Abstract
The present study aimed to investigate the influence of isometric training protocols with long- and short-duration contractions on the elasticity of human tendon structures in vivo. The elasticity was assessed through in vivo determination of the elongation (L) of the tendons and aponeuroses using ultrasonography, while the subjects performed ramp isometric exercise up to maximum voluntary contraction (MVC).
Eight young males completed 12 weeks (4 days per week) of a unilateral isometric training programme on knee extensors, which consisted of two different combinations of contraction and relaxation times at 70 % MVC: one leg was trained using a short-duration protocol (3 sets of 50 repetitions of contraction for 1 s and relaxation for 2 s), and the other leg was trained using a long-duration protocol (4 sets of a combination of contraction for 20 s and relaxation for 1 min). The training volume per session, expressed as the integrated torque, was the same for the two protocols.
Both protocols resulted in a significant increase in MVC: 31.8 ± 17.2 % for the short-duration protocol and 33.9 ± 14.4 % for the long-duration protocol. Moreover, the training produced significant increases in the muscle volume of the constituents of the quadriceps femoris, with similar relative gains for the two protocols: 7.4 ± 3.9 % for the short-duration protocol and 7.6 ± 4.3 % for the long-duration protocol.
The short-duration protocol produced no significant change in L values at any of the force production levels. For the long-duration protocol, however, the L values above 550 N were significantly shorter after training. Analysis revealed that the group × test time interaction effect on tendon stiffness was significant. Stiffness increased significantly for the long-duration protocol, but not for the short-duration protocol.
The present study demonstrates a greater increase in stiffness of human tendon structures following isometric training using longer duration contractions compared to shorter contractions. This suggests that the changes in the elasticity of the tendon structures after resistance training may be affected by the duration of muscle contraction.
Previous findings from animal experiments have shown that the elasticity of tendons can be altered by physical training (Woo et al. 1981; Simonsen et al. 1995). However, the influences of physical training on tendon elasticity differ depending on the exercise protocol used. Woo et al. (1981) indicated that the ultimate strength and stiffness of the pig tendon increased through 12 months ‘endurance training’. In contrast, Simonsen et al. (1995) reported that ‘strength training’ produced no significant changes in the ultimate tensile strength of the rat Achilles' tendon. Pousson et al. (1991) observed a decrease in the stiffness of the rat soleus muscle after 11 weeks of vertical ‘jumping training’.
With regard to the effect of physical training on human tendon structures, longitudinal observation has not yet been performed. Only cross-sectional information on trained individuals is available from previous research (Nakagawa et al. 1989; Kubo et al. 2000a,b). Nakagawa et al. (1989) observed that the cross-sectional area of the Achilles' tendon was similar in strength-trained athletes and untrained persons, while that of runners was greater. From recent findings obtained using real-time ultrasonography, the elasticity of the tendon structures in the knee extensors was more compliant in sprinters and stiffer in long-distance runners compared to untrained persons (Kubo et al. 2000a,b). Taking these in vivo observations into account together with those in vitro, it is hypothesized that adaptation of the tendon structures to physical training varies depending on the combinations of intensity, and contraction and relaxation times during exercise.
In an attempt to clarify our understanding of how physical training changes the elasticity of human tendon structures, real-time ultrasonic measurements were taken in the knee extensor muscles before and after isometric resistance exercises with different contraction times. The purpose of this study was to investigate the influence of isometric training protocols involving long- and short-duration contractions on the elasticity of human tendon structures in vivo.
METHODS
Subjects
Eight healthy males (age, 22.6 ± 2.8 years; height, 171.5 ± 6.1 cm; weight, 69.2 ± 5.8 kg; means ±s.d.) voluntarily participated as subjects. They were fully informed of the procedures to be used as well as the purpose of the study. Written informed consent was obtained from all subjects. None of the subjects were engaged in any sort of competitive exercise or regular exercise programmes. This study conformed to the Declaration of Helsinki and was approved by, and complied with the requirements for human experimentation of, the office of the Department of Sports Sciences, University of Tokyo.
Measurement of elasticity of tendon structures
Measurement of torque
Each subject was seated on the test bench of a dynamometer with hip joint angles of 80 deg flexed (full extension, 0 deg). The axis of the lever arm of the dynamometer was visually aligned with the centre of rotation of the knee joint. The foot was firmly attached to the lever arm of the dynamometer with a strap and fixed with knee joint angles of 80 deg flexed (full extension, 0 deg). After a standardized warm-up and submaximal contractions to enable the subjects to become accustomed to the tests, the subjects exerted isometric knee extension torque from zero (relaxed) to maximum voluntary contraction (MVC) within 5 s. The task was repeated two times per subject with at least 3 min between trials. Torque signals were A/D converted at a sampling rate of 1 kHz (MacLab/8, type ML780, AD Instruments, Japan) and analysed by computer (Macintosh Performa 630, Apple, USA). The measured values given are the means of the two trials.
Measurement of elongation of tendon structures
A real-time ultrasonic apparatus (SSD-2000, Aloka, Japan) was used to obtain a longitudinal ultrasonic image of the vastus lateralis muscle (VL) at a level of 50 % of thigh length, i.e. the distance from the greater trochanter to the lateral epicondyle of the femur. The ultrasonic images were recorded on videotape at 30 Hz, synchronized with recordings of a clock timer for subsequent analyses. The tester visually confirmed the echoes from the aponeurosis and VL fascicles. The point at which one fascicle was attached to the aponeurosis (P) was visualized on the ultrasonic image. P moved proximally during isometric torque development up to a maximum (Fig. 1). A marker (X) was placed between the skin and the ultrasonic probe as an indicator to confirm that the probe did not move during the measurements. The cross-point between the superficial aponeurosis and fascicles did not move. Therefore, the displacement of P (L) was considered to indicate the lengthening of the deep aponeurosis and the distal tendon (Kubo et al. 1999).
Figure 1. Ultrasonic images of the longitudinal sections of the vastus lateralis muscle at rest (A) and during isometric 50 % MVC contraction (B).

The point at which one fascicle was attached to the deep aponeurosis was defined as P. A marker (X) was placed between the skin and the ultrasonic probe as an indicator to confirm that the probe did not move during measurements. The cross-point between the superficial aponeurosis and fascicles did not move. Therefore, the distance travelled by P (P2– P1) was defined as the length change of the tendon and aponeurosis during contraction. VL, m. vastus lateralis; VI, m. vastus intermedius.
Calculation of elasticity
The knee joint torque (TQ) measured by the dynamometer was converted to muscle force (Fm) using the following equation:
where k (0.29 ± 0.04; range, 0.20–0.34) is the relative contribution of VL to the quadriceps femoris muscles in terms of the ratio of the muscle volume, and MA (44.1 ± 1.9 mm; range, 42.8–45.7 mm) is the moment arm length of the quadriceps femoris muscles at 80 deg of knee flexion, which was estimated from the thigh length of each subject (Visser et al. 1990).
Fm and L values above 50 % MVC were fitted to a linear regression equation, the slope of which was adopted as an index of the stiffness (Kubo et al. 1999). In addition, the elastic energy absorption by the tendon structures was proposed by calculating the area below the Fm-L curve from 0 to 100 % MVC, and is referred to as Ee. The intra-class correlation coefficients for the test-retest of the stiffness and Ee were 0.89 and 0.91, respectively. The coefficients of variation of the stiffness and Ee were 6.3 and 6.9 %, respectively.
Magnetic resonance imaging
Measurements of muscle and tendon cross-sectional areas were carried out using magnetic resonance imaging scans (Resona, 0.5 T system, General Electric). T1-weighted spin-echo and axial-plane imaging was performed using the following variables: TR (relaxation time), 450 ms; TE (echo time), 20 ms; matrix, 256 × 172; field of view, 300 mm; slice thickness, 10 mm; and interslice gap, 0 mm. The subjects were imaged in a prone position with the knee kept at 0 deg. Coronal plane images were taken to identify the spina illiaca anterior superior, which is the origin of the sartorius muscle. Consecutive axial images were obtained from the spina illiaca anterior superior to the extremitas distal of the tibia. The number of axial images obtained for each subject was 43–48. The muscles investigated were as follows: m. rectus femoris (RF), VL, m. vastus intermedius (VI) and m. vastus medialis (VM). Each muscle group was outlined using the original film onto tracing paper, which was subsequently scanned to create a digital image. The traced images were transferred to a Macintosh computer (Power Macintosh 7200/120, Apple Computer) and the anatomical cross-sectional area (CSA) calculated using a public domain National Institutes of Health (NIH) image software package (written by Wayne Rasband at NIH and available from the Internet by anonymous ftp from zippy.nimh.nih.gov). The muscle volume was determined by multiplying the anatomical CSA of each image by the thickness (10 mm). In addition, the measurement of the tendon CSA was taken at two sites, one above the patella and the other at 10 mm proximal from the patella. The average CSA at the two positions was calculated as representative of the tendon CSA.
The repeatability of the muscle volume and tendon CSA measurements was investigated on two separate days over a period of 12 weeks in a preliminary study involving six young males (24.2 ± 3.1 years, 169.5 ± 4.8 cm, 70.3 ± 5.4 kg). There were no significant differences between the test and retest values of the muscle volume and tendon CSA. The test-retest correlation coefficients (r) were 0.95 for the muscle volume and 0.97 for the tendon CSA. The coefficients of variation were 2.1 % for the muscle volume and 1.6 % for the tendon CSA.
Measurement of electromyogram
The electromyographic activity (EMG) was recorded during the ramp isometric contraction, i.e. measurement of the tendon properties. Bipolar surface electrodes (5 mm in diameter) were placed over the bellies of VL, RF, VM and biceps femoris (BF) muscles with a constant interelectrode distance of 25 mm. The positions of the electrodes were marked on the skin with small ink dots. These stained dots ensured the same electrode positioning in each test during the experimental period. The EMG signals were transmitted to a computer (Macintosh Performa 630, Apple) at a sampling rate of 1 kHz. The EMG was full-wave rectified and integrated for the duration of the contraction (from relaxed to MVC, 4.9 ± 0.2 s) to give an integrated EMG (iEMG).
Training
The subjects trained four times per week for 12 weeks. The posture of the subject and set-up were similar to those already described for the measurement of tendon elasticity. One leg was trained using a short-duration contraction (SC) protocol and the other leg using a long-duration contraction (LC) protocol. In each subject, the right and left legs were randomly allocated to the training protocols. Both protocols involved isometric knee extensions at 70 % MVC. The SC protocol consisted of three sets of 50 shorter contractions with a 2 s rest between each and a 1 min rest between each set. These shorter contractions involved rapid contraction followed by relaxation. The LC protocol consisted of four contractions of 20 s duration with a 1 min rest between each. The measurement of MVC was made every 2 weeks to adjust the training load. The exerted torque signals during training were integrated with respect to time, and the obtained integrated torque was defined as the iTQ value. In a training session a subject would train the LC protocol leg first then the SC protocol leg, and in the next session the order would be reversed.
Statistics
Descriptive data include means ±s.d. A two-way analysis of variance (ANOVA; 2 (groups) × 2 (test times)) was used to analyse the data. The F ratio for main effects and interactions was considered significant at P < 0.05. Significant differences among means at P < 0.05 were detected using a post hoc test.
RESULTS
The iTQ values during one training session increased significantly by 33.9 ± 18.5 % for the SC protocol and by 36.0 ± 13.8 % for the LC protocol. There were no significant differences in the iTQ values during all the training sessions between the SC (8.4 ± 1.3 × 105 N m s) and LC (8.6 ± 1.7 × 105 N m s) protocols.
The muscle volumes of the knee extensor muscles increased significantly from 2201 ± 441 to 2368 ± 497 cm3 (+7.4 ± 3.9 %; P = 0.018) for the SC protocol and from 2193 ± 448 to 2374 ± 502 cm3 (+7.6 ± 4.3 %; P = 0.023) for the LC protocol. No significant difference in the relative increase in muscle volume was found between the SC and LC protocols. There were no significant differences in the relative increase in the muscle volume among the constituents of the quadriceps femoris muscles (Fig. 2). Furthermore, no significant changes in the tendon CSA were found between both protocols (Table 1).
Figure 2. Relative change in the muscle volumes of m. rectus femoris (RF), VL, VI and m. vastus medialis (VM) before and after isometric training for 12 weeks.
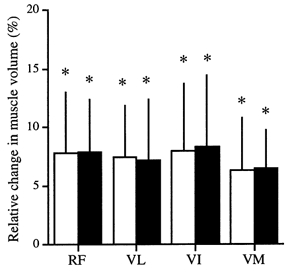
□, SC protocol; ▪, LC protocol. All the muscle volumes increased significantly. However, there appeared to be no differences in the degree of increase in the muscle volume among the knee extensor muscles. *Significantly greater than before at P < 0.05.
Table 1.
Measured parameters before and after training
| Maximum L (mm) | Tendon CSA (mm2) | Stiffness (N mm−1) | Elastic energy (J) | |||||
|---|---|---|---|---|---|---|---|---|
| Protocol | Before | After | Before | After | Before | After | Before | After |
| SC | 32.1 ± 4.8 | 33.4 ± 4.0 | 210 ± 16 | 213 ± 19 | 67.3 ± 23.4 | 79.1 ± 17.8 | 14.8 ± 2.2 | 18.6 ± 1.9* |
| LC | 32.6 ± 3.7 | 31.9 ± 3.7 | 212 ± 18 | 215 ± 21 | 67.5± 21.3 | 106.2 ± 33.4* | 15.0 ± 3.1 | 16.8 ± 3.1* |
Values are means ±s.d.
Significantly different from before.
Figure 3 shows the relationships between Fm and L before and after training. There were no significant differences in the activation levels (iEMG) of each knee extensor muscle before and after training. In addition, we also confirmed that little co-contraction of knee flexor muscles occurred during knee extension. The MVC value increased significantly from 222 ± 41 to 331 ± 47 N m (+31.8 ± 17.2 %; P < 0.001) for the SC protocol and from 219 ± 37 to 310 ± 45 N m (+33.9 ± 14.4 %; P < 0.001) for the LC protocol. The SC protocol produced no significant differences in the L values at all the force production levels before and after training. In the case of the LC protocol, the L values above 550 N were significantly shorter after training. A two-way ANOVA on the stiffness showed a significant effect (P = 0.013) of group × test time interaction. The stiffness increased significantly for the LC protocol (P = 0.003), but not for the SC protocol (P = 0.217; Table 1). The relative increase in stiffness was significantly greater for the LC than for the SC protocol (P = 0.045). In addition, the Ee values increased significantly by 26.3 ± 13.3 % for the SC protocol (P = 0.002) and by 12.9 ± 7.8 % for the LC protocol (P = 0.007), and the relative increase in Ee for the SC protocol appeared to be greater than that for the LC protocol (P = 0.056; Table 1).
Figure 3. Relationship between Fm and L before and after the two kinds of isometric training for 12 weeks.

The extent of elongation after the LC protocol tended to be shorter than that before. *Significantly greater than before at P < 0.05.
DISCUSSION
This study demonstrates a greater increment of stiffness of human tendon structures following isometric training using long-duration compared to short-duration contractions. To our knowledge, this is the first study to demonstrate changes in human tendon properties after resistance training in vivo. The present results suggest that the changes in the elasticity of the tendon structures may be affected by the duration of muscle contraction.
The Fm-L relationship should be converted to the stress-strain relationship to determine accurately the effect of training on the tendon structures. In this study, however, both training protocols produced no significant changes in the tendon CSA (Table 1). Most of the previous studies using animals reported that little change in the size of tendons was induced by immobilization (Amiel et al. 1982; Almeida-Silveira et al. 2000) or training (Rollhauser, 1954; Viidik, 1969; Woo et al. 1981). For example, Viidik (1969) observed that the energy absorbed before failure and the ultimate load of the peroneus brevis tendons of rabbits were higher for animals trained for 40 weeks than for untrained animals, but that the mass as well as the water and collagen content of these tendons did not differ. Similarly, Rollhauser (1954) reported that no increases were observed after 42 days training in the thickness of pig tendon, although the tensile strength increased by up to 12 %. Taking these findings into account together with the present results, we can say that the mass and/or collagen concentration of the quadriceps femoris tendons do not appear to be easily modified by only 12 weeks of training. At the same time, this justifies further discussion of the changes in the elasticity of the tendon structures after training using the Fm-L relationship instead of the stress-strain relationship.
A number of researchers have examined changes in the elasticity of tendon structures in vitro (e.g. Tipton et al. 1975; Woo et al. 1981). Most of these previous studies used endurance exercises as the training protocol, and revealed increases in the force at ultimate failure and stiffness. However, so far only a few attempts have been made at investigating the influences of strength training on the elasticity of tendon structures (Simonsen et al. 1995). According to the limited information available, strength (Simonsen et al. 1995), jumping (Nakagawa et al. 1988; Pousson et al. 1991) or sprint training (Tipton et al. 1975) produced no significant changes in the force at ultimate failure and stiffness, suggesting that an exercise protocol that required short-duration high-force production could not change the elasticity of the tendon structures. This supports our results using the SC protocol. For the LC protocol, however, the mechanisms that resulted in the increase in stiffness remain questionable, although the training protocol did not induce a significant hypertrophic change in the tendon structures. A possible explanation could be that the LC training protocol induced changes in the internal structure of the tendon and/or aponeurosis. Namely, the variability of the mechanical quality of the tendon structures originates from differences in the cross-link pattern or structure of the collagen fibres (Danielsen & Andreassen, 1988). Rollhauser (1954) suggested that, as a result of 42-day training for pigs, the only way in which mature tendons could make positive responses to the chronic exercise was to improve the internal structures of the tendons. This assumption had also been identified using animal models, which provided evidence indicating that excessive loading causes changes in the internal structures of the tendons (e.g. Michna, 1984). Michna (1984) demonstrated that the physical loading induced on rodent tendons caused changes in the degree of alignment of the constituent collagen fibres.
Some researchers have shown that the mean extension of wallaby tail tendons increased slowly during the fatigue test, but increased much faster just before rupture (Wang et al. 1995; Schechtman & Bader, 1997), suggesting that tendon failure would result from cumulative damage. Wang et al. (1995) pointed out that a ‘remodelling’ process of the tendon architecture could be involved during a transient period of mechanical weakness. Recently, we observed that repeated muscle contractions and static stretching had an acute effect and caused human tendon structures to become more compliant (Kubo et al. 2001a,b). Furthermore, while tasks involving the rapid repetition of isometric muscle actions with high-force production did not induce significant changes in the elasticity of tendon structures, the extent of elongation was significantly greater after the task with longer duration contraction at low-force production (Kubo et al. 2001c). This evidence indicates that the longer duration muscle contractions have an acute effect compared to the shorter duration contractions and cause the tendon structures to become more compliant. If the remodelling process, as suggested by Wang et al. (1995), can be applied to the present results, the observed change in the tendon elasticity after the LC protocol might be explained as a result of changes in the internal structure of tendons to compensate for the mechanical weakness induced by the repeated long-duration loading.
The force exerted by the muscle fibres stretches the tendon structures before it is transmitted to the bone. From a functional point of view, therefore, an increase in the stiffness of the tendon structures, as observed in the LC protocol, acts to improve the rate of torque development. This assumption can be indirectly supported by previous studies indicating that strength training increased the rate of force development (Hakkinen et al. 1985; Narici et al. 1996). Furthermore, the increased stiffness in the tendon structures seems to be an advantage for increasing the release of potential energy during stretch-shortening cycle exercises such as jumping or sprinting, since it shortens the coupling time, i.e. the time delay between stretching and shortening (e.g. Bosco et al. 1982). However, some previous researchers demonstrated that isometric training could not improve the vertical jumping ability (Berger, 1963; Ball et al. 1964). As a possible explanation for the smaller gain in jump performance, it has been suggested that the nervous command of contraction during isometric training differed from that during vertical jumping. In addition to this point, more recent observations have shown that the more compliant tendon structures favoured performances during jumping and/or sprinting exercises (Kubo et al. 1999, 2000b). Furthermore, we have shown that the stored elastic energy in the tendon structures was significantly correlated with the difference between the jumping performances with and without counter-movements (Kubo et al. 2000a). Cavagna (1977) indicated that compliant tendon structures would increase the contribution of elastic strain energy, allowing for a greater performance during stretch-shortening cycle exercises. Taking these points into account together with our results using the LC protocol, therefore, the previous evidence that isometric training did not improve the jumping ability could also be explained as a result of an offset of the positive and negative effects on muscle functions, which would be provided by increasing tendon stiffness. However, the SC protocol, compared to the LC protocol, can be characterized as training of a ‘ballistic’ or ‘explosive’ type on the basis of the mode of force production. This type of isometric training has a similar effect on the velocity-torque relationship as that produced by dynamic resistance training at high velocity contractions, i.e. a predominant increase in torque at the higher contraction velocities (Behm & Sale, 1993). Furthermore, it has been well documented that dynamic resistance training requiring ballistic force production increases athletic performance (van Oteghen, 1975; Blattner & Noble, 1979; Wilson et al. 1993). In addition to no gain in the tendon stiffness, the SC protocol tended to show a higher relative increase in Ee than the LC protocol. Hence, it might be assumed that, compared to the LC protocol, the SC protocol is more effective at improving the performance during stretch-shortening cycle exercises owing to the increased muscle strength.
In conclusion, the present results suggest that the adaptations of human tendon structures to isometric resistance training vary with the duration of contractions and, more precisely, the longer duration contraction protocol causes the tendon structures to be stiffer. With regard to the practical applications of these results, we suggest that the changes produced by resistance training in the tendon structures may affect physical performances during stretch-shortening cycle exercises. However, we have no definite information on how the changes induced by resistance training in the tendon properties are correlated to performances during stretch-shortening cycle exercises. Further investigations are needed to clarify this point.
References
- Almeida-Silveira MI, Lambertz D, Perot C, Goubel F. Changes in stiffness induced by hindlimb suspension in rat Achilles tendon. European Journal of Applied Physiology. 2000;81:252–257. doi: 10.1007/s004210050039. [DOI] [PubMed] [Google Scholar]
- Amiel D, Woo SLY, Harwood FL, Akeson WH. The effect of immobilization on collagen turnover in connective tissue: a biochemical-biomechanical correlation. Acta Orthopaedica Scandinavica. 1982;53:325–332. doi: 10.3109/17453678208992224. [DOI] [PubMed] [Google Scholar]
- Ball JR, Rich GQ, Wallis EL. Effects of isometric training on vertical jumping. Research Quarterly for Exercise and Sport. 1964;35:231–235. doi: 10.1080/10671188.1964.10613305. [DOI] [PubMed] [Google Scholar]
- Beger RA. Effects of dynamic and static training on vertical jumping ability. Research Quarterly for Exercise and Sport. 1963;34:419–424. [Google Scholar]
- Behm DG, Sale DG. Intended rather than actual movement velocity determines velocity-specific training response. Journal of Applied Physiology. 1993;74:359–368. doi: 10.1152/jappl.1993.74.1.359. [DOI] [PubMed] [Google Scholar]
- Blattner SE, Noble L. Relative effects of isokinetic and plyometric training on vertical jumping performance. Research Quarterly for Exercise and Sport. 1979;50:583–588. [Google Scholar]
- Bosco C, Vittasalo JT, Komi PV, Luhtanen P. Combined effect of elastic energy and myoelectrical potentiation during stretch-shortening cycle exercise. Acta Physiologica Scandinavica. 1982;114:557–565. doi: 10.1111/j.1748-1716.1982.tb07024.x. [DOI] [PubMed] [Google Scholar]
- Cavagna GA. Storage and utilization of elastic energy in skeletal muscle. Exercise and Sports Sciences Reviews. 1977;5:89–129. [PubMed] [Google Scholar]
- Danielsen CC, Andreassen TT. Mechanical properties of rat tail tendon in relation to proximal-distal sampling position and age. Journal of Biomechanics. 1988;21:207–212. doi: 10.1016/0021-9290(88)90171-6. [DOI] [PubMed] [Google Scholar]
- Hakkinen K, Alen M, Komi PV. Changes in isometric force- and relaxation-time, electromyographic and muscle fibre characterestics of human skeletal muscle during strength training and detraining. Acta Physiologica Scandinavica. 1985;125:573–585. doi: 10.1111/j.1748-1716.1985.tb07760.x. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Elastic properties of muscle-tendon complex in long distance runners. European Journal of Applied Physiology. 2000a;81:181–187. doi: 10.1007/s004210050028. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Elasticity of tendon structures of lower limbs in sprinters. Acta Physiologica Scandinavica. 2000b;168:327–335. doi: 10.1046/j.1365-201x.2000.00653.x. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Effects of repeated muscle contractions on the tendon structures in humans. European Journal of Applied Physiology. 2001a;84:162–166. doi: 10.1007/s004210000337. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Influence of static stretching on viscoelastic properties of human tendon structures in vivo. Journal of Applied Physiology. 2001b;90:511–519. doi: 10.1152/jappl.2001.90.2.520. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Influences of repetitive muscle contractions with different modes on tendon elasticity in vivo. Journal of Applied Physiology. 2001c;91:277–282. doi: 10.1152/jappl.2001.91.1.277. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kawakami Y, Fukunaga T. Influence of elastic properties of tendon structures on jump performance in humans. Journal of Applied Physiology. 1999;87:2090–2096. doi: 10.1152/jappl.1999.87.6.2090. [DOI] [PubMed] [Google Scholar]
- Michna H. Morphometric analysis of loading-induced changes in collagen-fibril populations in young tendons. Cell and Tissue Research. 1984;236:465–470. doi: 10.1007/BF00214251. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Ikegawa S, Abe T, Fukunaga T, Inoue T, Totsuka M, Sekiguchi O, Hirota K. The morphology and SEM in Achilles tendon. Japanese Journal of Physical Fitness and Sports Medicine. 1989;38:424. [Google Scholar]
- Nakagawa Y, Satoh T, Fukuda Y, Hirota K. Effect of aerobic and anaerobic training on collagen fibers of tendons in rats. Japanese Journal of Physical Fitness and Sports Medicine. 1988;37:100–108. [Google Scholar]
- Narici MV, Hoppeler H, Kayser B, Landoni L, Claassen H, Gavardi C, Conti M, Cerretelli F. Human quadriceps cross-sectional area, torque and neural activation during 6 months strength training. Acta Physiologica Scandinavica. 1996;157:175–186. doi: 10.1046/j.1365-201X.1996.483230000.x. [DOI] [PubMed] [Google Scholar]
- Pousson M, Perot C, Goubel F. Stiffness changes and fibre type transitions in rat soleus muscle produced by jumping training. European Journal of Physiology. 1991;419:127–130. doi: 10.1007/BF00372997. [DOI] [PubMed] [Google Scholar]
- Rollhauser H. Funktionelle Anpassung der Sehnenfaser im submikroskopischen Bereich. Anatomical Anzeiger. 1954;51:318–322. [Google Scholar]
- Schechtman H, Bader DL. In vitro fatigue of human tendons. Journal of Biomechanics. 1997;30:829–835. doi: 10.1016/s0021-9290(97)00033-x. [DOI] [PubMed] [Google Scholar]
- Simonsen RB, Klitgaard H, Bojsen-Moller F. The influence of strength training, swim training and aging on the Achilles tendon and m. soleus of the rat. Journal of Sports Science. 1995;13:291–295. doi: 10.1080/02640419508732242. [DOI] [PubMed] [Google Scholar]
- Tipton CM, Matthes RD, Maynard JA, Carey RA. The influence of physical activity on ligaments and tendons. Medicine and Science in Sports. 1975;7:165–175. [PubMed] [Google Scholar]
- Van Oteghen Sl. Two speeds of isokinetic exercise as related to the vertical jump performance of women. Research Quarterly for Exercise and Sport. 1975;46:78–84. [PubMed] [Google Scholar]
- Viidik A. Tensile strength properties of Achilles tendon systems in trained and untrained rabbits. Acta Orthopaedica Scandinavica. 1969;40:261–272. doi: 10.3109/17453676908989506. [DOI] [PubMed] [Google Scholar]
- Visser JJ, Hoogkamer JE, Bobbert MF, Huijing PA. Length and moment arm of human leg muscles as a function of knee and hip-joint angles. European Journal of Applied Physiology. 1990;61:453–460. doi: 10.1007/BF00236067. [DOI] [PubMed] [Google Scholar]
- Wang XT, Ker RF, Alexander RM. Fatigue rupture of wallaby tail tendons. Journal of Experimental Biology. 1995;198:847–852. doi: 10.1242/jeb.198.3.847. [DOI] [PubMed] [Google Scholar]
- Wilson GJ, Newton RU, Murphy AJ, Humphries BJ. The optimal training load for the development of dynamic athletic performance. Medicine and Science in Sports and Exercise. 1993;25:1279–1286. [PubMed] [Google Scholar]
- Woo SL, Gomez MA, Amiel D, Ritter MA, Gelberman RH, Akeson WH. The effects of exercise on the biomechanical and biochemical properties of swine digital flexor tendons. Journal of Biomechanical Engineering. 1981;103:51–56. doi: 10.1115/1.3138246. [DOI] [PubMed] [Google Scholar]


