the role of these Maxi Cl− channels remains unknown, our findings suggest strongly that their modulation by oestrogens and antioestrogens is linked to intracellular signalling pathways.
The function of Maxi Cl− channels in cell physiology remains unresolved. Single-channel events were first described in skeletal muscle and subsequently in many different cell types, including epithelia (Hanrahan et al. 1985; Velasco et al. 1989; Vaca & Kunze, 1992; Brown et al. 1993; Riquelme et al. 1995), muscle (Blatz & Magleby, 1983; Saigusa & Kokubun, 1988), macrophages (Schwarze & Kolb, 1984), nerve cells (Forshaw et al. 1993; Bettendorff et al. 1993) and glial cells (Dermietzel et al. 1994). Almost all Maxi Cl− channel recordings have been made following excision of the membrane patch containing the channel. The need for membrane excision might indicate the involvement of intracellular inhibitory factors rather than artefactual activation by membrane disruption. An early indication of Maxi Cl− channel modulation came from experiments showing that protein kinase C regulates Maxi Cl− channels (Saigusa & Kokubun, 1988), and that excised Maxi Cl− channels can be modulated by GTP, GDP and some of their analogues (McGill et al. 1993). Subsequently, the observations that Maxi Cl− channels could be reversibly activated under whole-cell (Hardy & Valverde, 1994; Diaz et al. 1999) and cell-attached (Hardy & Valverde, 1994; Li et al. 2000) recording conditions by triphenylethylene antioestrogens such as toremifene or tamoxifen provided a method of studying these channels in intact cells.
Oestrogens exert most of their actions by binding and activating their receptors, which function as transcription factors (Beato, 1989). Antioestrogens are generally considered to act by competing with the binding of oestrogens to the oestrogen receptors (Jordan, 1984). In addition to the classical effect of oestrogen and antioestrogen, some of their actions are related to their interaction with binding sites, which generally determine a rapid effect (seconds to minutes) as opposed to the long-term genomic effect (> 30 min). In most cases, the rapid effects represent the interaction of oestrogens with a plasma membrane target and/or the generation of intracellular signals (Ropero et al. 1999; Falkenstein et al. 2000; Nadal et al. 2000, 2001).
Oestrogens have been found to exert rapid effects on the electrical activity of cells in the central nervous system (Minami et al. 1990), vascular system (Ruehlmann et al. 1998) and endocrine system (Nadal et al. 1998), and in non-excitable cells such as fibroblasts (Hardy & Valverde, 1994). Some of these actions can be either mimicked (Ruehlmann et al. 1998) or antagonised (Wong & Moss, 1991) by antioestrogens. However, the mechanisms underlying the rapid modulation of membrane excitability by oestrogens and antioestrogens are still poorly understood. Recently, both the direct interaction of steroids with the subunits forming channel structures (Valverde et al. 1999) and the generation of intracellular signals which, in turn, would modulate the activity of different ion channels have been described (Ropero et al. 1999).
In the study presented here we have demonstrated that triphenylethylene antioestrogens activate Maxi Cl− currents by binding to an extracellular plasma membrane site. Such activation depends upon the activity of an okadaic acid-sensitive protein phosphatase. On the other hand, the activation of Maxi Cl− channels by antioestrogens can be prevented by the extracellular application of 17β-oestradiol, a process that appears to be dependent upon a phosphorylation step.
METHODS
Cell culture
C1300 mouse neuroblastoma cells, obtained from the Imperial Cancer Research Fund Laboratories, Clare Hall, UK, were maintained in culture using Dulbecco's modified Eagle's medium (DMEM; Sigma) supplemented with 10 % (v/v) fetal calf serum. Cells were grown at 37 oC in a humidified atmosphere of 5 % CO2. Cells were subcultured into 35 or 60 mm culture dishes when confluent and used for patch-clamp experiments within 48 h.
Electrophysiology
The intracellular solution contained (in mm) 140 N-methyl-d-glucamine chloride (NMDGCl), 1 EGTA, 1.2 MgCl2, 10 Hepes, pH 7.3, 2 Na2ATP and 0.5 Na2GTP, unless indicated otherwise. Mg2+-free intracellular solution ([Mg+2]free 50–100 nm; calculated using EqCal from Biosoft, Cambridge, UK, assuming an initial intracellular Mg2+ concentration of 100–200 μm) was obtained by removing MgCl2 and adding 5 mm EDTA to the standard pipette solution. The presence of intracellular nucleotides was required for channel activation by antioestrogens. Cells were bathed in a solution containing (in mm): 140 NMDGCl, 10 Hepes, 0.5 MgCl2, 1.2 CaCl2, pH 7.4. For anion substitutions experiments, NaI, NaF and sodium gluconate replaced NMDGCl in the bathing solution. Whole-cell patch-clamp recordings (Hardy & Valverde, 1994) were carried out at room temperature. Borosilicate pipettes were pulled and polished to a final resistance of 2–5 MΩ. Drugs were applied to the bathing solution using a U-tube applicator, as described previously (Bond et al. 1998). Data were recorded and analysed using WCP software (J. Dempster, Strathclyde University, UK).
With the exception of toremifene, okadaic acid and ethyl-bromide tamoxifen (EB-tamoxifen), all chemicals were obtained from Sigma. Toremifene was obtained from Farmos, Torku (Finland), okadaic acid was obtained from (Calbiochem) and EB-tamoxifen was synthesised at the University of Brighton (UK). Toremifene and tamoxifen were dissolved in dimethylsulphoxide and ethanol (5 mm stock solutions), respectively, and kept in the fridge for up to 5 days. 17β-Oestradiol was freshly prepared in ethanol. Occasionally, water-soluble 17β-oestradiol was used. Statistical comparisons were made using Student's t test.
Preparation of cell lysates
Plastic dishes containing cells at approximately 70–80 % confluency were washed three times with 2 ml of phosphate-free DMEM (Sigma). After the final wash, 3 ml of phosphate-free DMEM was added and the plates were incubated for 30 min at 37 °C in a 5 % CO2 atmosphere. After 30 min, the phosphate-free medium was replaced with 2 ml of fresh medium and the cells were exposed to one of the following protocols. Toremifene (5 μm) was added to the plates for 8 min before cell lysis. The plates were then washed three times with 2 ml of washing buffer (50 mm Tris-HCl, 120 mm NaCl, pH 7.4), while kept on ice. After the final wash, 600 μl of ice-cold cell lysis buffer (50 mm Tris-HCl, 120 mm NaCl, 0.5 % (v/v) Brij-35, 10 μg ml−1 phenylmethylsulphonyl fluoride, 10 μg ml−1 leupeptin and 10 μg ml−1 aprotinin, pH 8.0) was added per 60 mm plate (Li & Damuni, 1998). The cell lysate was transferred to a 2.0 ml Eppendorf tube, rocked for 15 min at 4 °C and subsequently centrifuged at 10000 g for 10 min at 4 °C. A 100 μl aliquot taken from the supernatant was stored at −20 °C for later determination of protein concentration, following the manufacturer's instructions for the Method DC Protein Assay (Bio-Rad). The remaining 500 μl was divided into aliquots and transferred to cryotubes, frozen rapidly in liquid nitrogen, and then stored at −70 °C.
Phosphatase assays
Phosphate substrate, myelin basic protein (MBP, 0.1–0.5 nmol mg−1 protein), was phosphorylated by protein kinase A (50 U) in the presence of 0.2 mm[γ-32P]ATP (5.5 × 106 c.p.m. nmol−1), 50 mm Tris-HCl, 0.1 m magnesium acetate and pH 7.5. The phosphatase assays were carried out for 30 min (at 30 °C), according to methods described previously (Posas et al. 1995) in a final volume of 30 μl, in Ca2+- and Mg2+-free conditions and in the absence or presence of 1 nm okadaic acid. The final concentration of substrate was 135 μg ml−1 and the protein concentration of the cell lysate was between 0.5 and 15 μg ml−1. One unit of phosphatase activity is defined as 1 μmol of phosphate released from MBP per minute. Phosphatase assays were also carried out in the presence of divalent cations (1 mm Mg2+ and/or 2.5 mm Mn2+). All experiments were carried out in quintuplicate for all conditions.
RESULTS
Activation of Maxi Cl− currents by extracellular antioestrogens
C1300 mouse neuroblastoma cells were studied using the whole-cell configuration of the patch-clamp technique. Under control conditions, with Cl− as the main charge carrier, currents were negligibly low (4.1 ± 3.9 pA pF−1, n = 49; measured at +80 mV). The addition of the triphenylethylene antioestrogen toremifene (Fig. 1A) or tamoxifen (Fig. 1B) induced a reversible activation of a Cl− current in 44 out of 49 cells. The current was characterised by inactivation at negative potentials and, unlike the Maxi Cl− currents that have been activated by antioestrogens in fibroblasts (Hardy & Valverde, 1994), presented little inactivation at positive potentials. The ionic selectivity of the whole-cell Cl− current (Fig. 1C) followed the same ionic selectivity as the Maxi Cl− current that is activated by antioestrogens in fibroblasts (Hardy & Valverde, 1994). Single-channel analysis of excised patches of C1300 cells revealed the presence of a Maxi Cl− channel with a single-channel conductance of 305 ± 16 pS (Fig. 1D, n = 12), which, like the antioestrogen-activated whole-cell currents, exhibited inactivation at negative potentials only. These observations are consistent with the antioestrogen-induced activation of Maxi Cl− channels in C1300 cells.
Figure 1. Activation of Maxi Cl− channels by toremifene (A) and tamoxifen (B) in C1300 neuroblastoma cells.

Cl− currents were obtained under control conditions and 5 min after exposure of the cells to the antioestrogens toremifene (A) and tamoxifen (B). Whole-cell currents were measured in response to voltage pulses from −80 to +80 mV in 40 mV increments from a holding potential of 0 mV. C, effect of ion replacements on toremifene-induced Maxi Cl− currents. The current-voltage curves were obtained by applying a ramp of voltage from −80 mV to +80 mV over a 1 s period. D, excised single-channel recording obtained from a C1300 cell.
The activation of Maxi Cl− currents by antioestrogens was rapid (Fig. 2A) and dose dependent (Fig. 2B). Similarly to the dose-response curve obtained for antioestrogen-activated Maxi Cl− currents in fibroblasts (Hardy & Valverde, 1994), toremifene was more potent than tamoxifen. The response to toremifene was also evaluated in the absence of intracellular Mg2+ ([Mg2+]free 50–100 nm) or in the presence of 1 mm adenosine 5′-(β,γ-imino) triphosphate (AMP-PCP), a non-hydrolysable analogue of ATP, instead of ATP. After 5 min in the whole-cell recording condition with an ATP/Mg2+-free or AMP-PCP/Mg2+-free pipette solution, the cells were exposed to 5 μm toremifene. The mean increase in current was 91 ± 9 pA pF−1 (n = 4) and 78 ± 17 pA pF−1 (n = 3), respectively.
Figure 2. Time course of toremifene-induced activation of Maxi Cl− currents, and demonstration of their concentration dependence.

A, time course of toremifene-induced activation of Maxi Cl− currents following addition of 5 μm toremifene at time = 0 min (Ipeak, peak current). B, concentration-dependent activation of Maxi Cl− currents by antioestrogens. Current amplitude was measured 5 min after addition of the antioestrogens (n≥ 3 for each concentration).
The activation of Maxi Cl− currents could not be reproduced when the antioestrogens were added to the intracellular compartment via the pipette solution (Fig. 3), suggesting the need for an intracellular binding site to trigger Maxi Cl− channel activation. This hypothesis was corroborated by the use of the non-permeant antioestrogen EB-tamoxifen (Jarman et al. 1986). Intracelullar EB-tamoxifen did not trigger the activation of the current for up to 5 min after breaking into the whole-cell configuration (Fig. 3A and B). Subsequent addition of EB-tamoxifen to the bathing solution resulted in the rapid activation of Maxi Cl− channels (Fig. 3C). These experiments suggest the presence of an external antioestrogen binding site that triggers the activation of Maxi Cl− channels.
Figure 3. Effect of the membrane-impermeant antioestrogen ethyl-bromide tamoxifen (EB-Tx).

Whole-cell currents recorded consecutively in a C1300 cell dialysed with an intracellular solution containing 5 μm EB-Tx (a and b). Cl− currents could only be activated when EB-Tx was added to the extracellular solution (c).
Intracellular signals involved in Maxi Cl− channel activation by antiestrogens
Role of protein phosphatases
The activity of many ion channels can be modulated by changes in the phosphorylation-dephosphorylation balance of the cell. In that respect, it is interesting to note that antioestrogens have been shown to induce phosphatase activity (Freiss & Vignon, 1994). Therefore, we tested the possibility that a dephosphorylation step could be involved in the antioestrogen-induced activation of Maxi Cl− channels.
A series of preliminary experiments indicated that, unlike general inhibitors of protein tyrosine phosphatases such as vanadate (Gordon, 1991) or Zn2+ (Brautigan et al. 1981), inhibitors of protein serine/threonine phosphatases (S/T PP) such as F− (Hawkinson et al. 1997) were effective in preventing the activation of Maxi Cl− channels by antioestrogens (Diaz et al. 1999). The participation of divalent cation-dependent phosphatases (of the family of PP2B and PP2C; Mumby & Walter, 1993; Cohen, 1997) could be excluded because intracellular Ca2+ and Mg2+ chelation to low nanomolar concentrations (n = 5) did not prevent the activation of Maxi Cl− currents. Furthermore, cyclosporin A at 100 μm, an inhibitor of PP2B (Hunter, 1995), was also ineffective (n = 7). Consequently, we examined the use of okadaic acid at a concentration that would distinguish between the two divalent cation-independent phosphatases, PP1 and PP2A. The IC50 for okadaic acid inhibition of PP1 is 60–600 nm, and that for PP2A is 0.1–2 nm (Cohen, 1991).
Figure 4A shows whole-cell currents obtained from a cell dialysed with the standard pipette solution containing 1 nm okadaic acid. The presence of intracellular okadaic acid prevented the activation of Maxi Cl− currents by antioestrogens. Figure 4B summarises the mean Cl− current amplitudes obtained in response to the application of 10 μm toremifene in the absence and presence of 1 nm okadaic acid in the pipette solution. From these experiments it could be postulated that protein phosphatases, possibly PP2A-like, are involved in the activation of Maxi Cl− by antioestrogens. Therefore, we studied further the protein phosphatase activity of C1300 cells. Figure 5A shows the percentage of phosphatase activity of cell extracts obtained from control cells and cells treated with 5 μm toremifene for 8 min. The overall phosphatase activity did not change with antioestrogen treatment. These assays were performed in the absence of Ca2+ and Mg2+. When the assays were performed under conditions that support the activity of cation-dependent phosphatases (i.e. in the presence of Mn2+ and Mg2+), similar responses were obtained (results not shown). The effect of okadaic acid on phosphatase activity in cell extracts was also tested (Fig. 5B). In the presence of 1 nm okadaic acid (a concentration at which it only blocks PP2A-like phosphatases) the activity was reduced to around 60 % of that observed in the control, untreated cells (see also Fig. 5D). No difference was observed between the okadaic acid-insensitive fraction (mostly PP1-like phosphatase activity) and the okadaic acid-sensitive fraction (which is the difference between control and okadaic acid-treated cells and represents mainly the PP2A-like phosphatase activity) in control and toremifene-treated cells (Fig. 5B).
Figure 4. Okadaic acid (OA) prevents the activation of Maxi Cl− channels by toremifene.
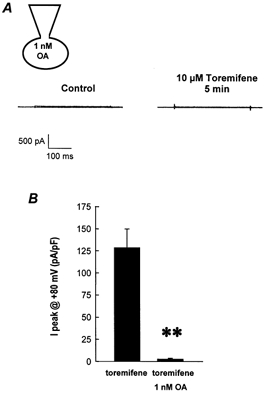
A, whole-cell Cl− currents recorded in a C1300 cell dialysed with an intracellular solution containing 1 nm OA. B, mean response to 10 μm toremifene in the presence of OA. Results are expressed as mean ±s.e.m. (n = 4 for each experimental condition) of normalised peak currents measured at +80 mV. ** Statistically different from control conditions with a probability value of P < 0.001.
Figure 5. Phosphatase activity in C1300 cells.
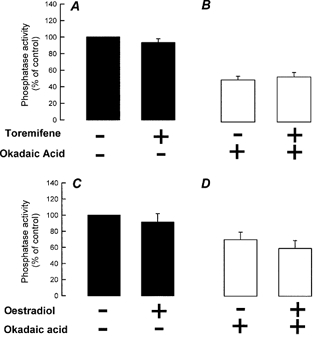
A, effect of 5 μm toremifene on phosphatase activity in the absence (-) of OA. B, effect of 5 μm toremifene on phosphatase activity in the presence of 1 nm OA (+). C, effect of 500 nm 17β-oestradiol on the phosphatase activity in C1300 cells in the absence (-) of OA. D, effect of 500 nm 17β-oestradiol on the phosphatase activity in C1300 cells in the presence of 1 nm OA (+). Phosphatase activity is presented as the percentage of that of control (untreated) cells. Bars represent the mean of quintuplicate determinations ±s.e.m. (n = 3). P > 0.05.
Modulation of Maxi Cl− channel activation by oestrogens
The activation of Maxi Cl− channels by antioestrogen can be prevented by pre-treatment with 17β-oestradiol in NIH3T3 fibroblasts (Hardy & Valverde, 1994) and endothelial cells (Li et al. 2000). This modulatory action of oestradiol was also investigated in C1300 neuroblastoma cells in the present study (Fig. 6A). Pre-exposure to 100–500 nm oestradiol in the bathing solution 5–10 min prior to the addition of toremifene did not modify the resting Cl− currents (Fig. 6Ab), but inhibited the activation of Maxi Cl− currents (Fig. 6Ac) by toremifene (in 10 out of 19 cells). In the cells in which Maxi Cl− currents were prevented by the presence of oestradiol, subsequent removal of oestradiol resulted in the prompt development of sizeable Maxi Cl− currents (Fig. 6Ae). The inhibitory effect of oestradiol was also checked in cells dialysed with Mg2+-free solutions ([Mg2+]free 50–100 nm; Fig. 6B). Under this condition, oestradiol did not prevent the toremifene-induced activation of Maxi Cl− currents.
Figure 6. Modulation of toremifene-activated Maxi Cl− channels by 17β-oestradiol.

A, pre-exposure of C1300 cells to 500 nm 17β-oestradiol prevented the activation of Maxi Cl− currents by toremifene. B, lack of effect of oestradiol in cells dialysed with Mg2+-free (50–100 nm) intracellular solutions.
The effect of 17β-oestradiol on phosphatase activity in C1300 cells was also tested. 17β-Oestradiol did not modify either the overall phosphatase activity (Fig. 5C) or the okadaic-acid-sensitive fraction (Fig. 5D).
Role of cAMP and phosphorylation on the oestrogen-mediated modulation of Maxi Cl− channels
Many of the actions of oestrogens can be mediated by the generation of intracellular signals, including the production of intracellular cAMP (Aronica et al. 1995; Doolan et al. 2000). We used a membrane-permeable cAMP derivative to try to mimic the effect of 17β-oestradiol. As shown in Fig. 7, a protocol similar to that described in Fig. 6 was used, but with dibutryl-cAMP instead of oestradiol. As with oestradiol, pre-incubation with cAMP significantly reduced the activation of Maxi Cl− currents by toremifene (Fig. 7A–c). To check whether the effect was due to an increase in cAMP levels or an increase in cAMP-dependent protein kinase activity, we repeated the experimental protocol in the same cell (Fig. 7D–g) but in the continuous presence of staurosporine, a general S/T protein kinase inhibitor (Werz et al. 1993). In the presence of cAMP and staurosporine, activation of Maxi Cl− currents by 10 μm toremifene was unaffected (90 ± 14 pA pF−1, n = 5). Staurosporine was also used in conjunction with oestradiol (Fig. 8), and similar results were obtained; in the presence of staurosporine, oestradiol did not prevent the toremifene-induced activation of Maxi Cl− currents. The mean responses to different experimental conditions are summarised in Fig. 9.
Figure 7. Effects of cAMP and staurosporine on toremifene-activated Maxi Cl− channels.

Representative current tracings taken from a single cell held at 0 mV and pulsed from −80 mV to +80 mV.
Figure 8. Effects of staurosporine on 17β oestradiol-induced modulation of Maxi Cl− channel activation.

Consecutive recordings were obtained from a single cell. E2, 17β-oestradiol.
Figure 9. Bar chart summarising the results of the incubation with 17β-oestradiol, and/or stauroporine on Maxi Cl− channel currents recorded under control conditions or in the presence of 10 μm toremifene.

The results are presented as the mean ±s.e.m. for Ipeak data obtained at +80 mV and normalised to cell capacitance. ** Statistically different from control conditions (in the absence of any drug) with a probability value of P < 0.001. NS, no significant differences with respect to the control conditions. The number of cells examined under each of the conditions is as follows: control (49), oestradiol (5), staurosporine (6), toremifene (18), oestradiol + toremifene (5) and staurospaurine + oestradiol + toremifene (4).
DISCUSSION
In the present study we report the modulation of Maxi Cl− channels in C1300 mouse neuroblastoma cells by both oestrogens and antioestrogens. Antioestrogen-activated Maxi Cl− channels in C1300 cells exhibited a similar conductance (∼300 pS) and ionic selectivity (I− > Cl− > F−) to the Maxi Cl− channels activated by antioestrogens in fibroblasts (Hardy & Valverde, 1994), although the voltage-dependent inactivation differed slightly from one cell type to another. While the channel in the fibroblasts shows marked inactivation at positive and negative potentials (Hardy & Valverde, 1994), the channel in the neuroblastoma cell line (this study) only presented inactivation at negative potentials, although the inactivation constant varied greatly from cell to cell. A Maxi Cl− channel activated by antioestrogens has also been described in endothelial cells, although its voltage dependence was not reported (Li et al. 2000). Differences in the voltage-dependent inactivation of Maxi Cl− channels have been observed among different cell types (Blatz & Magleby, 1983; Schwarze & Kolb, 1984; Kolb et al. 1985; Forshaw et al. 1993; Riquelme et al. 1995). In this respect, it is interesting to note that the mitochondrial voltage-dependent anionic channel (VDAC), also known as mitochondrial porin, exhibits bell-shaped voltage dependence, a property associated with the presence of different protein modulators (Liu & Colombini, 1991) or polyamines (Horn et al. 1998). VDAC have also been identified in the plasma membrane (Dermietzel et al. 1994). Thus, although there is still much debate regarding the molecular identification of plasma membrane Maxi Cl− channels and whether they can be considered members of the VDAC family, it would be interesting to investigate whether the differences in voltage-dependent inactivation between fibroblast and neuroblastoma Maxi Cl− channels are due to the presence of different structural features, regulatory subunits, or associations with low molecular-weight molecules such as polyamines.
Maxi Cl− channel activity has been seen in intact cells only rarely, although it generally appears following membrane-patch excision. This observation has suggested the existence of regulatory mechanisms that keep the channel closed under non-stimulatory conditions. Early evidence of such intracellular regulation came from Saigusa & Kokubun (1988), who showed the activation of Maxi Cl− channels by inhibiting protein kinase activity with H7. That work suggested that phosphorylation of the Maxi Cl− channel or a regulatory subunit kept the channel closed. The activation of Maxi Cl− channels under the whole-cell and cell-attached patch-clamp configurations by the addition of extracellular antioestrogens is interesting for two reasons. First, it supports the hypothesis that activation of the channel following patch excision is most likely the result of altered intracellular regulatory mechanisms rather than merely a physical disruption of the membrane caused by the process of patch excision itself. Second, the fact that activation of the channels only occurs when triphenylethylene antioestrogens were added to the extracellular solution indicates the presence of an antioestrogen binding site on the plasma membrane that is accessible from the external side. This is supported by the use of the membrane-impermeant derivative EB-tamoxifen (Jarman et al. 1986), which was only effective when applied to the extracellular solution. The presence of both oestrogen (Pietras & Szego, 1977; Bression et al. 1986; Pappas et al. 1995; Nadal et al. 2000) and antioestrogen (Sudo et al. 1983; Klinge et al. 1992) binding sites in the plasma membrane has been characterised in several cell preparations. We have demonstrated previously that the activation of Maxi Cl− channels in fibroblasts is independent of the activity of the so-called antioestrogen binding site (Hardy & Valverde, 1994).
The possibility that the activation of the Maxi Cl− channels by antioestrogens is related to the alteration of the phosphorylation-dephosphorylation balance was also investigated. We hypothesised that if phosphorylation of the channel, or a regulatory factor, keeps the channel closed under non-stimulatory conditions, as suggested by Saigusa & Kokubun (1988), the activation of the channel may be linked to a dephosphorylation reaction. The activation of Maxi Cl− currents by antioestrogens appears to be related to the activity of S/T PP, since general inhibitors of S/T PP (20 mm NaF), but not tyrosine phosphatases (2 mm ZnCl2 or 100 μm vanadate), prevented it. Of the four major S/T PPs, PP1, PP2A, PP2B and PP2C (reviewed by Cohen, 1991), the involvement of the divalent cation-dependent phosphatases, 2B and 2C, was excluded because current activation was preserved in the absence of intracellular Ca2+ or Mg2+ or in the presence of cyclosporin A, an inhibitor of PP2B (Hunter, 1995). The phosphatases PP1 and PP2A are both divalent cation independent and can be distinguished by their respective sensitivity to okadaic acid. PP2A is inhibited by 1 nm okadaic acid (IC50 < 1 nm; Cohen, 1991, 1997), while PP1 is unaffected by 1 nm okadaic acid, but is completely inhibited at 1 μm (IC50≈ 60–600 nm; Cohen, 1991, 1997). The fact that 1 nm okadaic acid totally inhibited the activation of Maxi Cl− channels by antioestrogens is consistent with the participation of a PP2A-like phosphatase. In accordance with this hypothesis, we detected phosphatase activity in C1300 cells that was inhibited by 1 nm okadaic acid. The okadaic acid-sensitive phosphatase activity represented around 30 % of the total cation-independent phosphatase activity. However, we did not observe changes in okadaic acid-sensitive or -insensitive phosphatase activity with either oestrogen or antioestrogens. The okadaic acid-sensitive PP2A-like phosphatases are heterotrimeric enzymes that are composed of a catalytic subunit (C) and two regulatory subunits (A and B: Mumby & Walter, 1993; Mayer-Jaekel & Hemmings, 1994; Price & Mumby, 2000). The characteristics of PP2A phosphatase (e.g. localisation, substrate specificity and kinetic properties) can be modulated by changes in the regulatory subunits, without a concomitant change in the total PP2A activity (Goldberg, 1999; Price & Mumby, 2000), that may represent a more subtle modulation of the enzyme than its massive activation. We speculate that the activation of Maxi Cl− channels, which depends upon an okadaic acid-sensitive step, represents a change in the substrate specificity by PP2A-like phosphatases in response to antioestrogens, enabling them to dephosphorylate Maxi Cl− channels or as yet unknown regulatory subunits of the channel.
Many of the rapid, non-genomic effects of oestrogens, including the modulation of ion channels, have been associated with increases in protein kinase activity via the activation of a membrane G-protein-coupled receptor (Minami et al. 1990; Lagrange et al. 1997; Gu & Moss, 1998; Kelly & Wagner, 1999). The effect of oestradiol on the prevention of Maxi Cl− activation by antioestrogens also appears to be linked to protein phosphorylation events. Several pieces of evidence support this assumption. First, the oestradiol effect can be mimicked by cAMP. Second, the effect of both cAMP and oestradiol can be prevented in the presence of staurosporine, a broad-spectrum S/T protein kinase inhibitor. Third, the Maxi Cl− channel activation effected by toremifene can be greatly reduced by including 100 μm GDPβS, a non-hydrolysable GDP analogue, in the pipette solution, and slightly increased by including 100 μm GppNHp (guanosine 5′-(β-imido) triphosphate), a non-hydrolysable analogue of GTP (Diaz et al. 1999). Finally, the inhibitory effect of oestradiol can be prevented by loading cells with Mg2+-free (50–100 nm) solutions, a situation in which transfer of the γ phosphate from ATP to the receptor protein by the kinase(s) is impaired. However, since no specific kinase inhibitors have been used we have no indication as to whether oestrogens use more than one kinase pathway to prevent channel activation, or whether they induce phosphorylation of the channel protein or other elements involved in the activation of Maxi Cl− channels.
The facts that antioestrogen-induced activation of Maxi Cl− channels depends upon the presence of intracellular GTP and is modulated by GDPβS and GppNHp suggest the participation of a G-protein in the signalling cascade. However, channel activation by antioestrogens is retained in Mg2+-free intracellular solutions, despite the fact that G-protein activation requires Mg2+. The different Mg2+ requirements of these two processes (G-protein activation and protein phosphorylation) might explain this apparent contradiction. While the concentration of Mg2+ required for the activation of many G-proteins (GTP hydrolysis and GTP binding in the presence of a ligand) is in the range of 10−9-10−8m (Brandt & Ross, 1986; Gilman, 1987; Higashijima et al. 1987), the [Mg2+]free required by different protein kinases is typically > 10−5m (Pickett-Gies & Walsh, 1985; Sun & Budde, 1997; Vinals et al. 1997; Rodriguez-Zavala & Moreno-Sanchez, 1998; Harvengt et al. 2000; Srivenugopal et al. 2000). Therefore, it is plausible that the actual intracellular [Mg2+]free (∼ 10−7m) present in the Mg2+-free solution is sufficient to activate G proteins but insufficient to support a phosphorylation process.
In summary, we propose that under non-stimulatory conditions the Maxi Cl− channels are kept closed by phosphorylation of the channel protein or a regulator of its activity. Upon the addition of triphenylethylene antioestrogens, a PP2A-like phosphatase is activated, probably via a G-protein-coupled receptor, leading to the dephosphorylation of the channel (or a regulatory subunit) and channel opening. On the other hand, oestrogen prevents channel activation by promoting phosphorylation of the channel or a key element in the channel activation pathway.
Acknowledgments
This work was supported by CICYT/FEDER 1FD97–1065-C03–01 (M.D.), Human Frontiers Science Program (M.A.V.) and Distinció de la Generalitat de Catalunya per a la Promocio de la Recerca Universitaria (M.A.V.).
References
- Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase activity and cAMP-regulated gene transcription. Proceedings of the National Academy of Sciences of the USA. 1995;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Bettendorff L, Kolb HA, Schoffeniels E. Thiamine triphosphate activates an anion channel of large unit conductance in neuroblastoma cells. Journal of Membrane Biology. 1993;136:281–288. doi: 10.1007/BF00233667. [DOI] [PubMed] [Google Scholar]
- Blatz AL, Magleby KL. Single voltage-dependent chloride-selective channels of large conductance in cultured rat muscle. Biophysical Journal. 1983;43:237–241. doi: 10.1016/S0006-3495(83)84344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond TD, Higgins CF, Valverde MA. P-Glycoprotein and swelling-activated chloride channels. Methods in Enzymology. 1998;292:359–370. doi: 10.1016/s0076-6879(98)92028-6. [DOI] [PubMed] [Google Scholar]
- Brandt DR, Ross EM. Catecholamine-stimulated GTPase cycle. Multiple sites of regulation by beta-adrenergic receptor and Mg2+ studied in reconstituted receptor-Gs vesicles. Journal of Biological Chemistry. 1986;261:1656–1664. [PubMed] [Google Scholar]
- Brautigan DL, Bornstein P, Gallis B. Phosphotyrosyl-protein phosphatase. Specific inhibition by Zn. Journal of Biological Chemistry. 1981;256:6519–6522. [PubMed] [Google Scholar]
- Bression D, Michard M, Le Dafniet M, Pagesy P, Peillon F. Evidence for a specific estradiol binding site on rat pituitary membranes. Endocrinology. 1986;119:1048–1051. doi: 10.1210/endo-119-3-1048. [DOI] [PubMed] [Google Scholar]
- Brown PD, Greenwood SL, Robinson J, Boyd RDH. Chloride channels of high conductance in the microvillous membrane of term human placenta. Placenta. 1993;14:103–115. doi: 10.1016/s0143-4004(05)80253-x. [DOI] [PubMed] [Google Scholar]
- Cohen P. Classification of protein-serine/threonine phosphatases: identification and quantitation in cell extracts. Methods in Enzymology. 1991;201:389–398. doi: 10.1016/0076-6879(91)01035-z. [DOI] [PubMed] [Google Scholar]
- Cohen PT. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends in Biochemical Sciences. 1997;22:245–251. doi: 10.1016/s0968-0004(97)01060-8. [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Hwang T-K, Buettner R, Hofer A, Dotzler E, Kremer M, Deutzman R, Thinnes FP, Fishman GI, Spray DC, Siemen D. Cloning and in situ localization of a brain-derived porin that constitutes a large-conductance anion channel in astrocytic plasma membranes. Proceedings of the National Academy of Sciences of the USA. 1994;91:499–503. doi: 10.1073/pnas.91.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M, Hardy SP, Valverde MA. Modulation of Maxi-Cl channels by oestrogen and antioestrogens. Journal of Physiology. 1999;517.P:7S–8S. [Google Scholar]
- Doolan CM, Condliffe SB, Harvey BJ. Rapid non-genomic activation of cytosolic cyclic AMP-dependent protein kinase activity and [Ca2+]i by 17beta-oestradiol in female rat distal colon. British Journal of Pharmacology. 2000;129:1375–1386. doi: 10.1038/sj.bjp.0703193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones — a focus on rapid, nongenomic effects. Pharmacological Reviews. 2000;52:513–556. [PubMed] [Google Scholar]
- Forshaw PJ, Lister T, Ray DE. Inhibition of a neuronal voltage-dependent chloride channel by the type II pyrethroid, deltamethrin. Neuropharmacology. 1993;32:105–111. doi: 10.1016/0028-3908(93)90089-l. [DOI] [PubMed] [Google Scholar]
- Freiss G, Vignon F. Antiestrogens increase protein tyrosine phosphatase activity in human breast cancer cells. Molecular Endocrinology. 1994;8:1389–1396. doi: 10.1210/mend.8.10.7854356. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annual Review of Biochemistry. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goldberg Y. Protein phosphatase 2A: who shall regulate the regulator? Biochemical Pharmacology. 1999;57:321–328. doi: 10.1016/s0006-2952(98)00245-7. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods in Enzymology. 1991;201:477–482. doi: 10.1016/0076-6879(91)01043-2. [DOI] [PubMed] [Google Scholar]
- Gu Q, Moss RL. Novel mechanism for non-genomic action of 17β-oestradiol on kainate-induced currents in isolated rat CA1 hippocampal neurones. Journal of Physiology. 1998;506:745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan JW, Alles WP, Lewis SA. Single anion-selective channels in basolateral membrane of a mammalian tight epithelium. Proceedings of the National Academy of Sciences of the USA. 1985;82:7791–7795. doi: 10.1073/pnas.82.22.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy SP, Valverde MA. Novel plasma membrane action of estrogen and antiestrogens revealed by their regulation of a large conductance chloride channel. FASEB Journal. 1994;8:760–765. doi: 10.1096/fasebj.8.10.8050676. [DOI] [PubMed] [Google Scholar]
- Harvengt P, Vlerick A, Fuks B, Wattiez R, Ruysschaert JM, Homble F. Lentil seed aquaporins form a hetero-oligomer which is phosphorylated by a Mg2+-dependent and Ca2+-regulated kinase. Biochemical Journal. 2000;352:183–190. [PMC free article] [PubMed] [Google Scholar]
- Hawkinson JE, Acosta-Burruel M, Ta ND, Wood PL. Novel phosphoserine phosphatase inhibitors. European Journal of Pharmacology. 1997;337:315–324. doi: 10.1016/s0014-2999(97)01304-6. [DOI] [PubMed] [Google Scholar]
- Higashijima T, Ferguson KM, Sternweis PC, Smigel MD, Gilman AG. Effects of Mg2+ and the beta gamma-subunit complex on the interactions of guanine nucleotides with G proteins. Journal of Biological Chemistry. 1987;262:762–766. [PubMed] [Google Scholar]
- Horn A, Reymann S, Thinnes FP. Studies on human porin. XVI: Polyamines reduce the voltage dependence of human VDAC in planar lipid bilayers — spermine and spermidine inducing asymmetric voltage gating on the channel. Molecular Genetics and Metabolism. 1998;63:239–242. doi: 10.1006/mgme.1997.2671. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Jarman M, Leung OT, Leclercq G, Devleeschouwer N, Stoessel S, Coombes RC, Skilton RA. Analogues of tamoxifen: the role of the basic side-chain. Applications of a whole-cell oestrogen-receptor binding assay to N-oxides and quaternary salts. Anticancer Drug Design. 1986;1:259–268. [PubMed] [Google Scholar]
- Jordan VC. Biochemical pharmacology of antiestrogens action. Pharmacological Reviews. 1984;36:245–276. [PubMed] [Google Scholar]
- Kelly MJ, Wagner EJ. Estrogen modulation of G-protein-coupled receptors. Trends in Endocrinology and Metabolism. 1999;10:369–374. doi: 10.1016/s1043-2760(99)00190-3. [DOI] [PubMed] [Google Scholar]
- Klinge CM, Bambara RA, Hilf R. What differentiates antioestrogen-liganded vs. estradiol-liganded estrogen receptor action? Oncology Research. 1992;4:145–150. [PubMed] [Google Scholar]
- Kolb HA, Brown CDA, Murer H. Identification of a voltage-dependent anion channel in the apical membrane of a Cl−-secretory epithelium (MDCK) Pflügers Archiv. 1985;403:262–265. doi: 10.1007/BF00583597. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Ronnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Molecular Pharmacology. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- Li M, Damuni Z. I1PP2A and I2PP2A. Two potent protein phosphatase 2A-specific inhibitor proteins. In: Ludlow JW, editor. Protein Phosphatase Protocols: Methods in Molecular Biology. Totowa: Humana; 1998. pp. 59–66. [DOI] [PubMed] [Google Scholar]
- Li Z, Niwa Y, Sakamoto S, Chen X, Nakaya Y. Estrogen modulates a large conductance chloride channel in cultured porcine aortic endothelial cells. Journal of Cardiovascular Pharmacology. 2000;35:506–510. doi: 10.1097/00005344-200003000-00023. [DOI] [PubMed] [Google Scholar]
- Liu MY, Colombini M. Voltage gating of the mitochondrial outer membrane channel VDAC is regulated by a very conserved protein. American Journal of Physiology. 1991;260:C371–374. doi: 10.1152/ajpcell.1991.260.2.C371. [DOI] [PubMed] [Google Scholar]
- McGill JM, Gettys TW, Basavappa S, Fitz JG. GTP-binding proteins regulate high conductance anion channels in rat bile duct epithelial cells. Journal of Membrane Biology. 1993;133:253–261. doi: 10.1007/BF00232024. [DOI] [PubMed] [Google Scholar]
- Mayer-Jaekel RE, Hemmings BA. Protein phosphatase 2A — a ‘menage a trois’. Trends in Cell Biology. 1994;4:287–291. doi: 10.1016/0962-8924(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Minami T, Oomura Y, Nabekura J, Fukuda A. 17B-estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Research. 1990;519:301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- Mumby MC, Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiological Reviews. 1993;73:673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- Nadal A, Diaz M, Valverde MA. The estrogen trinity: membrane, cytosolic and nuclear effects. News in Physiological Sciences (in the Press) 2001 doi: 10.1152/physiologyonline.2001.16.6.251. [DOI] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proceedings of the National Academy of Sciences of the USA. 2000;97:11603–11608. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A, Rovira JM, Laribi O, Leon-quinto T, Andreu E, Ripoll C, Soria B. Rapid insulinotropic effect of 17beta-estradiol via a plasma membrane receptor. FASEB Journal. 1998;12:1341–1348. doi: 10.1096/fasebj.12.13.1341. [DOI] [PubMed] [Google Scholar]
- Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB Journal. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- Pickett-Gies CA, Walsh DA. Subunit phosphorylation and activation of skeletal muscle phosphorylase kinase by the cAMP-dependent protein kinase. Divalent metal ion, ATP, and protein concentration dependence. Journal of Biological Chemistry. 1985;260:2046–2056. [PubMed] [Google Scholar]
- Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- Posas F, Bollen M, Stalmans W, Arino J. Biochemical characterization of recombinant yeast PPZ1, a protein phosphatase involved in salt tolerance. FEBS Letters. 1995;368:39–44. doi: 10.1016/0014-5793(95)00593-x. [DOI] [PubMed] [Google Scholar]
- Price NE, Mumby MC. Effects of regulatory subunits on the kinetics of protein phosphatase 2A. Biochemistry. 2000;39:11312–11318. doi: 10.1021/bi0008478. [DOI] [PubMed] [Google Scholar]
- Riquelme G, Stutzin A, Barros LF, Liberona JL. A chloride channel from human placenta reconstituted into giant liposomes. American Journal of Obstetrics and Gynecology. 1995;173:733–738. doi: 10.1016/0002-9378(95)90332-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Zavala JS, Moreno-Sanchez R. Modulation of oxidative phosphorylation by Mg2+ in rat heart mitochondria. Journal of Biological Chemistry. 1998;273:7850–7855. doi: 10.1074/jbc.273.14.7850. [DOI] [PubMed] [Google Scholar]
- Ropero AB, Fuentes E, Rovira JM, Ripoll C, Soria B, Nadal A. Non-genomic actions of 17β-oestradiol in mouse pancreatic beta-cells are mediated by a cGMP-dependent protein kinase. Journal of Physiology. 1999;521:397–407. doi: 10.1111/j.1469-7793.1999.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehlmann DO, Steinert JR, Valverde MA, Jacob R, Mann GE. Environmental estrogenic pollutants induce acute vascular relaxation by inhibiting L-type Ca2+ channels in smooth muscle cells. FASEB Journal. 1998;12:613–619. doi: 10.1096/fasebj.12.7.613. [DOI] [PubMed] [Google Scholar]
- Saigusa A, Kokubun S. Protein kinase C may regulate resting anion conductance in vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 1988;155:882–889. doi: 10.1016/s0006-291x(88)80578-3. [DOI] [PubMed] [Google Scholar]
- Schwarze W, Kolb HA. Voltage-dependent kinetics of an anionic channel of large unit conductance in macrophages and myotube membranes. Pflügers Archiv. 1984;402:281–291. doi: 10.1007/BF00585511. [DOI] [PubMed] [Google Scholar]
- Srivenugopal KS, Mullapudi SR, Shou J, Hazra TK, Ali-Osman F. Protein phosphorylation is a regulatory mechanism for O6-alkylguanine-DNA alkyltransferase in human brain tumor cells. Cancer Research. 2000;60:282–287. [PubMed] [Google Scholar]
- Sudo K, Monsma FJ, Jr, Katzenellenbogen BS. Antiestrogen-binding sites distinct from the estrogen receptor: subcellular localization, ligand specificity, and distribution in tissues of the rat. Endocrinology. 1983;112:425–433. doi: 10.1210/endo-112-2-425. [DOI] [PubMed] [Google Scholar]
- Sun G, Budde RJ. Requirement for an additional divalent metal cation to activate protein tyrosine kinases. Biochemistry. 1997;36:2139–2146. doi: 10.1021/bi962291n. [DOI] [PubMed] [Google Scholar]
- Vaca L, Kunze DL. Anion and cation permeability of a large conductance anion channel in the T84 human colonic cell line. Journal of Membrane Biology. 1992;130:241–249. doi: 10.1007/BF00240481. [DOI] [PubMed] [Google Scholar]
- Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- Velasco G, Prieto M, Alvarez-Riera J, Gascon S, Barros F. Characteristics and regulation of a high conductance anion channel in GBK kidney epithelial cells. Pflügers Archiv. 1989;414:304–310. doi: 10.1007/BF00584631. [DOI] [PubMed] [Google Scholar]
- Vinals F, Camps M, Testar X, Palacin M, Zorzano A. Effect of cations on the tyrosine kinase activity of the insulin receptor: inhibition by fluoride is magnesium dependent. Molecular Cell Biochemistry. 1997;171:69–73. doi: 10.1023/a:1006836001489. [DOI] [PubMed] [Google Scholar]
- Werz MA, Elmslie KS, Jones SW. Phosphorylation enhances inactivation of N-type calcium channel current in bullfrog sympathetic neurons. Pflügers Archiv. 1993;424:538–545. doi: 10.1007/BF00374919. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. Electrophysiological evidence for a rapid membrane action of the gonadal steroid, 17B-estradiol, on CA1 pyramidal neurons of the rat hippocampus. Brain Research. 1991;543:148–152. doi: 10.1016/0006-8993(91)91057-8. [DOI] [PubMed] [Google Scholar]


