Abstract
We tested the hypothesis that long-distance running activates parallel mitogen-activated protein kinase (MAPK) cascades that involve extracellular signal regulated kinase 1 and 2 (ERK1/2) and p38 MAPK and their downstream substrates.
Eleven men completed a 42.2 km marathon (mean race time 4 h 1 min; range 2 h 56 min to 4 h 33 min). Vastus lateralis muscle biopsies were obtained before and after the race. Glycogen content was measured spectrophotometrically. ERK1/2 and p38 MAPK phosphorylation was determined by immunoblot analysis using phosphospecific antibodies. Activation of the downstream targets of ERK1/2 and p38 MAPK, MAPK-activated protein kinase-1 (MAPKAP-K1; also called p90 ribosomal S6 kinase, p90rsk), MAPK-activated protein kinase-2 (MAPKAP-K2), mitogen- and stress-activated kinase 1 (MSK1) and mitogen- and stress-activated kinase 2 (MSK2) was determined using immune complex assays.
Muscle glycogen content was reduced by 40 ± 6 % after the marathon. ERK1/2 phosphorylation increased 7.8-fold and p38 MAPK phosphorylation increased 4.4-fold post-exercise. Prolonged running did not alter ERK1/2 and p38 MAPK protein expression. The activity of p90rsk, a downstream target of ERK1/2, increased 2.8-fold after the marathon. The activity of MAPKAPK-K2, a downstream target of p38 MAPK, increased 3.1-fold post-exercise. MSK1 and MSK2 are downstream of both ERK1/2 and p38 MAPK. MSK1 activity increased 2.4-fold post-exercise. MSK2 activity was low, relative to MSK1, with little activation post-exercise.
In conclusion, prolonged distance running activates MAPK signalling cascades in skeletal muscle, including increased activity of downstream targets: p90rsk, MAPKAP-K2 and MSK. Activation of these downstream targets provides a potential mechanism by which exercise induces gene transcription in skeletal muscle.
Intense interest has focused on delineating the exercise-induced signal transduction pathways that regulate transcription, growth and metabolism in skeletal muscle (Goodyear et al. 1995; Widegren et al. 1998; Sherwood et al. 1999; Wojtaszewski et al. 1999; Chibalin et al. 2000; Ryder et al. 2000). The cellular signalling mechanisms that mediate some of these exercise-induced adaptations in skeletal muscle may involve the mitogen-activated protein kinase (MAPK) signalling cascades, because they have been implicated in the activation of a variety of downstream kinases and transcription factors (reviewed in Cohen, 1997). Members of the MAPK family form at least three parallel signalling cascades that include extracellular signal regulated kinases (ERK1/2; p42/p44 MAPK), p38 MAPK and c-Jun NH2-terminal kinase (JNK; reviewed in Cano & Mahadevan, 1995). Evidence is emerging that MAPK signalling pathways are directly activated in human skeletal muscle in response to one acute bout of exercise (Aronson et al. 1997; Widegren et al. 1998). These MAPK cascades provide a molecular mechanism for exercise-induced regulation of transcription in skeletal muscle.
The MAPK enzymes are part of a large family of related protein kinases (reviewed in Cohen, 1997) and form a major signalling system that facilitates the transduction of extracellular signals into appropriate genomic responses (Blenis, 1993; Seger & Krebs, 1995). ERK1/2 were the first MAPK isoforms to be identified (Ray & Sturgill, 1987, 1988) and are activated primarily in response to mitogenic stimuli, including growth factors that act via receptor tyrosine kinases (Ray & Sturgill, 1988; Davis, 1993; Cohen, 1997), G-protein-coupled receptors (Crespo et al. 1994; van Biesen et al. 1995) and protein kinase C (PKC) (van Biesen et al. 1996). The activation of ERK1/2 is also important for differentiation in some cell types (Cowley et al. 1994). Several downstream substrates of ERK1/2 kinase signalling have been identified (Brunet & Pouyssegur, 1997), including MAPK-activated protein kinase-1 (MAPKAP-K1), also called p90 ribosomal S6 kinase (p90rsk; Stugill et al. 1988; Zhao et al. 1996), and the recently described enzymes mitogen- and stress-activated kinase 1 (MSK1) and mitogen- and stress-activated kinase 2 (MSK2; Deak et al. 1998). In isolated, electrically stimulated (contracting) skeletal muscle, activation of p90rsk appears to be under the control of ERK1/2, whereas activation of MSK1 requires the simultaneous activation of ERK1/2 and p38 MAPK (Ryder et al. 2000). These findings are important because they have identified contraction-responsive MAPK substrates that are activated by ERK1/2 and p38 MAPK pathways in skeletal muscle. However, to fully understand the physiological relevance of these intracellular signalling pathways, exercise-responsive MAPK substrates need to be identified and characterised in human skeletal muscle.
The extreme complexity of the MAPK family is due partly to the existence of parallel pathways, mediated via p38 MAPK and JNK, that can be activated simultaneously by environmental stress, such as UV damage, osmotic shock and heat shock (Freshney et al. 1994; Galcheva-Gargova et al. 1994; Han et al. 1994; Price et al. 1996), as well as by cytokines (Freshney et al. 1994). p38 MAPK is an upstream regulator of MAPK-activated protein kinase-2 (MAPKAP-K2; Cuenda et al. 1995; Beyaert et al. 1996), which in turn phosphorylates the small heat shock protein HSP27 (Cuenda et al. 1995). Exercise is a physiological means to increase the activity of p38 MAPK and JNK in human skeletal muscle (Aronson et al. 1997; Widegren et al. 1998). Recent evidence suggests that the activity of JNK and p38 MAPK and their upstream regulators, mitogen-activated protein kinase 4 (MKK4) and mitogen-activated protein kinase 6 (MKK6), is increased transiently after prolonged strenuous physical activity, such as marathon running (Boppart et al. 2000). These findings are important because they suggest that stress-activated protein kinase cascades might be responsible for the adaptation of skeletal muscle to strenuous exercise such as marathon running. However, the downstream substrates in these pathways have not been identified.
Several studies have assessed the effects of muscle contraction via electrical stimulation in rodent skeletal muscle (Goodyear et al. 1996; Aronson et al. 1997; Hayashi et al. 1999; Sherwood et al. 1999; Wojtaszewski et al. 1999; Ryder et al. 2000). Thus, there is a need to characterise MAPK cascades in response to different types of physical exercise and to validate the physiological role of these kinases in the exercise response. For example, whether ERK1/2 and downstream substrates are activated in response to long-term strenuous distance running is not currently known. Limited information is available regarding the effects of exercise on specific MAPK substrates in human skeletal muscle. Therefore, we tested the hypothesis that marathon running activates the parallel MAP kinase cascades that involve ERK1/2 and p38 and their downstream substrates. We measured changes in the phosphorylation of ERK1/2 and p38 MAPK, and the activity of the downstream targets p90rsk, MAPKAP-K2, MSK1 and MSK2, in muscle biopsies obtained prior to and after completion of a competitive marathon.
METHODS
Subjects
The Ethical Committee at Karolinska Institutet approved the study protocol and written informed consent was obtained from each subject. The study was performed according to the Declaration of Helsinki. Eleven healthy young male volunteers participated in the study. The subjects (Table 1) reported running 47 ± 5 km per week for not less than 2 months prior to the investigation. Subjects were asked to consume a carbohydrate-rich diet and refrain from strenuous exercise in the 48 h before participation in the study. Subjects consumed their usual breakfast and lunch before the marathon. Following local anaesthesia (Carbocain, Astra, Södertälje, Sweden; 1 ml of a 5 mg ml−1 solution), biopsies were obtained before and after completion of the marathon from the vastus lateralis portion of the quadriceps femoris muscle. Biopsies were immediately frozen in liquid nitrogen. Pre-race biopsies were taken at least 2 h before the start of the marathon. Post-race biopsies were taken within 23 min of completion of the marathon (mean time 17 ± 2 min; range 8–23 min). The pre and post-marathon biopsy was obtained from separate legs. After local anaesthesia (Carbocain; 1 ml of a 5 mg ml−1 solution), an incision (5 mm long, 10 mm deep) was made in the skin and muscle fascia, and a muscle biopsy (50–100 mg) was obtained by means of a Weil-Blakesley conchotome. Muscle biopsies were stored in liquid nitrogen until processed.
Table 1.
Subject characteristics
| Age (years) | 42 ± 4 |
| Weight (kg) | 76 ± 3 |
| Height (cm) | 181 ± 2 |
| Body mass index (kg m−2) | 24.4 ± 0.6 |
| Race time (h:min) | 4:01 ± 0:09 |
| Race time (range h:min)* | 2:56 to 4:33 |
Values (except *) are means ±s.e.m.
Materials
Phospho-ERK1/2, ERK1/2, phosho-p38 MAPK and p38 MAPK antibodies were purchased from New England Biolabs (Beverly, MA, USA). Characteristics of the p90rsk, MSK1, MSK2 and MAPKAP-K2 antibodies have been described previously (Alessi et al. 1995; Clifton et al. 1996; Deak et al. 1998). Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse immunoglobulin G was obtained from Bio-Rad Laboratories (Richmond, CA, USA). Reagents for enhanced chemiluminescence (ECL) were obtained from Amersham (Arlington Heights, IL, USA). All other reagents were of analytical grade (Sigma, St Louis, MO, USA).
Glycogen analysis
A portion of the muscle biopsy was utilised for biochemical determination of glycogen content. Glycogen content was measured after KOH digestion as previously described (Leighton et al. 1989). Results are expressed as millimoles per kilogram wet weight (mmol kg−1).
Immunoblot analysis
Skeletal muscle was homogenised in ice-cold buffer (50 mm Tris HCl, pH 7.5, 0.1 % Trition X-100, 1 mm EDTA, 1 mm EGTA, 50 mm NaF, 5 mm Na2P2O7, 10 mm glycerophosphate, 1 mm Na3VO4, 1 μm microcystin, 0.1 %β-mercaptoethanol). Homogenates were rotated for 60 min at 4 °C, centrifuged at 12 000 g for 10 min at 4 °C, and the protein concentration of the resulting supernatant measured using a commercially available kit (Bio-Rad). Aliquots of muscle lysate (20–40 μg) were mixed with Laemmli sample buffer, and proteins were separated by SDS-PAGE. Following electrophoresis, proteins were transferred to polyvinylidenedifluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Membranes were blocked in TBST (10 mm Tris, 100 mm NaCl, 0.02 % Tween-20) containing 7.5 % non-fat milk for 2 h at room temperature, washed with TBST for 10 min and incubated with an appropriate primary antibody overnight at 4 °C. To determine ERK1/2 phosphorylation, membranes were immunoblotted with a phosphospecific antibody that recognises ERK1/2 (p4244 MAPK) kinase when phosphorylated at Thr202 and Tyr204. To determine p38 MAPK phosphorylation, membranes were immunoblotted with a phosphospecific p38 MAPK antibody that recognises p38 MAPK phosphorylated at Thr180 and Tyr182. Thereafter, membranes were washed several times with TBST and incubated with an appropriate secondary antibody for 1 h at room temperature, followed by washing in TBST. Specific proteins were visualised by ECL and quantified by densitometric scanning. Following measurement of ERK1/2 or p38 MAPK phosphorylation, membranes were incubated in stripping buffer (62.5 mm Tris, pH 6.7, 2 % SDS and 100 mmβ-mercaptoethanol) for 30 min at 50 °C, washed extensively in TBST, and then used to determine ERK1/2 or p38 MAPK protein expression, respectively. Immunoreactive proteins were detected and quanified as described above.
Activity assays
Skeletal muscle was homogenised in an ice-cold buffer as described above (‘Immunoblot analysis’). Aliquots of the supernatant (500 μg) were immunoprecipitated with appropriate antibodies (MSK1, MSK2, p90rsk or MAPKAP-K2) coupled to protein G sepharose for 1 h at 4 °C. Immunoprecipitates were then washed three times in ice-cold buffer A (0.5 m NaCl, 50 mm Tris HCl, pH 7.5, 0.1 % Triton X-100, 1 mm EDTA, 1 mm EGTA, 50 mm NaF, 5 mm Na2P2O7, 10 mmβ-glycerophosphate, 1 mm Na3VO4, 1 μm microcystin, 0.1 %β-mercaptoethanol), twice in ice-cold buffer B (50 mm Tris HCl, pH 7.5, 0.03 % Brij 35, 0.1 mm EGTA, 0.1 %β-mercaptoethanol), and resuspended in 30 μl of buffer C (50 mm Tris HCl, pH 7.5, 16.7 mm Mg(C2H3O2)2.4H2O, 50 μm Crosstide, 0.1 mm EGTA, 0.1 %β-mercaptoethanol, 17 μm PKI, 20 μCi [γ-32P]ATP). For determination of MAPKAP-K2 activity, KKLNRTLSVA peptide was substituted for Crosstide peptide. After incubation for 10 min at 30 °C, the reaction was terminated on ice. Incorporation of γ-32P into the peptide substrate was determined by resolving the products on a 40 % polyacrylamide gel. The gel was visualised on a PhosphorImager (Bio-Rad), and the band corresponding to the peptide substrate was quantified.
Preparation of nuclear extracts
Nuclear extracts were prepared as described by Mora & Pessin (2000), with minor modifications. Skeletal muscle biopsies obtained before and after the marathon from a sub-group of subjects (n = 3) were pulverised in liquid nitrogen and homogenised in 10 volumes (wt/vol.) of buffer A (250 mm sucrose, 10 mm Hepes, pH 7.6, 25 mm KCl, 1 mm EDTA, 10 % glycerol, 0.15 mm spermine, 0.5 mm spermidine, 0.1 mm phenylmethylsulfonyl fluoride (PMSF), 2 μg ml−1 each of aprotinin, leupeptin and pepstatin A, and 6 μg ml−1 each of l-1-tosylamido-2-phenylethyl chloromethyl ketone and 1-chloro-3-tosylamido-7-amino-2-heptanone) with 20 strokes of a Pellet Pestle (Kebo Lab, Stockholm, Sweden), and filtered through gauze. The homogenate was centrifuged at 3900 g for 10 min at 4°C. The pellet was resuspended in 1 ml of buffer A and homogenised (10 s) with a motorised Pellet Pestle. The homogenate was layered over one-half volume of buffer B (1 m sucrose, 10 mm Hepes, pH 7.6, 25 mm KCl, 1 mm EDTA, 10 % glycerol, 0.15 mm spermine, 0.5 mm spermidine, 0.1 mm PMSF, 2 μg ml−1 each of aprotinin, leupeptin and pepstatin A, and 6 μg ml−1 each of l-1-tosylamido-2-phenylethyl chloromethyl ketone and 1-chloro-3-tosylamido-7-amino-2-heptanone) and centrifuged at 3900 g for 10 min at 4°C. The pellet was resuspended in buffer A/glycerol (9:1, w/w) and layered over one-third volume of buffer B/glycerol (9:1, w/w). The gradient was centrifuged at 48 000 g for 30 min at 4°C. The semi-purified nuclear pellet was resuspended in 100 μl of nuclear extraction buffer (10 mm Hepes, pH 7.6, 400 mm KCl, 3 mm MgCl2, 0.1 mm EDTA, 10 % glycerol, 1 mm DTT, 0.1 mm PMSF). Nuclear proteins were then extracted on ice for 30 min and samples were then centrifuged at 13 000 g for 10 min at 4°C. The supernatant was diluted 4 times with nuclear extraction buffer without KCl and assayed for total protein using the Bradford assay (Bio-Rad) and stored at −70°C.
Electrophoretic mobility shift assay
The myocyte enhancer factor 2 (MEF2) DNA-binding site oligonucleotide was commercially prepared (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The oligonucleotide was end-labelled with T4 polynucleotide kinase. The probes (0.5 ng) were incubated with 2 μg of nuclear extract in a 20 μl reaction containing 1 μg poly(dI-dC), 40 mm KCl, 5 mm MgCl2, 15 mm Hepes, pH 7.9, 1 mm EDTA, 0.5 mm DTT and 5 % glycerol for 20 min at room temperature. For competition studies, the extract was pre-incubated with a 10-fold molar excess of unlabelled oligonucleotide for 10 min before addition of the radiolabelled probe. The samples were then analysed on a non-denaturing 6 % polyacrylamide (38:1 acrylamide/bisacrylamide) gel buffered with Tris borate-EDTA (TBE; 22 mm Tris, 22 mm boric acid and 0.5 mm EDTA) and run at 200 V for 2 h at 4 °C. The dried gels were then exposed on the PhosphorImager.
Statistics
Results are presented as means ±s.e.m.. Differences between values were determined using Student's paired t test. Pearson's correlation analysis was used to determine associations between variables. Significance was assumed at P < 0.05. Where indicated in the text, a non-parametric sign test was used.
RESULTS
Muscle glycogen content
Pre-marathon muscle glycogen content was 133 ± 12 mmol kg−1, indicating that the pre-marathon dietary and exercise regime was sufficient to support a high level of glycogen storage. Muscle glycogen content was reduced by 40 ± 6 % (to 81.3 ± 11.9 mmol kg−1; P < 0.001) after completion of the marathon, reflecting the increased demand for glycogen by the working muscle during the 42.2 km marathon.
ERK1/2 MAPK phosphorylation and protein expression
We used a phosphospecific antibody to determine ERK1/2 phosphorylation in muscle biopsies pre- and post-exercise. The phosphospecific antibody recognised two bands (Fig. 1A), namely p42 and p44 MAPK, which correspond to ERK1 and ERK2, respectively. The results show densitometric analysis of both ERK bands (ERK1/2). ERK1/2 phosphorylation was markedly increased (7.1-fold) in skeletal muscle biopsies post-exercise (P < 0.001vs. pre-exercise; Fig. 1B). The magnitude the post-exercise increase ranged from 2- to 70-fold above pre-exercise levels. ERK protein expression was similar in pre- and post-exercise samples (19.5 ± 2.4. vs. 18.9 ± 1.9 arbitrary units, respectively). ERK protein expression and the absolute increase in phosphorylation after exercise were not significantly correlated (r = 0.24; n.s.).
Figure 1. Effect of prolonged marathon running on ERK1/2 phosphorylation.
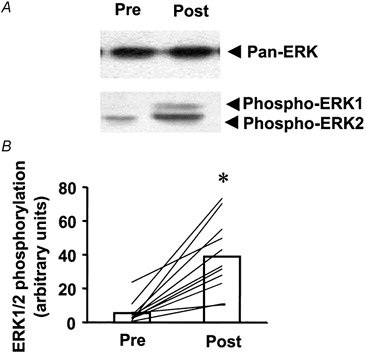
Skeletal muscle biopsies were obtained from subjects (n = 11) 2 h pre- or 17 ± 2 min post-exercise (a 42.2 km competitive marathon). A, immunoblot of pre- and post-exercise ERK1/2 MAPK phosphorylation in skeletal muscle from a representative subject. B, ERK1/2 MAPK phosphorylation for all subjects pre- and post-exercise. Data are presented as the mean response for the study group (□). Lines represent responses for individual subjects. *P < 0.001vs. pre-exercise.
p38 MAPK phosphorylation and protein expression
The phosphospecific p38 MAPK antibody used in the present study detected a single protein, with increased phosphorylation noted in post- vs. pre-exercise muscle (Fig. 2A). The magnitude of the exercise effect on p38 MAPK phosphorylation ranged from 1.2- to 27-fold, with an average increase of 4.4-fold over pre-exercise levels (P < 0.05, Fig. 2B). The protein expression of p38 MAPK was not altered by marathon running (10.8 ± 1.5 vs. 12.9 ± 1.8 arbitrary units, pre- vs. post-exercise, respectively; n.s.). The expression of p38 protein and the absolute increase in phosphorylation after exercise were not significantly correlated (r = 0.36; n.s.).
Figure 2. Effect of marathon running on p38 MAPK phosphorylation.
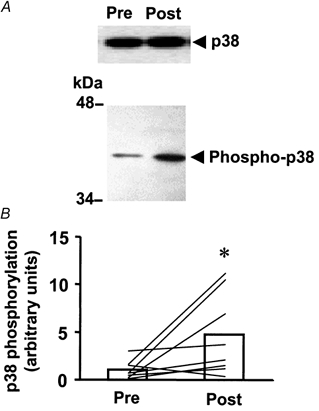
A, immunoblot of pre- and post-exercise p38 MAPK phosphorylation in skeletal muscle from a representative subject. B, p38 MAPK phosphorylation for all subjects (n = 11) pre- and post-exercise. Data are presented as the mean response for the study group (□). Lines represent responses for individual subjects. *P < 0.05vs. pre-exercise.
p90rsk and MAPKAP-K2 kinase activity
p90rsk is a downstream target of ERK1/2 (Stugill et al. 1988; Zhao et al. 1996), whereas MAPKAP-K2 is a downstream target of p38 MAPK (Cuenda et al. 1995; Beyaert et al. 1996). Marathon running increased p90rsk activity 2.4-fold over pre-exercise levels (P < 0.05; Fig. 3A). The magnitude of the post-exercise increase in p90rsk activity ranged from 1.4- to 27-fold. MAPKAP-K2 kinase activity also increased post-exercise (3.1-fold, P < 0.05; Fig. 3B). The increase in MAPKAP-K2 kinase activity after exercise ranged from 1.1- to 7.1-fold above pre-exercise levels. Using correlation analysis, a strong positive relationship was detected between the absolute difference in ERK phosphorylation and p90rsk activity after the marathon (r = 0.85, P < 0.01; Fig. 4). The absolute exercise-induced difference in p38 phosphorylation and MAPKAP-K2 activity was not significantly correlated and this is probably due to the small sample size (r = 0.82, n.s. for 5 subjects).
Figure 3. Effect of marathon running on p90rsk and MAPKAP-K2 kinase activity.
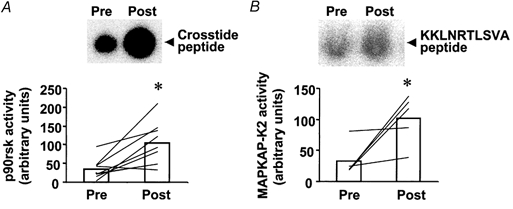
A, upper panel shows reaction products (phosphorylated Crosstide peptide) for the p90rsk activity assay from a representative subject. Lower panel is p90rsk activity for all subjects (n = 11) pre- and post-exercise. B, upper panel shows reaction products (phosphorylated KKLNRTLSVA peptide) from the MAPKAP-K2 activity assay from a representative subject. Lower panel is MAPKAP-K2 activity for all subjects pre- and post-exercise. Data are presented as the mean response for the study group (□). Lines represent responses for individual subjects. *P < 0.05vs. pre-exercise.
Figure 4. Correlation between ERK1/2 phosphorylation and p90rsk activity.
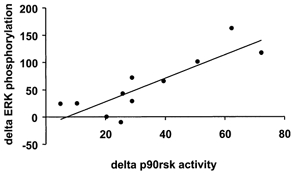
Correlation analysis was performed on the absolute post-exercise increase in ERK1/2 phosphorylation and p90rsk activity for all subjects (n = 11). Data are the difference (delta) between the post- and pre-exercise response. r = 0.85, P < 0.01.
MSK1 and MSK2 activity
MSK1 and MSK2 are newly described enzymes that are believed to function downstream of both ERK and p38 MAPK (Deak et al. 1998). MSK1 activity was increased 2.4-fold over pre-exercise levels (P < 0.05; Fig. 5A). The magnitude of the exercise-induced increase in MSK1 activity ranged from zero to 9.2-fold. By contrast, a weak induction of MSK2 after exercise was noted (Fig. 5B). Exercise increased MSK2 activity in 9 of 11 subjects (P < 0.1, Student's t test; P = 0.03 by non-parametric sign test). The magnitude of the exercise effect on MSK2 activity for the entire study population ranged from zero to 1.7-fold. The absolute increase in p90rsk or MAPKAP-K2 activity was not correlated with either MSK-1 or MSK-2 activity (post-exercise minus pre-exercise effects). However, a significant positive correlation between p90rsk and MSK-2 activity was noted (r = 0.59, P < 0.1).
Figure 5. Effect of marathon running on MSK1 and MSK2 activity.
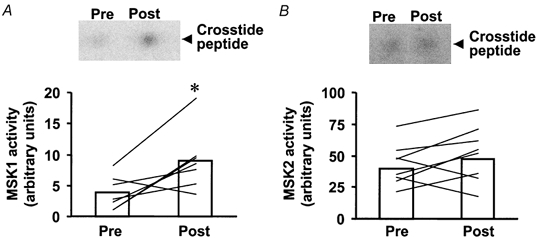
A, upper panel shows reaction products (phosphorylated Crosstide peptide) for the MSK1 activity assay from a representative subject. Lower panel is MSK1 activity for all subjects (n = 11) pre- and post-exercise. B, upper panel shows reaction products (phosphorylated Crosstide peptide) for the MSK2 activity assay from a representative subject. Lower panel is MSK2 activity for all subjects pre- and post-exercise. Data are presented as the mean response for the study group (□). Lines represent responses for individual subjects. P < 0.05vs. pre-exercise.
Effect of marathon running on MEF2 DNA binding
To assess the functional binding properties of the MEF2 transcription factor from skeletal muscle nuclear extracts, we determined MEF2 DNA binding by electrophoretic mobility shift assay (EMSA). In nuclear extracts isolated from skeletal muscle from three subjects, MEF2 DNA binding was significantly enhanced (1.7-fold, P < 0.05) after the marathon (Fig. 6). Binding activity was specifically blocked in the presence of a 10-fold molar excess of unlabelled MEF2 oligonucleotide probe (data not shown).
Figure 6. Effects of marathon running on MEF2 activation.
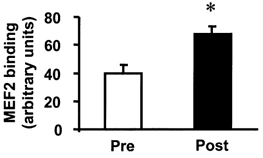
The consensus MEF2-binding oligonucleotide probe was labelled with 32P and incubated with 2 μg of total nuclear extract prepared from skeletal muscle obtained from 3 subjects before (□) and after (▪) a marathon. Samples were resolved by non-denaturing gel electrophoresis (EMSA). Data are mean + s.e.m.. arbitrary PhosphorImager units. *P < 0.05vs. pre-exercise.
DISCUSSION
Exercise/muscle contraction has long been recognised as an important regulator of protein synthesis and gene transcription in skeletal muscle (Booth & Thomason, 1991; Kim et al. 1995, 1999; Chibalin et al. 2000). Evidence is emerging to suggest that skeletal muscle contraction through physical exercise is a powerful stimulator of several parallel MAPK pathways including the ERK1/2, p38 MAPK and JNK signalling cascades (Aronson et al. 1997; Widegren et al. 1998; Boppart et al. 1999, 2000). Activation of the different MAPK pathways by physical exercise offers a candidate mechanism(s) by which transcriptional events could be regulated in skeletal muscle (Fig. 7). We hypothesised that prolonged endurance running leads to the activation of parallel MAPK signalling pathways in skeletal muscle. In the present study, we found that ERK1/2 and p38 MAPK phosphorylation was increased in skeletal muscle obtained from subjects after completion of a competitive marathon. These exercise-induced increases in phosphorylation were not due to increased ERK1/2 and p38 MAPK protein expression. Furthermore, these responses appear to be physiologically relevant, because they were associated with an in vivo, exercise-induced induction of p90rsk, MAPKAP-K2 and MSK enzymes, which are known downstream targets of ERK and p38 MAPK signalling cascades (Stugill et al. 1988; Cuenda et al. 1995; Beyaert et al. 1996; Zhao et al. 1996; Deak et al. 1998).
Figure 7.
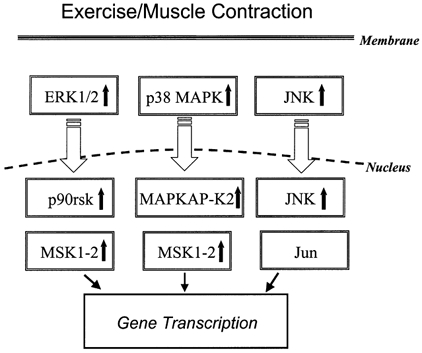
Presumed relationship among relevant MAPK signalling modules and downstream substrates in skeletal muscle in response to acute exercise or contraction.
Because we have studied the effects of exercise on MAPK signalling in humans, definitive conclusions regarding the precise interaction between these identified MAPK substrates and their upstream regulators are not possible. However, in a previous study (Ryder et al. 2000), we determined substrate specificity in MAPK signalling cascades by using an ex vivo system (electrical stimulation) to achieve contraction of isolated rodent skeletal muscle in combination with commonly used chemical inhibitors of ERK (Cuenda et al. 1995) and p38 MAPK (Alessi et al. 1995) (the MEK1 inhibitor PD98059 and the p38 MAPK inhibitor SB203580, respectively). Based on our previous study using specific ERK or p38 inhibitors (Ryder et al. 2000) and the correlation data reported here, contraction-mediated induction of p90rsk and MAPKAP-K2 is likely to occur largely via separate pathways that reflect ERK and p38 MAPK stimulation, respectively. By contrast, MSK1 induction requires simultaneous activation of both ERK and p38 MAPK (Ryder et al. 2000). These observations are consistent with findings in cultured cells, where activation of p90rsk and MAPKAP-K2 are primarily under the control of ERK (Stugill et al. 1988; Hazzalin et al. 1996) and p38 MAPK (Sánchez et al. 1994; Cuenda et al. 1995; Hazzalin et al. 1996), whereas induction of MSK1 requires activation of either ERK or p38 MAPK (Deak et al. 1998). The direct interaction between MSK2 and upstream regulators in contracting muscle has not been determined. However, in cultured cells, MSK2 is directly activated by either ERK or p38 MAP kinase (Deak et al. 1998). Future work will focus on understanding whether exercise-induced MAPK cascades are directly involved in the regulation of gene expression in skeletal muscle.
We found that in vitro MEF2 sequence-specific DNA-binding activity was increased in skeletal muscle in a sub-group of subjects (n = 3) after completion of a marathon. The MEF2 site appears to be essential for the expression of the glucose transporter GLUT4, because deletions or point mutations within the MEF2 consensus binding sequence of the human GLUT4 promoter completely prevent tissue-specific and hormonal/metabolic regulation of GLUT4 (Thai et al. 1998). Although these results do not link the MAPK cascades directly to changes in gene expression in response to exercise, evidence has been presented that MEF2 binding is necessary for p38 MAPK regulation of the muscle creatine kinase reporter gene in myoblasts (Zetser et al. 1999). Activated p90rsk phosphorylates several transcription factors, including Elk1 and cAMP responsive element binding protein (CREB; Blenis, 1993; Price et al. 1996; Xing et al. 1996). Activated MSK1 has been shown to phosphorylate histone H3, high mobility group nuclear protein 14 (HMG-14) (Hazzalin et al. 1996) and CREB (Deak et al. 1998). Therefore, these constituents of the MAPK signalling pathways appear to play a role in the regulation of gene expression in skeletal muscle.
p38 MAPK signal transduction can be mediated by four isoforms, each of which display differential sensitivity to chemical inhibitors; p38α and p38β are inhibited by SB203580, whereas p38δ and p38γ are not (Goedert et al. 1997). The existence of these different isoforms is likely to give rise to specificity in mediating downstream biological responses. For example, p38γ is highly expressed in human skeletal muscle and, although it does not activate activating transcription factor 2 (ATF-2) or MAPKAP-K2, it does phosphorylate myelin basic protein (Li et al. 1996). Recent attention has been directed towards understanding the differential regulation of p38 MAPK isoforms in skeletal muscle in response to exercise (Boppart et al. 2000). Using a phosphospecific p38 MAPK antibody, Boppart and co-workers (Boppart et al. 2000) detected two immunoreactive bands in human skeletal muscle, corresponding to p38α and p38γ MAPK. Marathon running increased p38γ phosphorylation 4-fold, whereas p38α phosphorylation and activity were not altered (Boppart et al. 2000). In our study, p38 isoform-specific responses were not determined. Although the phosphospecific p38 MAPK antibody used in the present study detected only a single protein, we noted a strikingly similar (4.4-fold) exercise-induced increase in p38 MAPK phosphorylation to that reported for p38γ by Boppart and co-workers (Boppart et al. 2000). However, at least two points should be considered. First, SB203580 does not inhibit p38γ activity in cultured cells (Cuenda et al. 1997; Goedert et al. 1997), but does inhibit contraction-induced p38 phosphorylation and MAPKAP-K2 activity in isolated rodent skeletal muscle (Ryder et al. 2000). Second, p38γ does not activate MAPKAP-K2 in vitro (Li et al. 1996), whereas muscle contraction (Ryder et al. 2000) and exercise (present study) increase MAPKAP-K2 activity in vivo. Together, these observations suggest that additional p38 MAPK isoforms might be involved in muscle contraction-/exercise-induced responses.
Studies of MAPK are complicated by the presence of parallel signalling pathways that can undergo simultaneous activation. In the present study, marathon running was associated with a parallel increase in ERK1/2 and p38 MAPK phosphorylation in skeletal muscle. Despite the difference in the timing of the post-race sampling, this was not related to the degree of activation of either p38 MAPK or ERK1/2 phosphorylation in the post-race muscle biopsy. Furthermore, ERK1/2 and p38 MAPK phosphorylation was not correlated with marathon performance time. Our present findings suggest that exercise might favour ERK over p38 MAPK signalling, because the effects of exercise on p38 MAPK phosphorylation were less robust. This finding is consistent with a previous study in humans (Widegren et al. 1998), where ERK and p38 MAPK phosphorylation was measured in skeletal muscle obtained 30 and 60 min after cycle ergometry at 70 %V̇O2,max. This differential response in the exercise-induced regulation of MAPK signalling and downstream substrates might reflect specific in vivo functions of these signalling cascades, substrate availability, time course differences in activation/deactivation of the enzymes, or sensitivity of the different reagents/antibodies and assays used in analyses.
p38 MAPK is activated in response to cellular stress induced by exposure to inflammatory cytokines (Freshney et al. 1994), ultraviolet light (Price et al. 1996) and hyperosmolarity (Han et al. 1994). Competitive marathon events cause a substantial amount of physiological stress, both to the working muscle and to whole body systems. In contrast to physiological exercise, contraction induced by electrical stimulation may favour p38 MAPK over ERK signalling, since the effects of electrical stimulation on p38 MAPK and MAPKAP-K2 in rodent muscle are more profound (Ryder et al. 2000). Although marathon running is associated with increased plasma creatine kinase and interleukin-6 levels, which are markers for skeletal muscle injury after exercise (Ostrowski et al. 1998), electrical stimulation might lead to more profound structural damage of the muscle tissue and, consequently, a greater induction of p38 MAPK signalling. Thus, the differential response of the MAPK signalling pathways to physical exercise vs. electrical stimulation might be related to differences in species (humans versus rodents) or, more likely, to the type of cellular stress induced during exercise versus electrical stimulation. For example, signal transduction through the JNK pathway in human skeletal muscle is greatly influenced by injury-inducing exercise, with greater effects noted after eccentric versus concentric exercise (Boppart et al. 1999). Similarly, p38 MAPK phosphorylation in human skeletal muscle is greater after 4 h of strenuous distance running (> 4-fold; Boppart et al. 2000 and present study) than after 30 min cycle ergometry at 70 %V̇O2,max (2.2-fold; Widegren et al. 1998).
In summary, ERK1/2 and p38 MAPK signalling cascades are activated in skeletal muscle after completion of a competitive marathon. This indicates that MAPK induction is not limited to acute exercise in untrained subjects, as profound effects were observed in subjects engaged in habitual exercise training programs. Moreover, exercise appears to favour ERK over p38 MAPK signalling, because the effects of exercise on p38 MAPK were less robust than for ERK1/2. This study also establishes that p90rsk, MAPKAP-K2 and MSK1, downstream targets of ERK1/2 and p38 MAPK, are activated after exercise. Activation of these downstream targets provides a potential physiological mechanism by which exercise induces gene transcription in skeletal muscle.
Acknowledgments
We are grateful to Dr Dario R. Alessi (Department of Biochemistry, University of Dundee, UK), for generously providing reagents used in the kinase assays. This study was supported by grants from the Swedish Medical Research Council, the Swedish Diabetes Association, the Foundation for Scientific Studies of Diabetology, the Swedish National Centre for Research in Sports and from the following Research Foundations: Thurings, Wibergs, Magnus Bergwalls, Tore Nilsons, Novo-Nordisk, Harald and Greta Jeansson, and Marcus and Amalia Wallenberg.
References
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. Journal of Biological Chemistry. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Aronson D, Dufresne SD, Goodyear LJ. Contractile activity stimulates the c-Jun NH2-terminal kinase pathway in rat skeletal muscle. Journal of Biological Chemistry. 1997;272:25636–25640. doi: 10.1074/jbc.272.41.25636. [DOI] [PubMed] [Google Scholar]
- Aronson D, Violan MA, Dufresne SD, Zangen D, Fielding RA, Goodyear LJ. Exercise stimulates the mitogenic-activated protein kinase pathway in human skeletal muscle. Journal of Clinical Investigation. 1997;99:1251–1257. doi: 10.1172/JCI119282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee J, Haegeman G, Cohen P, Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO Journal. 1996;15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proceedings of the National Academy of Sciences of the USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW, Thomason DB. Molecular and cellular adaptations of muscle in response to exercise: perspectives of various models. Physiological Reviews. 1991;71:541–585. doi: 10.1152/physrev.1991.71.2.541. [DOI] [PubMed] [Google Scholar]
- Boppart MD, Aronson D, Gibson L, Roubenoff R, Abad LW, Bean J, Goodyear LJ, Fielding RA. Eccentric exercise markedly increases c-Jun NH2-terminal kinase activity in human skeletal muscle. Journal of Applied Physiology. 1999;87:1668–1673. doi: 10.1152/jappl.1999.87.5.1668. [DOI] [PubMed] [Google Scholar]
- Boppart MD, Asp S, Wojtaszewski JFP, Fielding RA, Mohr T, Goodyear LJ. Marathon running transiently increases c-Jun NH2-terminal kinase and p38γ activities in human skeletal muscle. Journal of Physiology. 2000;526:663–669. doi: 10.1111/j.1469-7793.2000.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Pouyssegur J. Mammalian MAP kinase modules: how to transduce specific signals. Essays in Biochemistry. 1997;32:1–16. [PubMed] [Google Scholar]
- Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends in Biochemical Sciences. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- Chibalin AV, Yu M, Ryder JW, Song XM, Galuska D, Krook A, Wallberg-Henriksson H, Zierath JR. Exercise-induced changes in expression and activity of proteins involved in insulin signal transduction in skeletal muscle: differential effects on insulin receptor substrates 1 and 2. Proceedings of the National Academy of Sciences of the USA. 2000;97:38–43. doi: 10.1073/pnas.97.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton AD, Young PR, Cohen P. A comparison of the substrate specificity of MAPKAP kinase-2 and MAPKAP kinase-3 and their activation by cytokines and cellular stress. FEBS Letters. 1996;292:209–214. doi: 10.1016/0014-5793(96)00816-2. [DOI] [PubMed] [Google Scholar]
- Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends in Cell Biology. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Crespo P, Xu N, Simonds W, Gutkind J. Ras-dependent activation of MAP kinase pathway mediated by G-proteinβγ subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Cohen P, Buée-Scherrer V, Goedert M. Activation of stress-activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6); comparison of the specificities of SAPK3 and SAPK2 (RK/p38) EMBO Journal. 1997;16:295–305. doi: 10.1093/emboj/16.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher T-F, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Letters. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- Davis RJ. The mitogen-activated protein kinase signal transduction pathway. Journal of Biological Chemistry. 1993;268:14553–14556. [PubMed] [Google Scholar]
- Deak M, Clifton A, Lucocq L, Alessi D. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO Journal. 1998;15:6552–6563. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Galcheva-Gargova Z, Derijard B, Wu IH, Davis RJ. An osmosensing signal transduction pathway in mammalian cells. Science. 1994;265:806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- Goedert M, Cuenda A, Craxton M, Jakes R, Cohen P. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO Journal. 1997;16:3563–3571. doi: 10.1093/emboj/16.12.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear LJ, Chang P-Y, Sherwood DJ, Durfesne SD, Moller DE. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. American Journal of Physiology. 1996;271:E403–408. doi: 10.1152/ajpendo.1996.271.2.E403. [DOI] [PubMed] [Google Scholar]
- Goodyear JJ, Giorgino F, Balon TW, Condorelli G, Smith RJ. Effects of contractile activity on tyrosine phosphorylation and PI 3-kinase activity in rat skeletal muscle. American Journal of Physiology. 1995;268:E987–995. doi: 10.1152/ajpendo.1995.268.5.E987. [DOI] [PubMed] [Google Scholar]
- Han J, Lee J-D, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Dufresne SD, Goodyear LJ. Skeletal muscle contractile activity in vitro stimulates mitogen-activated protein kinase signaling. American Journal of Physiology. 1999;277:C701–707. doi: 10.1152/ajpcell.1999.277.4.C701. [DOI] [PubMed] [Google Scholar]
- Hazzalin CA, Cano E, Cuenda A, Barratt MJ, Cohen P, Mahadevan LC. p38/RK is essential for stress-induced nuclear responses: JNK/SAPKs and c-Jun/ATF-2 phosphorylation are insufficient. Current Biology. 1996;6:1028–1031. doi: 10.1016/s0960-9822(02)00649-8. [DOI] [PubMed] [Google Scholar]
- Kim Y, Inoue T, Nakajima R, Nakae K, Tamura T, Tokuyama K, Suzuki M. Effects of endurance training on gene expression of insulin signal transduction pathway. Biochemical and Biophysical Research Communications. 1995;210:766–773. doi: 10.1006/bbrc.1995.1725. [DOI] [PubMed] [Google Scholar]
- Kim YB, Inoue T, Nakajima R, Nakae K, Tamura T, Tokuyama K, Suzuki M. Effect of long-term exercise on gene expression of insulin signaling pathway intermediates in skeletal muscle. Biochemical and Biophysical Research Communications. 1999;254:720–727. doi: 10.1006/bbrc.1998.9940. [DOI] [PubMed] [Google Scholar]
- Leighton B, Blomstrand E, Challiss RAJ, Lozeman FJ, Parry-Billings M, Dimitriadis GD, Newsholme EA. Acute and chronic effects of strenuous exercise on glucose metabolism in isolated, incubated soleus muscle of exercise-trained rats. Acta Physiologica Scandinavica. 1989;136:177–184. doi: 10.1111/j.1748-1716.1989.tb08650.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Jiang Y, Ulevitch RJ, Han J. The primary structure of p38γ: a new member of p38 group of MAP kinase. Biochemical and Biophysical Research Communications. 1996;228:334–340. doi: 10.1006/bbrc.1996.1662. [DOI] [PubMed] [Google Scholar]
- Mora S, Pessin JE. The MEF2A isoform is required for striated muscle-specific expression of the insulin-responsive GLUT4 glucose transporter. Journal of Biological Chemistry. 2000;275:16323–16328. doi: 10.1074/jbc.M910259199. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Rhode T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. Journal of Physiology. 1998;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M, Cruzalegui F, Treisman R. The p38 and ERK MAP kinase pathways cooperate to activate ternary complex factors and c-fos transcription in response to UV light. EMBO Journal. 1996;15:6552–6563. [PMC free article] [PubMed] [Google Scholar]
- Ray LB, Sturgill TW. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proceedings of the National Academy of Sciences of the USA. 1987;84:1502–1506. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LB, Sturgill TW. Characterization of insulin-stimulated microtubule-associated protein kinase. Rapid isolation and stabilization of a novel serine/threonine kinase from 3T3-L1 cells. Journal of Biological Chemistry. 1988;263:12721–12727. [PubMed] [Google Scholar]
- Ryder JW, Fahlman R, Wallberg-Henriksson H, Alessi DR, Krook A, Zierath JR. Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle: involvement of the mitogen- and stress-activated protein kinase 1. Journal of Biological Chemistry. 2000;275:1457–1462. doi: 10.1074/jbc.275.2.1457. [DOI] [PubMed] [Google Scholar]
- Sánchez I, Hughes RT, Mayer BJ, Yee K, Woodgett JR, Avruch J, Kyriakis JM, Zon LI. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB Journal. 1995;9:726–735. [PubMed] [Google Scholar]
- Sherwood DJ, Dufresne SD, Markuns JF, Cheatham B, Moller DE, Aronson D, Goodyear LJ. Differential regulation of MAP kinase, p70S6K, and Akt by contraction and insulin in rat skeletal muscle. American Journal of Physiology. 1999;276:E870–878. doi: 10.1152/ajpendo.1999.276.5.E870. [DOI] [PubMed] [Google Scholar]
- Stugill TW, Ray LB, Erikson E, Maller JL. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988;334:715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Thai M, Guruswamy S, Cao K, Pessin J, Olson A. Myocyte enhancer factor 2 (MEF2)-binding site is required for GLUT4 gene expression in transgenic mice. Regulation of MEF2 DNA binding activity in insulin-deficient diabetes. Journal of Biological Chemistry. 1998;273:14285–14292. doi: 10.1074/jbc.273.23.14285. [DOI] [PubMed] [Google Scholar]
- Van Biesen T, Hawes BE, Luttrell DK, Krueger KM, Touhara K, Porfiri E, Sakaue E, Luttrell LM, Lefkowitz RJ. Receptor-tyrosine-kinase- and Gβγ-mediated MAP kinase activation by a common signaling pathway. Nature. 1995;376:781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- Van Biesen T, Hawes BE, Raymond JR, Luttrell LM, Koch WJ, Lefkowitz RJ. Go-protein α-subunits activates mitogen-activated protein kinase via a novel protein kinase C-dependent mechanism. Journal of Biological Chemistry. 1996;271:1266–1269. doi: 10.1074/jbc.271.3.1266. [DOI] [PubMed] [Google Scholar]
- Widegren U, Jiang XJ, Krook A, Chibalin AV, Björnholm M, Tally M, Roth RA, Henriksson J, Wallberg-Henriksson H, Zierath JR. Divergent effects of exercise on metabolic and mitogenic signaling pathways in human skeletal muscle. FASEB Journal. 1998;12:1379–1389. doi: 10.1096/fasebj.12.13.1379. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JFP, Lynge J, Jakobsen AB, Goodyear LJ, Richter EA. Differential regulation of MAP kinase by contraction and insulin in skeletal muscle: metabolic implications. American Journal of Physiology. 1999;277:E724–732. doi: 10.1152/ajpendo.1999.277.4.E724. [DOI] [PubMed] [Google Scholar]
- Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- Zetser A, Gredinger E, Bengal E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation: participation of the MEF2C transcription factor. Journal of Biological Chemistry. 1999;274:5193–5200. doi: 10.1074/jbc.274.8.5193. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Bjorbaek C, Moller DE. Regulation and interaction of pp90(RSK) isoforms with mitogen-activated protein kinase. Journal of Biological Chemistry. 1996;271:29773–29779. doi: 10.1074/jbc.271.47.29773. [DOI] [PubMed] [Google Scholar]


