Abstract
Natriuretic peptides have a major role in fluid and electrolyte homeostasis in vertebrates. Ambient temperature has a major influence on physiological processes in ectothermic animals. Here we have studied the mechanisms of regulation of a natriuretic peptide, sCP (salmon cardiac peptide), in salmon (Salmo salar) acclimatised and acclimated to varying temperatures.
The circulating and cardiac levels of sCP were found to be markedly upregulated in warm-acclimatised and warm-acclimated salmon. The release of sCP from isolated in vitro perfused salmon ventricle was, however, not increased by acclimation to higher temperatures, either in basal conditions or when stimulated by mechanical load.
Concomitant measurements of circulating sCP and the biologically inert N-terminal fragment of pro-sCP showed that the upregulation of circulating sCP at warm ambient temperature results from decreased elimination rather than increased secretion of sCP. This is the first direct evidence that changes in the elimination of a natriuretic peptide are used for important physiological regulation.
We found a paradoxical increase in cardiac sCP mRNA levels at cold temperatures which coincided with hypertrophy of the heart. sCP gene expression may therefore serve as a marker of cardiac hypertrophy in salmon, in analogy to that of atrial and brain natriuretic peptide (ANP and BNP, respectively) in mammals.
These results show that temperature has a major influence on the regulation of natriuretic peptide production and clearance in salmon. Salmon CP offers a novel model for the study of the endocrine function of the heart.
The natriuretic peptide system plays a major role in the fluid and electrolyte homeostasis in vertebrates, based on results from physiological studies (reviewed in Ruskoaho, 1992; Thibault et al. 1999) and experiments with transgenic animals (John et al. 1995; Lopez et al. 1995; Matsukawa et al. 1999). The regulation of the synthesis and secretion of cardiac A- and B-type natriuretic peptides, is, however, still incompletely understood. The ability of salmon (Salmo salar) to maintain volume and electrolyte balance despite the highly challenging osmoregulatory conditions of sea and fresh water prompted us to use it as a model for our studies on the regulation of natriuretic peptides. As an initial step, we recently cloned and sequenced from salmon heart the cDNA of a novel peptide hormone, salmon cardiac peptide (sCP) (Tervonen et al. 1998), which is stored in secretory granules and released by mechanical load, analogous to the mammalian natriuretic peptides (Kokkonen et al. 2000). Homologues of the novel peptide can be found in several teleost species (Tervonen et al. 2000). The evolutional position of fish make them ideal for studying the conserved characteristics of the natriuretic peptide system (Powers, 1989; Postlethwait et al. 1998; Wittbrot et al. 1998). They also make excellent models for studies on the general factors regulating the expression and secretion of natriuretic peptides. In keeping with this we have recently found that the promoter of the sCP gene is very active not only in salmon heart but also in mammalian cardiomyocytes, suggesting that, despite the large phylogenetic distance, the same transcription factors are responsible for the heart-specific expression of the peptides in mammals and salmon (Majalahti-Palviainen et al. 2000). Since sCP transcripts or peptide cannot be found in any tissues outside the heart (Majalahti-Palviainen et al. 2000) it can be used as a specific model for the endocrine function of the heart.
In fish and other ectothermic animals, thermal balance is governed by external sources of heat, and therefore ambient temperature has a major influence on most physiological processes. Many fishes are exposed seasonally to considerable changes in water temperature. Temperature acclimation is accompanied with compensatory changes in metabolic, contractile and morphological properties of muscle, which significantly offset the negative impact of reduced temperature on swimming performance (for example see Egginton & Sidell, 1989; Johnston et al. 1990; Keen & Farrell, 1994; Taylor et al. 1996). Thus many fish species, like salmonids, retain their locomotory activity at low temperatures (Nilssen et al. 1997; Aho & Vornanen, 1999). In these species therefore, cold can represent a significant challenge to the cardiovascular system. Low temperature directly affects the intrinsic properties of the heart, decreasing the rate of contractions and the power output. Adequate cardiac output can be maintained at low temperatures by enlargement of the ventricle and an increase of the stroke volume (reviewed in Driedzic & Gesser, 1994). Seasonal variation has also been observed in various blood parameters affecting the cardiovascular system indirectly, such as the viscocity, haematocrit, electrolytes, osmolality and thermal hysteresis (Nikinmaa et al. 1981; Audet & Claireaux, 1992; Duncker et al. 1995).
Based on the profile of the physiological effects of natriuretic peptides in mammals, we hypothesised that they might be important in the cardiovascular adaptation of salmon to the large seasonal variations in the ambient temperature. On the other hand, any changes in the function of the natriuretic peptide system of seasonally acclimatised salmon could help in understanding the general mechanisms regulating this conserved peptide hormone family. We have now tested these ideas by measuring the circulating levels of salmon cardiac peptide (sCP), as well as the cardiac levels of its prohormone and mRNA, from samples collected at monthly intervals for a period of 1 year from salmon kept under natural photoperiod and temperature. The possible effect of the stage of maturation was taken into account by sampling young salmon parrs and mature adult salmon separately. Since major seasonal variation was found in sCP levels, the mechanisms by which temperature could affect the sCP system were further studied in temperature acclimated salmon and in a ventricle perfusion system in vitro.
METHODS
animals
The experimental design was approved by the Animal Experimentation Review Board of the University of Oulu (permits 8/97 and 66/99 to O.V.). Cardiac tissue and plasma samples were collected monthly for a period of 1 year from salmon (Salmo salar) kept under field conditions with natural photoperiod and temperature. Samples were collected from young parrs and adult salmon to find out whether sCP gene expression is similar during the two major developmental stages. Fish of both sexes were used. Parrs were provided by the hatchery of Imatran Voima power plant in Montta, Muhos, Finland (26°0′ N, 64°8′ E). They were fed with commercial fish pellets, daily in the summer, and less frequently in the winter. The body weight of the parrs increased from 61 ± 8 to 262 ± 40 g during the study. When the sampling was started, the parrs were 2 years old. In the climatic conditions of northern Finland smoltification takes place at the average age of 3 years, that is during the sampling period. The fish are naturally exposed to high variations in ambient temperature. During the study the highest daily average water temperature was 20.4 °C and the lowest 0.2 °C (Fig. 1). The variation in the amount of ambient light was great as well. The light period of day varied from 3.5 to 22 h. The pH of the water was about 6.7, except in May when the pH declined to 6 due to acidic melt water draining from marshes surrounding the hatchery.
Figure 1. The average water temperature and day length the experimental animals would have been exposed to during the 1 year sampling period.
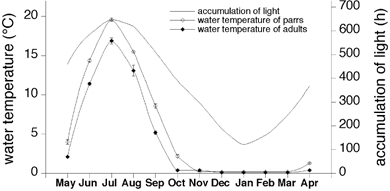
The water temperature given for each month is the average taken over the 14 day period prior to the sampling day. The accumulation of light is the sum of hours of daylight during the 30 days preceding the sampling day (data obtained from the airport of Oulu, N 64°55′ E 25°22′).
The adult salmon were provided by the Finnish Game and Fisheries Research Institute, Taivalkoski, Finland (28°3′ N, 65°6′ E). They were fed with commercial fish pellets three times a day during the summer and once a day during the winter. At the beginning of the sampling, the fish were 5 years old and weighed 464 ± 51 g. In April, the last sampling month, the salmon weighed 590 ± 78 g. During the study the highest daily average water temperature in July was 18.2 °C and the lowest 0.1 °C during the winter months. The seasonal changes in the photoperiod were the same as that for the parrs described above. The water pH was stable (6.3-6.6), except during May and June, when the pH varied between 5.19 and 6.0 for the reasons described above.
The parrs were sampled in the hatchery in which they were bred. Adult salmon were transported from Taivalkoski to the laboratory (Department of Physiology, University of Oulu), in plastic packages containing oxygenated water. Transportation took approximately 2 h, after which the fish were released into a tank containing 1500 l dechlorinated, recirculated, well-aerated tap water. The tank water temperature was adjusted to and maintained at the temperature of the outdoor water of the season using a cooling unit (Aqua Medic, Melle, Germany). The oxygen and nitrite content were monitored regularly with a portable dissolved oxygen meter (OxyGuard International A/S, Birkerød, Denmark) and Aqua Vital Multisticks (Aquarium Münster, Telgte, Germany). The sampling was started immediately after the arrival of the fish at the Department. At sampling all the fish appeared healthy. The condition factor (K) (Reimers, 1963) ranged in parrs from 0.7 ± 0.06 in May, to 1.1 ± 0.02 in the remaining months, and in adult salmon from 0.8 ± 0.03 to 1 ± 0.02. K was calculated as W× 100/L3, where W is the body mass in grams and L the body length in centimetres. The fish were killed with a sharp blow to the head. Each individual was weighed and the total body length was measured. The blood was drawn from the caudal vein into chilled EDTA tubes and centrifuged within 30 min to separate the plasma. Following decapitation the body cavity was opened and the sex of mature fish was determined. The heart was removed and the atrium and ventricle separated, weighed, and frozen in liquid N2. All the samples were stored at −70 °C.
Adult salmon (579 ± 42 g) of both sexes were used for the experiments in which the influence of different temperatures on plasma and tissue immunoreactive (ir)-sCP and cardiac sCP mRNA levels were studied, as well as for the in vitro ventricle perfusions. They originated from the stock of Finnish Game and Fisheries Research Institute, Taivalkoski. The salmon were transported and maintained as described above. During the acclimation the fish appeared healthy and the condition factor remained stable. At the water temperatures 0.2, 6 and 12 °C K was 1 ± 0.01, 0.97 ± 0.04 and 0.97 ± 0.05, respectively. The experiments were performed during the winter, when the fish were acclimatised to cold water (0.2 °C) and short periods of daylight. The term acclimatisation is used here to designate the process of adaptation under natural outdoor conditions, and acclimation designates the adaptation in the laboratory. The acclimation temperatures in the laboratory were 6 °C (range 5.2–6.6 °C), and 12 °C (11.2–12.6 °C). The stock of 30 fish was kept at the set temperature for 10 days, after which half the fish were used for experiments. The fish were killed with a sharp blow to the head and blood was drawn from the caudal vein into chilled EDTA tubes, and the ventricle was used for perfusion studies. For the remaining fish the water temperature was raised to the higher acclimation temperature. The photoperiod was 8 h light-16 h dark, which was an approximate average of the light-dark cycle of those months during which the experiments were performed.
Extraction of total RNA and Northern blot analysis
Total RNA was extracted by using the acidic phenol method (Chomczynski & Sacchi, 1987). Aliquots of the guanidine isothiocyanate homogenates were stored at −70 °C for use in radioimmunoassay and chromatography. Five microgram samples of total RNA from the atria and ventricle were size-fractioned on 1 % formaldehyde agarose gels, and blotted on nylon membranes. The membranes were hybridised with a full length (1234 bp) sCP cDNA probe (Tervonen et al. 1998), labelled with [32P]dCTP using random primed labelling. All the atrial RNA samples were processed in a single batch, as were the ventricular samples. The membranes were washed with 0.2 × saline-sodium citrate buffer (SSC), 0.2 % SDS at 65 °C for 1 h. X-Omat films (Kodak, Rochester, USA) were exposed for 36 h with an intensifying screen at −70 °C. Following the removal of the sCP probe by washing with 0.05 × SSC, 0.5 m EDTA, 10 % SDS at 95 °C for 30 min, the membranes were hydridised with a 482 bp r18S cDNA probe (Majalahti-Palviainen et al. 2000). The sCP mRNA hybridisation signals were normalised to those of ribosomal 18S RNA to account for potential differences in the RNA load. The films were digitised using a Umax flat-bed scanner, a Macintosh personal computer and Adobe Photoshop (Adobe Systems Inc., San Jose, CA, USA). The results were quantified with ScanAnalysis (Biosoft, Cambridge, UK).
Radioimmunoassay of plasma and tissue sCP
The plasma samples were extracted with SepPak C18 cartridges (Waters, Milford, MA, USA) as described previously (Tervonen et al. 2000) for use in sCP radioimmunoassay. Aliquots of the homogenates obtained during RNA purification (see above) were used to measure immunoreactive sCP (ir-sCP) in the tissue samples. No more than 1 μl of the solution was directly used in the radioimmunoassay because the strong chaotropic guanidine isothiocyanate solution interferes with the radioimmunoassay when present in amounts exceeding 2 μl. Details of the radioimmunoassay procedure have been described previously (Tervonen et al. 2000). The N-terminal fragment of pro-sCP was measured with the same procedure, with the exception that an antiserum raised against recombinant pro-sCP(1-97)-GST fusion protein was used. Pro-sCP(1-97) was used as a standard and for radioiodination. The sCP and NT-pro-sCP antisera have < 0.1 % cross-reactivity with mammalian ANP, BNP and CNP as well as with eel ANP and rainbow trout ventricular natriuretic peptide (VNP). The sCP antiserum does not recognise NT-pro-sCP, nor does the NT-pro-sCP antiserum sCP.
Perfusion of isolated salmon ventricle
The effect of temperature on sCP gene expression and release was studied using an isolated in vitro perfused salmon ventricle preparation. The ventricles were obtained from salmon acclimated to different temperatures as described above. The perfusion system was as described previously (Kokkonen et al. 2000) with the following modifications. The isolated ventricles were mounted in an organ bath and perfused with buffer, both of which were cooled to the temperature at which the fish had been acclimated (6 or 12 °C). Due to a practical limitation of the set-up, perfusion of ventricles from salmon acclimatised to 0.2 °C had to be performed at 6 °C (the lowest attainable temperature). We acknowledge that this is not ideal, but the present results show that the acute temperature change did not cause any detectable disturbance on the sCP system in the short-term experiment (see Fig. 5). The perfusate flow rate was 1.5 ml min−1. Collection of the 5 min perfusate fractions was started after a 60 min equilibration period. Basal samples without mechanical load were collected for 60 min, before applying the load of 13 cmH2O (1.3 kPa) for 120 min. The perfusate fractions were stored at −20 °C for use in radioimmunoassay. The ventricle tissue was blotted dry, weighed, frozen in liquid N2, and stored at −70 °C for use in RNA blots and radioimmunoassay.
Figure 5. The release of ir-sCP from the perfused salmon ventricle from animals acclimated to temperatures of 0.2, 6 and 12 °C.
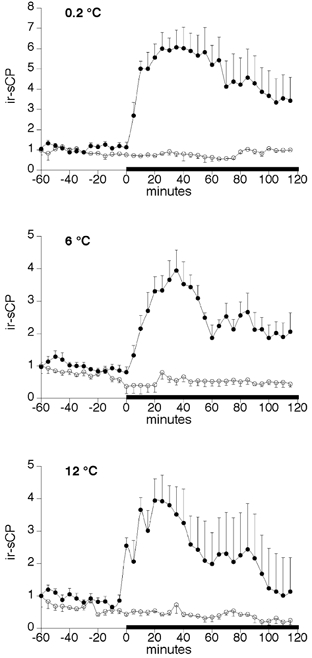
○, perfusions without mechanical load; •, perfusions in which mechanical load was imposed on the ventricle. The horizontal bar denotes the period during which a mechanical load of 13 cmH2O was applied. Results (means ±s.e.m., n = 6 for the mechanically loaded ventricles, n = 4 for the control ventricles), which are shown in relative terms, were calculated by dividing the ir-sCP level at each time point by the average ir-sCP level between time points −60 and −5 min.
Statistical analyses
One-way analysis of variance followed by Student-Newman-Keuls test (SigmaStat, SPSS Inc., Chicago, IL, USA) was used to assess the monthly differences in the hormone and relative mRNA levels. The results from parrs and adult salmon were tested using Student's unpaired t test, using InStat software (GraphPad Software Inc., San Diego, CA, USA). In case the variances were non-homogeneous, the non-parametric Mann-Whitney test was used. Two-way analysis of variance was used to assess the differences in the ventricular sCP mRNA and ir-sCP of the temperature-acclimated salmon. Base 10 logarithm transformation was applied to the results from temperature-acclimated salmon plasma immunoreactivity before ANOVA, since the data did not pass the normality test. Linear regression was used for correlation analysis. All results are expressed as means ±s.e.m. The statistical test results are expressed as the two-sided P value. Differences at the 95 % level were considered significant.
RESULTS
Levels of plasma and cardiac immunoreactive sCP during the year
We hypothesised that the novel vasoactive cardiac hormone sCP may be important for the adaptation of salmon cardiovascular system to the large annual variations in water temperature. It was found that the circulating levels of ir-sCP displayed marked seasonal changes, with highly elevated levels during the summer months and low levels during the winter (Fig. 2A and B). The levels correlated positively with the ambient temperature both in parrs and adult salmon (r = 0.65, P < 0.001 and r = 0.58, P < 0.001, respectively). The increase in plasma ir-sCP during the warm months was especially marked in the parrs, rising from the lowest level of 157 ± 25 pmol l−1 in March to 2805 ± 998 pmol l−1 in September (P < 0.05). In adult salmon the increase was not quite as great, the levels rising from 250 ± 46 pmol l−1 in May to 663 ± 46 pmol l−1 in June. As a consequence, plasma ir-sCP levels were significantly higher in parrs than adult salmon during the months of June, July and September (P < 0.001, P < 0.05 and P < 0.01, respectively).
Figure 2. The plasma concentration of immunoreactive sCP in parrs and adult salmon during the 1 year sampling period.
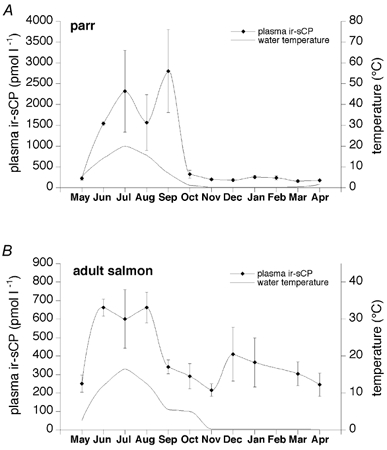
The animals were sampled at the end of each month. Data are given as means ±s.e.m. (n = 5). A, in parrs the plasma ir-sCP levels in July and September differed significantly from those in May, October, November, December, January, February or April (P < 0.05 and P < 0.001, respectively). B, in adult salmon the plasma ir-sCP levels were significantly higher in June and August than those in November (P < 0.05).
The monthly fluctuations of cardiac tissue ir-sCP levels resembled those observed in plasma sCP, although they were less extensive. The ir-sCP levels in adult salmon were significantly higher (P < 0.01) during the late summer and early autumn (July-September) than those during the remaining months (Fig. 3B). In general, the seasonal pattern of cardiac ir-sCP levels in parrs resembled that detected in the adult salmon (Fig. 3A). The concentration of ir-sCP in the parr atrium varied from 24 ± 3 nmol g−1 in May to 54 ± 6 nmol g−1 in September. In adult salmon the atrial ir-sCP concentrations were somewhat higher than those in the parrs, ranging from 36 ± 9 nmol g−1 in February to 73 ± 6 nmol g−1 in September. The ventricular ir-sCP concentration in parrs varied from 5.3 ± 0.7 nmol g−1 in July to 7.8 ± 0.8 nmol g−1 in August. In adult salmon the level of ventricular ir-sCP ranged from 4.3 ± 1 nmol g−1 in December to 14.7 ± 1.9 nmol g−1 in August. While the seasonal variations were similar in parrs and in adult salmon, the ir-sCP levels in adult salmon were significantly higher in the atrium in June and July (P < 0.05) and in the ventricle in August and September (P < 0.01) compared with those in the parrs.
Figure 3. The levels of ir-sCP in the atrium (□) and ventricle (▪) during the 1 year sampling period.
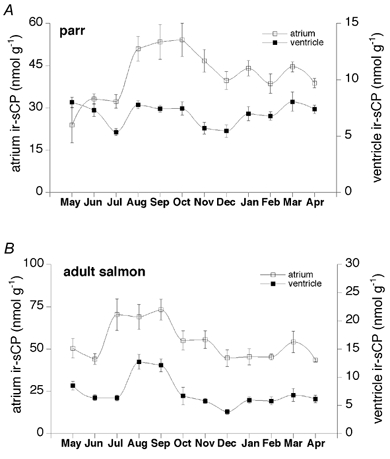
The animals were sampled at the end of each month. Data are given as means ±s.e.m. (n = 5). A, in parrs the levels of ir-sCP in the atrium were significantly higher in January and March (P < 0.05) as well as in August, September, October and November (P < 0.01), than those in May. B, in adult salmon the ir-sCP levels in July, August and September in the atrium and in August and September in the ventricle were significantly higher than those in the respective samples collected during any of the remaining months (P < 0.01).
Levels of cardiac sCP mRNA during the year
To find out whether the seasonal variations in circulating ir-sCP and cardiac tissue ir-sCP were associated with changes in sCP gene expression, the levels of sCP mRNA were measured from atrial and ventricular tissue from both parrs and adult salmon at the same time points as those of ir-sCP. Significant seasonal fluctuations were observed in both atrial and ventricular sCP mRNA (Fig. 4A and B). However, it was surprising that the mRNA levels tended to be higher during the cold period of the year when the plasma and cardiac ir-sCP levels were low. In keeping with this there was a significant inverse correlation between the plasma levels of ir-sCP and those of sCP mRNA in the ventricle of parrs and adults r =−0.3, P < 0.05 for both), and between temperature and ventricular sCP mRNA concentration of parrs and adults (r =−0.53, P < 0.001 and r =−0.5, P < 0.01, respectively). In the parrs also the atrial sCP mRNA level was inversely correlated with the water temperature (r =−0.49, P < 0.001). In addition, a significant peak in the ventricular sCP mRNA level in adult salmon was observed during the end of the warm period in August and September.
Figure 4. The relative concentration of sCP mRNA in the atrium (□) and in the ventricle (▪) during the 1 year sampling period.
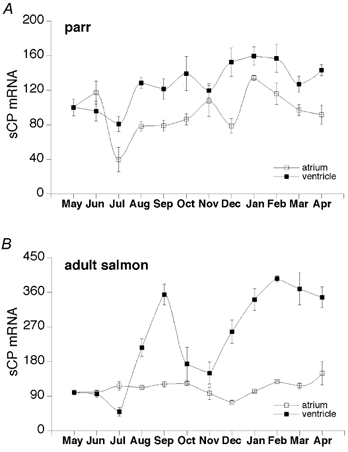
The animals were sampled at the end of each month. Data are given as means ±s.e.m. (n = 5). A, in parrs the atrial sCP mRNA levels were significantly lower in July than in June, November, January or February (P < 0.01). They were significantly higher in January than in August, September, October or December (P < 0.05). The ventricular sCP mRNA levels in parrs were significantly higher in December, January, and February than in June and July (P < 0.05). They were also significantly higher in October and April than in July (P < 0.05). B, in adult salmon the atrial sCP mRNA levels were significantly higher in April than in December (P = 0.001). The ventricular sCP mRNA levels in adult salmon were significantly higher in September, December, January, February, March and April than in May, June or July (P < 0.001). The sCP mRNA levels in August were significantly higher than those in May, June or July (P < 0.05) but significantly lower than those in September, January, February, March or April (P < 0.05). The October and November levels differed significantly from those of September, January, February, March or April (P < 0.001). Finally, the levels in October were significantly higher than those in July (P < 0.05).
Changes in the relative ventricle mass during the year
Both in parrs and adult salmon the lowest relative ventricle mass (ventricle mass/BW × 100) was observed in the warm-acclimatised fish, in August (0.065 ± 0.003 in parrs, 0.099 ± 0.008 in adult salmon), and the highest in March in fish that had acclimatised to temperatures near 0 °C for 5 months (0.12 ± 0.018 in parrs, 0.13 ± 0.007 in adult salmon). The increase was significant in both groups (P < 0.05).
The effect of different temperatures on sCP secretion and expression
To find out whether the variation in the water temperature was responsible for the major seasonal fluctuations sCP, the secretion of immunoreactive sCP was studied using an in vitro model. Isolated salmon ventricles, obtained from salmon acclimatised to cold (0.2 °C) and subsequently acclimated to 6 or 12 °C for 10 days, were perfused in a system which enables the adjustment of the mechanical load imposed on the ventricle. sCP levels in the perfusate were measured with a specific radioimmunoassay. Based on the seasonal variation of circulating ir-sCP (Fig. 2A and B) higher sCP release was expected when the temperature was raised. The amount of sCP secreted into the perfusate without any extra mechanical load was, however, not significantly different in salmon acclimated to the different temperatures (460 ± 94 pmol l−1 (g ventricle tissue)−1 at 0.2 °C, n = 10; 459 ± 16 pmol l−1 (g ventricle tissue)−1 at 6 °C, n = 10 and 314 ± 66 pmol l−1 (g ventricle tissue)−1 at 12 °C, n = 10). Moreover, in ventricles obtained from salmon acclimatised to 0.2 °C, the load of 13 cmH2O increased the release of ir-sCP 5.3 ± 0.6-fold compared with 3.5 ± 0.5-fold and 3.7 ± 0.7-fold in ventricles acclimated to 6 and 12 °C, respectively (n = 6, Fig. 5). We had to test the ventricles obtained from fish acclimatised to 0.2 °C at the perfusion temperature of 6 °C due to technical limitations of the perfusion system. The basal and load-stimulated secretion was, however, stable and similar in quantity to that found at the other temperatures, and there was not any indication of a disturbance caused by the acute increase in temperature. This suggests that the secretory mechanism is not sensitive for acute temperature changes. In absolute terms, the sCP release into the perfusate during the mechanical load was significantly lower (P < 0.05) from the 12 °C acclimated ventricles (993 ± 145 pmol l−1 (g ventricle tissue)−1, n = 6) than from 0.2 °C acclimatised ventricles (1478 ± 141 pmol l−1 (g ventricle tissue)−1, n = 6). This was probably due to the faster levelling off, at higher temperatures, of the sCP release despite continuous mechanical stimulus.
Although the sCP secretion was not increased at higher temperatures, the circulating level of ir-sCP was significantly higher in the warm-acclimated salmon used for ventricle perfusion studies than in salmon acclimatised to cold (Fig. 6A). In salmon acclimatised at 0.2 °C, the plasma ir-sCP was 148 ± 16 pmol l−1 and after 10 days at 6 °C it was 244 ± 46 pmol l−1. The level increased to 471 ± 102 pmol l−1 after 10 days at 12 °C (P < 0.05 compared with 6 °C; P < 0.001 compared with 0.2 °C). The level was 525 ± 116 pmol l−1 (n = 4), when the acclimation at 12 °C was extended to 23 days. The circulating ir-sCP was observed to correlate with the acclimation temperature (r = 0.63, P < 0.001). To rule out the possibility that the secretion of sCP was increased at elevated temperature in intact animals, despite the results from the in vitro model, we measured the plasma levels of the biologically inert N-terminal fragment of pro-sCP (NT-pro-sCP). The N-terminal peptide is released from the ventricle in equimolar quantities with sCP. Surprisingly, there was no elevation of plasma NT-pro-sCP levels associated with the acclimation to the higher temperatures, as was expected from the results with sCP. If anything, there was a tendency for a slight decrease (from 1428 ± 148 pmol l−1 at 0.2 °C to 1298 ± 140 pmol l−1 at 6 °C and 1393 ± 179 pmol l−1 at 12 °C, Fig. 6A). In salmon kept for 23 days at 12 °C the plasma level of the N-terminal fragment was 1124 ± 339 pmol l−1.
Figure 6. The effect of acclimation to different temperatures on immunoreactive sCP in salmon plasma and ventricle and sCP mRNA in salmon ventricle.
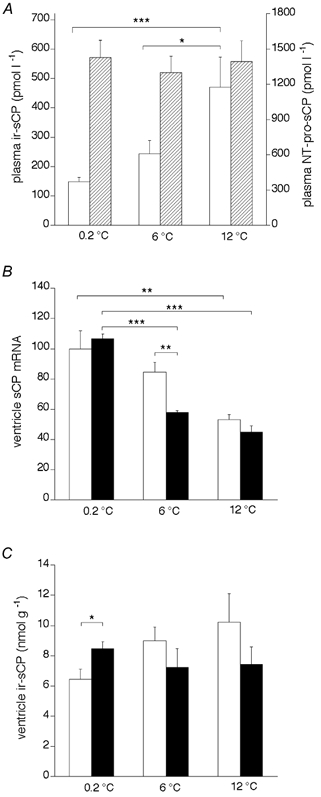
The ventricles were used for the perfusion experiments, as described in Fig. 5, after which the levels of sCP mRNA were determined by RNA blot analysis and those of ir-sCP by radioimmunoassay (n = 10, n = 6 and n = 8 for 0.2, 6 and 12 °C, respectively). A, plasma ir-sCP (open columns) and ir-NT-pro-sCP (hatched columns). B, sCP mRNA in the ventricle. C, ir-sCP in the ventricle. In B and C the open columns denote ventricles without mechanical load and filled columns those subjected to mechanical load of 13 cmH2O for 2 h. *P < 0.5, **P < 0.01 and ***P < 0.001.
In keeping with our findings on the seasonal variations of the ventricular sCP mRNA levels (Fig. 4), the sCP mRNA level in the ventricles used for the perfusion experiments was significantly lower when the tissue was obtained from salmon acclimated to 12 °C rather than from those acclimated to 6 °C or acclimatised to 0.2 °C (Fig. 6B). The difference was found to be associated with the temperature (r =−0.68, P < 0.005 in control experiments; r =−0.91, P < 0.001 in experiments with mechanical load). As we had observed previously (Kokkonen et al. 2000), loading tended to cause an initial decrease in the ventricular sCP mRNA level. However, the relatively short period of loading caused a significant decrease of sCP mRNA only in salmon acclimated to 6 °C (P < 0.01).
In contrast to the mRNA levels, but in accordance with our findings with the seasonal fluctuations in ventricular ir-sCP, the peptide levels were found to be higher in the ventricles obtained from warm-acclimated salmon than in those from the cold-acclimated salmon (10.2 ± 1.9 pmol l−1 (g ventricle tissue)−1 at 12 °C, n = 6; 8.9 ± 1.0 pmol l−1 (g ventricle tissue)−1 at 6 °C, n = 6; and 6.4 ± 0.7 pmol l−1 (g ventricle tissue)−1 at 0.2 °C, n = 4) (Fig. 6C). The level of immunoreactivity correlated significantly with temperature in non-loaded ventricles (r = 0.55, P < 0.05). Mechanical load decreased the ir-sCP concentration in the ventricles acclimated to 6 and 12 °C compared with the controls (from 9 ± 0.9 to 7.2 ± 1.2 pmol l−1 (g ventricle tissue)−1, and 10.2 ± 1.9 to 7.4 ± 1.1 pmol l−1 (g ventricle tissue)−1, respectively). In ventricles acclimatised to 0.2 °C the load was associated with significantly higher ir-sCP levels compared with non-loaded ventricles of the same acclimatisation temperature (6.4 ± 0.7 vs. 8.5 ± 0.5 pmol l−1 (g ventricle tissue)−1, P < 0.05). The acclimation to 6 or 12 °C was not associated with any significant changes in the relative ventricle mass (0.09 ± 0.01 at 0.2 °C, n = 9; 0.12 ± 0.01 at 6 °C, n = 7, and 0.1 ± 0.01 at 12 °C, n = 10).
DISCUSSION
Physiological functions are strongly influenced by temperature. Especially in ectothermic fishes, in which the thermal balance is predominated by external sources of heat, changes in ambient temperature challenge the cardiovascular system (reviewed in Driedzic & Gesser, 1994; Willmer et al. 2000). We have now studied the regulation of cardiac endocrine function using salmon exposed to extensive seasonal variation in ambient temperature. Our results would indicate that the plasma levels and production of a newly discovered salmon cardiac peptide hormone (sCP, Tervonen et al. 1998; Majalahti-Palviainen et al. 2000), belonging to the family of natriuretic peptides, display significant seasonal variation, with elevated activity associated with high ambient temperature. While the level of the expression of the sCP gene appears to be differently regulated in salmon acclimated to cold and warm, the major part of the upregulation of the plasma peptide levels can be explained by decreased elimination, rather than increased secretion, of the peptide at high ambient temperature.
We found that the plasma concentration of immunoreactive sCP is significantly higher in salmon adapted to warm ambient temperature. The possible contribution of the annual photoperiod to our present results is not known. It has been suggested to influence physiological processes in salmon, such as smoltification (Hoar, 1988). However, our observation that the elevation of sCP plasma levels associated with increasing water temperature can be detected both in animals studied under field conditions as well as in those acclimated in the laboratory argues strongly for a causal role of temperature in the regulation of sCP. An increased secretion of the peptide from the heart during the warm period of the year would be an obvious explanation for the increased plasma levels. In principle, however, a decreased rate of elimination during the period with increased ambient temperature could also result in elevation of plasma sCP. In mammals inhibition of the major routes of elimination of ANP, the type-C (or clearance) natriuretic peptide receptor or neutral endopeptidase (NEP), either pharmacologically (Kukkonen et al. 1992) or by gene inactivation (Matsukawa et al. 1999), have been shown to result in elevated basal plasma ANP levels and a diuretic response.
To differentiate between increased secretion and decreased elimination of the peptide as an explanation for the increased plasma concentration, we studied the effects of varying temperature within the physiological range on the release of immunoreactive sCP and concentration of plasma ir-sCP and NT-pro-sCP using homologous radioimmunoassays. We have previously shown that sCP is released by salmon ventricular myocytes via the regulated secretory pathway, and that the release is very sensitive to load (Tervonen et al. 1998; Kokkonen et al. 2000). Our present results show that the basal and load-induced release of immunoreactive sCP from perfused salmon ventricle is not augmented by increased temperature. In fact, there appears to be a slight decrease in the basal release and accelerated fading out of the response to constant loading with increased temperature. In mammalian ventricle (at +37 °C) the response to stretch has been found to level out even faster (Kinnunen et al. 1993). The apparent temperature dependence indicates that the levelling off is an active and regulated process, rather than a simple depletion of peptide stores, as has previously been suggested (Bloch et al. 1986; Kinnunen et al. 1992). It is unlikely that atrial secretion is responsible for temperature-associated increase of plasma sCP levels. We have found, using an in vitro perfused salmon atrium, that the basal and mechanical load-induced release of sCP is similar in the atrium to that observed in the ventricle (V. Tervonen & O. Vuolteenaho, unpublished results). Due to the fragility of the tissue, however, the atrial preparation is much more awkward to use than the ventricular preparation. Moreover, the seasonal profile of ir-sCP in the atrium is similar to that in the ventricle. Finally, the small size of salmon atrium argues against a major contribution of atrial release to circulating sCP.
Thus our results with the isolated perfused ventricle preparation were inconsistent with increased secretion as an explanation for the high plasma sCP levels in salmon during the warm months of the year. Therefore we wanted to test whether temperature-associated changes in the efficiency of elimination of sCP could explain the seasonal variation in plasma sCP. In mammals the biologically inert N-terminal fragments of proANP and proBNP can be used for the estimation of natriuretic peptide secretion (Sundsfjord et al. 1988; Hunt et al. 1995). They are produced and secreted in equimolar concentrations with the respective biologically active peptides originated from the same precursors, proANP or proBNP. The elimination of at least the N-terminal fragment of proANP, however, is much slower than that of ANP (Thibault et al. 1988). Specifically, it does not bind to the type-C (clearance) natriuretic peptide receptor nor is it degraded by neutral endopeptidase. We hypothesised that the measurement of the 97-amino-acid N-terminal fragment of pro-sCP could be used to assess the quantitative importance of the specific elimination pathways on the elevated levels of sCP in warm-adapted salmon, analogous to the use of the N-terminal fragment of ANP in mammalian experiments (Leskinen et al. 1997). So that this could be tested, we prepared a recombinant expression plasmid encoding the N-terminal fragment of pro-sCP and utilised the recombinant peptide to set up a specific radioimmunoassay. We were able to show that while warm-acclimation of salmon is associated with greatly elevated plasma sCP levels, the levels of the N-terminal fragment of pro-sCP remain stable. This indicates that the high plasma levels of sCP in salmon during the warm summer months result primarily from decreased rate of elimination, rather than increased secretion. While there are previous experimental data indicating that changes in elimination can affect the circulating natriuretic peptide levels (Kukkonen et al. 1992; Matsukawa et al. 1999), our present results are, to our knowledge, the first direct evidence that they are used for important physiological regulation.
In mammals, the cellular responses of ANP and BNP are mediated by type-A natriuretic peptide receptor (NPR-A) and of CNP by type-B natriuretic peptide receptor (NPR-B) (Garbers, 1990). The type-C natriuretic peptide receptor (NPR-C), by far the most abundant receptor type, binds all types of natriuretic peptides. The main role for NPR-C is believed to be the clearance of natriuretic peptides from the circulation (Maack, 1992; Cerra, 1994). Thus the clearance receptors may have an important role in regulating the half-life, and hence the bioavailability, of the natriuretic peptides as indicated by experimental evidence (Kukkonen et al. 1992; Matsukawa et al. 1999). In fish the distribution of natriuretic peptide receptors in the gills has received much attention, since the control of gill blood flow and epithelial function is critical for the osmotic and cardiovascular homeostasis. The gills of different fish species have been found to express both clearance type natriuretic peptide receptors as well as those mediating the physiological effects (Katafuchi et al. 1994; Kashiwagi et al. 1995, 1999; Takashima et al. 1995; Donald et al. 1997; Callahan et al. 2000). We have previously found that the plasma concentrations of sCP in salmon are much higher in the ventral aorta, receiving blood directly from the heart, than in the peripheral circulation, which receives its blood from the branchial circulation (Tervonen et al. 2000). These results, together with our present findings of diverging responses of the peripheral plasma levels of sCP and the N-terminal fragment of pro-sCP to changes in the ambient temperature, indicate that the expression of the clearance-type receptors is downregulated by acclimation to higher environmental temperatures. This would in turn cause an increased circulating half-life for natriuretic peptide secreted from the heart. In mammals, an important elimination pathway for circulating natriuretic peptides is proteolytic degradation catalysed by neutral endopeptidase (for review see Ruskoaho, 1992). Since we found an increased rather than decreased circulating level of sCP at warm temperatures a direct effect of temperature on the degradative enzyme(s) cannot explain our present results.
The levels of ventricular sCP mRNA and peptide appear to diverge completely during the winter, since the very high sCP mRNA levels are not associated with elevation of cardiac immunoreactive sCP levels. In accordance with these findings, increased ambient temperature was found to be associated with a decrease of sCP mRNA levels in the experiments with in vitro perfused salmon ventricles. The decrease was accentuated with concomitant mechanical loading of the perfused ventricle. The lack of concomitant decrease in the immunoreactive sCP levels could indicate that the scarcer sCP mRNA pool is translated more efficiently at higher temperatures. This is a surprising and unprecedented finding, although the stability of sCP mRNA could vary with temperature. The winter flounder antifreeze proteins (Duman & DeVries, 1974) demonstrate the potential importance of temperature for the stability of a specific mRNA. There is a remarkable seasonal variation of antifreeze protein mRNA level in winter flounder liver (Duncker et al. 1995), although temperature does not appear to regulate the transcription of the gene directly (Vaisius et al. 1989). Instead, the half-life of antifreeze protein mRNA is selectively decreased at elevated temperatures, resulting in low tissue and plasma antifreeze protein concentrations (Duncker et al. 1995). In the present study, however, possible changes in the mRNA stability cannot explain the divergent changes in ventricular sCP peptide levels, and especially those in the plasma levels of sCP, which appear not to have a connection with those of cardiac sCP mRNA. Our findings suggest that in cold-acclimatised and cold-acclimated salmon the production of pro-sCP is actively inhibited at the translational level, or that the half-life of pro-sCP in ventricular tissue is extraordinarily short. It is also possible that high circulating sCP levels exert a feedback inhibition on the transcription of sCP gene. Thus the high sCP mRNA levels in the winter could result from a release from the feedback inhibition. There is evidence, in a mammalian model, that ANP can inhibit its own secretion by an NPR-A mediated mechanism (Leskinen et al. 1997).
We found in the present study that the relative ventricle mass is increased in salmon acclimatised to cold. Ventricular hypertrophy has been observed in many teleosts as a response to cold-temperature acclimatisation, presumably as a compensation for the reduced cardiac contractility and heart rate associated with low temperatures (reviewed in Driedzic & Gesser, 1994). This helps the fish retain their locomotory activity at low ambient temperature. In mammals cardiac hypertrophy is associated with an induction of the A- and B-type natriuretic peptide genes (Nakao et al. 1992; Tamura et al. 1994; Pemberton et al. 1997). Thus our present observation of a concomitant increase in the relative ventricular mass and sCP mRNA levels during the winter could indicate that increased natriuretic peptide gene expression serves as a marker of cardiac hypertrophy in salmon. Our previous studies have shown that the promoter of the sCP gene is highly active in rat atrial myocytes, indicating that elements regulating atrial natriuretic peptide gene expression have been conserved over a considerable phylogenetic distance (Majalahti-Palviainen et al. 2000). Thus the same mechanisms could lead to hypertrophy-related upregulation of ANP and BNP in mammals and sCP in salmon. In view of markedly simpler in vitro and in vivo experimentation, salmon heart could therefore serve as a useful model to investigate general molecular mechanisms important for cardiac hypertrophy.
In addition to the effect of temperature, the importance of the stage of maturation was also examined. According to our present results parr-smolt transformation and maturation do not appear to be associated with major qualitative changes in the regulation of the sCP system. The cardiac and plasma levels of sCP showed similar seasonal profiles in parrs and adult salmon, but the extent of the changes varied between the different maturation stages. The seasonal fluctuations of the cardiac sCP levels had a higher amplitude in adult salmon than in parrs, but the increase in plasma sCP levels during acclimation to warm was in turn greater in parrs. This indicates that the parr- smolt transformation and sexual maturity could affect the sensitivity of sCP regulation.
In conclusion, the circulatory levels of sCP are markedly upregulated in salmon during the warm months of the year. While similar but considerably smaller changes can be detected in atrial and ventricular sCP levels, the cardiac sCP mRNA fluctuate in the opposite direction: high levels during the winter and lower during the summer. In experiments with salmon acclimated to different temperatures in the laboratory and in vitro perfused isolated ventricle preparations, we were able to show that the temperature-associated upregulation of plasma sCP levels results from decreased elimination rather than increased cardiac secretion. On the other hand, the increase in sCP mRNA levels coincides with low temperature-associated hypertrophy of the heart, indicating that sCP might serve as a marker of cardiac hypertrophy in salmon, analogous to ANP and BNP in mammals. Thus salmon offers an intriguing model for studying the general molecular and cellular pathways regulating the endocrine function of the heart, from transcription and translation to release and elimination of the hormones.
Acknowledgments
We gratefully acknowledge the staff of the Game and Fisheries Institute in Taivalkoski and the hatchery of Imatran Voima power plant in Muhos for providing the salmon used in this study. We wish to thank Tuula Taskinen, Seija Linnaluoto, Tuula Lumijärvi, and Alpo Vanhala for expert technical assistance, and Eero Kouvalainen, MSc (EE), for help in statistical analysis. This study was supported by grants from the Academy of Finland, Sigrid Jusélius Foundation, Finnish Cultural Foundation, and Research and Science Foundation of Farmos.
References
- Aho E, Vornanen M. Contractile properties of atrial and ventricular myocardium of the heart of rainbow trout Oncorhynchus mykiss: effects of thermal acclimation. Journal of Experimental Biology. 1999;202:2663–2677. doi: 10.1242/jeb.202.19.2663. [DOI] [PubMed] [Google Scholar]
- Audet C, Claireaux G. Diel and seasonal changes in resting levels of various blood parameters in blood parameters in brook trout (Salvelinus fontinalis) Canadian Journal of Fisheries and Aquatic Sciences. 1992;49:870–877. [Google Scholar]
- Bloch KD, Seidman JG, Naftilan JD, Fallon JT, Seidman CE. Neonatal atria and ventricles secrete atrial natriuretic factor via tissue-specific secretory pathways. Cell. 1986;47:695–702. doi: 10.1016/0092-8674(86)90512-x. [DOI] [PubMed] [Google Scholar]
- Callahan W, Forster M, Toop T. Evidence of a guanylyl cyclase natriuretic peptide receptor in the gills of the new zealand hagfish Eptatretus cirrhatus. Journal of Experimental Biology. 2000;203:2519–2528. doi: 10.1242/jeb.203.17.2519. [DOI] [PubMed] [Google Scholar]
- Cerra MC. Cardiac distribution of the binding sites for natriuretic peptides in vertebrates. Cardioscience. 1994;5:215–224. [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Donald JA, Toop T, Evans DH. Distribution and characterization of natriuretic peptide receptors in the gills of the spiny dogfish, Squalus acanthias. General and Comparative Endocrinology. 1997;106:338–347. doi: 10.1006/gcen.1997.6873. [DOI] [PubMed] [Google Scholar]
- Driedzic WM, Gesser H. Energy metabolism and contractility in ectodermic vertebrate hearts: Hypoxia, acidosis, and low temperature. Physiological Reviews. 1994;74:221–258. doi: 10.1152/physrev.1994.74.1.221. [DOI] [PubMed] [Google Scholar]
- Duman JG, DeVries AL. The effects of temperature and photoperoid on antifreeze production in cold water fishes. Journal of Experimental Zoology. 1974;190:89–98. doi: 10.1002/jez.1401900108. [DOI] [PubMed] [Google Scholar]
- Duncker BP, Koops MD, Walker VK, Davies PL. Low temperature persistence of type I antifreeze protein is mediated by cold-specific mRNA stability. FEBS Letters. 1995;377:185–188. doi: 10.1016/0014-5793(95)01340-7. [DOI] [PubMed] [Google Scholar]
- Egginton S, Sidell BD. Thermal acclimation induces adaptive changes in subcellular structure of fish skeletal muscle. American Journal of Physiology. 1989;256:R1–9. doi: 10.1152/ajpregu.1989.256.1.R1. [DOI] [PubMed] [Google Scholar]
- Garbers DL. The guanylyl cyclase receptor family. The New Biologist. 1990;2:499–504. [PubMed] [Google Scholar]
- Hoar WS. The physiology of smolting salmonids. In: Hoar WS, Randall DJ, editors. Fish Physiology. XIB. London: Academic Press Inc.; 1988. pp. 275–343. [Google Scholar]
- Hunt PJ, Yandle TG, Nicholls MG, Richards AM, Espiner EA. The amino-terminal portion of pro-brain natriuretic peptide (pro-BNP) circulates in human plasma. Biochemical and Biophysical Research Communications. 1995;214:1175–1183. doi: 10.1006/bbrc.1995.2410. [DOI] [PubMed] [Google Scholar]
- John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- Johnston IA, Fleming JD, Crockford T. Thermal acclimation and muscle contractile properties in cyprinid fish. American Journal of Physiology. 1990;259:R231–236. doi: 10.1152/ajpregu.1990.259.2.R231. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Katafuchi T, Kato A, Inuyama H, Ito T, Hagiwara H, Takei Y, Hirose S. Cloning and properties of a novel natriuretic peptide receptor, NPR-D. European Journal of Biochemistry. 1995;233:102–109. doi: 10.1111/j.1432-1033.1995.102_1.x. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Miyamoto K, Takei Y, Hirose S. Cloning, properties and tissue distribution of natriuretic peptide receptor-A of euryhaline eel, Anguilla japonica. European Journal of Biochemistry. 1999;259:204–211. doi: 10.1046/j.1432-1327.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Takashima A, Kashiwagi M, Hagiwara H, Takei Y, Hirose S. Cloning and expression of eel natriuretic-peptide receptor B and comparison with its mammalian counterparts. European Journal of Biochemistry. 1994;222:835–842. doi: 10.1111/j.1432-1033.1994.tb18930.x. [DOI] [PubMed] [Google Scholar]
- Keen JA, Farrell AP. Maximum prolonged swimming speed and maximum cardiac performance of rainbow trout, Onchorhynchus mykiss, acclimated to two different water temperatures. Comparative Biochemistry and Physiology. 1994;108A:287–295. [Google Scholar]
- Kinnunen P, Vuolteenaho O, Ruskoaho H. Passive mechanical stretch releases atrial natriuretic peptide from rat ventricular myocardium. Circulation Research. 1992;70:1244–1253. doi: 10.1161/01.res.70.6.1244. [DOI] [PubMed] [Google Scholar]
- Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: Effect of stretching. Endocrinology. 1993;132:1961–1970. doi: 10.1210/endo.132.5.8477647. [DOI] [PubMed] [Google Scholar]
- Kokkonen K, Vierimaa H, Bergström S, Tervonen V, Arjamaa O, Ruskoaho H, Järvilehto M, Vuolteenaho O. Novel cardiac peptide hormone is released by regulated secretory pathway. American Journal of Physiology. 2000;278:E285–292. doi: 10.1152/ajpendo.2000.278.2.E285. [DOI] [PubMed] [Google Scholar]
- Kukkonen P, Vuolteenaho O, Ruskoaho H. Basal and volume expansion-stimulated plasma atrial natriuretic peptide concentrations and haemodynamics in conscious rats: Effects of SCH 39. 370, an endopeptidase inhibitor, and C-ANF(4–23), a clearance receptor ligand. Endocrinology. 1992;130:755–765. doi: 10.1210/endo.130.2.1531129. [DOI] [PubMed] [Google Scholar]
- Leskinen H, Vuolteenaho O, Toth M, Ruskoaho H. Atrial natriuretic peptide (ANP) inhibits its own secretion via ANPA-receptors: Altered effect in experimental hypertension. Endocrinology. 1997;138:1893–1902. doi: 10.1210/endo.138.5.5120. [DOI] [PubMed] [Google Scholar]
- Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- Maack T. Receptors of atrial natriuretic factor. Annual Review of Physiology. 1992;54:11–27. doi: 10.1146/annurev.ph.54.030192.000303. [DOI] [PubMed] [Google Scholar]
- Majalahti-Palviainen T, Hirvinen M, Tervonen V, Ilves M, Ruskoaho H, Vuolteenaho O. Gene structure of a new cardiac peptide hormone: a model for heart-specific gene expression. Endocrinology. 2000;141:731–740. doi: 10.1210/endo.141.2.7312. [DOI] [PubMed] [Google Scholar]
- Matsukawa N, Grzesik WJ, Takahashi N, Pandey KN, Pang S, Yamauchi M, Smithies O. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proceedings of the National Academy of Sciences of the USA. 1999;96:7403–7408. doi: 10.1073/pnas.96.13.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K, Ogawa Y, Suga S-I, Imura H. Molecular biology and biochemistry of the natriuretic peptide system. I: Natriuretic peptides. Journal of Hypertension. 1992;10:907–912. [PubMed] [Google Scholar]
- Nikinmaa M, Soivio A, Railo E. Blood volume of Salmo gairdneri: influence of ambient temperature. Comparative Biochemistry and Physiology A. 1981;69:767–769. [Google Scholar]
- Nilssen KJ, Gulseth OA, Iversen M, Kjol R. Summer osmoregulatory capacity of the world's northernmost living salmonid. American Journal of Physiology. 1997;272:R743–749. doi: 10.1152/ajpregu.1997.272.3.R743. [DOI] [PubMed] [Google Scholar]
- Pemberton CJ, Yandle TG, Charles CJ, Rademaker MT, Aitken GD, Espiner EA. Ovine brain natriuretic peptide in cardiac tissues and plasma: effects of cardiac hypertrophy and heart failure on tissue concentration and molecular forms. Journal of Endocrinology. 1997;155:541–550. doi: 10.1677/joe.0.1550541. [DOI] [PubMed] [Google Scholar]
- Postlethwait J, Yan Y-L, Gates M, Horne S, Amores A, Brownlie A, Donovan A, Egan E, Force A, Gong Z, Goutel C, Fritz A, Kelsh R, Knapik E, Liao E, Paw B, Ransom D, Singer A, Thomson M, Abduljabbar T, Yelick P, Beier D, Joly J-S, Larhammar D, Rosa F, Westerfield M, Zon L, Johnson S, Talbot W. Vertebrate genome evolution and the zebrafish gene map. Nature Genetics. 1998;18:345–349. doi: 10.1038/ng0498-345. [DOI] [PubMed] [Google Scholar]
- Powers DA. Fish as model systems. Science. 1989;246:352–358. doi: 10.1126/science.2678474. [DOI] [PubMed] [Google Scholar]
- Reimers N. Body condition, water temperature, and over-winter survival of hatchery-reared trout in Convict Creek, California. Transactions of the American Fisheries Society. 1963;92:39–46. [Google Scholar]
- Ruskoaho H. Atrial natriuretic peptide: Synthesis, release, and metabolism. Pharmacological Reviews. 1992;44:479–601. [PubMed] [Google Scholar]
- Sundsfjord JA, Thibault G, Larochelle P, Cantin M. Identification and plasma concentrations of the N-terminal fragment of proatrial natriuretic factor in man. Journal of Clinical Endocrinology and Metabolism. 1988;66:605–610. doi: 10.1210/jcem-66-3-605. [DOI] [PubMed] [Google Scholar]
- Takashima A, Katafuchi T, Shibasaki M, Kashiwagi M, Hagiwara H, Takei Y, Hirose S. Cloning, properties, site-directed mutagenesis analysis of the subunit structure, tissue distribution and regulation of expression of the type-C eel natriuretic peptide receptor. European Journal of Biochemistry. 1995;227:673–680. doi: 10.1111/j.1432-1033.1995.tb20187.x. [DOI] [PubMed] [Google Scholar]
- Tamura N, Ogawa Y, Itoh H, Arai H, Suga S, Nakagawa O, Komatsu Y, Kishimoto I, Takaya K, Yoshimasa T, Shiono S, Nakao K. Molecular cloning of hamster brain and atrial natriuretic peptide cDNAs. Cardiomyopathic hamsters are useful models for brain and atrial natriuretic peptides. Journal of Clinical Investigation. 1994;94:1059–1068. doi: 10.1172/JCI117420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Eggington S, Taylor EW. Seasonal temperature acclimatisation of rainbow trout: cardiovascular and morphometric influences on maximal sustainable exercise level. Journal of Experimental Biology. 1996;199:835–845. doi: 10.1242/jeb.199.4.835. [DOI] [PubMed] [Google Scholar]
- Tervonen V, Arjamaa O, Kokkonen K, Ruskoaho H, Vuolteenaho O. A novel cardiac peptide hormone related to A-, B- and C-type natriuretic peptides. Endocrinology. 1998;139:4021–4025. doi: 10.1210/endo.139.9.6292. [DOI] [PubMed] [Google Scholar]
- Tervonen V, Ruskoaho H, Vuolteenaho O. Novel cardiac peptide hormone in several teleosts. Journal of Endocrinology. 2000;166:407–418. doi: 10.1677/joe.0.1660407. [DOI] [PubMed] [Google Scholar]
- Thibault G, Amiri F, Garcia R. Regulation of natriuretic peptide secretion by the heart. Annual Review of Physiology. 1999;61:193–217. doi: 10.1146/annurev.physiol.61.1.193. [DOI] [PubMed] [Google Scholar]
- Thibault G, Murthy KK, Gutkowska J, Seidah NG, Lazure C, Chretien M, Cantin M. NH2-terminal fragment of rat proatrial natriuretic factor in the circulation: identification, radioimmunoassay and half-life. Peptides. 1988;9:47–53. doi: 10.1016/0196-9781(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Vaisius A, Martin-Kearley J, Fletcher GL. Antifreeze protein gene transcription in winter flounder is not responsive to temperature. Cellular and Molecular Biology. 1989;35:547–554. [PubMed] [Google Scholar]
- Willmer P, Stone G, Johnston I. Environmental Physiology of Animals. London: Blackwell Science; 2000. [Google Scholar]
- Wittbrot J, Meyer A, Schartl M. More genes in fish? Bioessays. 1998;20:511–515. [Google Scholar]


