Abstract
The effects of sodium nitroprusside (SNP) and diethylenetriamine/nitric oxide adduct (DETA/NO), putative nitric oxide (NO) donors, on opossum oesophageal longitudinal smooth muscle were investigated using isometric tension and intracellular micro-electrode recordings.
SNP produced concentration-dependent contractions of oesophageal longitudinal smooth muscle with an EC50 of 239.6 ± 78.2 μm (mean ±s.e.m., n = 10). Maximal contraction induced by SNP (1 mm) was about 75.5 ± 8.5 % (n = 10) of the 60 mm KCl-induced contraction. The SNP-induced contraction was resistant to tetrodotoxin (TTX; 1 μm), but abolished by nifedipine (1 μm), as well as by niflumic acid (300 μm) and 9-anthroic acid (9-AC; 1 mm), Ca2+-activated Cl− channel blockers.
DETA/NO at concentrations of 100 and 500 μm induced 83.1 ± 24.4 and 104.1 ± 34.9 % of the 60 mm KCl-induced contraction (n = 4), respectively, which was abolished by nifedipine (1 μm), niflumic acid (300 μm) and 9-AC (1 mm).
Pre-application of 1H-[1,2,4]oxidiazolo[4,3,-α]quinoxalin-1-one (ODQ) (10 μm), a guanylate cyclase inhibitor, significantly inhibited the SNP-induced contraction, whereas 8-bromo-cGMP (1 mm), a membrane-permeable analogue of cGMP, mimicked the SNP-induced contraction.
Intracellular recordings revealed that SNP (300 μm) depolarized resting membrane potentials (RMPs) and increased the frequency of spontaneous spike-like action potentials. However, these electrical alterations were eliminated by pretreatment with niflumic acid (300 μm).
These results suggest that NO produces an excitation-contraction coupling in opossum oesophageal longitudinal smooth muscle via a cGMP-dependent signalling pathway. This contraction depends on extracellular Ca2+ entry through activation of L-type Ca2+ channels.
Several lines of evidence point to NO as being a non-adrenergic, non-cholinergic (NANC) inhibitory neurotransmitter in gastrointestinal smooth muscle (Bennett, 1997). Observations from mice lacking the neuronal nitric oxide (NO) synthase gene indicate that NO released from NANC nerve terminals in intestinal circular smooth muscle is responsible for slow inhibitory post-synaptic junction potentials (IJP), which leads to muscle relaxation (Mashimo et al. 1996; Kim et al. 1999). The slow IJP was suppressed by soluble guanylate cyclase inhibitors LY-83583 and 1H-[1,2,4]oxidiazolo[4,3,-α]quinoxalin-1-one (ODQ), suggesting the involvement of cGMP in the inhibitory effects of NO (Goyal & He, 1998). It was reported that these inhibitory effects of NO might be due to either opening of K+ channels or closing of Ca2+-activated Cl− channels (Cayabyab & Daniel, 1995; Koh et al. 1995; Zhang et al. 1998). However, the effects of NO on gastrointestinal longitudinal smooth muscle are paradoxical. It has been reported that NO has different effects on gastrointestinal longitudinal smooth muscle, causing a small transient relaxation, followed by sustained contraction, that can be either TTX sensitive or resistant, depending on the tissue studied (Bartho & Lefebvre, 1994, 1995; Olgart et al. 1997). By contrast, Corvera et al. (1997) showed that sodium nitroprusside (SNP) markedly inhibited spontaneous contractions of longitudinal smooth muscle of rat colon. The aforementioned observations were based on tension recordings, which make it difficult to determine whether the effects of NO are attributable to pharmaco-contraction coupling or excitation-contraction coupling.
The purpose of present study was to investigate the actions of NO on opossum oesophageal longitudinal smooth muscle, as well as the possible mechanisms involved, by using standard isometric tension and intracellular micro-electrode recordings. Our studies demonstrate that NO induces contraction of oesophageal longitudinal smooth muscle by excitation-contraction coupling via a cGMP-dependent signalling pathway, possibly by opening Ca2+-activated Cl− channels.
METHODS
Animals and tissues
The protocols were approved by the Animal Care Committee of Queen's University. Opossums (Didelphis virginiana) of either sex and weighing 2.5–5.0 kg were anaesthetized by tail vein injection of sodium pentobarbital (40 mg kg−1). The distal oesophagus and a short segment of attached stomach were then excised and placed in pre-oxygenated modified Krebs solution, following which the animals were killed by intracardiac injection of sodium pentobarbital. The oesophagus was opened longitudinally and pinned out with the mucosa side up in a dissecting dish and the mucosal layer was carefully removed by sharp dissection. As a standard preparation, a strip of longitudinal smooth muscle (with attached submucosa and circular muscle layers) of about 3 mm × 15 mm was cut along the long axis of the oesophagus for tension studies and a sheet of longitudinal smooth muscle of about 5 mm × 10 mm was excised for intracellular recordings. In some tension studies, special preparations of longitudinal smooth muscle were made as follows, in order to exclude the involvement of other tissue layers in the excitatory effect of SNP: (1) intact preparation including mucosal, submucosal, circular and longitudinal smooth muscle layers; (2) longitudinal and circular smooth muscle preparation (i.e. both mucosa and submucosa dissected free); and (3) isolated longitudinal smooth muscle preparation.
Isometric tension and micro-electrode intracellular recordings
Strips were suspended in a water-jacketed tissue bath containing 10 ml Krebs solution gassed with 5 % CO2-95 % O2 at 35 °C. Mechanical responses were recorded using standard isometric tension recordings as described previously (Saha et al. 1993; Uc et al. 1999). Silver wire electrodes, placed on either side of the muscle strip, were used to electrically stimulate intramural nerves using square-wave pulses generated by a Grass S88 stimulator (Grass Instrument Co., MA, USA). Conventional intracellular microelectrode techniques were used to record electrical responses as described previously (Zhang & Lang, 1994).
Solutions and drugs
The modified Krebs solution contained (mm): NaCl 118.07, NaHCO3 25.00, d(+)-glucose 11.10, KCl 4.69, CaCl2 2.52, MgSO4 1.00 and NaH2PO4 1.01. Niflumic acid was purchased from ICN Biochemicals Inc., tetrodotoxin (TTX) from Research Biochemicals Inc., and all other chemicals from Sigma. Niflumic acid and ODQ were dissolved in dimethyl sulphoxide as stock solutions. Nifedipine and 9-anthroic acid (9-AC) were dissolved in ethyl alcohol (100 %) and other chemicals in distilled water. These were diluted to final concentrations with the modified Krebs solution. SNP and DETA/NO were freshly dissolved in the modified Krebs solution and used within 5 min. The modified Krebs solution containing the drugs was gassed with 5 % CO2-95 % O2 to restore the pH to 7.4 prior to application.
Statistical analysis
Data are presented as means ±s.e.m. For tension recording studies, n refers to the number of animals, whereas for intracellular recording studies, n refers to the number of cells from which successful recordings were obtained. In tension recordings, the amplitude of contraction was standardized as a percentage of the maximal contraction induced by 60 mm KCl. Pre- and post-drug comparisons were made using Student's paired t test. P < 0.05 was considered statistically significant.
RESULTS
Isometric tension studies
None of the muscle strips developed basal tone, but in 6 of 67 preparations, spontaneous phasic contractions were recorded with an amplitude of 19.7 ± 8.1 % of maximal KCl (60 mm)-induced contraction and a frequency of 2.04 ± 0.62 min−1. Bath application of SNP produced a sustained concentration-dependent contraction with an EC50 of 239.6 ± 78.2 μm (n = 10; Fig. 1A). The maximum effective concentration of SNP (1 mm) evoked 75.5 ± 8.5 % (n = 10) of the KCl-induced contraction. DETA/NO, another putative NO donor, at a concentration of 100 and 500 μm induced 83.1 ± 24.4 and 104.1 ± 34.9 % of the KCl-induced contraction (n = 4), respectively (Fig. 2). The effects of SNP and DETA/NO were reversed after washing for 5–7 min.
Figure 1. Effects of SNP on standard longitudinal smooth muscle preparations of opossum oesophagus.
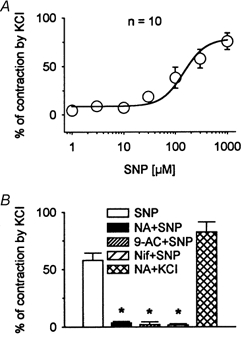
A, single concentration-response curve demonstrating concentration-dependent SNP-induced contraction. Curve was fitted with a Hill equation, which yielded EC50= 239.6± 78.2 μm (n = 10). B, histogram of effects of niflumic acid (NA, 300 μm), 9-AC (1 mm) and nifedipine (Nif, 1 μm) on the contraction produced by SNP (300 μm). Niflumic acid, 9-AC and nifedipine abolished the SNP-induced contraction (n = 7). However, in the presence of niflumic acid (300 μm) application of 60 mm KCl produced contraction to 82.7 ± 8.7 % of control induced by 60 mm KCl (n = 3), suggesting that inhibition of SNP-induced contraction was not due to the non-specific actions of niflumic acid on L-type Ca2+ channels or contractile protein processes. *P < 0.05.
Figure 2. DETA/NO-induced contractions.
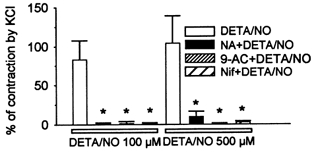
DETA/NO, a putative NO donor, at 100 and 500 μm induced contraction of oesophageal longitudinal smooth muscle in a concentration-dependent fashion (n = 4). Pretreatment with niflumic acid (300 μm), 9-AC (1 mm) and nifedipine (1 μm), respectively, blocked the DETA/NO-induced contraction. *P < 0.05.
Theoretically, either blockade of outward currents or activation of inward currents in smooth muscle could depolarize resting membrane potential (RMP). Membrane depolarization could activate voltage-gated, long-lasting Ca2+ channels, leading to excitation-contraction coupling (Sanders & Ozaki, 1994). In smooth muscle, the inward currents are usually carried by Ca2+, non-selective cation, or Cl− channels (Sanders & Ozaki, 1994; Large & Wang, 1996). These possibilities were tested using different channel blockers (Fig. 1B). Niflumic acid (300 μm) and 9-AC (1 mm), putative Ca2+-activated Cl− channel blockers, and nifedipine (1 μm), an L-type Ca2+ channel blocker, significantly inhibited the contraction induced by SNP (300 μm) from 57.9 ± 6.7 % to 3.6 ± 1.2, 2.1 ± 2.3 and 1.7 ± 1.0 %, respectively, of maximal contraction (P < 0.05, n = 7). However, DETA/NO-induced contractions at a concentration of 100 and 500 μm were significantly reduced from 83.1 ± 24.4 and 104.1 ± 34.9 % of the KCl-induced maximal contraction to 0.8 ± 0.6 and 2.6 ± 0.9 % by nifedipine (1 μm), to 0.9 ± 0.8 and 9.6 ± 6.9 % by niflumic acid (300 μm) and to 0.2 ± 1.4 and 1.2 ± 2.5 % by 9-AC (1 mm; Fig. 2). These data suggest that the NO-induced contraction might be due to the opening of Ca2+-activated Cl− channels, which, in turn, results in the activation of L-type Ca2+ channels. Niflumic acid (300 μm) did not significantly affect the KCl-induced contraction (Fig. 1B). Nifedipine (1 μm) substantially reduced the 60 mm KCl-induced contraction to 26.6 ± 4.4 % (n = 4). However, in the presence of 1 μm TTX in a control solution of 60 mm KCl and a solution of 60 mm KCl containing niflumic acid (300 μm), the KCl-induced contraction was unaffected by niflumic acid. The data establish that the KCl-induced contraction is largely mediated by L-type Ca2+ channels. They indicate that the inhibitory effect of niflumic acid was not via non-specific actions on L-type Ca2+ channels or contractile protein processes.
It was previously reported that SNP releases cyanide, which could produce toxic effects (Bates et al. 1991). To exclude the possibility that the contraction induced by SNP was due to the release of cyanide, we tested the effect of bath application of sodium cyanide on longitudinal muscle strips. At concentrations between 1 and 300 μm, no measurable contractions were evoked (n = 3; not shown).
To exclude the possibility that the SNP-induced contraction was due to mediators released from other layers of oesophageal wall, rather than via a direct action on the longitudinal smooth muscle, experiments were performed using longitudinal muscle strip preparations that included different components of the oesophageal wall (Fig. 3 and 4). SNP (300 μm) evoked virtually identical responses in four different preparations (n = 5). TTX (1 μm) failed to affect the SNP-induced contraction, whereas it abolished the contractile response induced by transmural nerve stimulation (20 Hz, 0.1 ms pulse duration, 60 V, 5 s). ODQ (10 μm), a guanylate cyclase inhibitor, markedly suppressed, whereas 8-bromo cGMP (1 mm), a membrane-permeable analogue of cGMP, mimicked the SNP-induced contraction. However, the contractile response evoked by nerve stimulation was not affected by ODQ, implying that the effects of ODQ on the SNP-induced contraction were not due to a non-specific direct inhibition of longitudinal smooth muscles.
Figure 3. Examples of effects of TTX (1 μm) and ODQ (10 μm) on contractions induced by SNP (300 μm) of different longitudinal smooth muscle preparations.
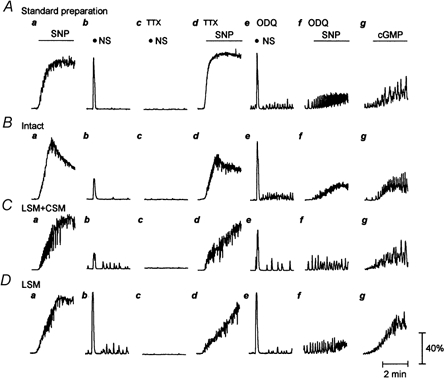
A, standard preparation. B, intact preparation. C, longitudinal smooth muscle (LSM) preparation with attached circular muscle (CSM) only. D, isolated longitudinal preparation. a, control contraction produced by SNP. b–d, TTX abolished nerve stimulation (NS)-induced contraction (20 Hz, 0.1 ms pulse duration, 60 V, 5 s), but failed to affect the contraction produced by SNP. e and f, ODQ significantly inhibited the SNP-induced contraction. However, in the presence of ODQ, nerve stimulation still evoked a contraction which was comparable to that without ODQ, implying that this inhibition was not due to the direct inhibition of ODQ on the longitudinal smooth muscle. g, 8-bromo-cGMP (1 mm) mimicked the SNP-induced contraction.
Figure 4. Histograms of effects of TTX (1 μm) and ODQ (10 μm) on the contraction induced by SNP (300 μm).
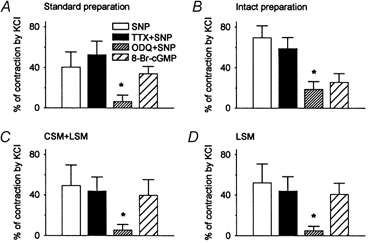
A, standard preparation. B, intact preparation. C, longitudinal smooth muscle preparation with attached circular muscle only. D, isolated longitudinal smooth muscle preparation. In each preparation, SNP produced a similar contraction, which was unaffected by pre-application of TTX, but significantly inhibited by ODQ. However, 8-bromo-cGMP (1 mm) mimicked the SNP-induced contraction. These data indicate that SNP has a direct excitatory effect on oesophageal longitudinal smooth muscle via a cGMP-dependent signalling pathway. n = 5, *P < 0.05.
Intracellular micro-electrode recordings
Intracellular micro-electrode recordings were employed to determine whether the SNP-induced contraction was due to pharmaco- or excitation-contraction coupling. Longitudinal smooth muscle exhibited spontaneous electrical activity consisting of slow-wave-like potentials with a RMP of −53.1 ± 1.6 mV, slow-wave-like potential amplitude of 4.4 ± 0.3 mV and slow-wave-like potential frequency of 2.32 ± 0.27 min−1. Superimposed on the slow-wave-like potentials were spike-like action potentials with a frequency of 4.4 ± 0.4 per slow-wave-like potential and an amplitude of 17.6 ± 0.9 mV (n = 26; Fig. 5A). Bath application of nifedipine (1 μm) led to membrane depolarization of 3.3 ± 0.4 mV vs. control (P < 0.05, n = 3) and abolished spontaneous electrical activity (Fig. 6). SNP (300 μm) significantly depolarized RMP by 2.2 ± 0.6 mV vs. control (P < 0.05, n = 6) and induced a sustained burst of spike-like action potentials at a frequency of 22.02 ± 4.57 min−1 (P < 0.05vs. control, n = 6) and with amplitude of 15.6 ± 3.0 mV (P > 0.05vs. control, n = 6; Fig. 7A). Spontaneous electrical activity returned to the basal state 5–7 min after washout. Niflumic acid (300 μm) hyperpolarized RMP by −9.8 ± 1.6 mV vs. control (P < 0.05, n = 4), gradually decreased the amplitude of spike-like action potentials and finally abolished spontaneous action potentials. Effects of niflumic acid on spontaneous electrical activity were reversible. The spontaneous spike-like action potentials quickly recovered 5–10 min after washout (Fig. 7Ba). In the presence of niflumic acid, concomitant application of SNP (300 μm) slightly depolarized RMP (1.6 ± 0.7 mV; P > 0.05vs. control, n = 3) and failed to induce a sustained bursting of spike-like action potentials (n = 3; Fig. 7B).
Figure 5. Spontaneous electrical activity of longitudinal smooth muscle of opossum oesophagus obtained by conventional intracellular recordings.
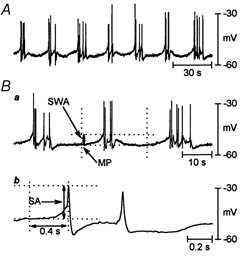
A, intracellular micro-electrode recording in the basal state revealed 1–6 spike-like action potentials usually superimposed on slow-wave-like potentials (n = 26). B, measurement of electrical parameters: a: SWA, amplitude of slow-wave-like potentials; MP, resting membrane potential; b: SA, amplitude of spike-like action potentials.
Figure 6. Effects of nifedipine on spontaneous electrical activity.
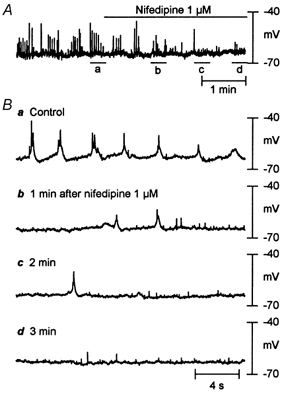
A, bath application of nifedipine (1 μm) led to membrane depolarization of 3.3 ± 0.4 mV over control (P < 0.05, n = 3) and elimination of spontaneous action potentials. B, snapshots of original recordings from A on an expanded time scale before and after application of nifedipine. Note that small upward deflections in original recordings are due to perfusion pump noise.
Figure 7. Effects of SNP (300 μm) on the electrical properties of the longitudinal smooth muscle.
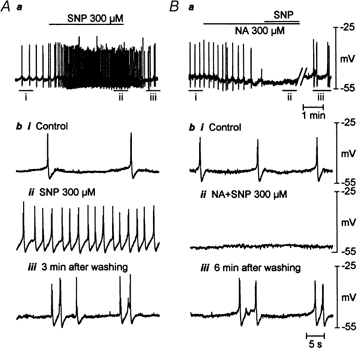
A and B, original intracellular micro-electrode recordings demonstrating effects of SNP in the absence and presence of niflumic acid (300 μm) from same cell. Ab and Bb, snapshots of original recordings from Aa and Ba on an expanded time scale. i, control. ii, drug application. iii, recovery after washout. SNP depolarized the resting membrane potential (RMP) and induced on-going spike-like action potentials (n = 6). Niflumic acid hyperpolarized RMP (n = 4), gradually decreased the amplitude of spike-like action potentials and finally abolished them. In the presence of niflumic acid, SNP failed to depolarize RMP and evoke on-going spike-like action potentials (n = 3).
DISCUSSION
The results of our study show that NO produced a direct, TTX-resistant contraction of oesophageal longitudinal smooth muscle, which was abolished by blockade of either L-type Ca2+ channels or Ca2+-activated Cl− channels. This unique contraction was inhibited by a guanylate cyclase inhibitor, and mimicked by a cGMP analogue. Moreover, intracellular recordings demonstrated that the SNP-induced contraction was associated with membrane depolarization and sustained bursts of spike-like action potentials, which were abolished by a Ca2+-activated Cl− channel blocker. These data suggest that NO opens Ca2+-activated Cl− channels via a cGMP-dependent pathway, leading to extracellular Ca2+ entry through L-type Ca2+ channels and contraction of opossum oesophageal longitudinal smooth muscle.
It was previously reported that NO elicited a biphasic response (a small relaxation followed by a marked contraction) in longitudinal smooth muscles of opossum oesophagus (Saha et al. 1993) and guinea-pig small intestine (Bartho & Lefebvre, 1994; Olgart et al. 1997). In the present study, no initial relaxation was observed, which is not surprising, because our longitudinal muscle strips did not develop resting tone. This might have occurred if additional stretch had been applied to the strips, although the preparations were stretched to 140 % of their original length to determine the ideal pre-load in our experiments (Saha et al. 1993; Uc et al. 1997). However, intracellular recordings also failed to demonstrate an initial inhibitory effect of SNP, in that we never observed membrane hyperpolarization upon application of SNP; invariably membrane depolarization and continuous spiking were recorded.
Alterations in several different ionic conductances could result in excitation-contraction coupling in smooth muscle (Sanders & Ozaki, 1994; Bolton et al. 1999). Because K+ conductance is a major contributor to the RMP, inhibition of K+ channels by different agents could be a powerful mechanism for inducing membrane depolarization. However, activation of one or more inward currents, usually carried by Ca2+, non-selective cation, or Cl− conductances (Benham et al. 1985; Vogalis & Sanders, 1990; Large & Wang, 1996) could also underlie the membrane depolarization. It is estimated that the reversal potential of the Cl− channel is between −30 and −20 mV (Aickin & Brading, 1982, 1983). At the resting state, activation of Cl− channels would allow outward movement of Cl− ions to carry an inward current, resulting in membrane depolarization. Previous reports have implicated Cl− channels in noradrenaline (norepinephrine)-induced constriction of vascular smooth muscle (Criddle et al. 1996; Lamb & Barna, 1998), and the myogenic tone of both cerebral arteries (Nelson et al. 1997) and tracheal smooth muscle (Janssen & Sims, 1994). Evidence also exists implicating the activation of Cl− channels in agonist-induced contraction of colonic smooth muscle (Kolbel et al. 1998). Our studies are the first to implicate Ca2+-activated Cl− channels in the NO-induced contraction of gastrointestinal longitudinal smooth muscle. In addition, the membrane hyperpolarization induced by niflumic acid suggests that the activity of Ca2+-activated Cl− channels also contributes to the RMP of this muscle.
Appropriate interpretation of our results rests on the selectivity of niflumic acid. Some studies have suggested that this drug might have non-selective effects, by affecting either K+ channels or Ca2+-dependent contractile processes (Large & Wang, 1996; Kato et al. 1999). It is unlikely that such mechanisms explain the blocking effect of niflumic acid on SNP-induced contraction in our experiments, because: (1) the non-specific effect of niflumic acid demonstrated in other studies was very slow to reverse, whereas the effect of niflumic acid was rapidly reversible in our experiments (Fig. 7B); (2) niflumic acid had no significant effect on KCl-induced longitudinal smooth muscle contraction, indicating that it did not affect contraction via a non-specific effect on voltage-gated Ca2+ channels or contractile proteins (Fig. 1B); (3) niflumic acid abolished both the membrane depolarization and sustained bursts of spontaneous action potentials produced by SNP. It is known that niflumic acid opens large conductance Ca2+-activated K+ channels at a higher concentration (> 50 μm; Greenwood & Large, 1995). It is possible that the inhibition of NO donor-induced contraction and of spontaneous electrical activity could contribute to the opening of large conductance Ca2+-activated K+ channels by niflumic acid. However, our experiments do not support this possibility, because niflumic acid relaxed both oesophageal longitudinal smooth muscle strips (data not shown) and circular smooth muscle of the lower oesophageal sphincter (Zhang et al. 2000) contracted by 1 mm TEA.
Niflumic acid has been used as a non-steroidal anti-inflammatory agent (Kulmacz et al. 1991), raising the possibility that the action of niflumic acid might be mediated by a reduction in eicosanoid production. In the present studies, it appeared that the action of niflumic acid on the inhibition of cyclo-oxygenase was insignificant relative to its blockade of Ca+-activated Cl− channels. This is because the effect of niflumic acid reported here was dramatically different from the effects of indomethacin on spontaneous contractile and electrical activity of the proximal renal pelvis smooth muscle of the guinea-pig, as reported by Zhang & Lang (1994). In their work, indomethacin abolished both the spontaneous mechanical and the electrical activity of this muscle and depolarized the RMP. It took 25–30 min for indomethacin to achieve a maximal response and 30–60 min to recover to its control state upon washing out. By contrast, our data showed that niflumic acid suppressed spontaneous electrical activity, but hyperpolarized RMP. Furthermore, the onset of the action of niflumic acid in oesophageal longitudinal smooth muscle is rapid, reaching a maximal response within 5 min of bath application and returning to a control state 5–7 min after washout (Fig. 7Ba). This is not consistent with an effect mediated via cyclo-oxygenase inhibition.
Activation of L-type Ca2+ or other cation channels could represent an ionic mechanism for the SNP-induced contraction. Our experiments do not support this idea, because nifedipine and niflumic acid abolished the SNP-induced contraction, whereas the KCl-induced contraction in the presence of niflumic acid was no different from control KCl-induced contractions (Fig. 1B). Furthermore, there is no direct evidence to date that niflumic acid blocks cation channels. Nevertheless, the possible involvement of NO in the activation of cation channels cannot be completely excluded. Further patch-clamp studies in dispersed single longitudinal smooth muscle cells are required to help clarify these issues.
An intriguing question that arises from our study is how SNP can work via cGMP to elicit contraction of longitudinal smooth muscle but relaxation of circular smooth muscle (Saha et al. 1993; Hirano et al. 1997; Olgart et al. 1997). This suggests that there are fundamental differences between the two muscle types in the second messenger systems downstream of cGMP. Zhang et al. (1998) showed that NO closed Ca2+-activated Cl− channels in oesophageal circular smooth muscle, although others (Cayabyab & Daniel, 1995; Koh et al. 1995) reported that NO opened K+ channels through the elevation of cGMP to relax circular smooth muscle. Based on current studies in longitudinal smooth muscle, we propose that NO activates Ca2+-activated Cl− channels via a cGMP-dependent pathway, which in turn activates L-type Ca2+ channels, leading to extracelluar Ca2+ entry and contraction of the muscle. This seems paradoxical, because NO elevates the same cGMP messenger in the two muscle types. However, it is possible that cGMP acts through diverging cellular signalling pathways to affect multiple ion channel processes, such as activation of Ca2+-activated Cl− channels in longitudinal muscle and inhibition of Ca2+-activated Cl− channels in circular muscle. This would be analogous to the divergence of signal transmission that has been described at single nerve terminals for the control of the radula closer muscle in Aplysia (Brezina et al. 1996). However, further studies are required to clarify different signal transmission processes downstream of cGMP in the two muscle types.
In summary, the present study is the first to report NO-evoked contraction via excitation-contraction coupling in gastrointestinal longitudinal smooth muscle. This contraction depends on extracellular Ca2+ entry through L-type Ca2+ channels, possibly resulting from the activation of Ca2+-activated Cl− channels through the elevation of cGMP. This might be of physiological significance to the coordination of motility between circular and longitudinal smooth muscle layers during peristalsis.
Acknowledgments
This work was supported by Medical Research Council of Canada grant no. MT9978. The authors would like to thank Mr David Miller for his technical assistance in conducting muscle strip studies.
References
- Aickin CC, Brading AF. Measurement of intracellular chloride in guinea-pig vas deferens by ion analysis, 36chloride efflux and micro-electrodes. Journal of Physiology. 1982;326:139–154. doi: 10.1113/jphysiol.1982.sp014182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin CC, Brading AF. Towards an estimate of chloride permeability in the smooth muscle of guinea-pig vas deferens. Journal of Physiology. 1983;336:179–197. doi: 10.1113/jphysiol.1983.sp014575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartho L, Lefebvre R. Nitric oxide induces acetylcholine-mediated contractions in the guinea-pig small intestine. Naunyn-Schmiedeberg's Archives of Pharmacology. 1994;350:582–584. doi: 10.1007/BF00173030. [DOI] [PubMed] [Google Scholar]
- Bartho L, Lefebvre RA. Nitric oxide-mediated contraction in enteric smooth muscle. Archives Internationales de Pharmacodynamie et de Therapie. 1995;329:53–66. [PubMed] [Google Scholar]
- Bates JN, Baker MT, Guerra RJ, Harrison DG. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochemistry and Pharmacology. 1991;42(suppl.):S157–S165. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- Benham CD, Bolton TB, Lang RJ. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985;316:345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Bennett MR. Non-adrenergic non-cholinergic (NANC) transmission to smooth muscle: 35 years on. Progress in Neurobiology. 1997;52:159–195. doi: 10.1016/s0301-0082(97)00012-9. [DOI] [PubMed] [Google Scholar]
- Bolton TB, Prestwich SA, Zholos AV, Gordienko DV. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annual Review of Physiology. 1999;61:85–115. doi: 10.1146/annurev.physiol.61.1.85. [DOI] [PubMed] [Google Scholar]
- Brezina V, Orekhova IV, Weiss KR. Functional uncoupling of linked neurotransmitter effects by combinatorial convergence. Science. 1996;273:806–810. doi: 10.1126/science.273.5276.806. [DOI] [PubMed] [Google Scholar]
- Cayabyab FS, Daniel EE. K+ channel opening mediates hyperpolarizations by nitric oxide donors and IJPs in opossum oesophagus. American Journal of Physiology. 1995;268:G831–842. doi: 10.1152/ajpgi.1995.268.5.G831. [DOI] [PubMed] [Google Scholar]
- Corvera CU, Dery O, McConalogue K, Bohm SK, Khitin LM, Caughey GH, Payan DG, Bunnett NW. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. Journal of Clinical Investigation. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criddle DN, De Moura RS, Greenwood IA, Large WA. Effect of niflumic acid on noradrenaline-induced contractions of the rat aorta. British Journal of Pharmacology. 1996;118:1065–1071. doi: 10.1111/j.1476-5381.1996.tb15507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal RK, He XD. Evidence for NO redox form of nitric oxide as nitrergic inhibitory neurotransmitter in gut. American Journal of Physiology. 1998;275:G1185–1192. doi: 10.1152/ajpgi.1998.275.5.G1185. [DOI] [PubMed] [Google Scholar]
- Greenwood IA, Large WA. Comparison of the effects of fenamates on Ca2+-activated chloride and potassium currents in rabbit portal vein smooth muscle cells. British Journal of Pharmacology. 1995;116:2939–2948. doi: 10.1111/j.1476-5381.1995.tb15948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano I, Kakkar R, Saha JK, Szymanski PT, Goyal RK. Tyrosine phosphorylation in contraction of opossum oesophageal longitudinal muscle in response to SNP. American Journal of Physiology. 1997;273:G247–252. doi: 10.1152/ajpgi.1997.273.1.G247. [DOI] [PubMed] [Google Scholar]
- Janssen LJ, Sims SM. Spontaneous transient inward currents and rhythmicity in canine and guinea-pig tracheal smooth muscle cells. Pflügers Archiv. 1994;427:473–480. doi: 10.1007/BF00374263. [DOI] [PubMed] [Google Scholar]
- Kato K, Evans AM, Kozlowski RZ. Relaxation of endothelin-1-induced pulmonary arterial constriction by niflumic acid and NPPB: mechanism(s) independent of chloride channel block. Journal of Pharmacology and Experimental Therapeutics. 1999;288:1242–1250. [PubMed] [Google Scholar]
- Kim CD, Goyal RK, Mashimo H. Neuronal NOS provides nitrergic inhibitory neurotransmitter in mouse lower oesophageal sphincter. American Journal of Physiology. 1999;277:G280–284. doi: 10.1152/ajpgi.1999.277.2.G280. [DOI] [PubMed] [Google Scholar]
- Koh SD, Campbell JD, Carl A, Sanders KM. Nitric oxide activates multiple potassium channels in canine colonic smooth muscle. Journal of Physiology. 1995;489:735–743. doi: 10.1113/jphysiol.1995.sp021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbel CB, Holtmann G, McRoberts JA, Scholer S, Aengenvoordt P, Singer MV, Mayer EA. Involvement of chloride channels in the receptor-mediated activation of longitudinal colonic muscle. Neurogastroenterology and Motility. 1998;10:489–498. doi: 10.1046/j.1365-2982.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- Kulmacz RJ, Palmer G, Tsai AL. Prostaglandin H synthase: perturbation of the tyrosyl radical as a probe of anticyclooxygenase agents. Molecular Pharmacology. 1991;40:833–837. [PubMed] [Google Scholar]
- Lamb FS, Barna TJ. Chloride ion currents contribute functionally to norepinephrine-induced vascular contraction. American Journal of Physiology. 1998;275:H151–H160. doi: 10.1152/ajpheart.1998.275.1.H151. [DOI] [PubMed] [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. American Journal of Physiology. 1996;271:C435–454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Mashimo H, He XD, Huang PL, Fishman MC, Goyal RK. Neuronal constitutive nitric oxide synthase is involved in murine enteric inhibitory neurotransmission. Journal of Clinical Investigation. 1996;98:8–13. doi: 10.1172/JCI118781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Conway MA, Knot HJ, Brayden JE. Chloride channel blockers inhibit myogenic tone in rat cerebral arteries. Journal of Physiology. 1997;502:259–264. doi: 10.1111/j.1469-7793.1997.259bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olgart C, Wiklund NP, Gustafsson LE. Blockade of nitric oxide-evoked smooth muscle contractions by an inhibitor of guanylyl cyclase. NeuroReport. 1997;8:3355–3358. doi: 10.1097/00001756-199710200-00032. [DOI] [PubMed] [Google Scholar]
- Saha JK, Hirano I, Goyal RK. Biphasic effect of SNP on opossum oesophageal longitudinal muscle: involvement of cGMP and eicosanoids. American Journal of Physiology. 1993;265:G403–407. doi: 10.1152/ajpgi.1993.265.2.G403. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Ozaki H. Excitation-contraction coupling in gastrointestinal smooth muscles. In: Szekeres L, Papp JG, editors. Pharmacology of Smooth Muscle. Berlin: Springer-Verlag; 1994. pp. 331–404. [Google Scholar]
- Uc A, Murray JA, Conklin JL. Effects of calcitonin gene-related peptide on opossum oesophageal smooth muscle. Gastroenterology. 1997;113:514–520. doi: 10.1053/gast.1997.v113.pm9247471. [DOI] [PubMed] [Google Scholar]
- Uc A, Oh ST, Murray JA, Clark E, Conklin JL. Biphasic relaxation of the opossum lower oesophageal sphincter: roles of NO, VIP, and CGRP. American Journal Physiology. 1999;277:G548–554. doi: 10.1152/ajpgi.1999.277.3.G548. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Sanders KM. Cholinergic stimulation activates a non-selective cation current in canine pyloric circular muscle cells. Journal of Physiology. 1990;429:223–236. doi: 10.1113/jphysiol.1990.sp018253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lang RJ. Effects of intrinsic prostaglandins on the spontaneous contractile and electrical activity of the proximal renal pelvis of the guinea-pig. British Journal of Pharmacology. 1994;113:431–438. doi: 10.1111/j.1476-5381.1994.tb17007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Miller DV, Paterson WG. Opposing roles of K+ and Cl− channels in maintenance of opossum lower oesophageal sphincter tone. American Journal Physiology. 2000;279:G1226–1234. doi: 10.1152/ajpgi.2000.279.6.G1226. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Vogalis F, Goyal RK. Nitric oxide suppresses a Ca2+-stimulated Cl− current in smooth muscle cells of opossum oesophagus. American Journal of Physiology. 1998;274:G886–890. doi: 10.1152/ajpgi.1998.274.5.G886. [DOI] [PubMed] [Google Scholar]


