Abstract
Single unit activity was recorded with the microneurography technique from sixteen spindle afferents and one Golgi tendon organ afferent originating from the forearm extensor muscles. Impulse rates were studied while subjects performed unobstructed aiming movements at the wrist in eight different directions 45 deg apart. In addition, similar imposed movements were performed while the subject was instructed to remain relaxed. Movement amplitudes were about 5 deg and the speed 10–30 deg s−1. Joint movements were translated to movements of a cursor on a monitor to provide visual feedback.
Individual spindle afferents modulated their activity over a number of targets, i.e. were broadly tuned, during these aiming movements. The preferred direction for a spindle afferent was the same during both passive and active movements, indicating that the fusimotor effects associated with active contractions had little or no effect on the direction of tuning.
The direction of tuning of individual spindle afferents could be predicted from the biomechanically inferred length changes of the parent muscle. Thus spindle afferents responded as stretch receptors, i.e. impulse rates increased with lengthening and decreased with shortening, in active as well as passive movements.
Spindles from muscles, which continuously counteracted gravity exhibited a stretch response and directional tuning during the phase of movement alone whereas their position sensitivity was poor. In contrast, spindle afferents from the muscles that had no or minimal antigravity role were directionally tuned during both the dynamic and the static phase of the aiming task and their position sensitivity was substantially higher.
In spite of the limited data base from three extensor muscles it could be demonstrated that wrist joint position was remarkably well encoded in the ensemble muscle spindle data. In some cases the ensemble muscle spindle data encoded the instantaneous trajectory of movement as well.
It is well established that sensory activity arising from muscle afferents contributes not only to segmental feedback control of movement, but also to the conscious coding of position and movement in humans (Goodwin et al. 1972; Gandevia, 1996; Prochazka, 1996). Theoretical models of motor control and psychophysical results posit roles for such sensory feedback in higher order processes such as motor planning, motor prediction, state estimation and motor learning (Jeannerod, 1988; Scott & Loeb, 1994; Wolpert, 1997). However very little is known about the expected patterns of sensory information arising from human muscle afferents when volitional movements make use of several degrees of freedom available at a given joint. For example, it is not clear how the data from simple flexion/extension movements at the finger should be extrapolated to more complex movements. This paper presents the first report of human muscle afferent activity during voluntary multi-degree of freedom hand movements that give rise to a complex pattern of active length changes. Muscle afferent responses to imposed movements about two axes of the ankle have been described in a recent study (Bergenheim et al. 2000).
Previous studies have established that in a resting human muscle the spindle afferents behave like stretch receptors with distinct response patterns for primary and secondary afferents. During active movements the simplicity of a stretch response may be masked by the simultaneous activation of the fusimotor systems (Burke et al. 1978; Hulliger et al. 1982; Al-Falahe et al. 1990). While to date the human neurography data have emphasized the co-activation of the skeletomotor and fusimotor systems (e.g. Kakuda et al. 1996; Gandevia et al. 1997), experiments from freely moving cats have clearly demonstrated that the two systems can be modulated independently according to the motor task (Prochazka et al. 1985). It is not known how the human fusimotor systems may be engaged in a more complex paradigm such as the aiming experiments described here.
The goals of the present study were to determine if the sensory information from muscle spindles coded for the direction and position of the hand in wrist joint space, to determine how the fusimotor system may modify muscle spindle coding during these movements and to investigate the possible ensemble population coding of muscle spindles in different forearm muscles during voluntary precision hand movements. In light of psychophysical experiments (Roll & Gilhodes, 1995; Verschueren et al. 1998), our a priori prediction was that proprioceptive coding of hand movements would be expressed by a series of vectors related to the biomechanics of the wrist musculature. The results of the present study support this hypothesis. Part of this work has previously been reported in abstract form (Jones et al. 1998).
METHODS
Subjects
Data were collected from twelve subjects, four males and eight females, aged 21–31 years. Informed consent was obtained according to the Declaration of Helsinki; the Ethical Committee for the Medical Faculty of Göteborg University approved the study. Seventeen muscle afferents were recorded from the finger and wrist extensor muscles of the left arm using the microneurography method.
Experimental set-up and movement recording system
Each subject was seated in a semi-reclined position with the left forearm resting on a horizontal platform that extended to the wrist leaving the hand free. The forearm was stabilized in a neutral position 45 deg supinated from full pronation using a vacuum splint. The splint was carefully adjusted to permit free wrist rotation around the flexion/extension (Flex/Ext) and radial/ulnar deviation (Rad/Uln) axes (Brumbaugh et al. 1982; Moore et al. 1993).
An optoelectronic position tracking system monitored the kinematics of the hand movements (REMAC, Department of Physiology, Umeå University, Sweden; Sandström et al. 1996). A triad of reflective markers (each a 7 mm diameter sphere) was mounted to a lightweight frame and fixed to the back of the hand. The camera viewed the hand from above so the three markers were visible in the field of view directly as well as indirectly from the lateral side via the reflection in a mirror (float glass, 20 cm × 20 cm). The REMAC system tracked the positions of the markers that were then sampled at 100 Hz using SC/ZOOM (Department of Physiology, Umeå University; Sandström et al. 1996). A calibration frame consisting of eight reflective markers at the corners of a precision-machined cube was used to calibrate the REMAC signals. All hand movements occurred within the volume of the calibration cube (for review see Allard et al. 1995).
Signals from the REMAC system were used to control the movement of a cursor on a monitor placed in front of the subject. With the forearm positioned 45 deg supinated from full pronation, Flex/Ext movements produced diagonal cursor movements roughly between the top left and bottom right corner of the monitor while Rad/Uln movements tracked perpendicular to those of Flex/Ext.
Electromyography recording
Electromyographic (EMG) signals were recorded from the extensor carpi radialis (ECR), extensor digitorum communis (EDC) and flexor carpi radialis (FCR) muscles with surface electrodes. EMG signals were sampled at 1600 Hz and for some analysis were digitally root mean square rectified off-line in SC/ZOOM.
Microneurographic recording, unit identification and unit sample
An insulated tungsten electrode (impedance 400–700 kΩ at 1 kHz) was inserted in the radial nerve approximately 7–12 cm proximal to the elbow. The electrode was manipulated until single unit impulses clearly stood out from the background noise (Vallbo et al. 1979). The nerve signal was sampled at 12.8 kHz and off-line analysis was performed to discriminate single unit activity.
Only slowly adapting units whose receptors were localized by palpation over the extensor muscles and responded to either passive wrist movements or to isometric extension of the wrist or fingers were recorded. Care was taken to identify the muscle of origin and, in the case of EDC units, the finger that elicited the best passive response from the afferent was identified. Units were classified as Golgi tendon organ afferents or spindle afferents by their responses firstly to intrafascicular electrical stimulation, where a Golgi tendon organ will show increased firing during the muscle twitch due to loading of the tendon, whereas a muscle spindle will be unloaded (McKeon & Burke, 1980; Burke et al. 1987). In addition, passive ramp stretches and active isometric contractions were also used to distinguish Golgi tendon organs from muscle spindles (Edin & Vallbo, 1990a,b). Data from the same tests were used to tentatively classify spindle afferents as primary or secondary afferents. Recordings from 17 muscle afferents were analysed, five spindle afferents from EDC, two from ECU, and nine from ECR, and, in addition, one Golgi afferent from ECR.
Experimental protocol
The main experimental protocol consisted of a visual targeting task. The subjects were asked to move a cursor displayed on a monitor from a central home position, to one of eight targets arranged in circle (on-screen radius of 5 cm, which translated to 5–6 deg of joint rotation) around the home position (Fig. 1). Immediately before the experiment proper, the subjects had a short training session to make sure that they understood the task. The posture of the hand was aligned to an initial neutral wrist position, corresponding to the home position on the monitor, where the long axis of the third metacarpal was parallel with the long axis of the radius while the forearm was 45 deg supinated from full pronation. To hold the hand against gravity in this neutral position, the subject had to maintain a constant low-level muscular contraction, mainly in the ECR muscles. No extra load or obstacle to movement was added so the hand was free to move in the wrist joint space. At the beginning of a set of tests the cursor was lit and the subject was asked to move the cursor to the home position and be prepared to perform targeting movements from this position. Following a period of 4 s in the home position, one of eight targets was lit and an audio cue given to prompt the subject to move the cursor into the target box. The primary instruction was to move accurately to the target whilst maintaining a reasonable speed. The size of the target box compared with the cursor was relatively small, thus the task required a high degree of precision to acquire the target (Fitts, 1954). The emphasis on accuracy necessarily resulted in relatively slow movements with an average duration of 634 ± 19 ms (mean ±s.d.) giving an average rotational velocity of 9–10 deg s−1 with peaks in the range 20–30 deg s−1. The subject was then asked to hold the cursor within the target box for a period that varied between 3 and 4 s. Subsequent to this period both the target and cursor were extinguished while the subject was asked to maintain the same position without visual feedback for a period of 3 s. The trial concluded when the cursor was re-lit and an audio cue was given to prompt the subject to return to the home position and prepare for the presentation of another target.
Figure 1. Experimental set-up and protocol.
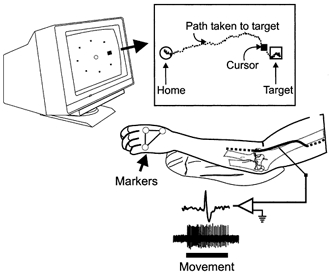
A monitor was placed directly in front of the subject. Movements of the subject's hand were translated into movements of a cursor on the monitor. The subject was required to move the cursor into the central circle (home position) in response to visual and audio cues for a period of about 2 s after which an unknown target was lit. The subject was requested to move directly to the target and hold that position. The inset illustrates the path of the cursor movement from the home position to the target. Simultaneously, single unit activity was recorded from the radial nerve.
In addition to the active movements to the eight targets, the experimenter manually imposed wrist movements of similar trajectories while the subject was requested to remain relaxed.
Data analysis
The raw data describing marker movements in two dimensions was used to reconstruct the three-dimensional (3-D) movements using the direct linear transformation (DLT) technique (Marzan, 1975; Woltring & Huiskes, 1990). The standard deviation of the wrist angles in the home position was ±0.2 deg over a series of aiming trials. This confirmed that the static positional accuracy of our tracking system was adequate. A fixed 3-D co-ordinate system was established by calibrating the camera-mirror system with the calibration cube. The forearm was fixed in position with respect to this co-ordinate system thus permitting the calculation of Flex/Ext angles about a fixed axis relative to the neutral wrist position. Similarly, Rad/Uln angles were calculated relative to the hand in the neutral wrist position.
Each trial (moving from the home position to a target) was divided into six separate phases (Fig. 2): (1) home hold time (HHT); (2) reaction time (RT); (3) movement time (MT); (4) position holding with vision (PHv), when the subject was holding the cursor within the target box and had visual feedback of both the target and cursor; (5) position holding without vision (PH-v; in some cases the analysis of the position holding phase did not distinguish between the presence or absence of visual feedback and in those cases is referred to as PH); and (6) return home time (RH). The onset and termination of movement, which determined the duration of the MT phase, was set at the points where the tangential velocity record exceeded the mean plus two standard deviations of the tangential velocity during HHT. During the RH phase the subjects returned to the central starting position in a casual manner resulting in often circuitous and disjointed kinematics. This phase of the trials was not analysed further. The bars in the lower part of Fig. 2 indicate the time during which the cursor and target were visible.
Figure 2. Phases of the aiming task.
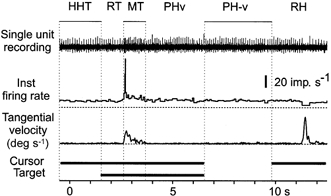
Traces show, from above, the raw nerve recording, the instantaneous firing rate of the unit and the tangential velocity during an aiming movement. At the bottom of the figure the horizontal bars indicate the times during the task when the cursor and the target were visible. The movement to a single target is broken into six separate phases: HHT, home hold time; RT, reaction time; MT, movement time; PHv, position hold time with visual feedback; PH-v, position hold time without visual feedback; RH, return to the home position.
The neutral wrist position was defined as the origin of a Cartesian co-ordinate system whose area was determined by the extent of Flex/Ext movements (along the x-axis) and Rad/Uln movements (along the y-axis). This Cartesian system was transformed into a polar co-ordinate system. In the polar system pure flexion movements pointed toward the right at 0 deg, pure extension movements to the left at 180 deg, radial deviation movements pointed upwards at 90 deg and ulnar deviation movements pointed downwards at 270 deg. The activity of a single unit was represented by a vector with a length, determined either by the instantaneous or mean firing rate, and direction, determined either by the direction of wrist movement or the target location.
The firing rate of the unit with movements to different targets was tested for directional tuning using re-sampling methods (Fisher, 1993). The advantages of these procedures are that no assumptions need to be made about the form of the probability distribution from which the data are drawn and that the methods are particularly effective for small data sets. The first stage is to use vector averaging to calculate the direction and length of the resultant mean vector. The direction of the mean vector is the preferred direction for activation of the sensory receptor. The second stage involves a random re-sampling, or shuffling, of the original data followed by vector averaging to calculate an estimate of the resultant mean vector. This was repeated 4000 times to generate 4000 estimates of the length of the resultant mean vector. The length of the mean vector from the original data was compared with the 4000 estimates. The unit was considered directionally tuned if the length of the mean vector from the original data was greater than 95 % of the estimated lengths (P < 0.05).
RESULTS
Sixteen muscle spindle afferents and one Golgi tendon organ afferent were analysed for modulation of firing rates during active and/or passive movements of the hand about the two axes of the wrist. The subjects participated in an aiming task with eight targets arranged in a circular fashion around the neutral wrist position (Fig. 3). Fourteen afferents were recorded during active trials to all eight targets, one afferent to six targets (EDC IV) and nine units during imposed passive movements to the same nine targets. In addition, active repeat trials to single targets were studied in 10 units. The total number of active trials was 163 with a range of one to seven trials to a single target with the individual afferent. The total number of passive trials was 72.
Figure 3. Modulation of muscle spindle firing with movements to the eight target locations.
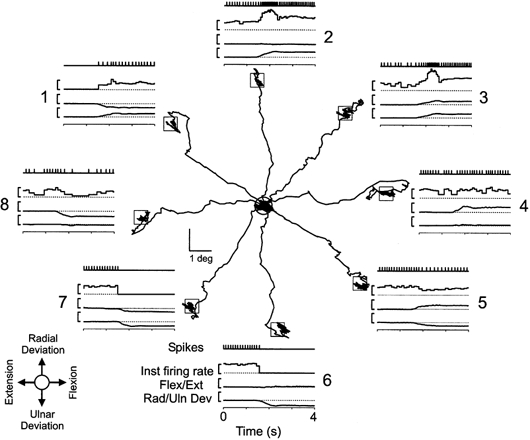
The trajectories of the movements from the central home position to the eight target locations in wrist joint space while recording from an ECU primary muscle spindle afferent. The inset (lower left) gives the directions of wrist movement with respect to the home position. Around the circumference are eight insets that illustrate the temporal occurrence of spikes, instantaneous firing rate, flexion/extension and radial/ulnar deviation during the movement to the associated target. Each inset is 4 s in duration, centred on the initiation of movement, i.e. the MT phase. The vertical calibration bars to the right of the instantaneous firing rate and wrist rotation traces represent 10 impulses s−1 and 10 deg, respectively. The targets were presented in the order 1, 8, 3, 4, 6, 7, 2 and 5.
Afferent response during active movements to different targets
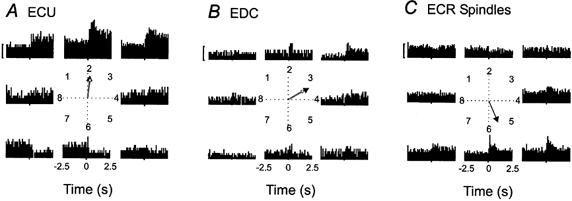
In general, the firing rate of a particular afferent increased with movements to some targets and decreased with movements to other targets, as illustrated in Fig. 3 for an ECU primary muscle afferent. When the subject moved the cursor to targets 2 or 3, the parent muscle was stretched, and the unit responded with an increased firing rate during the dynamic phase of the task followed by an elevated steady-state discharge rate in the target position. In contrast, the unit immediately silenced upon initiation of movement to targets 6 and 7, which involved shortening of the parent muscle.
Qualitative observations with the other spindle afferents implied that the units from a particular muscle had preferred targets. These were targets 1–3 for ECU spindles, targets 4–7 for ECR spindles, and targets 2–6 for EDC spindles. With movements in opposite directions the firing often decreased. These findings indicated that spindle firing generally increased with active movements to targets that involved lengthening of the muscle and vice versa. Finally, the single Golgi afferent of the sample exhibited a reverse target response pattern compared with the spindles of the parent muscle, as will be detailed below.
Directional tuning of single muscle afferents
The modulation of the firing rate of a muscle afferent with movements to the separate targets, illustrated in Fig. 3, was reminiscent of directional tuning as described in cortical and spinal neurons (Georgopoulos et al. 1982; Bosco & Poppele, 1993; Scott & Kalaska, 1997; Herrmann & Flanders, 1998). A vector analysis approach, similar to that used in these studies, was undertaken to quantify the relationship between direction and muscle afferent activity. This analysis is illustrated in Fig. 4 for the ECU primary unit of Fig. 3, for three phases of the task, i.e. movement time (MT), position holding with vision (PHv) and position holding without vision (PH-v). Instantaneous rate vectors were calculated for the occurrence of each spike during the appropriate phase. Each vector had an angle determined from the Cartesian co-ordinates of the wrist joint angle at the moment of spike occurrence and a length equivalent to the instantaneous firing rate. The dynamic increase in firing rate, evident in Fig. 3 with movements to targets 2 and 3, can be seen in the MT phase as the presence of long vectors in the direction of these targets. In addition, the lack of firing during movements to targets 6 and 7 is reflected by the absence of vectors towards these targets. Differences between the response during movement and position holding are seen as an increased variability of the vector angles and longer length during the movement time compared with position holding. On the other hand the difference between vision and no-vision during position holding was negligible. Further analyses of the total sample indicated that there was no statistical difference between the vision and no-vision phases (repeated measures ANOVA) and the two were considered together as the PH phase in all other analyses. This finding confirms a previous study demonstrating that fusimotor activity is not substantially modified when subjects are denied visual information on joint and target position (Wessberg & Vallbo, 1995).
Figure 4. Vector analysis for testing directional tuning during different phases of the aiming task.
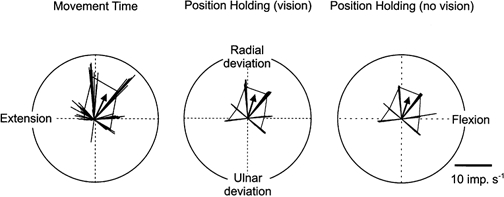
Polar plots demonstrating afferent activity in terms of vectors for an ECU primary spindle afferent in relation to wrist joint position. The thin lines represent vectors calculated at the occurrence of each spike. The length of the vector is equal to the instantaneous firing rate at the time of the spike while the angle of the vector is determined by hand position at that time. Average intratarget vectors were calculated from the instantaneous vectors related to a given target. The tips of these average intratarget vectors are at the cusps of the underlying polygon. A global mean intertarget vector (arrow) shows the directional tuning of the unit.
The statistical distribution of the total number of instantaneous rate vectors was tested for directional tuning of the individual muscle afferents. First, average intratarget vectors were calculated for each phase to each target. The corners of the polygons in Fig. 4 indicate the directions and lengths of these average intratarget vectors. An overall intertarget mean vector was then calculated for each phase. The arrow shows the mean intertarget vector (Fig. 4). Upon evaluation, using a non-parametric re-sampling test, as described in Methods, this ECU primary muscle spindle afferent was considered directionally tuned during the three phases of the aiming task that are illustrated in Fig. 4, whereas it did not exhibit directional tuning during the other phases that were tested, i.e. HHT and RT.
Directional tuning during active aiming movements
Tuning data of 17 units are summarized in Table 1. Fourteen afferents were tested during active movements to all eight targets. The majority exhibited directional tuning during the movement phase (MT) and some during position holding (PH) on the targets as well. In contrast, none of the afferents exhibited directional tuning during the phases preceding the movement when the subjects were holding on the home position, i.e. the HHT and the RT phase (cf. Flament et al. 1992). However, there was a difference between spindles from the separate muscles, i.e. none of the ECR spindle afferents exhibited directional tuning during the PH phase, whereas most of the EDC and ECU afferents did. The direction of tuning is given as the theta angle in Table 1. It may be appreciated that spindles from a particular muscle had similar preferred directions. A comparison between the two groups of putative primary and secondary muscle spindle afferents revealed no clear difference with regard to tuning. The single Golgi tendon organ afferent from the ECR muscle was directionally tuned during both the MT and PH phases.
Table 1.
Directional tuning of wrist muscle afferents
| Active | Passive | ||||
|---|---|---|---|---|---|
| Muscle | Unit type | Phase(s) | Theta (deg) | Phase(s) | Theta (deg) |
| EDC [V] | Ia | MT, PH | 38, 42 | n.p. | — |
| [IV] | Ia | n.s. | — | n.p. | — |
| [III] | Ia | MT, PH | 17, 12 | MT, PH | 16, 12 |
| [III] | II | PH | 337 | n.p. | — |
| [II] | II | MT, PH | 69, 77 | n.p. | — |
| ECU | Ia | MT, PH | 107, 108 | MT, PH | 89, 90 |
| II | PH | 52 | MT, PH | 85, 95 | |
| ECR | Ia | MT (MT) | 286 (277) | MT, PH | 300, 301 |
| Ia | MT | 215 | n.p. | — | |
| Ia | MT | 307 | n.p. | — | |
| Ia | n.p. | — | MT, PH | 281, 277 | |
| Ia | n.p. | — | MT, PH | 289, 287 | |
| II | MT | 309 | n.p. | — | |
| II | MT | 267 | MT | 276 | |
| II | n.s. | — | n.p. | — | |
| II | n.s. | n.s. | n.p. | — | |
| Ib | MT, PH | 116, 123 | n.s. | — | |
The entries in the columns marked ‘Phase(s)’ give the codes for the phases with significant directional tuning, ‘n.s.’ indicates that significant tuning was not significant in all phases of the trial whereas ‘n.p.’ indicates the test was not performed. Unit type abbreviations: Ia, muscle spindle primary afferent; II, muscle spindle secondary afferent; Ib, Golgi tendon organ afferent. In the case of one ECR Ia afferent, trials to all eight targets were completed twice with the results from the second trial given in parentheses. Theta values are given in degrees with 0/360 deg being the direction of a pure flexion movement, 90 deg pure radial deviation, 180 deg pure extension and 270 deg a pure ulnar deviation movement as illustrated in Fig. 3.
Directional tuning during passive versus active movements
Imposed movements were carried out on eight units to compare spindle response in two conditions that presumably involved different fusimotor drives. The subjects were encouraged to relax completely and allow the experimenter to manually move their hand. An example from an ECR primary unit is illustrated in Fig. 5A, which also shows its response during active aiming movements (thick lines). The ECR muscle is stretched during the movement to target 6 and shortened during the movement to target 2. The records are representative in showing qualitatively similar kinematics during passive and active movements although the spindle firing was stronger during the latter. Most units exhibited a stronger bias during active compared with passive movements indicating that active trials were associated with an increased fusimotor drive.
Figure 5. Comparing directional tuning during passive and active aiming in an ECR primary afferent.
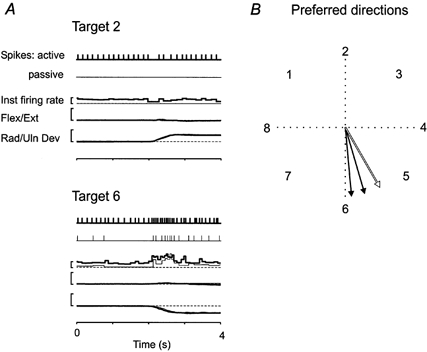
A, trials for two of the eight targets are illustrated during active (thick lines) and passive (thin lines) movements. The occurrence of spikes, instantaneous firing rate, flexor/extensor and radial/ulnar is shown for both an active and a passive trial. The similarity of trajectories of the active and passive movements is evident from the overlap of the Flex/Ext and Rad/Uln traces. The active trial is associated with an increase bias in the activity of the afferent producing stable firing prior to movement and compensating for muscle shortening during the movement to target 2. Calibration bars represent 10 impulses s−1 and 10 deg joint rotation. B, the location of the mean vectors for two trials of active aiming (filled arrows, both during the MT phase) and the passive aiming trial (open arrow, the same arrow for both MT and PH phases) are illustrated on the polar plot. The preferred direction of the unit, indicated by the arrows, is similar for the two active trials that were separated by a 5 min interval and is not significantly different during the passive trial.
Data from two sets of active aiming movements and one set of passive movements to all eight targets were collected from this unit. The phases exhibiting significant directional tuning are shown in Fig. 5B. Significant mean vectors during active movements (filled arrows) occurred only during the MT phase. During passive movement the mean vectors during both the MT and PH phase were significant (the vectors for the two phases were virtually identical with regard to length as well as direction, thus only one outline arrow is indicated). This finding suggests a weaker position response in active compared with passive trials in spite of a stronger fusimotor drive in the former.
The polar plot of Fig. 5B is representative in demonstrating the similarity of preferred directions during active and passive movements (Table 1). The difference in the angle of the individual unit's active and passive vectors (theta) ranged from 0.1 to 42.8 deg with a mean (±s.d.) of 15.8 ± 12.8 deg (5 units, 7 phases). Thus it seems that the influence of any fusimotor activity associated with the active aiming movements had a relatively small effect on the preferred direction.
Pooled activity of spindle afferents in active movements
Since the spindle afferents from a given muscle tended to have similar preferred directions, it seemed reasonable to pool the afferent activity recorded from the individual muscles. The data for a given muscle were grouped by constructing histograms of spike occurrence centred on the initiation of movement to a particular target (Fig. 6).
Figure 6. Pooled activity of muscle spindle afferents during active aiming movements.
Histograms are centred on the initiation of movement and include 2.5 s prior to movement and 2.5 s following movement onset. The phases of movement to a target in the pooled data were defined as: HHT, -2.5-0 s; MT, 0-0.7 s; PH, 0.8-2.5 s. The polar plot in the centre of each panel illustrates the mean vectors for the MT (filled arrow) and PH (open arrow) phases. The calibration bars are equivalent to 10 impulses s−1. A, pooled activity for two ECU muscle spindle afferents with an average of 6.6 trials per target. B, pooled activity for five EDC muscle spindle afferents with an average of 6.9 trials per target. C, the pooled activity of eight ECR muscle spindle afferents with an average of 8.9 trials per target. The central polar plot illustrates the mean vector during the MT phase, as this was the only directionally tuned phase of the task.
ECU and EDC muscle spindle afference
The pooled data from two ECU and five EDC muscle spindles are shown in Fig. 6A and B, respectively. The pooled impulse rates of the ECU spindles increased substantially for movements that involved a radial deviation (targets 1–3) whereas impulse rates decreased for movements in opposite directions (targets 6 and 7). The static response was particularly prominent, i.e. the difference between the PH phase and the HHT. For the EDC muscle spindles, movements to targets 2–4 were associated with a moderately elevated activity (Fig. 6B).
To quantify the relationship between pooled spindle activity and wrist joint position, mean vectors were calculated and are illustrated in the polar plots of Fig. 6. The pooled ECU and EDC samples were directionally tuned towards radial deviation and wrist flexion, respectively, during both the dynamic (MT) and static (PH) phases of the active aiming task as shown by the arrows in the polar plots (Fig. 6A and B). For comparison, tuning during passive movements was computed as well, and it turned out that the vectors were nearly identical to those for the active movements with regard to direction as well as length.
ECR muscle spindle afference
The modulation of the activity of the pooled ECR muscle spindles during the active aiming task is illustrated in Fig. 6C. The response increased during movements that involved ulnar deviation (targets 5–7) as well as pure flexion (target 4). The impulse rate did not decrease much for movements in the directions opposite to the optimal targets. In fact, the quantitative analysis demonstrated that the pooled ECR spindle data were directionally tuned during the dynamic (MT) phase alone but not during the static (PH) phase of the active aiming task. The polar plot of Fig. 6C shows the vector during the MT phase of active aiming. On the other hand, during passive imposed movements, directional tuning was significant during both the MT and PH phases in pooled data from ECR muscle spindles (not shown). This would imply that active aiming might be accompanied by a loss of directional tuning for ECR muscle spindle afferents during the static phase of position holding due to fusimotor activity associated with the task.
Spindle afferent activity related to tendon excursion
As muscle spindles are basically length receptors it seemed of interest to relate their response and the directional tuning data to tendon excursion of the parent muscles. Estimates of tendon excursion were developed from the literature describing the biomechanics of the wrist musculature (Loren et al. 1996; Gonzalez et al. 1997). The directions of maximum tendon excursion according to these estimates are illustrated in Fig. 7A as the filled circles on the circumference of the polar plot. In addition, full-drawn lines indicate the mean vectors during the MT phase of the three separate muscle spindle samples. The correspondence between directions of the afferent vectors and directions of maximal tendon excursion estimates is obvious. This result suggested that the response of the muscle spindles was dominated by stretch even in active movements when the fusimotor system was engaged. It was interesting that the pooled data yielded vectors of similar length for the two prime wrist movers (ECR and ECU), whereas the length of the EDC vector was considerably shorter. The length of a mean vector is a measure of how strongly tuned the activity is, i.e. largely the amount of dispersion between the individual unit vectors. This increased dispersion of EDC vectors, as indicated by the relatively short mean vector, is in keeping with the dispersed tendon excursion estimates for the different digits indicated in Fig. 7A.
Figure 7. Comparison of the pooled muscle afferent activity and tendon excursion estimates.
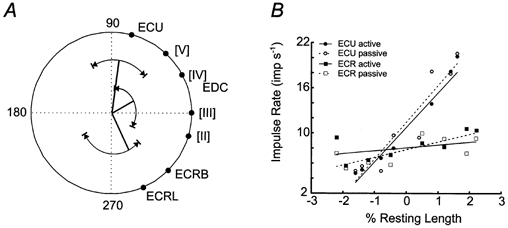
A, mean vectors are shown for the MT phase of spindle afferents from three muscles based on pooled data. Each mean vector of the spindle sub-samples is capped by an arc that illustrates the range of the directional tuning from the single unit data. Around the perimeter of the polar plot the directions of maximum tendon excursion for the forearm muscles are indicated. The spatial congruence of the population vectors and the tendon excursion estimates for ECU and ECR suggest that the muscle spindles in the respective muscles monitor muscle length in wrist joint space. B, impulse rate during position holding (PH phase) at each target is plotted in relation to the estimated tendon excursion in the parent muscle. Tendon excursion was normalized to percentage resting length to show the position sensitivity of the spindles.
The tuning vector of the single ECR Golgi tendon organ afferent was found to be almost exactly opposite to the direction of the maximal tendon excursion of the parent muscle. This implies, that the vector of the tendon organ coincides with the optimal direction of active muscle shortening (not illustrated). This finding fits with the interpretation that the tendon organ is activated in proportion to the amount of active muscle contraction, rather then movements of the tendon.
The relationship between tendon excursion estimates and pooled activity of the muscle afferents was further investigated by plotting impulse rate during the PH phase against tendon excursion at each target (Fig. 7B, data from EDC has been omitted due to lack of sufficient anthropometrical data during radial/ulnar deviation). Tendon excursion estimates were normalized to the resting length of the respective muscles (ECU resting muscle length 182 mm; average ECR resting muscle length 110 mm; from Lieber et al. 1990). Significant linear relationships between impulse rate and normalized length changes were found for ECU spindles (active, P < 0.001, r2 = 0.92; passive, P < 0.001, r2 = 0.88) and ECR spindles in the passive condition (active, P = 0.3, r2 = 0.16; passive, P = 0.006, r2 = 0.74). The slopes, i.e. the position sensitivity (Matthews, 1972), for the ECU spindles in the passive and active conditions were not significantly different and had values of 5.0 and 4.7 impulses s−1 (% resting length)−1, respectively. The slope of the line for the pooled passive ECR spindle data was 1.0 impulses s−1 (% resting length)−1.
Muscle afferent co-ordinate systems
Although we did not have access to spindle data from more than three of the 15 muscles crossing the wrist joint, it seemed of interest to explore to what extent our limited sample would be sufficient to compute the direction of movement of the wrist joint. Therefore the ensemble response pattern was calculated based on pooled data from all muscle spindle afferents.
Figure 8 illustrates the outcome of this analysis for movements to target 2. The thin lines in Fig. 8A represent the average vectors from the entire sample of muscle spindles calculated at 100 ms intervals starting at the onset of movement and continuing through the PH phase. The filled arrow illustrates the mean vector calculated from the thin lines occurring during the MT phase. Similarly, the open arrow is the mean vector calculated from the thin lines occurring during the PH phase. It can be seen that the mean MT and PH vectors for the ensemble data are pointing very much in the direction of the target. This analysis was repeated for all eight targets and it was found that the vectors during the MT and PH phases pointed very accurately towards the target in the case of targets 2–6. For the remaining targets the ensemble data were not good estimates of the direction of movement, as may be expected considering that many afferents were silent with movements to these targets involving shortening of the parent muscles. A reasonable assumption is that spindles in the wrist flexor muscles might provide the information required to encode movements to targets 1, 7 and 8, i.e. movements that include wrist extension.
Figure 8. Population analysis of muscle spindle activity according to the vector hypothesis.
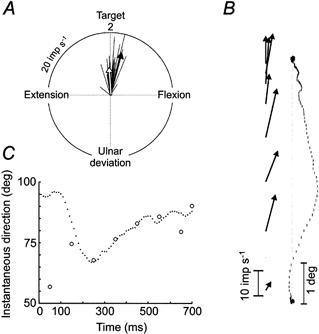
A, mean vectors were calculated each 100 ms (thin lines) based on the activity and directional tuning of the grouped muscle spindle data in Fig. 6A, B and C during movements to target 2. The filled arrow is the mean vector during the dynamic (MT) phase of the movement and the open arrow is the mean vector during the static (PH) phase of the movement. B, relationship between details of the direction of the ongoing movement trajectory and the direction of the population vector calculated at 100 ms intervals during the MT phase. The curvature of the averaged trajectory seems to be reflected in the directions of the population vectors. C, overlay plot of instantaneous direction of the ongoing trajectory and the angle of the population vector. Apart from the discrepancy of the initial angle of the population vector, the direction of the population vector resembles the direction of the ongoing trajectory.
In Fig. 8A the directions of the MT vector (filled arrow) and the PH vector (open arrow) were not identical, i.e. the former was slightly skewed to the right. A sequential analysis of the dynamic phase (Fig. 8B) revealed an interesting relationship, i.e. that the directions of spindle vectors derived from 100 ms time windows match the instantaneous directions of the movement trajectory. In Fig. 8C the instantaneous direction of the movement trajectory is plotted with the angle of the ensemble spindle vector. After an initial large discrepancy between the two, the direction of the spindle vector superimposed the direction of the instantaneous trajectory. These findings suggest that our sample of spindle afferents may, in fact, predict movement trajectory remarkably well.
DISCUSSION
During the process of sensorimotor integration, a variety of sensory signals are merged to provide a spatial reference frame upon which motor plans are constructed. To determine the neurophysiological basis for encoding a two-dimensional reference frame using muscle afferents, spindle afferents were recorded from extensor muscles crossing the wrist joint. It was reasoned that the control parameters that co-ordinate the large number of muscles that cross the wrist joint were substantially more complex than parameters controlling simple flexion and extension movements at the finger or ankle joints. Hence it seemed of interest to explore whether voluntary precision movements at the wrist joint were associated with a different pattern of fusimotor activation compared with finger flexion/extension movements. We found that spindle impulse rates were similarly low as in previous human studies and the sensitivity of the afferents was not generally higher. Perhaps disappointingly, the present data provide further evidence that human spindle afferents fire at substantially lower rates than spindles in the hindlimb muscles of behaving cats. Nevertheless, because the wrist joint is of significant importance for most precision movements of the upper extremity in man, it seems that spindle afference from wrist muscles during natural movements may be particularly relevant to our understanding of the role played by proprioceptive activity in the control of a complex and highly encephalized motor system in man.
Stretch response of spindle afferents
It was obvious that most spindles responded as stretch receptors to muscle length changes not only during imposed movements but during active aiming movements as well (Fig. 7B). As a group ECU and EDC spindle afferents provided a clear stretch response in both the dynamic and the static phase of the trial (MT and PH phases, Fig. 6) although the response was generally larger during the dynamic phase (cf. Kakuda & Nagaoka, 1998). The quantitative analysis indicated that the ECU spindle afferents exhibited high position sensitivity that was similar during active and passive trials (Fig. 7B).
The ECR spindle afferents, on the other hand, clearly deviated from the others because their stretch response was essentially limited to the movement phase (MT) whereas it was very small or lacking altogether in the phase of position holding (Fig. 6). Thus the position sensitivity of the ECR spindle afferents was small in the passive state and insignificant during active movement (Fig. 7B). One factor that might be relevant for the poor position response of the ECR spindle is the unique role of the parent muscle in the task. Since the subject's forearm was fixed at 45 deg from full pronation, a continuous activity in ECR muscle was required to keep the hand in any of the eight target positions to cope with the effect of gravity. The ECU and EDC, on the other hand, contributed less or not at all to the antigravity force. Perhaps the continuous contraction of the ECR muscle during active aiming trials to the eight targets was associated with a continuous fusimotor drive that might have a different profile than any fusimotor activity to the ECU and EDC muscles. On the other hand, this would not explain the low sensitivity of the ECR spindle afferents in passive trials.
Apart from the effects of fusimotor modulation on impulse rates, muscle spindles are susceptible to variation due to the effect of contractile history of the extra- and intrafusal muscle fibres (Proske et al. 1993). This effect is particularly apparent when comparing responses to stretch following manoeuvres designed to make or break crossbridges prior to the stretch. It is unlikely that this effect will play much of a role in the variation of impulse rate with movements to different targets in the present data as each movement follows at least 2 s of active contraction in the central home position. Thus each stretch is preceded by a manoeuvre designed to ensure a similar amount of crossbridge formation.
Some research has indicated that self-generated movements with low velocities, below 0.2 resting lengths per second (RL s−1) in the cat, are associated with a pattern of fusimotor activity that fully compensates for muscle length changes so that spindle impulse rates remain invariant during the movement or even increase with shortening (Hulliger et al. 1982; Prochazka, 1999). Since a dynamic stretch response was present in most spindle afferents, it was obvious that the fusimotor effects in the present data were not strong enough to dominate over effects of muscle length changes even though the velocity of the movements rarely exceeded 0.1 RL s−1. However, a certain degree of fusimotor compensation for length changes might account for the poor position sensitivity and the lack of directional tuning during active position holding in the ECR spindles. This finding suggests a balanced α-γ co-activation in the major muscle acting to hold the hand against gravity that maintains firing rate constant at different positions (Hulliger et al. 1982).
Directional tuning of spindle afferents
Directional tuning was analysed using a similar approach as described in studies of cortical and spinal neurons (Georgopoulos et al. 1982; Bosco & Poppele, 1993; Scott & Kalaska, 1997; Herrmann & Flanders, 1998). Although the data collected from an individual spindle afferent in the present study were smaller than commonly used with central neurons, the analysis seems justified because the variation between repeat trials was small, confirming previous demonstrations that the response of the individual spindle afferent is remarkably uniform when voluntary contractions are repeated (Edin & Vallbo, 1990b; Vallbo & Al-Falahe, 1992).
The quantitative analysis indicated that most of the single muscle spindles exhibited directional tuning, i.e. a distribution of activity that was statistically different from a uniform distribution. In fact, as many as 79 % or 11 of 14 were directionally tuned during some phase of active aiming movements (Table 1). As illustrated in Figs 3–5 the tuning of the individual afferent was fairly broad in the sense that firing was modified with movements to a large number of targets. Moreover, it was obvious that the direction of tuning of individual spindle afferents could be roughly predicted from the biomechanically inferred length changes of the parent muscle (Fig. 7).
Directional tuning was common not only in the active trials when the subjects themselves performed the movements but in passive trials as well, i.e. when similar movements were imposed while subjects were instructed to remain relaxed. Moreover, it was striking that the afferents tested during both active and passive trials (n = 5) had the same preferred direction in the two conditions. The similar tuning was not due to identical fusimotor drive because higher spindle impulse rates indicated higher fusimotor activity in active movements (Fig. 5A). Thus it seemed that the preferred direction was not substantially modified by the increased fusimotor activity associated with the voluntary movements.
Ensemble data predicted direction of movement
The analysis of the ensemble data indicated that direction of movement was predicted remarkably well on the basis of the limited database available. Thus for a range of targets (5 of 8) that involved stretch of the extensor muscles, the target direction was effectively encoded in the ensemble activity of the sample of extensor muscle spindles. It remains to be assessed if the remaining targets would be covered if a wider database had been available including afferents from the wrist flexor muscles. Moreover, it was demonstrated that not only the direction of the target was predicted in the ensemble spindle afference but details of the unfolding movement could be accurately encoded as well (Fig. 8). In active aiming movements to target 2 the average trajectory was conspicuously curved in one direction. The analysis of the ensemble data demonstrated that the shifts in instantaneous direction of the ongoing wrist joint movement were well reflected in the instantaneous direction of the ensemble spindle vector. In contrast, details of movement did not seem to be well reflected in the single unit traces (Fig. 3). Hence, the ensemble analysis emphasizes that a population approach, rather than single unit analyses, might be necessary to reveal essential features of the proprioceptive afference. The recent study by Bergenheim et al. (2000) has also emphasized the ensemble response in encoding of passive movements at the ankle.
Behavioural and perceptual implications
The broad and overlapping tuning of extensor muscle afferents demonstrated in the present study is interesting in relation to perceptual and behavioural effects of tendon vibration, a stimulus known to effectively excite muscle spindle primary afferents (Hagbarth & Eklund, 1966; Goodwin et al. 1972). It has been demonstrated that vibration of the wrist muscle tendons produces illusions of movements as well as errors in performance of a motor co-ordination task (Roll & Gilhodes, 1995; Verschueren et al. 1998). These findings strongly suggest that the ensemble spindle afference from the muscles crossing the joint is a crucial input to derive joint position and movements. Our analysis of pooled spindle data from a few muscles suggests that the discrimination of movements in different directions is well represented by the shifts in the pattern of spindle activity evoked across the population, supporting the emphasis on the ensemble response (Roll & Gilhodes, 1995; Verschueren et al. 1998). Furthermore, the role of spindle afference from an individual muscle for the ensemble pattern suggested by our data seems to fit with the behavioural analysis. For instance, the mean vector for the EDC spindles in our sample is most closely oriented toward wrist flexion whereas the vectors for the ECU and ECR spindles diverge from flexion toward radial and ulnar deviation, respectively. The differential orientations of the vectors imply that vibration of the EDC tendons would produce a larger error with regard to wrist flexion than a similar vibration of the ECU or ECR tendons. This implication has been verified during differential vibration of the wrist tendons (Verschueren et al. 1998).
Psychophysical data have shown that the sense of position is more accurate during active movement compared with passive movement about a joint (reviewed in Jeannerod, 1988). One explanation for the difference in acuity of position sense in the two conditions has been that, in the relaxed state, the fusimotor output is weak or absent, thus, the gain of the muscle spindles is low. Our data suggest that there is little statistical difference in the directional tuning of muscle spindles in the passive and active conditions. This would not support the idea of reduced gain in spindle afference being the cause of the difference in sensory acuity. If the gain of the afferent system is not the underlying cause of the discrepancy, perhaps the presence of an efference copy, or corollary discharge, during active movements and its absence in passive movements is a more likely source for the psychophysical result.
Acknowledgments
This study was supported by the Swedish Medical Research Council (project 3548) and The Bank of Sweden Tercentenary Foundation. K.E.J. was supported by a long-term fellowship from the Human Frontier Science Program Organization. We would like to thank Sven-Öjvind Swahn for technical support and Maria Setterbom and Karin Göthner for help with the figures.
References
- Al-Falahe NA, Nagaoka M, VallboÅ B. Response profiles of human muscle afferents during active finger movements. Brain. 1990;113:325–346. doi: 10.1093/brain/113.2.325. [DOI] [PubMed] [Google Scholar]
- Allard P, Stokes IAF, Blanchi J-P. Threedimensional Analysis of Human Movement. Champaign, IL, USA: Human Kinetics; 1995. [Google Scholar]
- Bergenheim M, Ribot-Ciscar E, Roll JP. Proprioceptive population coding of two-dimensional limb movements in humans: I. Muscle spindle feedback during spatially oriented movements. Experimental Brain Research. 2000;134:301–310. doi: 10.1007/s002210000471. [DOI] [PubMed] [Google Scholar]
- Bosco G, Poppele RE. Broad directional tuning in spinal projections to the cerebellum. Journal of Neurophysiology. 1993;70:863–866. doi: 10.1152/jn.1993.70.2.863. [DOI] [PubMed] [Google Scholar]
- Brumbaugh RB, Crowninshield RD, Blair WF, Andrews JG. An in-vivo study of normal wrist kinematics. Journal of Biomechanical Engineering. 1982;104:176–181. doi: 10.1115/1.3138345. [DOI] [PubMed] [Google Scholar]
- Burke D, Aniss AM, Gandievia SC. In-parallel and in-series behaviour of human muscle spindle endings. Journal of Neurophysiology. 1987;58:417–426. doi: 10.1152/jn.1987.58.2.417. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth K-E, Löfstedt L. Muscle spindle activity in man during shortening and lengthening contractions. Journal of Physiology. 1978;277:131–142. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, VallboÅ B. Dynamic response of human muscle spindle afferents to stretch. Journal of Neurophysiology. 1990a;63:1297–1306. doi: 10.1152/jn.1990.63.6.1297. [DOI] [PubMed] [Google Scholar]
- Edin BB, VallboÅ B. Muscle afferent responses to isometric contractions and relaxations in humans. Journal of Neurophysiology. 1990b;63:1307–1313. doi: 10.1152/jn.1990.63.6.1307. [DOI] [PubMed] [Google Scholar]
- Fisher NI. Statistical Analysis of Circular Data. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movements. Journal of Experimental Psychology. 1954;47:381–391. [PubMed] [Google Scholar]
- Flament D, Fortier PA, Fetz EE. Response patterns and postspike effects of peripheral afferents in dorsal root ganglia of behaving monkeys. Journal of Neurophysiology. 1992;67:875–889. doi: 10.1152/jn.1992.67.4.875. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Kinesthesia: roles for afferent signals and motor commands. In: Rowell LB, Sheperd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 128–172. [Google Scholar]
- Gandevia SC, Wilson LR, Inglis JT, Burke D. Mental rehearsal of motor tasks recruits α-motoneurones but fails to recruit human fusimotor neurones selectively. Journal of Physiology. 1997;505:259–266. doi: 10.1111/j.1469-7793.1997.259bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. Journal of Neuroscience. 1982;2:1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez RV, Buchanan TS, Delp SL. How muscle architecture and moment arms affect wrist flexion-extension moments. Journal of Biomechanics. 1997;30:705–712. doi: 10.1016/s0021-9290(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. Proprioceptive illusions induced by muscle vibration: contribution by muscle spindles to perception? Science. 1972;175:1382–1384. doi: 10.1126/science.175.4028.1382. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Eklund G. Motor effects of vibratory muscle stimuli in man. In: Granit R, editor. Muscular Afferents and Motor Control. Stockholm: Almqvist & Wiksell; 1966. pp. 177–186. [Google Scholar]
- Herrmann U, Flanders M. Directional tuning of single motor unit. Journal of Neuroscience. 1998;18:8402–8416. doi: 10.1523/JNEUROSCI.18-20-08402.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M, Nordh E, VallboÅ B. The absence of position response in spindle afferent units from human finger muscles during accurate position holding. Journal of Physiology. 1982;322:167–179. doi: 10.1113/jphysiol.1982.sp014030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M. The neural and behavioural organization of goal-directed movements. Oxford: Oxford University Press; 1988. [Google Scholar]
- Jones KE, Wessberg J, VallboÅ B. Response characteristics of human muscle afferents during unrestrained wrist movements. Society for Neuroscience Abstracts. 1998;652:15. [Google Scholar]
- Kakuda N, Nagaoka M. Dynamic response of human muscle spindle afferents to stretch during voluntary contraction. Journal of Physiology. 1998;513:621–628. doi: 10.1111/j.1469-7793.1998.621bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda N, VallboÅ B, Wessberg J. Fusimotor and skeletomotor activities are increased with precision finger movements in man. Journal of Physiology. 1996;492:921–929. doi: 10.1113/jphysiol.1996.sp021358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber RL, Fazeli BM, Botte MJ. Architecture of selected wrist flexor and extensor muscles. Journal of Hand Surgery. 1990;15A:244–250. doi: 10.1016/0363-5023(90)90103-x. [DOI] [PubMed] [Google Scholar]
- Loren GJ, Shoemaker SD, Burkholder TJ, Jacobson MD, Friden J, Lieber RL. Human wrist motors: Biomechanical design and application to tendon transfers. Journal of Biomechanics. 1996;29:331–342. doi: 10.1016/0021-9290(95)00055-0. [DOI] [PubMed] [Google Scholar]
- McKeon B, Burke D. Identification of muscle spindle afferents during in vivo recordings in man. Electroencephalography and Clinical Neurophysiology. 1980;48:606–608. doi: 10.1016/0013-4694(80)90297-7. [DOI] [PubMed] [Google Scholar]
- Marzan GT. Rational design for close-range photogrammetry. 1975. PhD Thesis, University of Illinois at Urbana-Champaign. [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and their Central Actions. London: Edward Arnold Publishers Ltd; 1972. [Google Scholar]
- Moore JA, Small CF, Bryant JT, Ellis RE, Pichora DR, Hollister AM. A kinematic technique for describing wrist joint motion: analysis of configuration space plots. Proceedings of the Institution of Mechanical Engineers. 1993;207:211–218. doi: 10.1243/PIME_PROC_1993_207_299_02. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Rowell LB, Sheperd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 89–127. [Google Scholar]
- Prochazka A. Quantifying proprioception. Progress in Brain Research. 1999;123:133–142. [PubMed] [Google Scholar]
- Prochazka A, Hulliger M, Zangger P, Appenteng K. Fusimotor set: new evidence for α-independent control of γ-motoneurones during movement in the awake cat. Brain Research. 1985;339:136–140. doi: 10.1016/0006-8993(85)90632-8. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal-muscle and in muscle-spindles: A review. Progress in Neurobiology. 1993;41:705–721. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Roll JP, Gilhodes JC. Proprioceptive sensory codes mediating movement trajectory perception: human hand vibration-induced drawing illusions. Canadian Journal of Physiology and Pharmacology. 1995;73:295–304. doi: 10.1139/y95-040. [DOI] [PubMed] [Google Scholar]
- Sandström G, Bäckström A, Olsson K Å. REMAC: A video-based motion analyzer interfacing to an existing flexible sampling system. Journal of Neuroscience Methods. 1996;69:205–211. doi: 10.1016/S0165-0270(96)00079-9. [DOI] [PubMed] [Google Scholar]
- Scott SH, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. I. Activity of individual cells in motor cortex. Journal of Neurophysiology. 1997;77:826–852. doi: 10.1152/jn.1997.77.2.826. [DOI] [PubMed] [Google Scholar]
- Scott SH, Loeb GE. The computation of position sense from spindles in mono- and multiarticular muscles. Journal of Neuroscience. 1994;14:7529–7540. doi: 10.1523/JNEUROSCI.14-12-07529.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VallboÅ B, Al-Falahe NA. Human muscle spindles in sampling and reproduction of precision finger movements. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle Afferents and Spinal Control of Movement. Oxford: Pergamon Press; 1992. pp. 143–150. [Google Scholar]
- VallboÅ B, Hagbarth K-E, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiological Reviews. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Verschueren SMP, Cordo PJ, Swinnen SP. Representation of wrist joint kinematics by the ensemble of muscle spindles from synergistic muscles. Journal of Neurophysiology. 1998;79:2265–2276. doi: 10.1152/jn.1998.79.5.2265. [DOI] [PubMed] [Google Scholar]
- Wessberg J, VallboÅ B. Human muscle spindle afferent activity in relation to visual control in precision finger movements. Journal of Physiology. 1995;482:225–233. doi: 10.1113/jphysiol.1995.sp020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM. Computational approaches in motor control. Trends in Cognitive Science. 1997;1:209–216. doi: 10.1016/S1364-6613(97)01070-X. [DOI] [PubMed] [Google Scholar]
- Woltring HJ, Huiskes R. Stereophotogrammetry. In: Berme N, Capposso A, editors. Biomechanics of Human Movement: Applications in Rehabilitation, Sports and Ergonomics. Worthington, OH, USA: Bertec Corporation; 1990. pp. 108–127. [Google Scholar]


