Abstract
The effects of external divalent cations on spontaneous single non-selective cation channel currents were studied in outside-out patches from rabbit portal vein smooth muscle cells in K+-free conditions.
In an external medium containing 1.5 mm Ca2+ () the majority of spontaneous channel currents had a unitary conductance of 23 pS, reversal potential (Vr) of +10 mV and a low open probability (Po) at negative patch potentials. Some channels opened to a lower conductance state of about 13 pS suggesting that the cation channels have two conductance states. Open time and burst duration distributions could both be described by two exponentials with time constants of about of 1 ms and 7 ms for open times and 3 ms and 16 ms for burst durations.
In 0 the majority of spontaneous cation channels had a unitary conductance of 13 pS and Vr was shifted to +4 mV. Moreover the longer open time and longer burst duration time constants were both reduced to approximately half the values in 1.5 mm .
Compared to 0 the single channel currents in 3 μm and 100 μm had a 5- to 6-fold increase in Po which was accompanied by increases in both open times and burst durations. In 3 μm and 100 μm the unitary conductance of the single channel currents was between 22 and 26 pS.
At positive membrane potentials the single channel currents had an increased Po compared to negative potentials which was associated with increased open times and burst durations but these values were similar in 3 μm, 100 μm and 1.5 mm .
In 1.5 mm and 1.5 mm channels opened to the higher conductance state of about 22–25 pS and had a 3- to 7-fold greater Po than in 0 .
In conclusion, external divalent cations have marked effects on the unitary conductance and kinetic behaviour of non-selective cation channels in rabbit portal vein smooth muscle cells.
In rabbit portal vein myocytes stimulation of α1-adrenoceptors by noradrenaline evokes a non-selective cation current (Icat) in addition to Ca2+-activated Cl− and K+ conductances (Byrne & Large, 1988). The proposed physiological role of Icat is to produce membrane depolarisation with subsequent opening of voltage-dependent Ca2+ channels (VDCCs) to induce contraction of vascular smooth muscle. In addition, reversal potential measurements indicated that this conductance is highly permeable to divalent cations and it was proposed that Ca2+ may enter the cell through this channel to produce contraction independently of VDCCs (Byrne & Large, 1988; Wang & Large, 1991). Recent evidence suggests that a member of the transient receptor potential (TRP) family, TRPC6, is an essential component of Icat (Inoue et. al. 2001). This is an important observation because it provides strong support for a physiological role for a TRP protein in native mammalian vascular smooth muscle cells. That is to say, the TRP channel is involved in the vasoconstrictor response to noradrenaline released from sympathetic neurons.
We have shown previously that external Ca2+ ions () have both facilitatory and inhibitory actions on the amplitude of Icat (Helliwell & Large, 1996). In 0 noradrenaline activated Icat with a peak amplitude of approximately 25 pA showing that is not obligatory for stimulating Icat. Increasing from 0 to micromolar concentrations increased the peak amplitude of Icat by about 8-fold. However, increasing further (> 200 μm) decreased the amplitude of Icat so that the amplitude of Icat in 1.5 mm was similar to that in 0 . This produced a bell-shaped relationship between the amplitude of Icat and concentration with estimated equilibrium constants of about 6 μm and 400 μm for, respectively, the facilitatory and inhibitory effects (Helliwell & Large, 1996). The vastly different half-maximal values of concentration suggest that the facilitatory and inhibitory effects may be mediated by two distinct binding sites. In a fluctuation analysis study, the facilitatory effect of on Icat was shown to be associated with changes in both the kinetics and amplitude of the unitary conductance underlying the whole-cell current (Helliwell & Large, 1998). In addition, a further study showed that external Sr2+ () and Ba2+ () also had facilitatory effects on the amplitude of Icat when evoked in 0 , albeit with a potency and efficacy less than Ca2+, which were associated with changes in the kinetics and single channel conductance of the cation channels (Aromolaran & Large, 1999). To provide a more detailed understanding of the facilitatory and inhibitory effects of , and on Icat we have studied their effects at the single channel level.
A recent study from our laboratory showed that approximately 50 % of outside-out patches contained spontaneous cation channel activity which had properties very similar to noradrenaline- and 1-oleoyl-2-acetyl-sn-glycerol-evoked single cation currents (Albert & Large, 2001), indicating that the spontaneous channel currents are Icat. In the present study we describe the effects of external divalent cations on spontaneous single cation currents recorded in outside-out patches. A preliminary report has been given to the Physiological Society (Albert & Large, 2000).
METHODS
Cell isolation
New Zealand White rabbits (2–3 kg) were killed by an i.v. injection of sodium pentobarbitone (120 mg kg−1) and the portal vein was removed and placed in normal physiological salt solution (PSS). The tissue was dissected free of connective tissue and fat before being cut into strips and placed in ‘Ca2+-free’ PSS. The tissue was enzymatically dispersed in two sequential enzyme steps. First, the strips of tissue were incubated in ‘Ca2+-free’ PSS with 0.3 mg ml−1 protease type VIII (Sigma) for 5 min and then the strips were washed in ‘Ca2+-free’ PSS. In the second step the strips were incubated with 1 mg ml−1 collagenase type IV (Sigma) in 100 μm Ca2+-PSS for 10 min and were then washed in 100 μm Ca2+-PSS. All enzyme and wash procedures were carried out at 37 °C. After the enzyme treatments the strips were incubated in 100 μm Ca2+-PSS at room temperature (20–25 °C) for 10 min before the cells were released into the solution by gentle mechanical agitation of the strips of tissue using a wide-bore Pasteur pipette. The suspension of cells was then centrifuged (1000 r.p.m.) to form a loose pellet which was resuspended in 0.75 mm Ca2+-PSS. The cells were then plated onto glass coverslips and stored at 4 °C before use (1–6 h).
The normal PSS contained (mm): NaCl (126), KCl (6), CaCl2 (1.5), MgCl2 (1.2), glucose (10), and Hepes (11) and the pH was adjusted to 7.2 with 10 m NaOH. ‘Ca2+-free’ PSS, 100 μm Ca2+-PSS and 0.75 mm Ca2+-PSS had the same composition except that either Ca2+ was omitted or 1.5 mm CaCl2 was replaced by 100 μm CaCl2 and 0.75 mm CaCl2, respectively.
Electrophysiology
Single non-selective cation channel currents were recorded with a List L/M-PC patch clamp amplifier at room temperature using the standard isolated outside-out patch configuration (Hamill et al. 1981) and with recording techniques described previously (Albert & Large, 2001). Patch pipettes were manufactured from borosilicate glass and were routinely coated in Sylgard (Dow Corning, Germany) to reduce stray capacitance and fire polished to increase seal resistance giving pipette resistances between 6 and 10 MΩ when filled with the standard internal patch pipette solution. All experiments were carried out at a holding potential of −50 mV. To evaluate the unitary single cation channel current- voltage (I–V) characteristics, the membrane potential was manually stepped from −50 mV to between −90 mV and +40 mV.
Single cation channel currents were initially recorded onto digital audiotape (DAT) using a CDATA digital tape-recorder (Cygnus Technology Inc., Delaware, PA, USA) at a bandwidth of 5 kHz (−3 dB). For off-line analysis single cation channel current records were filtered at 1 kHz (−3 dB, lowpass 8-pole Bessel filter, Frequency Devices, model LP02, Scensys Ltd, Aylesbury, UK) and acquired using a CED 1401plus interface and CED Patch and Voltage Clamp Software (Version 6.0, Cambridge Electronic Design Ltd, Cambridge, UK) at a sampling rate of 10 kHz. Data were captured with a Pentium (P5–100) personal computer (Gateway, Ireland).
Single cation channel current amplitudes were calculated from idealised traces of at least 10 s duration using the half-amplitude crossing method. I–V relationships were calculated from single channel amplitude histograms in two ways; first, Gaussian curves were fitted to the histograms and the amplitudes of the major peaks, defined by the areas under the curves and reflecting the amplitude of the majority of events, were plotted and the unitary conductance was calculated from the slope of the relationship using linear regression (Origin software, Microcal, USA). The reversal potential (Vr) was calculated directly from the I–V relationship by interpolation. In some experiments, Vr was calculated by extrapolation and these values were similar to those obtained by interpolation. The unitary conductance and Vr from all the individual patches tested were then used to calculate mean conductance and mean Vr values. Secondly, in some experiments the amplitude of the majority of events from individual patches at different membrane potentials were pooled together and the unitary conductance and Vr were determined by linear regression. Lifetime distributions containing events of both the lower and higher conductance states were plotted using either a 0.5 ms or a 1 ms bin width and where appropriate were fitted with one or more exponential functions using the maximum likelihood method. Events which lasted for < 0.664 ms were excluded from analysis. Po was calculated using the equation:
We have previously carried out burst duration analysis by determining a critical value (Tcrit) from closed time distributions to distinguish between closures between bursts of channel activity and closures within a burst of activity (Albert & Large, 2001). In a method similar to Magleby & Pallotta (1983), Tcrit was calculated as 3–4 times the fast closed time constant from closed time distributions with more than one time constant and where the fast time constant was at least 20 times smaller than any other time constant. Figure preparation was carried out using Origin software.
Solutions and drugs
The cells were perfused in a standard K+-free external solution containing (mm): NaCl (126), CaCl2 (1.5), Hepes (11) and glucose (10), pH to 7.2 with 10 m NaOH. In 0 solution CaCl2 was omitted and 1 mm BAPTA was added (< 10 nm free as calculated by EQCAL software, Biosoft, Feguson, MO, USA). In the 3 μm solution 0.934 mm CaCl2 and 1 mm BAPTA were added (calculated using EQCAL software, Biosoft, Ferguson, MO, USA) and in the 100 μm solution 100 μm CaCl2 was added without added BAPTA. For the 1.5 mm and 1.5 mm solutions 1.5 mm CaCl2 was replaced with 1.5 mm SrCl2 or 1.5 mm BaCl2, respectively, and 1 mm BAPTA was added. The standard internal patch pipette solution contained (mm): CsCl (18), caesium aspartate (108), MgCl2 (1.2), Hepes (10), glucose (11), BAPTA (10) and CaCl2 (1) (free internal calcium concentration approximately 14 nm as calculated using EQCAL software); pH to 7.2 with Tris. Under these conditions voltage-gated Ca2+ currents, K+ currents and Ca2+-activated conductances are abolished at the holding potential of −50 mV and single non-selective cation channels could be recorded in isolation. Moreover, with the anion gradient the chloride equilibrium potential (ECl) is about −50 mV, i.e. the holding potential. All drugs were purchased from Sigma (UK). The values are the means of n cells ±s.e.m. Statistical analysis was carried out using Student's t test with the level of significance set at P < 0.05.
RESULTS
Properties of spontaneous single cation channel currents in 1.5 mm and 0
We have shown previously in a fluctuation analysis study that an external solution containing 0, 3 μm or 100 μm produced distinctive effects on the amplitude and kinetic behaviour of the unitary conductance underlying Icat (Helliwell & Large, 1998). Therefore in the present study we have investigated the effects of these concentrations on the properties of spontaneous single non-selective cation channel currents. We initially recorded single cation channel currents in a 1.5 mm external solution and then exchanged the solution for one containing different divalent cation concentrations. This procedure created more stable outside-out patches with a better signal to noise ratio than recording directly with a lower solution.
In the first series of experiments, we compared the activity of single channel currents in 1.5 mm and 0 . Figure 1A(i) illustrates typical spontaneous single cation channel currents recorded in 1.5 mm from an isolated outside-out patch at a holding potential of −50 mV. The appearance of single channel currents was observed after the excision of the outside-out patch from the whole-cell configuration as described previously (Albert & Large, 2001). The majority of the single channel currents had peak amplitudes of approximately −1 to −2 pA at −50 mV although Fig. 1A(i) also shows that there were events which had lower amplitudes. To measure the amplitudes of the channel currents we fitted Gaussian curves to single channel current amplitude histograms. Figure 1B (i) shows that the channel currents from the patch shown in Fig. 1A(i) could be fitted with three Gaussian curves with peaks at −0.75, −1.2 and −1.9 pA. The areas under the curves consisted of 195, 441 and 52 events, respectively, for the −0.75, −1.2 and −1.9 pA peaks indicating that the peak at −1.2 pA is the main open state of the channel currents in 1.5 mm . The smaller currents had similar amplitudes when the single channel currents were lowpass filtered at 2 kHz (−3 dB) indicating their amplitudes were not truncated by over-filtering of brief events. The observation of two amplitude events suggests a possible equilibrium between two conductance states of the cation channel. Figure 1B (i) also shows there were a few openings with a larger amplitude (−1.9 pA) which probably represented multiple openings of both the lower and higher conductance states, indicating there was more than one cation channel in the patch. The higher amplitude events (−1.2 pA) had either brief discrete openings or longer openings containing flickerings between the open and closed states indicating a bursting behaviour (see Colquhoun & Hawkes, 1995). The lower amplitude events (−0.75 pA) opened for brief periods of time with less obvious ‘flickery’ behaviour. In addition, the single channel currents had a low activity at −50 mV (Po < 0.01, see Table 1).
Figure 1. Effect of 0 on spontaneous single cation currents.
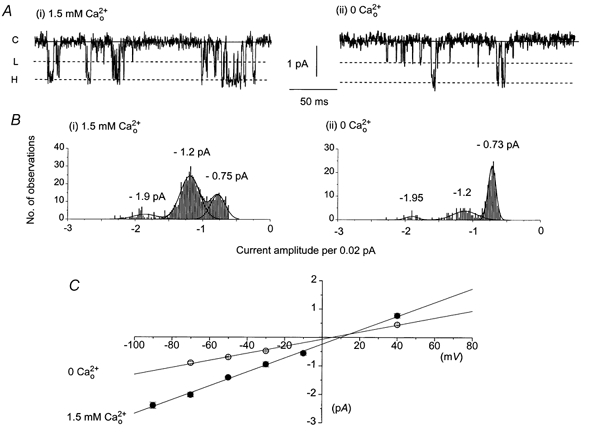
A(i), spontaneous single inward cation currents recorded from an outside-out patch at −50 mV in 1.5 mm . The continuous line represents the closed level (C) and the dashed lines represent open levels. The dashed line marked ‘L’ represents the level of a lower conductance state and ‘H’ the higher conductance state. Note the flickering between closed and open levels denotes bursting behaviour; A(ii), effect of 0 on the single cation currents recorded from the same patch as shown in A(i). B (i), single cation channel current amplitude histogram for the events shown in A(i); B (ii), single channel current amplitude histogram for the channels shown in A(ii). Both histograms could be fitted by three Gaussian curves with different proportions of peaks at approximately −0.7, −1.2 and −2 pA. C, current-voltage (I–V) relationships created from pooled main peak amplitude data for single cation currents recorded in 1.5 mm (•) and 0 (○) .
Table 1.
Characteristics of spontaneous single cation channel currents in different concentrations at a holding potential of −50 mV
| [Ca2+]o | γ (pS) | Vr (ms) | Po | Oτ1 (ms) | Oτ2 (ms) | Bτ1 (ms) | Bτ2 (ms) | Frequency (bursts s−1) | n |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 12 ± 2 L** | +4 ± 2 * | 0.02 ± 0.01 | 0.7 ± 0.1 * | 3.9 ± 0.4 * | 2.9 ± 1.4 | 5.8 ± 1.1 * | 12 ± 3 | 6 |
| 3 μm | 22 ± 2 | +10 ± 2 | 0.11 ± 0.03** | 1.3 ± 0.3 | 17 ± 2.3** | 2.8 ± 0.4 | 43 ± 4** | 17 ± 4 * | 7 |
| 100 μm | 26 ± 2 | +12 ± 2 | 0.15 ± 0.03** | 1.1 ± 0.2 | 6.2 ± 1.1 | 3.9 ± 0.7 | 42 ± 9* | 45 ± 8** | 6 |
| 1.5 mm | 23 ± 1 | +10 ± 1 | 0.05 ± 0.02 | 1.2 ± 0.3 | 7.4 ± 0.2 | 3.1 ± 0.3 | 16 ± 1.4 | 14 ± 1 | 19 |
γ is the single channel conductance.
P < 0.05
P < 0.01 compared with values in 1.5 mM .
Lower conductance state. The kinetic parameters were determined using openings to both the lower and higher conductance states.
Figure 1A(ii) shows the effect of 0 on the channel currents from the same patch as shown in Fig. 1A(i). Changing from 1.5 mm to 0 altered both the amplitude distribution and kinetic behaviour of the single channel currents. In 0 the most noticeable feature is that whereas a few channel currents reach the higher open state most of the channel currents have a smaller amplitude. This is shown in the amplitude histogram which could be fitted with three Gaussian curves with peaks of −0.73, −1.2 and −1.95 pA (Fig. 1B (ii)). The areas under the curves consisted of 163, 80 and 5 events respectively for the −0.73, −1.2 and −1.95 pA peaks indicating that the −0.73 pA peak is the main open state in 0 . These results suggest that the channel exists in two states and that alters the equlibrium between the two states with high favouring the higher conductance state. The small number of larger openings (−1.95 pA, Fig. 1B (ii)) probably represent multiple openings as in Fig. 1B (i).
Figure 1C shows the I–V relationships of the single channel currents. For 1.5 mm the relationship of the larger conductance channel current was plotted since this represented the major event. In 0 the lower conductance channel current was measured as this was the predominant event. In 1.5 mm the I–V relationship behaved ohmically between −90 mV and +40 mV, with a unitary conductance of 23 pS and Vr of +10 mV. In 0 the I–V relationship had an unitary conductance of 13 pS and Vr of +4 mV (Fig. 1C). Table 1 shows that the mean Vr in 0 was significantly different to the mean Vr of the single channel currents in 1.5 mm .
Table 1 shows the mean values of unitary conductance and Vr of single cation currents recorded from individual patches in 1.5 mm or 0 . The mean data were not different from the pooled values. Table 1 shows that, compared to 1.5 mm , in 0 there was no significant difference in Po but there were marked changes in other kinetic parameters of channel activity which are described below.
It was apparent that has an effect on open time distributions. Figure 2A illustrates open time histograms created using both the lower and higher conductance states of the single channel currents shown in Fig. 1A. In 1.5 mm the open time distribution was fitted by two exponentials with time constants of 1.4 ms (Oτ1) and 7.2 ms (Oτ2), i.e. similar to our previous work (Albert & Large, 2001). In 0 the majority of events were open for briefer durations (< 2 ms) and the open lifetime distributions could also be fitted with two time constants of 0.7 ms (Oτ1) and 4.3 ms (Oτ2, Fig. 2A). The mean data for six patches are shown in Table 1. The open time distributions suggest that the cation channels have at least two open states which are both significantly reduced on removing .
Figure 2. The effect of 0 on open time and burst duration distributions.
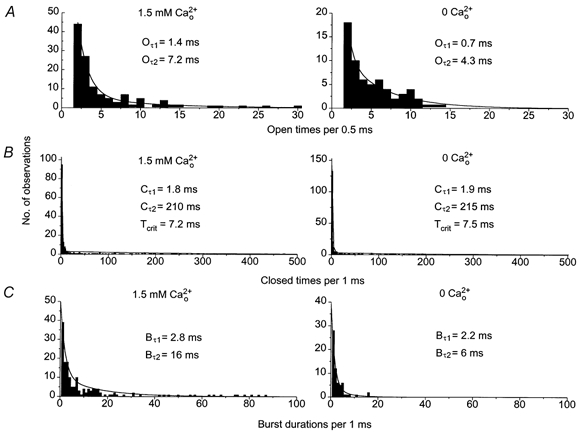
A, open time distributions of the single channel currents shown in Fig. 1A. In 1.5 mm and 0 the distributions could be fitted by the sum of two exponentials with time constants Oτ1 and Oτ2. Changing from 1.5 mm to 0 reduced Oτ1 from 1.4 ms to 0.7 ms and Oτ2 from 7 ms to 4 ms. B, closed time distributions of the single channel currents shown in Fig. 1A could be described by two exponentials with markedly different time constants of approximately 2 ms and 200 ms. The faster time constant (Cτ1) was used to calculate Tcrit (see Methods). Changing from 1.5 mm to 0 did not significantly alter any of these values. C, burst duration distributions of the single channel currents shown in Fig. 1A could both be fitted by the sum of two exponentials with time constants Bτ1 and Bτ2. Changing from 1.5 mm to 0 did not alter Bτ1 whereas Bτ2 was reduced from 16 ms to 6 ms.
Previously, we have shown that these cation channel currents exhibited bursting behaviour and the burst duration histograms were fitted by two exponentials (Albert & Large, 2001). Closures between bursts of single cation channel activity were distinguished from closures within a burst of activity by calculating a critical value (Tcrit) from closed time distributions as shown previously (see Albert & Large, 2001). Figure 2B shows the closed time distributions created from the single channel currents shown in Fig. 1A. In both 1.5 and 0 the closed time distributions could be fitted by the sum of two exponentials with markedly different time constants of approximately 2 ms (Cτ1) and 200 ms (Cτ2), suggesting Cτ1 represents brief closures within a burst of channel activity and Cτ2 represents long closures between bursts. The marked differences between Cτ1 and Cτ2 make it unlikely that the calculated Tcrit values are seriously affected by underestimation of the closed states due to more than one channel in the patch. Figure 2C shows burst duration histograms created from the single channel currents shown in Fig. 1A. In 1.5 mm the burst duration histogram was composed of brief events (< 5 ms) and a similar number of bursts with durations of between 5 ms and 90 ms (Fig. 2C). The histogram could be fitted by two exponentials with time constants of 2.8 ms (Bτ1) and 16 ms (Bτ2). The mean data from 19 patches in 1.5 mm are shown in Table 1 and these values are similar to those reported previously (Albert & Large, 2001).
In Figure 1A(ii) it is not immediately obvious that the lower conductance cation channel currents in 0 exhibit bursting behaviour compared to the channel currents in 1.5 mm . However in 0 the closed time distributions could also be fitted by the sum of two exponentials with mean time constants of 2.6 ± 0.3 ms (Cτ1) and 188 ± 48 ms (Cτ2, n= 6, Fig. 2B). These two vastly different time constants indicate that Cτ1 represents closures within a burst of channel activity and Cτ2 represents closures between bursts of activity and therefore in 0 the cation channels do exhibit bursting behaviour. Figure 2C shows that in 0 the majority of the bursts had brief durations (< 5 ms) with only a few events having burst durations longer than 10 ms and that the distribution could be fitted by the sum of two exponentials with time constants of 2.2 ms (Bτ1) and 6 ms (Bτ2, Fig. 2B). The mean data from six patches are shown in Table 1 and it is evident that reducing from 1.5 mm to a Ca2+-free solution significantly decreased the longer burst duration but did not affect the faster burst period. Therefore it can be concluded that there are marked differences in single channel conductance, open times and burst durations of these single channel currents in 0 and 1.5 mm .
Properties of single cation channel currents recorded in 3 μm and 100 μm
In the present study, we investigated the effects of 3 μm since this concentration is close to the half-maximal facilitatory effect on the amplitude of noradrenaline-evoked Icat recorded with whole-cell recording with little apparent inhibitory effect. We also studied the effect of 100 μm since at this concentration the facilitatory effects of on Icat is near maximal (Helliwell & Large, 1998). In addition, using 3 μm and 100 μm allowed us to compare the properties of the single cation channels with the kinetic and single channel conductance values determined with fluctuation analysis of the whole-cell current (Helliwell & Large, 1998).
Figure 3A shows typical effects of reducing from 1.5 mm to 3 μm on single channel activity in the same patch at −50 mV. In 3 μm the main peak amplitudes of the single channel currents, between 1 and 2 pA at −50 mV, were similar to the amplitudes in 1.5 mm and the unitary conductance was 22 pS (Table 1). Figure 3A also shows that reducing from 1.5 mm to 3 μm increased the time for which the channel currents were in the open state which was manifest as an increase in Po from 0.05 to 0.15 (see Table 1 for mean values). It can be seen in Fig. 3A that the open time of the channel appears to be longer in which is confirmed in Fig. 3B where it can be seen that the longer open time (Oτ2) increases from 7 ms to 19 ms on changing from 1.5 mm to 3 μm but there is little change in the faster open time constant (Oτ1). Also in 3 μm there was an increase in the longer burst duration time constant (Bτ2, 46 ms compared to 14 ms in 1.5 mm ), with no change in the faster burst time constant (Bτ1). Also burst frequency was increased from 6 bursts s−1 in 1.5 mm to 23 bursts s−1 in 3 μm (Fig. 3C and see Table 1).
Figure 3. Effect of reducing concentration from 1.5 mm to 3 μm on single cation channel current activity.
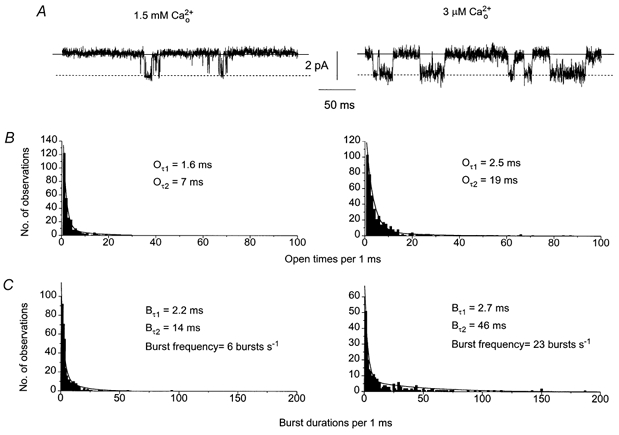
A, effect of reducing from 1.5 mm to 3 μm on single channel currents recorded from the same patch. Note the increased channel activity and longer openings in 3 μm . B, effect of changing from 1.5 mm to 3 μm on the open time distribution of the single cation channels shown in Fig. 3A. Both open time distributions could be described by the sum of two time constants, Oτ1 and Oτ2. Note that reducing to 3 μm did not alter Oτ1 but it did increase Oτ2 from 7 ms to 19 ms. C, effect of reducing from 1.5 mm to 3 μm on the burst duration distributions of the single channel currents shown in Fig. 3A. The burst duration distributions could be described by two time constants, Bτ1 and Bτ2. In 3 μm Bτ1 was not changed whereas Bτ2 was increased from 14 ms to 46 ms.
Changing from 1.5 mm to 100 μm significantly increased channel activity (Fig. 4A) with an increased Po (from 0.06 in 1.5 mm to 0.22 in 100 μm in this patch, also see Table 1). This increased activity was not due to a change in open times (Oτ1 and Oτ2, Fig. 4B, Table 1) but to significant increases in the longer burst duration time constant (Fig. 4C, Bτ2, from 16 ms in 1.5 mm to 45 ms in 100 μm , Table 1) and the burst frequency (from 9 bursts s−1 to 34 bursts s−1, Fig. 4C, Table 1).
Figure 4. Single cation channel activity in 100 μm .
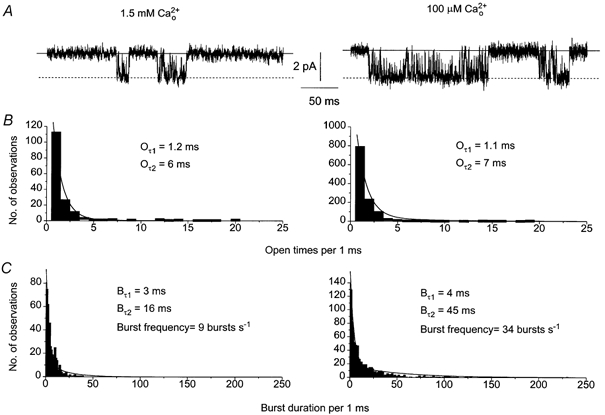
A, effect of reducing from 1.5 mm to 100 μm on channel currents recorded from the same patch. In 100 μm the channel currents showed significantly longer bursts of channel activity. B, effect of reducing from 1.5 mm to 100 μm on the open time distributions of the single channel currents shown in Fig. 4A. Changing from 1.5 mm to 100 μm did not alter Oτ1 or Oτ2. C, burst duration distributions of the single channel currents shown in Fig. 4A. Reducing from 1.5 mm to 100 μm did not change Bτ1 but increased the Bτ2 from 16 ms to 52 ms.
The effects of on the kinetic behaviour of single cation channel currents are voltage dependent
In a recent study we have shown that the spontaneous single cation currents have a higher Po and increased open time and burst duration at +40 mV compared to −50 mV (Albert & Large, 2001) and therefore we have compared the effects of different concentrations on single cation channel activity at −50 mV and +40 mV. Figure 5A, B and C shows the effect of voltage on single cation channel currents recorded from three different patches in different concentrations. Increasing from 3 μm to 1.5 mm had a dramatic effect on the kinetic behaviour of cation channel activity at −50 mV as described previously (Fig. 3 and Fig. 4, Table 1). However, at +40 mV increasing from 3 μm to 1.5 mm had no effect on single cation channel activity (Fig. 5A, B and C) with the single channel currents having similar Po, open and closed times and burst durations (Table 2). Therefore the effect of on the channel current kinetics was voltage dependent and only observed at negative potentials.
Figure 5. Effect of membrane potential on single channel currents in different concentrations.
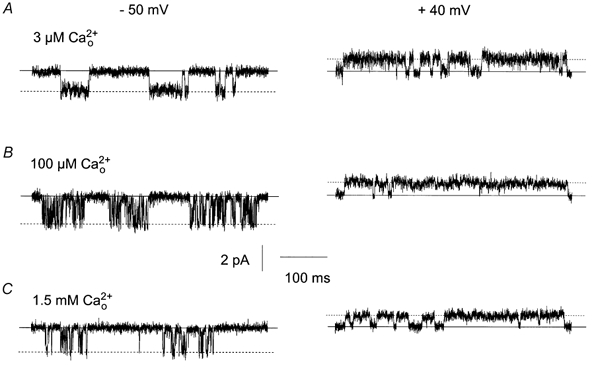
A, B and C, effects of different concentrations on single channel currents recorded from three different patches at −50 mV and +40 mV. Note that single channel currents behave similarly at +40 mV in 3 μm (A), 100 μm (B) and 1.5 mm (C) .
Table 2.
The effects of different concentrations on spontaneous single cation channel currents at a holding potential of +40 mV
| [Ca2+]o | Po | Oτ1 (ms) | Oτ2 (ms) | Bτ1 (ms) | Bτ2 (ms) | n |
|---|---|---|---|---|---|---|
| 3 μm | 0.51 ± 0.14 | 1.4 ± 0.3 | 37 ± 4 | 3.2 ± 0.4 | 128 ± 22 | 4 |
| 100 μm | 0.47 ± 0.11 | 1.7 ± 0.3 | 43 ± 2 | 2.9 ± 0.2 | 112 ± 15 | 4 |
| 1.5 mm | 0.62 ± 0.15 | 1.5 ± 0.2 | 40 ± 8 | 3.8 ± 0.5 | 104 ± 19 | 6 |
The effects of and on spontaneous single cation channel currents
We have shown previously that 1.5 mm and 1.5 mm increased the amplitude of Icat by approximately 8- and 3-fold, respectively, when Icat was evoked in 0 (Aromolaran & Large, 1999); i.e. and also have facilitatory effects on Icat. Therefore we investigated the effect of and on spontaneous single channel currents. Figure 6A shows a typical response of single channel currents produced by the substitution of 1.5 mm with 1.5 mm at −50 mV. In 1.5 mm the single cation channels spent more time in the open state reflecting a significant increase in Po from 0.03 in 1.5 mm to 0.12 in 1.5 mm (see also Table 3). Figure 6B shows the I–V relationship of the cation channel currents recorded in 1.5 mm shown in Fig. 6A. The relationship was linear between −90 and −30 mV with a slope conductance of 25 pS and an extrapolated Vr of +9 mV. Figure 6C shows the open time histogram of the single cation currents shown in Fig. 6A. In 1.5 mm the open time distribution could be described by the sum of two exponentials with time constants of 1.2 ms (Oτ1) and 7 ms (Oτ2), i.e. similar to the values in 1.5 mm . Figure 6D illustrates the burst durations distribution of the single channel currents shown in Fig. 6A. The burst duration histogram could be described by the sum of two exponentials with time constants of 2.5 ms (Bτ1) and 31 ms (Bτ2) indicating that the longer burst duration (Bτ2) was significantly increased on changing from 1.5 mm to 1.5 mm . Table 3 shows the mean values of unitary conductance, Po, mean open and closed times and burst durations in 1.5 mm .
Figure 6. The effect of replacing of 1.5 mm by 1.5 mm on the properties of single cation channel currents.
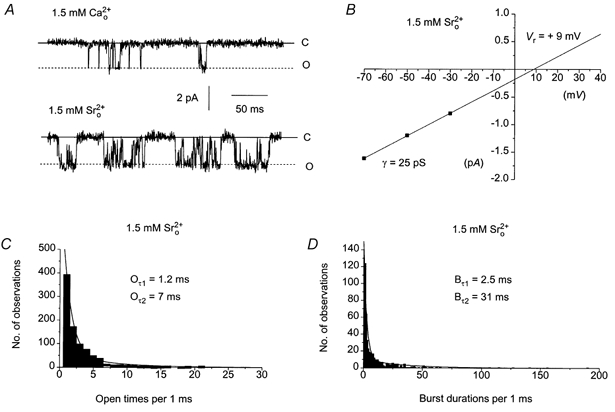
A, effects of replacing 1.5 mm by 1.5 mm on single channel currents recorded from the same patch at −50 mV. B, I–V relationship of the single channel currents shown in A in 1.5 mm had an unitary conductance of 25 pS and an extrapolated Vr of +9 mV. C, the open time distribution of the single channel currents in shown in A could be described by two exponentials with time constants of 1.2 ms (Oτ1) and 7 ms (Oτ2). D, the burst duration distribution of the single channel currents in shown in A could be described by two exponentials with time constants of 2.5 ms (Bτ1) and 31 ms (Bτ2).
Table 3.
Comparison of the properties of the noradrenaline-evoked Icat obtained from spectral density functions and single spontaneous cation channel currents in the presence of various external divalent cations concentrations
| Single channel data | Spectral density analysis of whole-cell current | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Divalent cation concentration | γ (pS) | Po | Oτ1 (ms) | Oτ2 (ms) | Bτ1 (ms) | Bτ2 (ms) | Frequency (bursts s−1) | γ (pS) | τ1† (ms) | τ2† (ms) |
| 0 | 12 ± 2 ** | 0.02 ± 0.01 | 0.7 ± 0.1* | 3.9 ± 0.4* | 3.9 ± 0.7 | 5.8 ± 1* | 12 ± 3 | 11 ± 2 | 1.4 ± 0.1 | 11 ± 1 |
| 3 μm | 22 ± 2 | 0.11 ± 0.03 ** | 1.3 ± 0.3 | 17 ± 2.3 ** | 2.8 ± 0.4 | 43 ± 4 ** | 17 ± 4* | 22 ± 2 | 1.2 ± 0.2 | 44 ± 3 |
| 100 μm | 26 ± 2 | 0.15 ± 0.03 ** | 1.1 ± 0.2 | 6.2 ± 1.1 | 2.9 ± 1.4 | 42 ± 9* | 45 ± 8** | 24 ± 2 | 1.6 ± 0.4 | 45 ± 4 |
| 1.5 mm | 23 ± 1 | 0.05 ± 0.02 | 1.2 ± 0.3 | 7.4 ± 0.2 | 3.1 ± 0.3 | 16 ± 1 | 14 ± 1 | 19 ± 2 | 1.6 ± 0.3 | 15 ± 1 |
| 1.5 mm | 25 ± 2 | 0.14 ± 0.04* | 1.1 ± 0.2 | 6.0 ± 0.7 | 3.6 ± 0.4 | 30 ± 5* | 19 ± 3 | 22 ± 1 | 1.5 ± 0.1 | 24 ± 2 |
| 1.5 mm | 22 ± 1 | 0.06 ± 0.02 | 1.0 ± 0.1 | 5.3 ± 0.8 | 3.7 ± 0.4 | 13 ± 2 | 19 ± 4 | 20 ± 1 | 1.8 ± 0.3 | 12 ± 1 |
These values were obtained from τ=½πfc, where fc is the corner frequency (Helliwell & Large, 1998; Albert et. al. 2001). All mean values are calculated from at least five patches.
P < 0.05
P < 0.01 compared to values in 1.5 mm .
Figure 7A illustrates a typical response of spontaneous single channel currents in 1.5 mm . Changing from 1.5 mm to 1.5 mm increased Po of the single channel currents from 0.05 to 0.10 but although an increase in Po was observed in all patches tested the increase was not statistically significant. Figure 7B shows the I–V relationship of the single channel currents shown in Fig. 7A. The relationship had a unitary conductance of 20 pS and Vr of +11 mV. Figure 7C and D shows the open time and burst duration distributions of the single channel currents shown in Fig. 7A. Both distributions could be described by the sum of two exponentials with time constants of 1.4 ms (Oτ1) and 7 ms (Oτ2) for open times and 2.6 ms (Bτ1) and 19 ms (Bτ2) for burst durations respectively; i.e. the open time and burst duration time constants are similar to the values in 1.5 mm . The mean data are shown in Table 3.
Figure 7. The effect of replacing 1.5 mm by 1.5 mm on the properties of single cation channel currents.
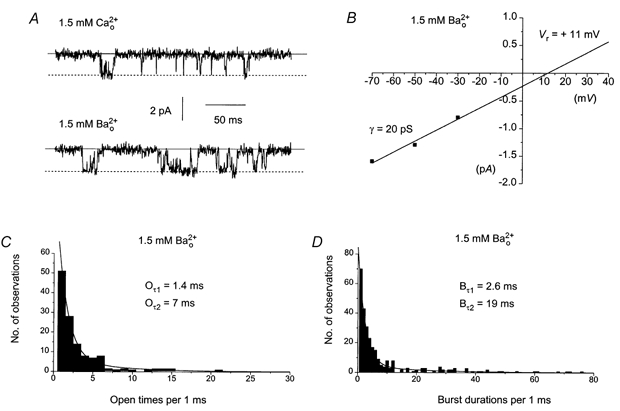
A, effects of replacing 1.5 mm by 1.5 mm on single cation currents recorded from the same patch at −50 mV. B, I–V relationship of the single cation currents shown in A in 1.5 mm had a unitary conductance of 20 pS and an extrapolated Vr of +11 mV. C, the open time distribution of the single channel currents shown in A could be described by two exponentials with time constants of 1.4 ms (Oτ1) and 7 ms (Oτ2). D, the burst duration distribution of the single channel currents shown in A could be described by two exponentials with time constants of 2.6 ms (Bτ1) and 19 ms (Bτ2)
DISCUSSION
Previously we have shown that external divalent cations have a profound effect on the amplitude of the noradrenaline-evoked whole-cell current (Icat) in rabbit portal vein smooth muscle cells (Helliwell & Large, 1996, 1998; Aromolaran & Large, 1999). The present work was undertaken to elucidate the basis of these actions at the single channel current level. In the present study we have shown that external divalent cations have a marked effect on the amplitude and kinetic behaviour of non-selective cation channel currents recorded with isolated patches in the outside-out configuration. In external divalent cation-free conditions the channel currents have a number of distinguishing properties with two conductance levels, at least two open states and a bursting behaviour. Increasing the concentration of external divalent cations alters these properties to produce facilitatory and inhibitory effects on the single channel currents. Here we will discuss the effect of external divalent cations on the properties of single channel currents and how this relates to both the amplitude of Icat and the properties of the unitary conductance estimated by noise analysis of the whole-cell current.
Facilitatory effect of on single non-selective cation channel currents
In all concentrations we observed two conductance states, one of about 12 pS and another of approximately 23 pS. In 0 the majority of the cation channels opened to a conductance state of about 12 pS whereas in higher concentrations most of the channels opened to the higher conductance state. Therefore there seems to be an equilibrium between the two conductance levels which is shifted in favour of the higher conductance state by increasing . Table 3 shows that increasing from 0 to 3 μm almost doubled the unitary conductance to 22 pS. Fluctuation analysis of the noradrenaline-evoked whole-cell current with ≥ 3 μm yields an underlying unitary conductance value of 19–24 pS (Table 3) which suggests that the higher conductance state is predominant in these conditions. Moreover, increasing from 0 to 3 μm increased Po, mainly due to marked increases in the longer open time (Oτ2), from 4 ms to 17 ms, and the longer burst duration (Bτ2), from 6 ms to 43 ms (Table 3). The increases in single channel conductance and Po on increasing from 0 to 3 μm accounts for the 4- to 5-fold increase in the amplitude of the noradrenaline-evoked whole-cell current shown previously (Helliwell & Large, 1998).
Interestingly, in 0 the smaller conductance channel current had a Vr of about +4 mV whereas the Vr of the higher conductance state was about +10 mV. Thus the larger conductance state in 0 had similar characteristics to channels recorded in ≥ 3 μm. It is possible that with 1 mm BAPTA in the bathing solution there may still be pockets of Ca2+ ions in microdomains surrounding the external mouth of some of the channels. In such cases the causes the channel to open to the full conductance state and also Ca2+ permeates the channel with the result that Vr is shifted to more positive values. However, in the majority of cases there are no Ca2+ ions near the channel mouth and therefore the channel only opens to the lower conductance level and no Ca2+ ions permeate the channel with the consequence that the Vr is relatively negative. According to the Boltzmann principle, it would not be expected that the concentration at the channel mouth would be similar in bathing solutions containing 0 and 1.5 mm . However, there might be structural characteristics of the external surface of the channel causing regional differences in concentration at the channel mouth compared to the bulk solution, i.e. a form of compartmentalisation.
With whole-cell recording, the amplitude of noradrenaline-evoked Icat in 100 μm is about double the value in 3 μm (Helliwell & Large, 1998). With single channel recording there are marked changes in channel activity and compared to 3 μm there was an increase in burst frequency, from 17 bursts s−1 to 45 bursts s−1, and a reduction in the longer open time (Oτ2, Table 3) from 17 ms to 6 ms in 100 μm . Presumably, therefore, the doubling of the amplitude of Icat on changing from 3 μm to 100 μm is accounted for by the increase in burst frequency which outweighs the reduction in Oτ2.
Inhibitory effect of on single cation channel currents
With whole-cell recording, increasing above 100 μm reduces the amplitude of Icat and the apparent equilibrium constant for the inhibitory effect of is about 400 μm (Helliwell & Large, 1996). In the present study with single channel recording, the main effect of increasing from 100 μm to 1.5 mm is to reduce the long burst duration (Bτ2) from 42 ms to 16 ms and the burst frequency from 45 bursts s−1 to 14 bursts s−1 (Table 3). This produces a marked reduction of Po (Table 3) which is responsible for the smaller amplitude of Icat in 1.5 mm compared to 100 μm .
The effects of and on single cation channel currents
Table 3 shows that compared to 0 the single cation channel currents in 1.5 mm or 1.5 mm had a 2-fold increase in unitary conductance, indicating that and can substitute for in potentiating single channel conductance. Compared to 0 in 1.5 mm there is a marked increase in Po associated with an increase in the longer burst duration (Bτ2) from 6 ms to 30 ms (Table 3). With whole-cell recording the amplitude of Icat in 1.5 mm is about 7 times larger than in 0 (Aromolaran & Large, 1999) which is accounted for by the increase in single channel conductance and Bτ2. In contrast, Icat is only 2- to 3-fold larger in 1.5 mm compared to 0 (Aromolaran & Large, 1999) which is mainly due to the increase in single channel conductance and a doubling of the longer burst duration (Bτ2).
Mechanisms of action of the facilitatory and inhibitory effects of external divalent cations
The present study shows unequivocally that the facilitatory effect of , and is associated with two distinct effects on single cation channel currents, namely increasing single channel conductance and altering kinetic behaviour. As the experiments were carried out using isolated outside-out patches these effects are probably due to , and binding to sites on the external surface of the membrane. Changing from external divalent-free conditions to 1.5 mm increased single channel conductance but had little effect on the kinetic properties of the cation channels which was also apparent with noise analysis of whole-cell currents (Aromolaran & Large, 1999). It is therefore tempting to speculate that the facilitatory effects of external divalent cations on single channel conductance and kinetic behaviour are mediated by two distinct binding sites. Spectral density analysis of the noradrenaline-evoked Icat also suggested that the facilitatory effect of may be due to Ca2+ binding to two sites since in 1 μm single channel conductance was the same as in 0 but there were marked differences in kinetics (Helliwell & Large, 1998).
A notable feature was that at positive membrane potentials the single cation currents had similar amplitudes and kinetic properties in 3 μm, 100 μm and 1.5 mm . In our experiments the internal Ca2+ concentration was buffered to about 14 nm with 10 mm BAPTA. Consequently the inhibitory effects of on channel activity at negative potentials is due to external Ca2+ ions affecting inward movement of cations through the channel. At −50 mV increasing from 3 μm to 100 μm induced a reduction in mean open time and increased flickerings between the open and closed states during bursts of channel activity. Increasing further from 100 μm to 1.5 mm reduced Po. These are characteristics of a molecule causing channel block (see Hille, 1992). Since we have shown that divalent cations can permeate this conductance (Byrne & Large, 1988; Wang & Large, 1991) it is possible that Ca2+ ions produce an apparent block as they permeate the channel.
Comparison of the single channel data with spectral density analysis data
Table 3 compares the single channel data from the present study with the characteristics of the unitary conductance estimated by spectral density analysis of the whole-cell current evoked by noradrenaline. The values of the single channel conductance predicted by noise analysis of whole-cell current were extremely accurate in all the divalent cation concentrations used. The ‘noise’ spectrum also was described by two Lorentzian components predicting two open states. Single channel analysis confirms this prediction. The fast open time was the same in the two studies but the corner frequency of the second Lorentzian reflects the burst duration and not the longer of the mean open times in all divalent solutions (Table 3).
Acknowledgments
This work was supported by the British Heart Foundation and The Wellcome Trust.
References
- Albert AP, Aromolaran AS, Large WA. Agents that increase tyrosine phosphorylation activate a non-selective cation current in single rabbit portal vein smooth muscle cells. Journal of Physiology. 2001;530:207–217. doi: 10.1111/j.1469-7793.2001.0207l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Large WA. The effect of external calcium ions on spontaneous non-selective cation channels in rabbit portal vein myocytes. Journal of Physiology. 2000;527.P:108P. doi: 10.1111/j.1469-7793.2001.0457k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Large WA. Comparison of spontaneous and noradrenaline-evoked non-selective cation channels in rabbit portal vein myocytes. Journal of Physiology. 2001;530:457–468. doi: 10.1111/j.1469-7793.2001.0457k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromolaran AS, Large WA. Comparison of the effects of divalent cations on the noradrenaline-evoked cation current in rabbit portal vein smooth muscle cells. Journal of Physiology. 1999;520:771–782. doi: 10.1111/j.1469-7793.1999.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne NG, Large WA. Membrane ionic mechanisms activated by noradrenaline in cells isolated from the rabbit portal vein. Journal of Physiology. 1988;404:557–573. doi: 10.1113/jphysiol.1988.sp017306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG. The principles of the stochastic interpretation of ion channel mechanisms. In: Sakmann B, Neher E, editors. Single Channel Recording. New York: Plenum Press; 1995. [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. Dual effect of external Ca2+ on nordrenaline-activated cation current in rabbit portal vein smooth muscle cells. Journal of Physiology. 1996;492:75–88. doi: 10.1113/jphysiol.1996.sp021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. Facilitatory effect of Ca2+ on the noradrenaline-evoked cation current in rabbit portal vein smooth muscle cells. Journal of Physiology. 1998;512:731–741. doi: 10.1111/j.1469-7793.1998.731bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. MA, USA: Sinauer Associates; 1992. [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular αi-adrenoceptor-activated Ca2+-permeable cation channel. Circulation Research. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Magleby KL, Pallotta BS. Burst kinetics of single calcium-activated potassium channels in cultured rat muscle. Journal of Physiology. 1983;344:605–623. doi: 10.1113/jphysiol.1983.sp014958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Large WA. Noradrenaline-evoked cation conductance recorded with the nystatin whole-cell method in rabbit portal vein cells. Journal of Physiology. 1991;435:21–39. doi: 10.1113/jphysiol.1991.sp018496. [DOI] [PMC free article] [PubMed] [Google Scholar]


