Abstract
We investigated the effect of oral creatine supplementation during leg immobilization and rehabilitation on muscle volume and function, and on myogenic transcription factor expression in human subjects.
A double-blind trial was performed in young healthy volunteers (n=22). A cast was used to immobilize the right leg for 2 weeks. Thereafter the subjects participated in a knee-extension rehabilitation programme (3 sessions week−1, 10 weeks). Half of the subjects received creatine monohydrate (CR; from 20 g down to 5 g daily), whilst the others ingested placebo (P; maltodextrin).
Before and after immobilization, and after 3 and 10 weeks of rehabilitation training, the cross-sectional area (CSA) of the quadriceps muscle was assessed by NMR imaging. In addition, an isokinetic dynamometer was used to measure maximal knee-extension power (Wmax), and needle biopsy samples taken from the vastus lateralis muscle were examined to asses expression of the myogenic transcription factors MyoD, myogenin, Myf5, and MRF4, and muscle fibre diameters.
Immobilization decreased quadriceps muscle CSA (∼10 %) and Wmax (∼25 %) by the same magnitude in both groups. During rehabilitation, CSA and Wmax recovered at a faster rate in CR than in P (P < 0.05 for both parameters). Immobilization changed myogenic factor protein expression in neither P nor CR. However, after rehabilitation myogenin protein expression was increased in P but not in CR (P < 0.05), whilst MRF4 protein expression was increased in CR but not in P (P < 0.05). In addition, the change in MRF4 expression was correlated with the change in mean muscle fibre diameter (r=0.73, P < 0.05).
It is concluded that oral creatine supplementation stimulates muscle hypertrophy during rehabilitative strength training. This effect may be mediated by a creatine-induced change in MRF4 and myogenin expression.
Muscle disuse due to physical inactivity, ageing and many disease conditions results in muscle atrophy and reduced muscle functional capacity, which may impair activities of daily living. Strategies that may prevent or reverse these effects are of considerable functional significance to the individual. Recent evidence suggests that oral creatine supplementation is an effective intervention to prevent or reverse neuromuscular degeneration in humans.
Since Harris and his co-workers (1992) reported that high-dose oral creatine supplementation can significantly increase muscle total creatine content, the potential of creatine supplementation to boost muscular performance has been widely acknowledged. It has become well established in healthy subjects that oral creatine supplementation can improve muscle power output during high-intensity exercise (Balsom et al. 1993; Greenhaff et al. 1993; Vandenberghe et al. 1996; American College of Sports Medicine, 2000) and enhance the increments of muscle mass and muscle strength that result from heavy resistance training (Vandenberghe et al. 1997; Kreider et al. 1998). These findings, which were obtained in experiments involving athletes and healthy subjects, have also prompted interest in the potential of oral creatine supplementation to treat muscular pathologies. In this respect, creatine supplementation was recently found to enhance muscle functional capacity in patients with various forms of neuromuscular diseases or muscular dystrophies (Tarnopolsky & Martin, 1999; Walter et al. 2000) as well as in McArdle's disease (Vorgerd et al. 2000). Conversely, creatine supplementation was found to be ineffective in improving functional capacity in patients afflicted by chronic progressive external ophthalmoplegia or mitochondrial myopathy (Klopstock et al. 2000). Still, the precise physiological mechanism underlying the described beneficial effects of creatine supplementation on skeletal musculature remains largely unexplained.
There is evidence from experiments in rats that the expression of the myogenic transcription factors MyoD, myogenin, Myf5 and MRF4 is involved in determining structural and metabolic phenotype in adult skeletal muscle cells (Eftimie et al. 1991; Voytik et al. 1993; Megeney & Rudnicki, 1995; Hughes et al. 1999), in particular during episodes of muscle catabolism or anabolism (Hughes et al. 1993; Loughna & Brownson, 1996; Marsh et al. 1997; Mozdziak et al. 1998; Adams et al. 1999). Thus, disuse atrophy was found to cause fibre-specific alterations in mRNA expression of the various myogenic factors (Loughna & Brownson, 1996). Compensatory hypertrophy in overloaded plantaris muscle led to markedly increased expression of myogenin, whereas the expression of MyoD was only slightly and transiently increased (Adams et al. 1999). The regeneration of muscle fibres after bupivacaine injection led to a rapid and marked increase in mRNA expression of myogenin and MyoD, whereas the expression of MRF4 first decreased and then increased at the time when expression of myogenin and MyoD decreased (Marsh et al. 1997). Furthermore, long-term administration of the anabolic compound clenbuterol and/or thyroid hormone decreased the expression of myogenin (Loughna & Brownson, 1996; Mozdziak et al. 1998). However, the protein expression of myogenic factors has yet to be determined in human skeletal muscle; nor has the effect of altered activity level on this expression been studied.
It is thus well established that oral creatine supplementation can stimulate muscle hypertrophy. In addition, the expression of myogenic transcription factors has been implicated in the regulation of muscle fibre adaptations during hypertrophy. Therefore, in the present study we investigated the effects of oral creatine supplementation on both the functional and structural adaptations of skeletal muscle and the expression of myogenic transcription factors during leg-immobilization-induced disuse atrophy and subsequent exercise rehabilitation. The data presented in this paper demonstrate for the first time that oral creatine supplementation is an effective therapeutic strategy with which to enhance rehabilitation from muscle disuse atrophy. This effect may be mediated by a creatine-induced change in myogenic transcription factor expression.
METHODS
Subjects
Healthy students (n= 22: 13 males and 9 females), ranging in age from 20 to 23 years, gave their informed written consent to take part in the study. Exclusion criteria on admission were: prior renal pathology, albuminuria, prior oral creatine supplementation, intake of other nutritional supplements or medication and any medical condition that might contra-indicate leg immobilization or rehabilitation training. Three of the female subjects who enrolled in the study were taking oral contraceptive medication. The subjects were asked to avoid changes in their diet and level of physical activity during the period of the study. Two subjects from the placebo (P) group (one female and one male) withdrew within 5 weeks of the follow-up due to lack of time to participate in the rehabilitation sessions. One subject from the creatine (CR) group dropped out due to an urgent medical problem that was unrelated to the study protocol. At the end of the study the subjects were asked whether they had any notion of the treatment they had received. Irrespective of the supplement received, all were unsure. No side effects were reported during the entire duration of the study.
Study protocol
A double-blind study was performed over a 12 week period after the local ethics committee had approved the study protocol. The experiments were carried out according to the guidelines issued by the ethics committee, which conform with the Declaration of Helsinki. During the 1st week of the study, baseline measurements were performed (session 1, week 0). On day 1, after a light standardized meal (2500 kJ, 60 % carbohydrates, 25 % fat, 15 % proteins) the cross-sectional area (CSA) of the quadriceps muscle was measured by NMR imaging, after which a percutaneous needle biopsy sample of the right vastus lateralis muscle was taken for biochemical and histochemical analyses. On day 4, isometric and dynamic maximal knee-extension torque of the right and left leg was evaluated using an isokinetic dynamometer. Subsequently, subjects were coupled into pairs, matched for gender, quadriceps muscle CSA and maximal isometric knee-extension torque. Thereafter, each pair was divided in a double-blind randomized manner, with subjects being assigned to either a CR group (n=11) or a P group (n=11). From the next day, CR subjects ingested 5 g of creatine monohydrate four times per day. The creatine supplements were flavoured by the addition of citrate (60 mg g−1 creatine) and maltodextrin (940 mg g−1 creatine), while the P group ingested only maltodextrin containing citrate (40 mg g−1 maltodextrin). The creatine and placebo powders were identical in taste and appearance. The subjects' right leg was then immobilized at a knee angle of ∼160 deg by a light polyester cast, extending from groin to ankle. One week later the cast was removed for 1 h, after which a new cast was fitted for the 2nd week of immobilization. On the 1st and 2nd day following removal of the cast the subjects participated in a 30 min physiotherapy session aimed at restoring normal knee-joint mobility, after which the post-immobilization measurements were performed (session 2, week 2). Session 2 was identical to session 1. Immediately after session 2, a 10 week rehabilitation programme was started. Subjects participated in a training programme at a rate of three sessions per week. Each training session consisted of four series of 12 unilateral knee extensions ranging from a knee angle of 90 deg to full extension, interspersed by 2 min rest intervals, on a knee-extension apparatus (Technogym). The workload was set at 60 % of maximum isometric knee-extension torque, which was measured at the start of each session. During the last 7 weeks of the training period, the number of contraction series was increased from four to six. The dose of creatine or placebo ingested was reduced from four times 5 g per day during immobilization, to three times 5 g per day during the initial 3 weeks of rehabilitation, and thereafter to a single daily dose of 5 g. After 3 weeks (session 3, week 5) and 10 weeks (session 4, week 12) of rehabilitation and at least 48 h following the last training session, subjects returned to the laboratory for an evaluation session. All measurements (sessions 1–4) were performed on the same day of the week and at the same time of the day for each subject.
Determination of quadriceps muscle CSA
NMR imaging was performed in a 1.5 T scanner (Vision, Siemens) using a phased-array body coil positioned over the upper legs. Subjects lay in a supine position with a plastic leg mould fitted, which allowed accurate positioning of the subjects' legs with reference to the coil and magnet during different imaging sessions. T1-weighted images were acquired with a spin-echo sequence (TR/TE=500/12 ms). Three axial slices with 10 mm thickness and 30 mm spacing, were positioned on coronal slices at 17, 20 and 23 cm proximal to the reference point. The CSA of the quadriceps muscle was determined by digitization of the images using Visual Basic software (Microsoft, USA). Quadriceps muscle CSA (cm2) was defined as the mean of the three axial scan images.
Determination of maximal dynamic knee-extension power (Wmax) and isometric force (Fmax)
Maximal voluntary torque and Wmax of the knee extensors was evaluated on an isokinetic dynamometer, which consists of a computer-controlled asynchronous electromotor (AMK Dynasyn, 19 kW) instrumented with a torque transducer (Lebow, maximal torque 565 N m, 0.05 % precision). The exercise test consisted of unilateral knee extensions performed in a sitting position on the dynamometer. After a 5 min standardized warm-up, the subjects performed three voluntary maximal isometric contractions (3 s), interspersed by 2 min rest intervals, at a knee angle of 110 deg. Maximal isometric torque (N m) was then obtained from the smoothed curve of the static torque. On the next day, and again after a standardized 5 min warm-up, subjects performed a bout of 30 dynamic maximal voluntary knee extensions at a constant velocity of 180 deg s−1, starting from 90 deg to full extension (180 deg). After each contraction, the leg was returned (180 deg s−1) passively to the starting position from which the next contraction was immediately initiated. Torque and angular velocity were measured during each contraction and were digitized simultaneously (250 Hz) by an on-line computer. Power was calculated from the registered torque and velocity measurements.
Muscle biochemistry and histochemistry
The needle biopsy technique was used to obtain muscle samples from the vastus lateralis muscle of the right leg. Incisions were made through the skin and muscle fascia following the administration of local anaesthesia (2–3 ml of 1 % lidocaine). Following removal from the limb, a piece of each muscle biopsy sample was immediately freed from blood and visible connective tissue, rapidly frozen in liquid N2, and stored at -80 °C for subsequent biochemical analysis. The remaining muscle was mounted in embedding medium, frozen in isopentane, cooled to its freezing point in liquid N2, and stored at -80 °C until analyses were performed at a later date. For the immunochemical and biochemical assays, muscle samples were freeze-dried and washed twice in petroleum ether to remove fat. An aliquot of the freeze-dried muscle was homogenized in an Omni homogenizer for 30 s on ice in RIPA extraction buffer (150 mm NaCl; 1 % NP40; 0.5 % deoxycholate; 0.1 % SDS; 50 mm Tris; pH 8), which facilitates the dissolution of the nuclear envelope, thus freeing the transcription factors. The homogenate was incubated on ice for 1 h, spun for 15 min at 13 000 g and the supernatant (hereafter called the extract) was collected for analysis. An aliquot (100 μg) of the extract was resolved by SDS-PAGE before electroblotting to polyvinylidene fluoride membranes. Specific proteins were detected by incubation with specific antibodies in Tris-buffered saline plus Tween 20 (150 mm NaCl, 50 mm Tris, 0.1 % Tween 20) after blocking in 1 % bovine serum albumin followed by incubation with an alkaline phosphatase-labelled antibody. Visualization and quantification of the protein bands was enabled using a Storm phosphoimager. The primary antibodies were all affinity-purified polyclonal antibodies raised in rabbits and purchased from Santa Cruz. Myf5 was raised against the C-terminal end of the peptide, MyoD against amino acids 1–318, myogenin against amino acids 1–225 and MRF4 against amino acids 1–242. For the biochemical assays, a portion of each sample was dissected free of visible blood and connective tissue and then pulverized. Part of the powdered extract (3–5 mg) was then used for spectrophotometric determination of ATP, phosphocreatine (PCr) and free creatine concentrations (Harris et al. 1974). For the histochemical analyses, a freezing microtome (-20 °C) was used to cut serial transverse sections (10 μm) of the biopsy samples; these sections were stained for myofibrillar ATPase to allow identification of the different fibre types (Brooke & Kaiser, 1970). The biochemical, immunochemical and histochemical measurements were only performed in a subgroup of 16 subjects (P: n=8; CR: n=8) from whom good quality muscle biopsy material was obtained at all times of the study.
Statistical analysis
The effects observed during immobilization were evaluated by a two-way analysis of variance that was covariate-adjusted for the baseline values. The effects observed during rehabilitation were evaluated by a two-way analysis of variance that was covariate-adjusted for the post-immobilization values. To evaluate the effects observed in the contralateral leg, a single two-way analysis of variance was used that was covariate-adjusted for the baseline values. In addition to these primary analyses, we carried out a one-way analysis of variance to compare within groups the values after immobilization and after rehabilitation in the immobilized leg, or after 2, 5 and 12 weeks of creatine supplementation in the contralateral leg, with the corresponding baseline values. Data from the three dropout subjects were not included in the statistical analyses. Statistical significance was taken at a two-sided significance level of P < 0.05. All data are expressed as means ±s.e.m.
RESULTS
The immobilized leg
Muscle mass and functional capacity (Table 1)
Table 1.
Effect of oral creatine supplementation on muscle cross-sectional area and muscle force and power during immobilization and rehabilitation
| Immobilization | Rehabilitation | |||||
|---|---|---|---|---|---|---|
| Baseline | After | P value | 3 weeks | 10 weeks | P value | |
| CSA quadriceps muscle (cm2) | ||||||
| Placebo | 90.3 ± 4.6 | 81.8 ± 4.8 * | 89.3 ± 4.8 | 93.5 ± 6.0 * | ||
| Creatine | 92.5 ± 4.9 | 82.3 ± 4.8 * | 0.30 | 94.6 ± 5.7 | 99.8 ± 6.0 * | 0.01 |
| Wmax (W) | ||||||
| Placebo | 160 ± 16 | 122 ± 13 * | 156 ± 17 | 165 ± 18 | ||
| Creatine | 152 ± 17 | 113 ± 12 * | 0.61 | 160 ± 17 | 172 ± 17 * | 0.05 |
| Fmax (N m) | ||||||
| Placebo | 151 ± 12 | 117 ± 9 * | 155 ± 12 | 166 ± 13 * | ||
| Creatine | 141 ± 10 | 112 ± 8 * | 0.70 | 154 ± 11 * | 168 ± 13 * | 0.42 |
The cross-sectional area (CSA) of the right quadriceps muscle was measured by NMR imaging, and the dynamic power (Wmax) and isometric torque (Fmax) of the knee extensor muscles of the right leg were assessed on an isokinetic dynamometer. A cast was used to immobilize the right leg for a period of 2 weeks. Thereafter the subjects participated in a 10 week rehabilitation programme for the knee extensors of the same leg. The subjects ingested either supplementary creatine monohydrate (creatine group) or placebo (placebo group). Values are means ±s.e.m. of 10 observations in the creatine group and 9 observations in the placebo group. The P values refer to the treatment effect (creatine versus placebo) during immobilization and rehabilitation
significant difference compared with the corresponding baseline value (P < 0.05). See Methods for further details.
The quadriceps muscle CSA, and knee-extension Wmax and Fmax at baseline were similar in the two groups. The 2 week immobilization period decreased quadriceps muscle CSA, Wmax and Fmax by ∼10 %, 25 % and ∼22 %, respectively (P < 0.05), with no significant differences between the two treatment groups. The rehabilitation programme increased quadriceps muscle CSA, Wmax and Fmax in all subjects (Table 1). Compared with the post-immobilization value, 3 and 10 weeks of rehabilitation in P increased quadriceps muscle CSA by 9 % and 14 %, respectively (P < 0.05). The corresponding increases in CR were significantly greater (15 % and 21 %, respectively; P < 0.05). Wmax also increased more during rehabilitation in CR (+42 % and +52 % at 3 and 10 weeks, respectively; P=0.05) than in P. In contrast, the increment in Fmax due to rehabilitation training was not significantly smaller in P than in CR (+32 % and +42 % at 3 and 10 weeks, respectively in P, versus+38 % and +50 %, respectively, in CR; P < 0.05). At the end of the 10 week rehabilitation period, quadriceps muscle CSA and Fmax were higher than at baseline in both treatment groups (P < 0.05). Wmax was higher only in the CR group (P < 0.05).
Myogenic transcription factors (Fig. 1)
Figure 1. Protein expression of the myogenic factors before and after leg immobilization and after rehabilitation training.
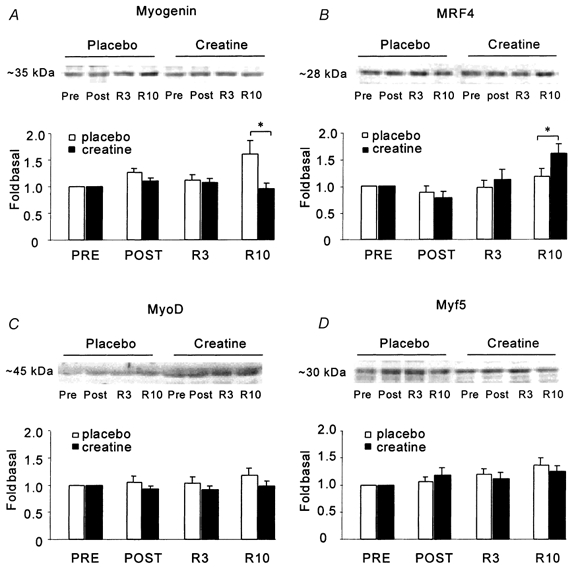
A, myogenin; B, MRF4; C, MyoD; D, Myf5. Pre, before leg immobilixation; Post, 2 weeks after leg immobilization. R3, 3 weeks of rehabilitation training; R10, 10 weeks of rehabilitation training. Values are means ±s.e.m. of eight observations and are expressed relative to the baseline values (Pre). *Significant treatment effect compared with placebo.
We detected all four myogenic factors at the protein level. The average protein expression of the various myogenic factors was similar in the two groups at the onset of the experiment (data not shown). There were no significant changes in expression of the myogenic factors after 2 weeks of immobilization or after 3 weeks of rehabilitation training in either group. However, after 10 weeks of rehabilitation, myogenin protein content had increased in P but not in CR (P < 0.05). Furthermore, 10 weeks of rehabilitation training increased MRF4 protein content in CR only (P < 0.05versus P). Neither training, immobilization nor creatine supplementation significantly changed the protein expression of Myf5 and MyoD.
ATP and creatine content (Table 2)
Table 2.
Effect of creatine supplementation on creatine content and muscle fibre CSA during immobilization and rehabilitation
| Immobilization | Rehabilitation | |||||
|---|---|---|---|---|---|---|
| Baseline | After | P value | 3 weeks | 10 weeks | P value | |
| Phosphocreatine (μmol (g dry wt)−1) | ||||||
| Placebo | 76.5 ± 1.8 | 64.9 ± 3.1 * | 73.8 ± 2.6 | 71.6 ± 2.2 | ||
| Creatine | 82.4 ± 6.2 | 80.2 ± 5.8 | 0.01 | 89.7 ± 6.8 | 75.1± 6.3 | 0.01 |
| Free creatine (μmol (g dry wt)−1) | ||||||
| Placebo | 31.3 ± 3.3 | 41.3 ± 3.6 * | 43.5 ± 5.4 * | 37.7 ± 2.9 | ||
| Creatine | 30.6 ± 2.9 | 48.5 ± 4.5 * | 0.16 | 53.9 ± 5.4 * | 43.4 ± 4.0 | |
| Total creatine (μmol (g dry wt)−1) | ||||||
| Placebo | 108.8 ± 2.8 | 106.2 ± 5.7 | 117.3 ± 5.1 | 109.3 ± 3.4 | ||
| Creatine | 113.9 ± 8.4 | 128.7 ± 9.9 * | 0.04 | 143.6 ± 11.6 * | 118.5 ± 8.0 | 0.01 |
| CSA type I fibres (μm2) | ||||||
| Placebo | 4483 ± 358 | 4116 ± 123 | 4266 ± 168 | 4940 ± 391 | ||
| Creatine | 3932 ± 381 | 4037 ± 542 | 0.68 | 4285 ± 573 | 5889 ± 1141 * | 0.21 |
| CSA type IIa fibres (μm2) | ||||||
| Placebo | 5033 ± 294 | 4479 ± 223 | 4919 ± 256 | 5812 ± 541 | ||
| Creatine | 4175 ± 301 | 4191 ± 695 | 0.77 | 4522 ± 621 | 6666 ± 1188 * | 0.21 |
| CSA type IIb fibres (μm2) | ||||||
| Placebo | 4240 ± 342 | 3860 ± 309 | 4478 ± 403 | 5297 ± 529 | ||
| Creatine | 3485 ± 485 | 3784 ± 745 | 0.65 | 4054 ± 657 | 5902 ± 914 * | 0.12 |
Values are means ±s.e.m. of eight observations and represent concentrations (μmol (g dry wt)−1) or muscle fibre CSAs (μm2) measured in needle biopsy samples obtained from the vastus lateralis muscle. Total creatine concentration was calculated as the sum of the free creatine and phosphocreatine concentrations measured. Measurements were made before and after 2 weeks of immobilization of the right (ipsilateral) leg, and after 3 and 10 weeks of rehabilitation of the right leg knee extensors only. The P values refer to the treatment effect (creatine versus placebo) during immobilization and rehabilitation
significant difference compared with the corresponding baseline value (P < 0.05). See Methods for further details.
The baseline characteristics were similar in the two groups (Table 2). During immobilization, PCr concentration decreased to about 15 % below the baseline in P (P < 0.05). This fall was completely prevented by oral creatine supplementation (P < 0.05). In P, the muscle PCr concentration returned to the pre-immobilization baseline value within the initial 3 weeks of rehabilitation, after which the level was maintained. Conversely, in CR the muscle PCr concentration increased to ∼12 % above the baseline value after 3 weeks of rehabilitation compared with P (P < 0.05). However, this PCr ‘overshoot’ was reversed during the final stage of the rehabilitation period. The muscle free creatine concentration was not significantly different between P and CR throughout the study. In P, muscle total creatine concentration was not significantly changed compared with the baseline value during either immobilization or rehabilitation. Conversely, in CR muscle total creatine concentration was higher after the immobilization period and during the initial 3 weeks of knee-extension training compared with P (P < 0.05). However, together with the declining muscle PCr content, muscle total creatine had returned to the baseline value by the end of the rehabilitation schedule. Muscle ATP concentration ranged from 17.5 ± 0.6 to 21.5 ± 5 mmol kg−1 dry weight and was not significantly affected by oral creatine supplementation or by immobilization or rehabilitation per se.
Fibre types
There were no significant differences at baseline between the two groups for the CSAs of type I, type IIa and type IIb muscle fibres (Table 2). The statistical analyses did not reveal a significant therapeutic effect of oral creatine supplementation on muscle fibre CSA for any fibre type during either immobilization or rehabilitation. However, compared with the pre-immobilization baseline value, CSA in all fibre types at the end of the rehabilitation period was significantly higher in CR (P < 0.05), but not in P.
Effects in the contralateral leg (Table 3)
Table 3.
Effect of creatine supplementation on muscle CSA and muscle force and power in the contralateral leg
| Duration of creatine supplementation | |||||
|---|---|---|---|---|---|
| Baseline | 2 weeks | 5 weeks | 12 weeks | P value | |
| CSA quadriceps muscle (cm2) | |||||
| Placebo | 91.0 ± 4.3 | 92.2 ± 4.2 | 94.3 ± 4.5 * | 94.0 ± 4.2 * | |
| Creatine | 93.2 ± 4.8 | 94.9 ± 5.1 | 97.7 ± 5.6 * | 99.5 ± 6.0 * | 0.07 |
| Wmax (W) | |||||
| Placebo | 153 ± 15 | 158 ± 17 | 156 ± 17 | 158 ± 16 | |
| Creatine | 143 ± 15 | 150 ± 14 | 160 ± 17 * | 163 ± 17 * | 0.01 |
| Fmax (N m) | |||||
| Placebo | 149 ± 13 | 146 ± 14 | 153 ± 14 | 160 ± 16 * | |
| Creatine | 135 ± 9 | 135 ± 10 | 155 ± 11 * | 159 ± 12 * | 0.01 |
Values are means ±s.e.m. of 10 observations in the creatine group and 9 observations in the placebo group. The CSA of the left quadriceps muscle (contralateral to the immobilized leg), as measured by NMR imaging, and Wmax and Fmax of the left knee extensor muscles were assessed on an isokinetic dynamometer. Measurements were made before and after 2 weeks of immobilization of the right (ipsilateral) leg, and after 3 and 10 weeks of rehabilitation of the right leg knee extensors only. The P values refer to the treatment effect (creatine versus placebo)
significant difference compared with the corresponding baseline value (P < 0.05). See Methods for further details.
The baseline contralateral quadriceps muscle CSA, Wmax and Fmax were similar in the two groups (Table 3). During the 12 week creatine supplementation period, during which the other (ipsilateral) leg was immobilized (2 weeks) and then subject to rehabilitation (10 weeks), contralateral quadriceps muscle CSA did not significantly change in P, but tended to increase in CR (+7 %; P=0.07). At the end of the study, the contralateral leg Wmax was significantly increased in CR (+14 %, P < 0.05), but not in P. Furthermore, contralateral Fmax increased more (+18 %) in CR than in P (+7 %) (P < 0.05).
Body mass and training workload
At the start of the study body mass was 66.9 ± 2.7 kg in P and 65.9 ± 3.1 kg in CR. Over the course of the study, body mass slightly increased (68.5 ± 3.2 kg in P and 68.4 ± 3.5 kg in CR) in the two treatment groups (P < 0.05). However, there were no significant differences in this parameter between P and CR. The training workload during rehabilitation was similar for the two groups over the entire rehabilitation period. Initial knee-extension one-repetition maximum was 67 ± 9 kg in P and 70 ± 8 kg in CR; this increased to 103 ± 10 and 98 ± 10 kg, respectively, during the final stage of the rehabilitation period.
DISCUSSION
The 2 weeks of leg immobilization induced the expected (Appell, 1990) reduction in quadriceps muscle CSA (∼10 %), and the 10 weeks of rehabilitation training increased (∼+15 %) muscle CSA (see Table 1). This increase was accounted for by hypertrophy of both type I and type II muscle fibres (see Table 2). Interestingly, oral creatine supplementation enhanced the recovery of muscle mass during rehabilitation. Thus, our data indicate that creatine supplementation is capable of shortening the duration of rehabilitation needed to restore muscle mass following an episode of disuse atrophy. Furthermore, the current study demonstrates that the ‘anabolic’ action of creatine loading impinges on both fast- and slow-twitch muscle fibre types. Creatine intake hypertrophied type I, type IIa and type IIb fibres in the vastus lateralis muscle to a similar degree during the 10 weeks of rehabilitative knee-extension training. This indicates that the individual's muscle fibre distribution is probably not critical to the impact of creatine supplementation on muscle mass during weight training.
There is substantial evidence from experiments in rats to suggest that the myogenic transcription factors are involved in regulating the metabolic processes intrinsic to muscle catabolism or anabolism (Hughes et al. 1993; Loughna & Brownson, 1996; Marsh et al. 1997; Mozdziak et al. 1998; Adams et al. 1999). This is the first time that protein expression of the myogenic transcription factors myogenin, MRF4, Myf5 and MyoD has been measured in human muscle samples; all four were detected at the protein level. Rehabilitative exercise training increased the protein expression of myogenin, whereas expression of the other myogenic factors was unaffected (Fig. 1). Interestingly, oral creatine supplementation, which increased muscle fibre hypertrophy during rehabilitation, increased the protein expression of MRF4 but prevented the training-induced increase in myogenin protein expression. In addition, the change in MRF4 protein content from day 0 to 10 weeks of rehabilitation was closely correlated (r=0.73, Fig. 2) with the concomitant change in average muscle fibre size. These findings thus suggest that MRF4 in particular may play an important role in regulating the muscle hypertrophy that results from resistance training. Whether the increase in MRF4 (versus the decrease in myogenin) plays any causative role in the effect of creatine supplementation to stimulate muscle hypertrophy during rehabilitation training cannot be determined conclusively with the present data. However, in keeping with published work showing that myogenin expression is decreased in rat skeletal muscle following long-term administration of the anabolic compound clenbuterol and/or thyroid hormone, our study demonstrates clearly that the expression of myogenin and MRF4 depends upon both muscle activity level and creatine supplementation. A recent study in rats has provided preliminary evidence to suggest that creatine supplementation in combination with increased functional load results in increased satellite cell mitotic activity (Dangott et al. 1999). Whether this finding applies to human muscle during strengthening after atrophy, and is relevant to the changes in myogenic factor protein expression observed here, remains to be investigated. However, clearly the present study provides for the first time a putative mechanism to explain the ‘anabolic’ action of creatine supplementation.
Figure 2.
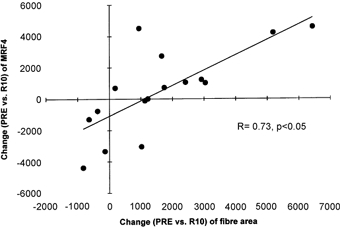
Correlation between the individual changes (R10 minus Pre) in MRF4 protein expression (arbitrary units) and average muscle fibre size (μm2) from the start (Pre) to the end (R10) of the study
It is well established that oral creatine supplementation (20–25 g daily) can increase muscle total creatine content by up to 50 % within 4–5 days (Harris et al. 1992). A fraction of the creatine accumulated is phosphorylated to increase the intramuscular PCr content, whilst muscle ATP remains constant (Harris et al. 1992; Greenhaff et al. 1994; Hultman et al. 1996; Vandenberghe et al. 1997). In the experiments described here, we here also found muscle ATP content to be constant and muscle total creatine content to increase in response to creatine supplementation. A novel finding is that oral creatine supplementation was capable of preventing the anticipated fall (Appell, 1990) in muscle PCr concentration due to immobilization (Table 2). Still, creatine supplementation did not prevent the structural and functional changes induced by 2 weeks of immobilization (Table 1). However, the 2 week immobilization period may conceivably have been too short to allow for a significant impact of creatine supplementation on disuse atrophy. Oral supplementary creatine of 12 weeks duration, but not 2 and 5 weeks, increased both quadriceps muscle CSA and knee-extension Wmax in the contralateral leg by 10–15 % (P < 0.05; Table 3). This indicates that exercise training is not essential for creatine supplementation to induce muscle hypertrophy. This finding is consistent with observations in patients with neuromuscular disease or muscle dystrophies showing increased muscle functional capacity due to creatine supplementation in the absence of a consistent rehabilitation programme.
After the immobilization, muscle PCr content in the creatine group increased to ∼10 % above the baseline, yet reverted to the pre-immobilization baseline value within the next 7 weeks of rehabilitation training in conjunction with creatine intake. Whether this drop in muscle PCr content was caused by the higher creatine dose used in the current study (> 10 g day−1 for 5 weeks) than in previous studies (Vandenberghe et al. 1997; Kreider et al. 1998; Volek et al. 1999), and/or was due to the higher training workload and volume used beyond week 3 of rehabilitation, is unclear. However, studies in rats have indicated that long-term high-dose creatine supplementation can cause downregulation of the expression of muscle creatine transporters (Guerrero-Ontiveros & Wallimann, 1998), which might eventually cause the intracellular creatine store to decrease (Neubauer et al. 1999).
In conclusion, the present study proves the efficacy of oral creatine supplementation to stimulate muscle hypertrophy and to enhance the rehabilitation of muscle functional capacity after disuse. In addition, it is shown for the first time that creatine supplementation can alter the response of myogenin and MRF4 protein expression to exercise training. It will be worthwhile to investigate further creatine supplementation as a potential strategy to treat or prevent muscle disuse atrophy in various clinical therapeutic conditions.
Acknowledgments
The authors wish to express their gratitude to Betina Bolmgreen, Irene Beck-Nielsen, Monique Ramaekers and Francis Vandebuerie for providing skilled technical assistance throughout this work. This study was supported by grants from the ‘Onderzoeksraad K.U.-Leuven’ (grant no. OT 94/31), from the Flemish ‘Fonds voor Wetenschappelijk Onderzoek’ (FWO grant no. G.0331.98) and from the Danish National Research Foundation (grant no. 504–14). The knee-extension training apparatus was kindly provided by Technogym, Gambettola, Italy.
References
- Adams GR, Haddad F, Baldwin KM. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. Journal of Applied Physiology. 1999;87:1705–1712. doi: 10.1152/jappl.1999.87.5.1705. [DOI] [PubMed] [Google Scholar]
- American Collegeof Sports Medicine. (American College of Sports Medicine roundtable. The physiological and health effects of oral creatine supplementation) Medicine and Science in Sports and Exercise. 2000;32:706–717. doi: 10.1097/00005768-200003000-00024. [DOI] [PubMed] [Google Scholar]
- Appell HJ. Muscular atrophy following immobilisation. A review. Sports Medicine. 1990;10:42–58. doi: 10.2165/00007256-199010010-00005. [DOI] [PubMed] [Google Scholar]
- Balsom PD, Ekblom B, Söderlund K, Sjödin B, Hultman E. Creatine supplementation and dynamic high-intensity intermittent exercise. Scandinavian Journal of Medicine and Science in Sports. 1993;3:143–149. [Google Scholar]
- Brooke MH, Kaiser KK. Three “myosin adenosine triphosphatase” systems: the nature of their pH lability and sulfhydryl dependence. Journal of Histochemistry and Cytochemistry. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Dangott B, Schultz E, Mozdziak PE. Dietary creatine monohydrate supplementation increases satellite cell mitotic activity during compensatory hypertrophy. International Journal of Sports Medicine. 1999;20:13–16. doi: 10.1055/s-2000-8848. [DOI] [PubMed] [Google Scholar]
- Eftimie R, Brenner HR, Buonanno A. Myogenin and MyoD join a family of skeletal muscle genes regulated by electric activity. Proceedings of the National Academy of Sciences of the USA. 1991;88:1349–1353. doi: 10.1073/pnas.88.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhaff PL, Bodin K, Söderlund K, Hultman E. Effect of oral creatine supplementation on skeletal muscle phosphocreatine resynthesis. American Journal of Physiology. 1994;266:E725–730. doi: 10.1152/ajpendo.1994.266.5.E725. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Casey A, Short AH, Harris R, Söderlund K. Influence of oral creatine supplementation on muscle torque during repeated bouts of maximal voluntary exercise in man. Clinical Science. 1993;84:565–571. doi: 10.1042/cs0840565. [DOI] [PubMed] [Google Scholar]
- Guerrero-Ontiveros ML, Wallimann T. Creatine supplementation in health and disease. Effects of chronic creatine ingestion in vivo: downregulation of the expression of creatine transporter isoforms in skeletal muscle. Molecular and Cellular Biochemistry. 1998;184:427–437. [PubMed] [Google Scholar]
- Harris R, Hultman E, Nordesjø L-O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scandinavian Journal of Clinical and Laboratory Investigation. 1974;33:109–120. [PubMed] [Google Scholar]
- Harris RC, Söderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clinical Science. 1992;83:367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Chi MMY, Lowry OH, Gundersen K. Myogenin induces a shift of enzyme activity from glycolytic to oxidative metabolism in muscles of transgenic mice. Journal of Cell Biology. 1999;145:633–642. doi: 10.1083/jcb.145.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SM, Taylor JM, Tapscott SJ, Gurley CM, Carter WJ, Peterson CA. Selective accumulation of MyoD and myogenin in fast and slow skeletal muscle is controlled by innervation and hormones. Development. 1993;118:1137–1147. doi: 10.1242/dev.118.4.1137. [DOI] [PubMed] [Google Scholar]
- Hultman E, Söderlund K, Timmons JA, Cederblad G, Greenhaff PL. Muscle creatine loading in men. Journal of Applied Physiology. 1996;81:232–237. doi: 10.1152/jappl.1996.81.1.232. [DOI] [PubMed] [Google Scholar]
- Klopstock T, Querner V, Schmidt F, Gekeler F, Walter M, Hartard M, Hennig M, Gasser T, Pongratz D, Straube A, Dieterich M, Müller-Felber W. A placebo-controlled crossover trial of creatine in mitochondrial diseases. Neurology. 2000;55:1748–1751. doi: 10.1212/wnl.55.11.1748. [DOI] [PubMed] [Google Scholar]
- Kreider RB, Ferreira M, Wilson M, Grinstaff P, Plisk S, Reinardy J, Cantler E, Almada AL. Effects of creatine supplementation on body composition, strength, and sprint performance. Medicine and Science in Sports and Exercise. 1998;30:73–82. doi: 10.1097/00005768-199801000-00011. [DOI] [PubMed] [Google Scholar]
- Loughna PT, Brownson C. Two myogenic regulatory factors transcripts exhibit muscle-specific responses to disuse and passive stretch in adult rats. FEBS Letters. 1996;390:304–306. doi: 10.1016/0014-5793(96)00681-3. [DOI] [PubMed] [Google Scholar]
- Marsh DR, Criswell DS, Carson JA, Booth FW. Myogenic regulatory factors during regeneration of skeletal muscle in young, adult, and old rats. Journal of Applied Physiology. 1997;83:1270–1275. doi: 10.1152/jappl.1997.83.4.1270. [DOI] [PubMed] [Google Scholar]
- Megeney LA, Rudnicki MA. Determination versus differentiation and the MyoD family of transcription factors. Biochemistry and Cell Biology. 1995;73:723–732. doi: 10.1139/o95-080. [DOI] [PubMed] [Google Scholar]
- Mozdziak PE, Greaser ML, Schultz E. Myogenin, MyoD, and myosin expression after pharmacologically and surgically induced hypertrophy. Journal of Applied Physiology. 1998;84:1359–1364. doi: 10.1152/jappl.1998.84.4.1359. [DOI] [PubMed] [Google Scholar]
- Neubauer S, Remkes H, Spindler M, Horn M, Wiesmann F, Prestle J, Walzel B, Ertl G, Hasenfuss G, Wallimann T. Downregulation of the Na+-creatine cotransporter in failing human myocardium and in experimental heart failure. Circulation. 1999;100:1847–1850. doi: 10.1161/01.cir.100.18.1847. [DOI] [PubMed] [Google Scholar]
- Tarnopolsky M, Martin J. Creatine monohydrate increases strength in patients with neuromuscular disease. Neurology. 1999;52:854–857. doi: 10.1212/wnl.52.4.854. [DOI] [PubMed] [Google Scholar]
- Vandenberghe K, Gillis N, Van Leemputte M, Van Hecke P, Vanstapel F, Hespel P. Caffeine counteracts the ergogenic action of muscle creatine loading. Journal of Applied Physiology. 1996;80:452–457. doi: 10.1152/jappl.1996.80.2.452. [DOI] [PubMed] [Google Scholar]
- Vandenberghe K, Goris M, Van Hecke P, Van Leemputte M, Vangerven L, Hespel P. Long-term creatine intake is beneficial to muscle performance during resistance training. Journal of Applied Physiology. 1997;83:2055–2063. doi: 10.1152/jappl.1997.83.6.2055. [DOI] [PubMed] [Google Scholar]
- Volek JS, Duncan ND, Mazzetti SA, Staron RS, Putukian M, Gómez AL, Pearson DR, Fink WJ, Kraemer WJ. Performance and muscle fibre adaptations to creatine supplementation and heavy resistance training. Medicine and Science in Sports and Exercise. 1999;31:1147–1156. doi: 10.1097/00005768-199908000-00011. [DOI] [PubMed] [Google Scholar]
- Vorgerd M, Grehl T, Jäger M, Müller K, Freitag G, Patzold T, Bruns N, Fabian K, Tegenthoff M, Mortier W, Luttmann A, Zange J, Malin JP. Creatine therapy in myophosphorylase deficiency (McArdle disease): a placebo-controlled crossover trial. Archives of Neurology. 2000;57:956–963. doi: 10.1001/archneur.57.7.956. [DOI] [PubMed] [Google Scholar]
- Voytik SL, Przyborski M, Badylak SF, Konieczny SF. Differential expression of muscle regulatory factor genes in normal and denervated adult rat hindlimb muscles. Development Dynamics. 1993;198:214–224. doi: 10.1002/aja.1001980307. [DOI] [PubMed] [Google Scholar]
- Walter MC, Lochmüller H, Reilich P, Klopstock T, Huber R, Hartard M, Hennig M, Pongratz D, Müller-Felber W. Creatine monohydrate in muscular dystrophies: a double-blind, placebo-controlled clinical study. Neurology. 2000;54:1848–1850. doi: 10.1212/wnl.54.9.1848. [DOI] [PubMed] [Google Scholar]


