Abstract
We investigated the effect of acidosis on the sarcomere length (SL) dependence of tension generation, in comparison with the effect of inorganic phosphate (Pi), in rat skinned ventricular trabeculae. The shift of the mid-point of the pCa-tension relationship associated with an increase in SL from 1.9 to 2.3 μm (ΔpCa50) was studied.
Decreasing pH from 7.0 to 6.2 lowered maximal and submaximal Ca2+-activated tension and increased ΔpCa50 in a pH-dependent manner (from 0.21 ± 0.01 to 0.30 ± 0.01 pCa units). The addition of Pi (20 mm) decreased maximal tension and enhanced the SL dependence, both to a similar degree as observed when decreasing pH to 6.2 (ΔpCa50 increased from 0.20 ± 0.01 to 0.29 ± 0.01 pCa units).
Further experiments were performed using 6 % (w/v) Dextran T-500 (molecular weight ∼500 000) to osmotically reduce interfilament lattice spacing (SL, 1.9 μm). Compared with that at pH 7.0, in the absence of Pi the increase in the Ca2+ sensitivity of tension induced by osmotic compression was enhanced at pH 6.2 (0.18 ± 0.01 vs. 0.25 ± 0.01 pCa units) or in the presence of 20 mm Pi (0.17 ± 0.01 vs. 0.24 ± 0.01 pCa units).
H+, as well as Pi, has been reported to decrease the number of strongly binding cross-bridges, which reduces the co-operative activation of the thin filament and increases the pool of detached cross-bridges available for interaction with actin. It is therefore considered that during acidosis, the degree of increase in the number of force-generating cross-bridges upon reduction of interfilament lattice spacing is enhanced, resulting in greater SL dependence of tension generation.
Our results suggest that the Frank-Starling mechanism may be enhanced when tension development is suppressed due to increased H+ and/or Pi under conditions of myocardial ischaemia or hypoxia.
It is well known that twitch tension in myocardium is enhanced as muscle length (i.e. sarcomere length, SL) is increased within the physiological range (SL from ∼1.8 to ∼2.3 μm), and is accompanied by an increase in the Ca2+ sensitivity of tension (Allen & Kurihara, 1982; Allen & Kentish, 1985; Lakatta, 1991). This, in part, is the basis for the Frank-Starling law of the heart. At the myofilament level, there is an increasing amount of evidence suggesting that length dependence is primarily caused by a change in interfilament lattice spacing (Harrison et al. 1988; McDonald & Moss, 1995; Fuchs & Wang, 1996; Fitzsimons & Moss, 1998; Fukuda et al. 2000). A possible consequence of decreased lattice spacing is an increase in the probability of myosin attaching to the thin filament, resulting in an increase in the number of force-generating cross-bridges (Ishiwata & Oosawa, 1974; McDonald & Moss, 1995; Fukuda et al. 2000).
An alternative hypothesis suggested that SL-dependent activation of the myofilament was caused by cardiac troponin C (TnC), which possibily acts as a ‘length sensor’ (Babu et al. 1988; Gulati et al. 1990). However, McDonald et al. (1995) demonstrated that the expression of skeletal TnC in ventricular myocytes of transgenic mice did not alter the SL dependence of the Ca2+ sensitivity of tension in skinned myocytes. Hence making it unlikely that cardiac TnC acts as a length sensor (Moss et al. 1991; McDonald et al. 1995).
It is well known that contractile dysfunction is one of the earliest consequences of myocardial ischaemia/hypoxia, during which intracellular H+ and inorganic phosphate (Pi) are markedly increased (for review see Allen & Orchard, 1987). The effects of acidosis on the contractile functions of cardiac muscle have been extensively studied (for review see Orchard & Kentish, 1990). Acidosis decreases both maximal Ca2+-activated tension and the Ca2+ sensitivity of tension (Godt & Nosek, 1989; Orchard & Kentish, 1990; Komukai et al. 1998; Fujita & Ishiwata, 1999; Fukuda & Ishiwata, 1999). Acidosis also decreases maximum shortening velocity and lowers maximum power output (Ricciardi et al. 1994). In intact ventricular preparations, it has been reported that a reduction in extracellular pH results in a change in the length-tension relationship which is similar to that seen with a decrease in the extracellular Ca2+ concentration (Ricciardi et al. 1986; Orchard et al. 1991), exerting little influence on the slope of the length-tension relationship when normalized to peak tension (Orchard et al. 1991). In those studies, however, twitch contraction was used to examine the effects of acidosis at limited extracellular Ca2+ concentrations, and the degree of presumed pH reduction inside the cell was limited to within a narrow range. Therefore, a more systematic study is needed to clarify the effect of acidosis on the SL dependence of tension generation using skinned muscle preparations.
In the present report, we studied the SL-dependent shift of the pCa-tension relationship using skinned ventricular trabeculae over a large pH range to investigate the effects of acidosis on the SL dependence of tension generation. We compared the effect of acidosis with that of inorganic phosphate (Pi), which acts on the actin-myosin interaction, but not directly on TnC, resulting in a decrease in the number of attached cross-bridges (Hibberd et al. 1985; Kentish, 1986, 1991; Kawai et al. 1988; Fukuda et al. 1998). Our results demonstrate that acidosis, as well as Pi, enhances the SL dependence of tension generation.
METHODS
Experimental procedure
Male Wistar rats (250–300 g) were anaesthetized with sodium pentobarbitone (50 mg kg−1i.p.) and the hearts were removed. The rats were supplied by Saitama Experimental Animals Supply (Saitama, Japan), and the present study conforms with the Guiding Principles for the Care and Use of Animals approved by the Council of the Physiological Society of Japan. Thin trabecula muscles (diameter 100–150 μm) were dissected from the right ventricle in oxygenated Tyrode solution without Ca2+ at 30 °C (135 mm Na+, 5 mm K+, 1 mm Mg2+, 98 mm Cl−, 20 mm HCO3−, 1 mm HPO42-, 1 mm SO42-, 20 mm acetate, 10 mm glucose, 5 i.u. l−1 insulin, pH 7.35 when equilibrated with 5 % CO2-95 % O2) (Komukai et al. 1998; Fukuda et al. 2000). The preparations were skinned by superfusion with 1 % (v/v) Triton X-100 in relaxing solution (composition (mm): MgATP 4, Mops 10, EGTA 10, free Mg2+ 1, ionic strength 180 (pH 7.0)) for 60 min at about 2 °C. The ionic strength (IS) was adjusted with KCl. The preparations were stored at −20 °C in relaxing solution containing 50 % (v/v) glycerol and 2 mm leupeptin for 1 week or less. Both ends of the preparation were tied to thin tungsten wires with silk threads (Fukuda et al. 2000). One end was attached to a tension transducer (BG-10; Kulite Semiconductor Products, Inc., Leonia, NJ, USA) and the other to a micromanipulator (Narishige, Tokyo). The SL was adjusted to either 1.9 or 2.3 μm by measuring laser light diffraction in the relaxing solution. Isometric tension was measured in solutions containing 4 mm MgATP, 10 mm Mops (or 10 mm Mes for pH 6.2), 1 mm free Mg2+, various concentrations of free Ca2+ (adjusted with CaCl2), 10 mm EGTA, 0.1 mm P1,P5-di(adenosine-5′)pentaphosphate (AP5A), 15 mm creatine phosphate (CP), 15 IU ml−1 creatine phosphokinase (CPK) and 180 mm IS (adjusted with KCl). The pH of each solution was finally adjusted to within error of 0.02 of the desired pH.
The control pCa-tension relationship (at pH 7.0 without Pi) was first obtained at SL 1.9 μm and then at 2.3 μm. Subsequently, the pCa-tension relationships at a lower pH (6.8, 6.6 or 6.2) or in the presence of 20 mm Pi (pH 7.0) were obtained at the two SLs using the same preparation. In another series of experiments, the effect of acidosis or Pi on maximal Ca2+-activated tension was measured at SLs of 1.9 and 2.3 μm according to a previously described procedure (Fukuda et al. 1998; Fukuda & Ishiwata, 1999). At the end of the experiments, maximal Ca2+-activated tension was measured at the two SLs under control conditions (pH 7.0, no Pi). We only used data in which the final tension values were greater than 90 % of those measured at the beginning of the experiment.
The muscle width was measured under a microscope (SMZ645, Nikon, Tokyo) at a magnification of × 225. The concentrations of ionic species in solutions were estimated by computer calculation (Horiuti, 1986). All experiments were carried out at 20 ± 0.2 °C.
Data and statistical analyses
The pCa-tension relationship was fitted to the Hill equation: log(P/(100 −P)) =nH (pCa50− pCa), where P is the relative tension expressed as a percentage of the maximum, nH is the Hill coefficient and pCa50 is -log[Ca2+] at P = 50. All data are expressed as means ±s.e.m. Student's paired t test was used and significance verified at P < 0.05.
RESULTS
Effect of acidosis on the length dependence of the Ca2+ sensitivity of tension
The effect of acidosis on the SL dependence of the Ca2+ sensitivity of tension in the absence of Pi is shown in Fig. 1. The pH value was decreased from 7.0 to 6.8 (A), 6.6 (B) or 6.2 (C). At pH 7.0, maximal Ca2+-activated tension values were 50.8 ± 4.0 and 83.1 ± 6.7 mg (P < 0.001, n = 21) at SL 1.9 and 2.3 μm, respectively, and the SL-dependent shift of pCa50 (ΔpCa50) was ∼0.2 pCa units (see Fukuda et al. 2000). The muscle width was decreased from 120.4 ± 7.2 to 107.1 ± 6.0 μm (P < 0.001, n = 5) when SL was increased from 1.9 to 2.3 μm during relaxation (i.e. ∼11 % decrease in the width). Acidosis did not change the muscle width (119.4 ± 7.3 and 105.8 ± 6.3 μm (P < 0.001, n = 5) at SL 1.9 and 2.3 μm, respectively, at pH 6.2). Consistent with previous studies using skinned cardiac muscle preparations (Godt & Nosek, 1989; Orchard & Kentish, 1990; Fujita & Ishiwata, 1999; Fukuda & Ishiwata, 1999), acidosis shifted the pCa-tension relationship to the right (Fig. 1). It was also found that the SL-dependent shift of the pCa-tension relationship was enhanced with acidosis in a pH-dependent manner (P < 0.05 and P < 0.01 for pH 6.6 and 6.2, respectively). At pH 6.8, ΔpCa50 was not significantly different from that at pH 7.0 (Fig. 1A, inset); however, it increased from 0.21 ± 0.01 to 0.30 ± 0.01 pCa units at pH 6.2 (Fig. 1C, inset).
Figure 1. Effects of acidosis on pCa-tension relationships at different SLs.

Solvent conditions: 4 mm MgATP, 10 mm Mops (or 10 mm Mes for pH 6.2), 1 mm free Mg2+, various concentrations of free Ca2+ (pCa adjusted using Ca-EGTA), 0.1 mm AP5A, 15 mm CP, 15 IU ml−1 CPK and IS maintained at 180 ± 1 mm. Temperature, 20 ± 0.2 °C. A–C show the effect of lowering pH from 7.0 to 6.8, 6.6 and 6.2, respectively, on the pCa-tension relationships at SLs of 1.9 μm (circles) and 2.3 μm (squares). Note that different preparations were used in A, B and C. Continuous lines and open symbols, pCa-tension relationships at pH 7.0; dotted lines and filled symbols, those at a lower pH. Data obtained for each preparation were fitted to the Hill equation, and the results were simulated by the Hill equation with the mean values of pCa50 and nH. Each inset represents ΔpCa50 (difference between the values of pCa50 at long and short SLs) at pH 7.0 and at a lower pH. pCa-tension relationships were normalized to the maximal tension at pCa 4.8 for pH 7.0 and at 4.4, 4.1 and 3.8, respectively, for pH 6.8, 6.6 and 6.2. Error bars, s.e.m. of 5–6 data points. N.S., not significant compared with pH 7.0.
Table 1 summarizes the nH values of the pCa-tension relationships of Fig. 1. Decreasing pH to 6.2 significantly increased nH at SL 2.3 μm. However, there was no definitive correlation between the magnitude of nH at either SL and that of ΔpCa50 (i.e. at pH 6.6, ΔpCa50 was significantly increased with no significant change in nH at either SL, Fig. 1B and Table 1) (see also Fukuda et al. 2000). The nH values obtained here are relatively high in comparison with those reported in previous studies using rat skinned ventricular muscle (e.g. Kentish, 1986; Harrison et al. 1988; McDonald & Moss, 1995). One reason for this may lie in the different experimental conditions. It is also possible that the high nH values came about because of our measurement protocol for the pCa-tension relationship. In accordance with our previous report (Fukuda et al. 2000), we measured the pCa-tension relationship by cumulatively raising the free Ca2+ concentration from the relaxing condition. It is thus possible that the thin filament is readily activated upon solution change, because of pre-activation at the preceding lower Ca2+. This may result in enhanced co-operative activation of the thin filament (i.e. high nH).
Table 1.
Change in the Hill coefficient with acidosis
| Fig. 1A | Fig. 1B | Fig. 1C | ||||
|---|---|---|---|---|---|---|
| SL | pH 7.0 | pH 6.8 | pH 7.0 | pH 6.6 | pH 7.0 | pH 6.2 |
| 1.9 μm | 5.81 ± 0.21 | 5.79 ± 0.28 | 5.49 ± 0.28 | 4.86 ± 0.30 | 5.48 ± 0.38 | 6.02 ± 0.52 |
| 2.3 μm | 4.18 ± 0.29 | 4.38 ± 0.31 | 4.70 ± 0.19 | 4.80 ± 0.18 | 4.59 ± 0.21 | 5.70 ± 0.43 * |
Values were obtained from Fig. 1A–C (mean ±s.e.m.; n = 5–6)
P < 0.05 compared with the corresponding value of pH 7.0.
Effect of Pi on the length dependence of the Ca2+ sensitivity of tension
Figure 2 shows the effect of Pi (20 mm) on the SL dependence of the Ca2+ sensitivity of tension at pH 7.0. Consistent with previous studies using skinned cardiac muscle (Godt & Nosek, 1989; Kentish, 1986; Fukuda et al. 1998), Pi shifted the pCa-tension relationship to the right. It was found that ΔpCa50 was significantly increased in the presence of Pi (0.20 ± 0.01 vs. 0.29 ± 0.01 pCa units), and the magnitude of increase was comparable to that obtained by lowering pH from 7.0 to 6.2 (see Fig. 1C), although the rightward shift of the pCa curve was smaller. The nH values in the absence and presence of Pi were 6.26 ± 0.24 and 6.94 ± 0.36 (P > 0.05, n = 5), respectively, at SL 1.9 μm, and 5.08 ± 0.33 and 6.40 ± 0.43 (P > 0.05, n = 5), respectively, at SL 2.3 μm. Consistent with the work of Kentish (1986) using rat ventricular muscles, nH did not change significantly with Pi.
Figure 2. Effects of Pi on pCa-tension relationships at different SLs.
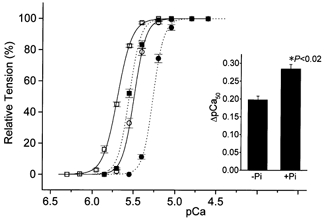
Solvent conditions: the same as in Fig. 1 except that pH was maintained at 7.0 and 20 mm Pi was added, maintaining IS at 180 mm. Continuous lines and open symbols, pCa-tension relationships in the absence of Pi; dotted lines and filled symbols, those in the presence of Pi. Circles, SL 1.9 μm; squares, SL 2.3 μm. Inset represents ΔpCa50 in the absence (-Pi) and presence (+Pi) of Pi. pCa-tension relationships were normalized to the maximal tension at pCa 4.8 in the absence of Pi and at 4.6 in the presence of Pi. Error bars, s.e.m. of 5 data points.
Effects of acidosis and Pi on tension induced by osmotic compression
Dextran T-500 (6 % w/v) reduced the muscle width from 129.3 ± 8.1 to 111.6 ± 6.8 μm (P < 0.001, n = 10) under relaxing conditions at SL 1.9 μm. The degree of reduction (∼13.5 %) was similar to that observed when increasing SL to 2.3 μm without Dextran T-500 (∼11 %). Osmotic compression shifted the pCa-tension relationship leftward (0.17–0.18 pCa units), similar to that observed when increasing SL to 2.3 μm (∼0.2 pCa units) (Fukuda et al. 2000). Maximal tension was also increased by about 15 % with compression, but this change was smaller than that obtained by lengthening (McDonald & Moss, 1995; Fukuda et al. 2000); in contrast, increasing SL from 1.9 to 2.3 μm in the absence of Dextran T-500 increased maximal tension by about 60 % (see above). It was found that the Dextran-induced increase in the apparent Ca2+ sensitivity was significantly increased at pH 6.2 (0.18 ± 0.01 vs. 0.25 ± 0.01 pCa units) or in the presence of 20 mm Pi (0.17 ± 0.01 vs. 0.24 ± 0.01 pCa units) (Fig. 3). The nH values at pH 7.0 and 6.2 were 6.12 ± 0.36 and 6.36 ± 0.40 (P > 0.1, n = 5), respectively, without Dextran T-500 and 5.63 ± 0.26 and 6.08 ± 0.54 (P > 0.1, n = 5), respectively, with Dextran T-500. Those in the absence and presence of Pi were 6.18 ± 0.54 and 6.43 ± 0.39 (P > 0.1, n = 5), respectively, without Dextran T-500 and 6.18 ± 0.38 and 7.08 ± 0.80 (P > 0.1, n = 5), respectively, with Dextran T-500.
Figure 3. Effects of acidosis or Pi on pCa-tension relationships in the absence and presence of Dextran T-500.

Solvent conditions: the same as in Figs 1C and 2 for acidosis and Pi, respectively, except that 6 % (w/v) Dextran T-500 (molecular weight ∼500 000, Amersham Pharmacia Biotech AB, Sweden) was used instead of increasing SL to 2.3 μm. Circles, in the absence of Dextran T-500; squares, in the presence of Dextran T-500. SL was fixed at 1.9 μm. A, effect of acidosis (pH 6.2). Pi was absent. Continuous lines and open symbols, pCa-tension relationships at pH 7.0; dotted lines and filled symbols, those at pH 6.2. Inset represents ΔpCa50 (difference between the values of pCa50 with and without Dextran T-500) at pH 7.0 and 6.2. B, effect of Pi (20 mm). pH was held at 7.0. Continuous lines and open symbols, pCa-tension relationships in the absence of Pi; dotted lines and filled symbols, those in the presence of Pi. Inset represents ΔpCa50 in the absence (-Pi) and presence (+Pi) of Pi. pCa-tension relationships in A and B were normalized to the maximal tension at the values of pCa shown in Figs 1C and 2, respectively. Error bars, s.e.m. of 5 data points.
Effects of acidosis and Pi on maximal Ca2+-activated tension
Finally, we studied maximal Ca2+-activated tension as functions of pH and Pi at SLs of 1.9 and 2.3 μm. As reported previously in skinned skeletal and cardiac muscle preparations (Godt & Nosek, 1989; Kentish, 1991; Fujita & Ishiwata, 1999; Fukuda & Ishiwata, 1999), maximal tension decreased almost linearly with the reduction of pH (Fig. 4A). We found that the inhibitory effect was SL dependent, and that the effect was weaker at the longer SL. The inhibitory effect of Pi on maximal tension was also SL dependent; the effect was less at the longer SL (Fig. 4B).
Figure 4. Effects of acidosis or Pi on maximal Ca2+-activated tension at different SLs.

Conditions: the same as in Figs 1 and 2 for acidosis and Pi, respectively. Circles, SL 1.9 μm; squares, SL 2.3 μm. A, effect of acidosis in the absence of Pi. The values of pCa at pH 7.0, 6.8, 6.6 and 6.2 for obtaining maximal Ca2+-activated tension were set based on Fig. 1 (i.e. 4.8, 4.4, 4.1 and 3.8 for pH 7.0, 6.8, 6.6 and 6.2, respectively) and estimated to be 4.0 for pH 6.4 by drawing the pCa curve. Tension was normalized with respect to pH 7.0. B, effect of Pi. pH was held at 7.0. Tension was normalized with respect to 0 mm Pi. The value of pCa was held at 4.8 in all conditions, because there was little difference between the tension at pCa 4.8 and that at pCa < 4.8 in the presence of 30 mm Pi. Error bars, s.e.m. of 3–4 data points. *P < 0.05 compared with the corresponding values for SL 1.9 μm.
DISCUSSION
We found that in rat skinned ventricular trabeculae, both acidosis and Pi exhibited a similar inhibitory effect on maximal Ca2+-activated tension and enhanced the magnitude of SL-dependent changes of the pCa-tension relationship. We discuss these results of the effects of interfilament lattice spacing, acidosis and Pi in relation to the formation of force-generating cross-bridges.
First, an increase in SL results in a decrease in the lateral separation between the thick and thin filaments both in living (Matsubara & Millman, 1974) and skinned (Irving et al. 2000) myocardium. A recent X-ray study using rat skinned ventricular trabeculae confirmed that interfilament lattice spacing (d10) decreases with an increase in SL, maintaining a nearly constant lattice volume over the physiological range of SL (i.e. from ∼1.9 to ∼2.4 μm) (Irving et al. 2000). Consistent with our previous study (Fukuda et al. 2000), an increase in SL from 1.9 to 2.3 μm produced a similar muscle width reduction as osmotic compression using 6 % (w/v) Dextran T-500, and both procedures increased the Ca2+ sensitivity of isometric tension to a similar degree (Figs 1–3). Thus, we consider that the change in the lattice spacing is the primary cause of the modulation of the SL-dependent Ca2+ sensitivity of tension in the cardiac myofilament (McDonald & Moss, 1995; Fuchs & Wang, 1996; Fukuda et al. 2000).
A decrease in pH towards the isoelectric point of the myofilaments decreases the charge distribution on the filaments, causing a reduction of electrostatic repulsion force between filaments, which in turn causes interfilament lattice spacing to shrink (Millman, 1998). In the present study, a decrease in pH from 7.0 to 6.2 did not significantly alter the muscle width at a SL of 1.9 or 2.3 μm. It is thus reasonable to consider that interfilament lattice spacing is not directly influenced by the change in pH in the range examined here, presumably because the isoelectric point is far below 6.2 (i.e. < 5.0; see Kawai et al. 1988; Millman, 1998).
The inhibitory effect of acidosis on Ca2+-activated tension is in part attributable to a decrease in the number of force-generating cross-bridges (Metzger & Moss, 1988, 1990; Kentish, 1991; Fujita & Ishiwata, 1999; Fukuda & Ishiwata, 1999). Kentish (1991) postulated that H+ is released during the transition between the non-force-generating states preceding the Pi release (force-generating) step. An increased concentration of H+ will thus reverse the H+ release step and decrease the number of force-generating cross-bridges (Kentish, 1991). Pi is believed to reverse the Pi release step in the cross-bridge cycle by shifting the distribution of cross-bridges toward a state with a full complement of bound products (ADP and Pi) (i.e. non-force-generating state) (Hibberd et al. 1985; Kentish, 1986, 1991; Kawai et al. 1988). It is thus realized that both acidosis and Pi reduce the co-operative activation of the thin filament by decreasing the number of strongly binding cross-bridges. In the present study, lowering pH to 6.2 and the addition of 20 mm Pi reduced maximal tension and enhanced the SL-dependent increase in the Ca2+ sensitivity of tension to a similar degree (Figs 1C, 2 and 4). However, the rightward shift of the pCa50-tension curve was substantially larger for acidosis (Figs 1C and 2), which is reasonable because Pi decreases the number of force-generating cross-bridges with no direct effect on TnC, whereas acidosis reduces the affinity of TnC for Ca2+ in addition to its inhibitory effect on cross-bridges (Orchard & Kentish, 1990). It is therefore suggested that a change in the cross-bridge distribution, shown by a decrease in maximal tension, but not a reduced affinity of TnC for Ca2+, underlies the mechanisms of enhanced SL-dependent tension generation by acidosis (see Fukuda et al. 2000). Once the number of strongly binding cross-bridges is decreased by either H+ or Pi, co-operative activation of the thin filament will be reduced, resulting in an increase of detached cross-bridges that are available for interaction with actin. Consequently, the fraction of cross-bridges recruited to the force-generating state on lengthening (i.e. lattice shrinkage) will be increased, resulting in a greater SL dependence of isometric tension (Ishiwata & Oosawa, 1974). This interpretation is supported by the fact that both acidosis and Pi enhance the increase in the Ca2+ sensitivity of tension due to osmotic compression to a degree similar to that observed in the study of varying SL (Fig. 3). Also, this interpretation is consistent with the recent finding that SL-dependent tension generation is attenuated by an enhanced co-operative activation of the thin filament by strongly binding cross-bridges produced by the addition of N-ethylmaleimide-modified myosin subfragment 1 (Fitzsimons & Moss, 1998) or ADP (Fukuda et al. 2000).
We observed SL dependence in the inhibitory effects of acidosis and Pi (Fig. 4). Assuming that the formation of the force-generating cross-bridges is accelerated with an increase in SL, it is expected that the inhibitory effect of H+ or Pi becomes less pronounced at a longer SL. Since maximal tension was dramatically increased by lengthening compared with osmotic compression (i.e. ∼60 %vs.∼15 %) (see also McDonald & Moss, 1995; Fukuda et al. 2000), the reduction of interfilament lattice spacing may not fully account for the increase in maximal tension due to an increase in SL. There may be additional mechanisms modulating the SL-dependent activation in the cardiac myofilament, such as extension of titin/connectin (Fukuda et al. 2001), in addition to the reduction of the lattice spacing, and therefore the reduction of the lattice spacing alone may not be sufficient to produce force-generating cross-bridges. Alternatively, it is possible that osmotic compression and lengthening may not be precisely equivalent such that changes in lattice packing may differ in the two cases; the shape of the muscle may be altered differently by mechanical stretch compared with osmotic compression (McDonald & Moss, 1995; Fukuda et al. 2000).
Alterations of SL-dependent shift of the pCa-tension relationship are predicted to affect the relation between muscle length and tension production (Fuchs, 1995). The present finding that acidosis enhances the SL dependence of the Ca2+ sensitivity of tension (Fig. 1) leads us to conclude that as pH falls, the slope of the length-tension relationship at physiologically submaximal levels of Ca2+ is increased. This is in disagreement with an earlier finding that acidosis does not essentially change the slope of the length-tension relationship in intact myocardium (Ricciardi et al. 1986; Orchard et al. 1991). The difference in the effect of acidosis on length-dependent tension generation may lie in the buffering capacity of pH by the cell membrane in intact preparations (Allen & Orchard, 1987; Ricciardi et al. 1994), resulting in values of intracellular pH between 6.6 and 6.8 in those studies (Ricciardi et al. 1986; Orchard et al. 1991), which are higher than that used in the present study (6.2). Other differences include ionic conditions and experimental protocols.
The manifestation of a change in the pCa-tension relationship in the ventricular volume-end-systolic pressure (ESP) relation of the intact ventricle is complex. However, a simplified prediction of how a change in SL-dependent activation may affect the slope of the volume-ESP relation can be made (Arteaga et al. 2000). Arteaga et al. (2000) reported that the slope of the volume-ESP relation may depend on the degree of activation of the myofilament on SL. We have reported that the volume-ESP relation may be impaired during the late phase of myocardial ischaemia/hypoxia (Fukuda et al. 2000), in which the ratio of the intracellular concentration of ADP to that of ATP is dramatically increased and subsequently augments tension development (Kammermeier et al. 1982; Allen & Orchard, 1987; Ventura-Clapier & Veksler, 1994; Fukuda et al. 1998). In contrast, the present results indicate that during the early phase of myocardial ischaemia/hypoxia, in which accumulation of ADP is minimal and tension development is suppressed by the increased H+ and/or Pi (Allen & Orchard, 1987; Ventura-Clapier & Veksler, 1994), the SL-dependent activation is enhanced. It can therefore be predicted that in the beating heart, the slope of the volume-ESP relation may be increased during the initial phase of myocardial ischaemia/hypoxia, thereby enhancing the Frank-Starling mechanism.
Acknowledgments
We would like to thank Drs David Allen, Masataka Kawai, and Henk Granzier for critical reading of the manuscript, and Naoko Tomizawa for technical assistance. This work was supported in part by Grants-in-Aid for Scientific Research, for Scientific Research on Priority Areas, and for High-Tech Research Center Project from the Ministry of Education, Science, Sports and Culture of Japan, from Japan Heart Foundation, and from the Vehicle Racing Commemorative Foundation.
References
- Allen DG, Kentish JC. The cellular basis for length-tension relation in cardiac muscle. Journal of Molecular and Cellular Cardiology. 1985;17:821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Allen DG, Kurihara S. The effect of muscle length on intracellular calcium transients in mammalian cardiac muscle. Journal of Physiology. 1982;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Orchard CH. Myocardial contractile function during ischemia and hypoxia. Circulation Research. 1987;60:153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Arteaga GM, Palmiter KA, Leiden JM, Solaro RJ. Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I. Journal of Physiology. 2000;526:541–549. doi: 10.1111/j.1469-7793.2000.t01-1-00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu A, Sonnenblick E, Gulati J. Molecular basis for the influence of muscle length on myocardial performance. Science. 1988;240:74–76. doi: 10.1126/science.3353709. [DOI] [PubMed] [Google Scholar]
- Fitzsimons DP, Moss RL. Strong binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes. Circulation Research. 1998;83:602–607. doi: 10.1161/01.res.83.6.602. [DOI] [PubMed] [Google Scholar]
- Fuchs F. Mechanical modulation of the Ca2+ regulatory protein complex in cardiac muscle. News in Physiological Sciences. 1995;10:6–12. [Google Scholar]
- Fuchs F, Wang YP. Sarcomere length versus interfilament spacing as determinants of cardiac myofilament Ca2+ sensitivity and Ca2+ binding. Journal of Molecular and Cellular Cardiology. 1996;28:1375–1383. doi: 10.1006/jmcc.1996.0129. [DOI] [PubMed] [Google Scholar]
- Fujita H, Ishiwata S. Tropomyosin modulates pH dependence of isometric tension. Biophysical Journal. 1999;77:1540–1546. doi: 10.1016/S0006-3495(99)77001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda N, Fujita H, Fujita T, Ishiwata S. Regulatory roles of MgADP and calcium in tension development of skinned cardiac muscle. Journal of Muscle Research and Cell Motility. 1998;19:909–921. doi: 10.1023/a:1005437517287. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Ishiwata S. Effects of pH on spontaneous tension oscillation in skinned bovine cardiac muscle. Pflügers Archiv. 1999;438:125–132. doi: 10.1007/s004240050889. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Kajiwara H, Ishiwata S, Kurihara S. Effects of MgADP on length dependence of tension generation in skinned rat cardiac muscle. Circulation Research. 2000;86:E1–6. doi: 10.1161/01.res.86.1.e1. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Sasaki D, Ishiwata S, Kurihara S. Length dependence of tension generation in rat skinned cardiac muscle: Role of titin in the Frank-Starling mechanism of the heart. Circulation. 2001 doi: 10.1161/hc3901.095898. (in the Press) [DOI] [PubMed] [Google Scholar]
- Godt RE, Nosek TM. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. Journal of Physiology. 1989;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J, Sonnenblick E, Babu A. The role of troponin C in the length dependence of Ca2+-sensitive force of mammalian skeletal and cardiac muscles. Journal of Physiology. 1990;441:305–324. doi: 10.1113/jphysiol.1991.sp018753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, Lamont C, Miller DJ. Hysteresis and the length dependence of calcium sensitivity in chemically skinned rat cardiac muscle. Journal of Physiology. 1988;401:115–143. doi: 10.1113/jphysiol.1988.sp017154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd MG, Dantzig JA, Trentham DR, Goldman YE. Phosphate release and force generation in skeletal muscle fibers. Science. 1985;228:1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Horiuti K. Some properties of the contractile system and sarcoplasmic reticulum of skinned slow fibres from Xenopus muscle. Journal of Physiology. 1986;373:1–23. doi: 10.1113/jphysiol.1986.sp016032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving TC, Konhilas J, Perry D, Fischetti R, De Tombe PP. Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. American Journal of Physiology – Heart and Circulatory Physiology. 2000;279:H2568–2573. doi: 10.1152/ajpheart.2000.279.5.H2568. [DOI] [PubMed] [Google Scholar]
- Ishiwata S, Oosawa F. A regulatory mechanism of muscle contraction based on the flexibility change of the thin filaments. Journal of Mechanochemistry and Cell Motility. 1974;3:9–17. [PubMed] [Google Scholar]
- Kammermeier H, Schmidt P, Jungling E. Free energy change of ATP-hydrolysis: a causal factor of early hypoxic failure of the myocardium? Journal of Molecular and Cellular Cardiology. 1982;14:267–277. doi: 10.1016/0022-2828(82)90205-x. [DOI] [PubMed] [Google Scholar]
- Kawai M, Guth K, Cornacchia TW. The role of monovalent phosphate anions in the crossbridge kinetics of chemically skinned rabbit psoas fibers. In: Sugi H, Pollack GH, editors. Molecular MEchanism of Muscle Contraction. New York, NY, USA: Plenum Publishing Corporation; 1988. pp. 203–214. [PubMed] [Google Scholar]
- Kentish JC. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. Journal of Physiology. 1986;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish JC. Combined inhibitory actions of acidosis and phosphate on maximum force production in rat skinned cardiac muscle. Pflügers Archiv. 1991;419:310–318. doi: 10.1007/BF00371112. [DOI] [PubMed] [Google Scholar]
- Komukai K, Ishikawa T, Kurihara S. Effects of acidosis on Ca2+ sensitivity of contractile elements in intact ferret myocardium. American Journal of Physiology. 1998;43:H147–154. doi: 10.1152/ajpheart.1998.274.1.H147. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Length modulation of muscle performance: Frank-Starling law of the heart. In: Fozzard HA, editor. The Heart and Cardiovascular System. New York, NY, USA: Raven Press Publishers; 1991. pp. 1325–1352. [Google Scholar]
- McDonald KS, Field LJ, Parmacek MS, Soonpaa M, Leiden JM, Moss RL. Length dependence of Ca2+ sensitivity of tension in mouse cardiac myocytes expressing skeletal troponin C. Journal of Physiology. 1995;483:131–139. doi: 10.1113/jphysiol.1995.sp020573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KS, Moss RL. Osmotic compression of single cardiac myocytes eliminates the reduction in Ca2+ sensitivity of tension at short sarcomere length. Circulation Research. 1995;77:199–205. doi: 10.1161/01.res.77.1.199. [DOI] [PubMed] [Google Scholar]
- Matsubara I, Millman BM. X-ray diffraction patterns from mammalian heart muscle. Journal of Molecular Biology. 1974;82:527–536. doi: 10.1016/0022-2836(74)90246-0. [DOI] [PubMed] [Google Scholar]
- Metzger JM, Moss RL. Depression of Ca2+ insensitive tension due to reduced pH in partially troponin-extracted skinned skeletal muscle fibers. Biophysical Journal. 1988;54:1169–1173. doi: 10.1016/S0006-3495(88)83052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Moss RL. Effects of pH and stiffness due to reduced pH in mammalian fast- and slow-twitch skinned skeletal fibers. Journal of Physiology. 1990;428:737–750. doi: 10.1113/jphysiol.1990.sp018238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman BM. The filament lattice of striated muscle. Physiological Reviews. 1998;78:359–391. doi: 10.1152/physrev.1998.78.2.359. [DOI] [PubMed] [Google Scholar]
- Moss RL, Nwoye LO, Greaser ML. Substitution of cardiac troponin C into rabbit muscle does not alter the length dependence of Ca2+ sensitivity of tension. Journal of Physiology. 1991;440:273–280. doi: 10.1113/jphysiol.1991.sp018708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard CH, Hamilton DL, Astles P, McCall E, Jewell BR. The effect of acidosis on the relation between Ca2+ and force in isolated ferret cardiac muscle. Journal of Physiology. 1991;436:559–578. doi: 10.1113/jphysiol.1991.sp018567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard CH, Kentish JC. Effects of changes of pH on the contractile function of cardiac muscle. Americal Journal of Physiology. 1990;27:C967–981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- Ricciardi L, Bottinelli R, Canepari M, Reggiani C. Effects of acidosis on maximum shortening velocity and force-velocity relation of skinned rat cardiac muscle. Journal of Molecular and Cellular Cardiology. 1994;26:601–607. doi: 10.1006/jmcc.1994.1072. [DOI] [PubMed] [Google Scholar]
- Ricciardi L, Bucx JJJ, Ter Keurs HEDJ. Effects of acidosis on force-sarcomere length and force-velocity relations of rat cardiac muscle. Cardiovascular Research. 1986;20:117–123. doi: 10.1093/cvr/20.2.117. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Veksler V. Myocardial ischemic contracture. Metabolites affect rigor tension development and stiffness. Circulation Research. 1994;74:920–929. doi: 10.1161/01.res.74.5.920. [DOI] [PubMed] [Google Scholar]


