Abstract
In mechanically dissociated rat spinal cord substantia gelatinosa (SG) neurones attached with native presynaptic nerve endings, glycinergic miniature inhibitory postsynaptic currents (mIPSCs) were recorded using nystatin perforated patch recording mode under voltage-clamp conditions. Under these conditions, it was tested whether the changes in P2X receptor subtype on the glycinergic presynaptic nerve terminals occur during postnatal development.
ATP facilitated glycinergic mIPSC frequency in a concentration-dependent manner through all developmental stages tested, whereas αβ-methylene-ATP (αβ-me-ATP) was only effective at later developmental stages.
αβ-me-ATP-elicited mIPSC frequency facilitation was completely occluded in the Ca2+-free external solution, but it was not affected by adding 10−4m Cd2+.
αβ-me-ATP still facilitated mIPSC frequency even in the presence of 10−6m thapsigargin, a Ca2+ pump blocker.
In later developmental stages, ATP-elicited presynaptic or postsynaptic responses were reversibly blocked by 10−5m pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), but only partially blocked by 10−7m 2′,3′-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP). However, αβ-me-ATP-elicited presynaptic or postsynaptic responses were completely and reversibly blocked by either 10−5m PPADS or 10−7m TNP-ATP.
αβ-me-ATP significantly reduced the evoked glycinergic IPSC amplitude in postnatal 28–30 day neurones, whereas it had no effect in 10–12 day neurones.
It was concluded that αβ-me-ATP-sensitive P2X receptors were functionally expressed on the glycinergic presynaptic nerve terminals projecting to SG neurones in later developmental stages. Such developmental changes of presynaptic P2X receptor subtypes might contribute to synaptic plasticity such as the regulation of neuronal excitability and the fine controlling of the pain signal in spinal dorsal horn neurones.
P2X receptors activated by ATP are non-selectively permeable to cations, including Na+, K+ and Ca2+ (Chen et al. 1995; Buell et al. 1996; Taschenberger et al. 1999). Seven different P2X receptor subunit genes have been cloned from various tissues (Valera et al. 1994; Chen et al. 1995; Lewis et al. 1995; Buell et al. 1996; Collo et al. 1996; Garcia-Guzman et al. 1997; North & Barnard, 1997). Of these cloned receptors, the P2X1, P2X2, P2X3, P2X4, P2X5 and P2X6 subunits are expressed in the CNS (Collo et al. 1996; Vulchanova et al. 1996, 1998), and the P2X2, P2X4 and P2X6 subunits are also expressed in the superficial laminae of the spinal cord dorsal horn (Collo et al. 1996; Vulchanova et al. 1997; Le et al. 1998b).
ATP acts as a fast excitatory neurotransmitter through the ionotropic P2X receptors in the superficial laminae of the dorsal horn (Edwards et al. 1992; Bardoni et al. 1997) and the peripheral nervous system (Evans et al. 1992; Galligan & Bertrand, 1994). ATP also acts as a neuromodulator through the presynaptic P2X receptors. For example, ATP modulates glutamate release from the nerve terminals of dorsal root ganglion (DRG) neurones co-cultured with dorsal horn neurones (Gu & MacDermott, 1997). Li et al. (1998) also suggested that P2X2 receptors modulate excitatory synaptic transmission between primary afferent fibres and spinal dorsal horn neurones. Recently, Hugel & Schlichter (2000) reported that ATP, through P2X2 receptors, facilitates the GABAergic inhibitory transmission among cultured rat dorsal horn neurones. We have previously shown that ATP facilitates glycine release through P2X2 receptors in the dorsal horn lamina II neurones (Rhee et al. 2000). Thus, the P2X2 receptor on the inhibitory presynaptic nerve terminals has an important role in the negative feedback system, to protect against excessive excitation of substantia gelatinosa (SG) neurones in response to nociceptive input.
However, a question still remains as to whether other P2X receptors might act functionally on the modulation of neurotransmitter release in the dorsal horn of the spinal cord during postnatal development. In fact, most of the binding or immunohistochemical studies which demonstrated the existence of P2X2, P2X4 and P2X6 subunit RNAs in the dorsal horn have been conducted in adult rats (Collo et al. 1996; Vulchanova et al. 1996, 1997). Accordingly, in this study, we have investigated whether different kinds of P2X receptor subtypes appear during postnatal development using a synaptic bouton preparation (Rhee et al. 1999, 2000). This preparation allows us to focus selectively on the P2X receptor-mediated effects, because in slice preparation exogenously applied ATP is often hydrolysed to adenosine by extracellular nucleotidases (Dunwiddie et al. 1997).
METHODS
Mechanical dissociation
Wistar rats (10–30 days old) were decapitated under pentobarbital anaesthesia (50 mg kg−1, i.p.). The lumbar enlargement of the spinal cord was quickly removed and transversely sliced at a thickness of 400 μm using a microslicer (VT1000S, Leica, Germany). Slices were kept in the control incubation medium (see below) saturated with 95 % O2 and 5 % CO2 at room temperature (22–24 °C) for at least 1 h. For dissociation, slices were transferred to a 35 mm culture dish (Primaria 3801, Becton Dickinson, NJ, USA) and the dorsal horn region of the spinal cord was identified under a binocular microscope (SMZ-1, Nikon, Tokyo, Japan). The details of mechanical dissociation have been described previously (Rhee et al. 1999, 2000). Briefly, mechanical dissociation was accomplished using a custom-built vibration device and a fire-polished glass pipette oscillating at about 3–5 Hz (0.1–0.2 mm). The tip of the fire-polished glass pipette was lightly placed on the surface of the dorsal horn region (layer II) with a micromanipulator. The tip of the glass pipette was vibrated horizontally for about 2 min. Slices were removed and the mechanically dissociated neurones allowed to settle and adhere to the bottom of the dish for about 15 min. Such neurones undergoing dissociation retained a short portion of their proximal dendrites.
Slice preparation
Wistar rats (10–30 days old) were decapitated under pentobarbital anaesthesia (50 mg kg−1, i.p.). The lumbar enlargement of the spinal cord was quickly removed and transversely sliced at a thickness of 250 μm using a microslicer (VT1000S, Leica, Germany) in a cold low-Na+ medium (see below). The slices were kept in an incubation medium saturated with 95 % O2 and 5 % CO2 at 30–35 °C for at least 1 h. Thereafter the slices were transferred to a recording chamber, and lamina II of the dorsal horn was identified under an upright microscope (Axioscope, Zeiss, Germany). The bath solution was perfused at 8–10 ml min−1.
All experiments conformed to the guiding principles for the care and use of animals approved by The Council of The Physiological Society of Japan, and all efforts were made to minimize the number of animals and their suffering.
Electrical measurements
Electrical measurements were performed on the dissociated neurones using the nystatin perforated patch recording mode and on the slice preparation using the conventional whole cell patch recording mode under voltage-clamp conditions. When the nystatin perforated patch recording mode was applied to the dissociated neurones, the technique allowed a long time for recordings, which was not possible using the conventional whole cell patch recording mode. In the slice preparation, however, the conventional whole cell patch technique was applied because this technique is easier and more convenient, and gave sufficient time to record cell response. All voltage-clamp recordings were made at a holding potential (Vh) of −60 mV (CEZ-2300, Nihon Kohden, Tokyo, Japan). Patch pipettes were made from borosilicate capillary glass (1.5 mm o.d., 0.9 mm i.d.; G-1.5, Narishige, Tokyo, Japan) in two stages on a vertical pipette puller (PB-7, Narishige, Japan). The resistance of the recording pipettes when filled with internal solution was 5–6 MΩ. Neurones were viewed under phase contrast on an inverted microscope (Diaphot, Nikon, Japan). Current and voltage were continuously monitored on an oscilloscope (VC-6023, Hitachi, Japan), a pen recorder (RECTI-HORIT-8K, Sanei, Tokyo, Japan) and recorded on a digital-audio tape recorder (RD-120TE, TEAC, Japan). Membrane currents were filtered at 1 kHz (E-3201A Decade Filter, NF Electronic Instruments, Tokyo, Japan), digitized at 4 kHz, and stored on an off-line computer equipped with pCLAMP 8.0 (Axon Instruments, Germany). All experiments were performed at room temperature (22–24 °C), except for the slice preparation (32–35 °C).
Electrical stimulations used to obtain evoked glycinergic IPSCs (eIPSCs) were performed by applying short (100 μs) voltage pulses at 0.1 Hz through a glass pipette (i.d., 10 μm). The pipette was placed in the lamina II region and filled with the incubation medium using the PULSE program on a computer. The signals were filtered at 3 kHz and digitized at 10 kHz.
Data analysis
Spontaneous miniature inhibitory postsynaptic currents (mIPSCs) were counted and analysed using the MiniAnalysis Program (Synaptosoft, Decatur, GA, USA). Spontaneous events were screened automatically using an amplitude threshold of 5 pA and then visually accepted or rejected based upon rise and decay times. The average value of mIPSC frequency during the control period (10–15 min) was calculated, and the frequency of all the events during agonist application (1–2 min) was normalized to this value. The effect of the drug was quantified as a percentage increase in mIPSC frequency compared to the control value, and the results were discarded when agonist actions showed apparent desensitization to repeated applications. The decay time was analysed for mIPSC events with a rise time of 1.0–1.5 ms, and this was well fitted to a single exponential function. The amplitudes and inter-event intervals of large numbers of spontaneous IPSCs obtained from a single neurone were examined by constructing cumulative probability distributions and compared using the Kolmogorov-Smirnov (K-S) test. The continuous curves for concentration- response relationships were constructed according to a modified Michaelis-Menten equation, using a least-squares fitting routine:
where F is the agonist-elicited facilitation ratio of mIPSC frequency and C is the corresponding agonist concentration. EC50 and n denote the half-effective concentration and Hill coefficient, respectively. Differences in amplitude and frequency distribution were tested by Student's paired two-tailed t test using their absolute values. Numerical values were provided as the mean ± standard error of the mean (s.e.m.). Values of P < 0.05 were considered significant.
Solutions
The incubation medium consisted of (mm): 124 NaCl, 5 KCl, 1.2 KH2PO4, 24 NaHCO3, 2.4 CaCl2, 1.3 MgSO4 and 10 glucose bubbled with 95 % O2 and 5 % CO2. The pH was about 7.45. The standard external solution consisted of (mm): 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose and 10 Hepes. The low-Na+ medium consisted of 230 sucrose, 2.5 KCl, 1.25 Na2HPO4, 10 MgSO4, 0.5 CaCl2, 26 NaHCO3 and 30 glucose. Ca2+-free external solution consisted of (mm): 150 NaCl, 5 KCl, 5 MgCl2, 2 EGTA, 10 glucose and 10 Hepes. These external solutions were adjusted to pH 7.4 with Tris-base. In recording mIPSCs, external solutions routinely contained 3 × 10−7m tetrodotoxin (TTX) to block voltage-dependent Na+ channels and 3 × 10−6m 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 10−5mdl-2-amino-5-phosphovaleric acid (AP5) and 3 × 10−6m bicuculline to block glutamatergic and GABAergic currents, respectively. The ionic composition of the internal (patch pipette) solution for the nystatin-perforated patch recording was (mm): 40 caesium methanesulfonate, 5 MgCl2, 100 CsCl and 10 Hepes with pH adjusted to 7.2 with Tris-base. Nystatin was dissolved in acidified methanol at 10 mg ml−1. This stock solution was diluted with the internal solution just before use to a final concentration of 100–200 μg ml−1. The ionic composition of the internal solution for whole cell patch recording was (mm): 92 caesium methanesulfonate, 43 CsCl, 5 TEA-Cl, 2 EGTA, 4 ATP-Mg and 10 Hepes with pH adjusted to 7.2 with Tris-base.
Drugs
The drugs used in the present study were AP5, bicuculline, CNQX, TTX, EGTA, nystatin, Na-ATP, pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), reactive blue-2 (RB-2), strychnine, thapsigargin, substance P (SP) and αβ-methylene-ATP (αβ-me-ATP) from Sigma (USA); 2-methylthio-ATP (2MeS-ATP), calcitonin gene-related peptide (CGRP) and trans-(±)-1-amino-1,3-cyclopentanedicarboxylic acid (t-ACPD) from RBI (USA); 2′,3′-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP) from Molecular Probes (USA). CNQX and bicuculline were dissolved in dimethyl sulfoxide at 10 mm as a stock solution. All solutions containing drugs were applied using the ‘Y-tube’ perfusion system for rapid solution exchange within 20 ms (Akaike & Harata, 1994). In all experiments, either ATP or related agonists were applied at intervals of more than 20 min to allow the full recovery of presynaptic effects (see also Results).
RESULTS
Glycinergic mIPSCs
To examine whether ATP receptor subtypes do change during postnatal development, we divided the specimens into three age groups: postnatal days 10–12 (P10–12), P16–18 and P28–30. The reasons for this division are as follows. First, many physiological systems develop quickly in the first 2 postnatal weeks. Second, previously our results suggested that only the P2X2 receptor subtype is involved in the modulation of spontaneous glycinergic transmission. Third, most of the binding or immunohistochemical studies showing that multiple P2X subunit RNAs are expressed in the dorsal horn have been conducted in adult rats (Collo et al. 1996; Vulchanova et al. 1996, 1997).
In the presence of 3 × 10−7m TTX, 3 × 10−6m CNQX, 10−5m AP5 and 3 × 10−6m bicuculline, the spontaneous miniature postsynaptic currents recorded from P28–30 neurones were completely blocked by 10−6m strychinine (Fig. 1A). Figure 1B shows typical mIPSCs at various holding potentials and their current-voltage (I–V) relationship. The reversal potential of these mIPSCs, as estimated from the I–V relationship, was about −10.0 ± 0.32 mV (n = 4), which was almost identical to the theoretical Cl− equilibrium potential (ECl) of −10.4 mV calculated by the Nernst equation using extra- and intracellular Cl− concentrations (161 mm[Cl−]o and 110 mm[Cl−]i, respectively). The results indicate that the spontaneous postsynaptic currents are glycinergic miniature inhibitory postsynaptic currents (mIPSCs).
Figure 1. Glycinergic mIPSCs.
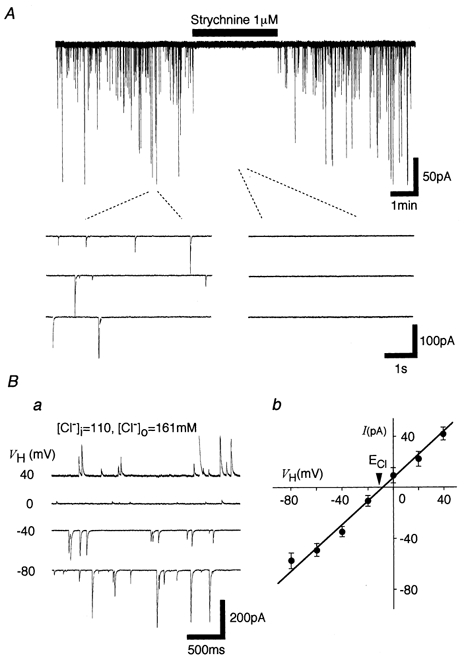
A, typical spontaneous miniature postsynaptic currents (mIPSCs) observed before, during and after the application of 10−6m strychnine in the presence of 3 × 10−7m TTX, 3 × 10−6m CNQX, 10−5m AP5 and 3 × 10−6m bicuculline. Insets indicate the current recordings with an expanded time scale. Ba, typical traces recorded from a neurone at various holding potentials. Bb, the I–V relationship in which each point is the mean of 4 neurones.
Table 1 shows the properties of glycinergic mIPSCs obtained from each developmental stage. Both the mIPSC frequencies and amplitudes showed no significant difference among these three groups (P > 0.05, Student's unpaired t test). In addition, there is no significant correlation between the decay time constants and the postnatal stage when fitted by linear regression (r2= 0.139, P > 0.05, Student's unpaired t test). In summary, the sensitivity of glycine receptors in postsynaptic neurones and the glycine release from the presynaptic nerve terminals hardly changed during all the stages tested.
Table 1.
The properties of glycinergic mIPSCs in SG neurones
| Developmental stages | Frequency (Hz) | Amplitude (pA) | Decay time (ms) |
|---|---|---|---|
| P10–12 | 0.4 ± 0.15 (n = 37) | 69.3 ± 7.1 (n = 37) | 12.3 ± 0.7 (n = 10) |
| P16–18 | 0.5 ± 0.12 (n = 67) | 71.8 ± 6.9 (n = 67) | 12.2 ± 0.9 (n = 10) |
| P28–30 | 0.5 ± 0.13 (n = 84) | 70.7 ± 6.4 (n = 84) | 10.1 ± 0.7 (n = 10) |
Values are means ±s.e.m.
Multiple effects of ATP or αβ-me-ATP on SG neurones during maturation
Previous results indicated that ATP activates presynaptic P2X receptors on the glycinergic nerve terminals and/or postsynaptic P2X receptors on the SG neurone soma (Rhee et al. 2000). In order to understand more fully the changes in ATP response during neuronal maturation, we have investigated the effects of ATP or αβ-me-ATP in three developmental stages. In the majority of neurones tested, regardless of stage of maturation, ATP (10−4m) markedly increased the mIPSC frequency without eliciting any postsynaptic inward currents (Fig. 2Aa). In a small number of neurones tested, ATP elicited postsynaptic inward currents in addition to an increase of mIPSC frequency (Fig. 2Ab). In a subset of neurones, ATP elicited only postsynaptic inward currents (Fig. 2Ac). In the remaining neurones, ATP had no effect (Fig. 2Ad). Table 2 shows the results obtained from each developmental stage. The number of neurones which exhibit postsynaptic responses increased while ATP-insensitive neurones decreased. These results indicate that ATP-sensitive neurones consistently increase with maturation. αβ-me-ATP (10−4m) did not elicit either presynaptic or postsynaptic responses in P10–12 neurones (Table 2, see also Rhee et al. 2000). In later developmental stages, however, αβ-me-ATP mimicked ATP-elicited presynaptic or postsynaptic effects, and αβ-me-ATP-sensitive neurones also increased with maturation (Fig. 2B and Table 2). In the P28–30 group, all αβ-me-ATP-sensitive neurones tested (38/38, 100 %) were sensitive to ATP, while some of the ATP-sensitive neurones tested (11/76, 14 %) were insensitive to αβ-me-ATP. This suggests that a subset of P28–30 neurones expresses αβ-me-ATP-insensitive ATP receptors. It should be noted that αβ-me-ATP elicited quickly desensitizing postsynaptic currents, whereas ATP elicited biphasic currents with a non-desensitizing plateau phase during the application (Fig. 2Bb and c).
Figure 2. ATP- and αβ-me-ATP-elicited multiple responses.
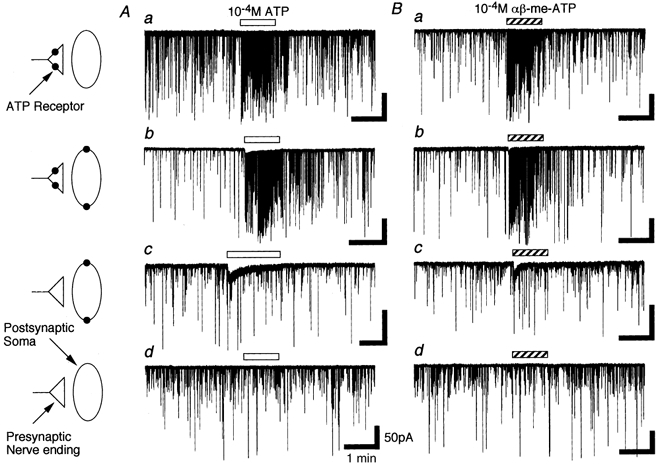
A, the incidence of four kinds of ATP-elicited responses including only presynaptic action (a), presynaptic and postsynaptic actions (b), only postsynaptic action (c) and no action (d) in P28–30 neurones. B, the incidence of four kinds of αβ-me-ATP-elicited responses including only presynaptic action (a), presynaptic and postsynaptic actions (b), only postsynaptic action (c) and no action (d). Note that αβ-me-ATP-elicited postsynaptic currents were more quickly desensitized than ATP-elicited postsynaptic currents.
Table 2.
The incidence of ATP or αβ-me-ATP-elicited responses during postnatal development
| ATP sensitive | αβb-me-ATP sensitive | |||||
|---|---|---|---|---|---|---|
| P10–12 | P16–18 | P28–30 | P10–12 | P16–18 | P28–30 | |
| Presynaptic | 58 (65.2) | 78 (64.5) | 61 (61.7) | 0 (0) | 12 (19.3) | 92 (46.9) |
| Postsynaptic | 6 (6.7) | 8 (6.6) | 14 (14.1) | 0 (0) | 7 (11.3) | 24 (12.2) |
| Pre- and postsynaptic | 5 (5.6) | 10 (8.3) | 14 (14.1) | 0 (0) | 6 (9.7) | 28 (14.3) |
| No effect | 20 (22.5) | 25 (20.6) | 10 (10.1) | 42 (100) | 37 (59.7) | 52 (26.5) |
| Total number of responses | 89 (100) | 121 (100) | 99 (100) | 42 (100) | 62 (100) | 196 (100) |
Values in parentheses are a percentage of the total number of responses.
Presynaptic effects of ATP and related agonists on SG neurones during maturation
To identify what kinds of ATP receptor subtypes are responsible for mIPSC frequency facilitation during maturation, the effects of alternative agonists were examined at each postnatal stage (Fig. 3A). ATP (10−4m) increased the mIPSC frequency to 621.4 ± 115.3 % in P10–12, 557.3 ± 86.5 % in P16–18 and 594.1 ± 141.3 % in P28–30 neurones. 2MeS-ATP (10−4m) mimicked the ATP action and increased mIPSC frequency to 864.1 ± 170.6 %, 765.4 ± 129.1 % and 851.6 ± 100.3 % in P10–12, P16–18 and P28–30 neurones, respectively. Another P2X receptor agonist, αβ-me-ATP (10−4m), had no effect on the mIPSC frequency and amplitude in P10–12 (see also Rhee et al. 2000). However, the facilitatory effect of αβ-me-ATP on mIPSC frequency was noted from P16–18 and increased with maturation. αβ-me-ATP increased mIPSC frequency to 340.5 ± 87.6 % and 649.3 ± 83.6 % in P16–18 and P28–30 neurones, respectively (Fig. 3Ba). On the other hand, the mean amplitude of mIPSCs elicited by each agonist did not change with maturation (Fig. 3Bb).
Figure 3. Developmental changes of agonist-elicited presynaptic responses.
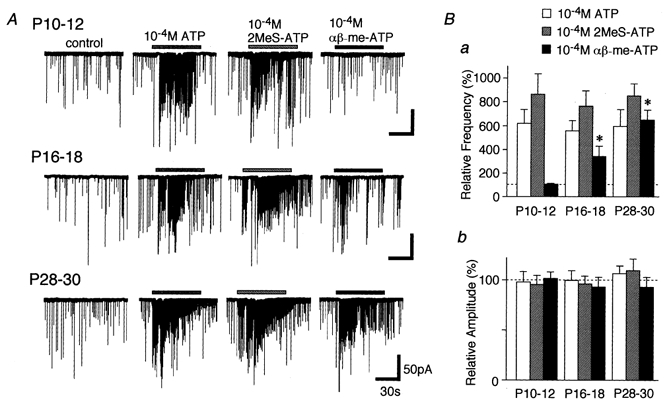
A, typical traces of mIPSCs induced by application of 10−4m ATP, 2MeS-ATP and αβ-me-ATP. All recordings were obtained from a single neurone at different developmental stages. B, each column is the mean of 5–12 neurones and normalized to the respective control. *P < 0.05.
To quantify ATP- and αβ-me-ATP-elicited presynaptic responses, we examined the effects of the repeated applications of agonists on mIPSC frequency. The results might be important particularly for determining concentration-response relationships or analysing the pharmacological properties of ATP receptor subtypes. Figure 4 shows how time intervals influenced agonist-elicited presynaptic responses during the repeated applications in P28–30 neurones. ATP- and αβ-me-ATP-elicited presynaptic responses were fully recovered at a interval of 20 min (Fig. 4Aa and b). In addition, αβ-me-ATP-elicited presynaptic responses were reproducible during the repeated applications, although they slightly decreased in the fourth application (Fig. 4Ba and b). In all experiments, therefore, either ATP or related agonists were applied at intervals of more than 20 min to allow the full recovery of their presynaptic responses.
Figure 4. Time-dependent recovery of ATP- and αβ-me-ATP-elicited presynaptic responses.
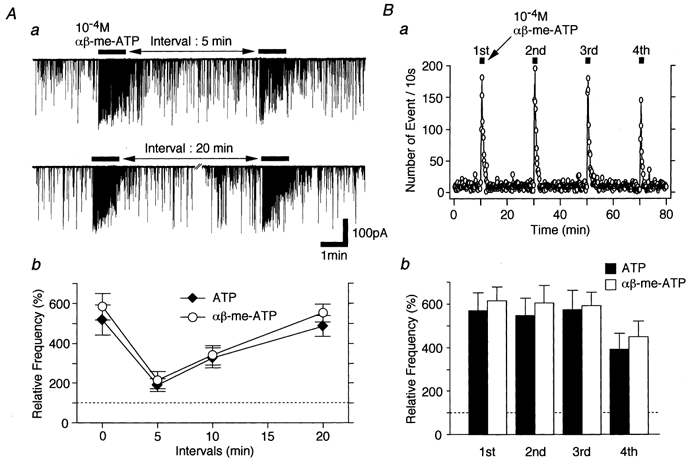
Aa, typical traces of mIPSCs induced by repeated applications of 10−4m ATP at different time intervals. Ab, summary of time-dependent recovery of ATP- and αβ-me-ATP-elicited presynaptic responses. Note that ATP and αβ-me-ATP actions on mIPSC frequency were fully recovered after an interval of 20 min. B, the reproducibility of ATP- or αβ-me-ATP-elicited presynaptic responses. Ba, successive applications of 10−4mαβ-me-ATP at 19 min intervals increased mIPSC frequency in a reproducible manner. The number of events every 10 s is plotted. Bb, each column is the mean of 4 neurones and normalized to the respective control.
Figure 5 shows how ATP and related agonists increase mIPSC frequency in a concentration-dependent manner at three developmental stages. The EC50 values of ATP were 4.9 × 10−6m, 2.1 × 10−6m and 1.7 × 10−6m and those of 2MeS-ATP were 9.3 × 10−6m, 1.7 × 10−5m and 5.6 × 10−6m in P10–12, P16–18 and P28–30 neurones, respectively. The potencies of ATP or 2MeS-ATP were the same throughout the three different stages. On the other hand, the facilitatory effect of αβ-me-ATP on mIPSC frequency was noted at P16–18. The EC50 values of αβ-me-ATP were 1.6 × 10−5m in P16–18 and 6.2 × 10−6m in P28–30 neurones (Fig. 5). The data suggest that αβ-me-ATP-sensitive ATP receptors appear with maturation. Accordingly, all of the following pharmacological experiments were performed with P28–30 neurones to determine the properties of αβ-me-ATP-sensitive ATP receptors.
Figure 5. Concentration-response relationships for ATP and its agonists.
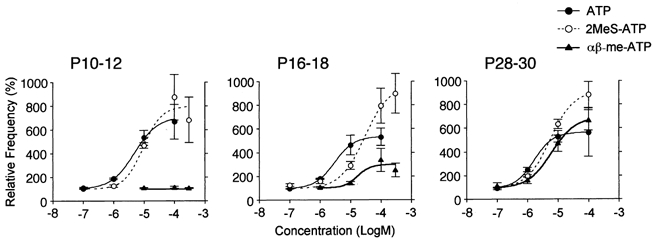
Concentration-response relationships for ATP, 2MeS-ATP and αβ-me-ATP in P10–12, P16–18 and P28–30 neurones, respectively. All points (n = 5–12 neurones) are normalized to the respective control. The continuous lines represent the fit of the Hill function (see Methods).
Ionotropic action of αβ-me-ATP-elicited mIPSC frequency facilitation
We examined whether voltage-dependent Ca2+ channels (VDCCs) in the glycinergic nerve terminals participate in the facilitatory effect of αβ-me-ATP on mIPSC frequency in later developmental stages (Fig. 6). In the presence of 10−4m Cd2+, a concentration which completely blocks VDCCs, glycinergic mIPSC frequency was significantly reduced by 54.7 ± 10.7 % of the control (P < 0.01, n = 5). This suggests that the VDCCs contribute to spontaneous glycine release from these presynaptic nerve terminals. In the presence of Cd2+, either 10−4m ATP or 10−4mαβ-me-ATP could still facilitate the mIPSC frequency to 551.6 ± 102.0 and 590.1 ± 130.5 % of the control Cd2+ condition (P < 0.05, n = 5), respectively (Fig. 6Aa and b). The results suggest that the ATP- and αβ-me-ATP-sensitive ATP receptors may be permeable to Ca2+ and that Ca2+ influxes through ATP receptors may directly contribute to the facilitation of glycine release. Thus, the effects of ATP and αβ-me-ATP were examined in P28–30 neurones immersed in the Ca2+-free external solution (Fig. 6B and C). Ca2+-free external solution itself also reduced the mIPSC frequency to 55.6 ± 7.1 % of the control (P < 0.01, n = 5, Fig. 6Bb and Cb). The decrease of mIPSC frequency was nearly the same as that observed in the Cd2+ solution. In the Ca2+-free external solution, however, the facilitatory effects of either 10−4m ATP or αβ-me-ATP on the mIPSC frequency were completely abolished (Fig. 6B and C, P > 0.05, n = 5).
Figure 6. Effects of Cd2+ and Ca2+-free external solution on ATP- and αβ-me-ATP-elicited glycinergic mIPSC frequency facilitation.
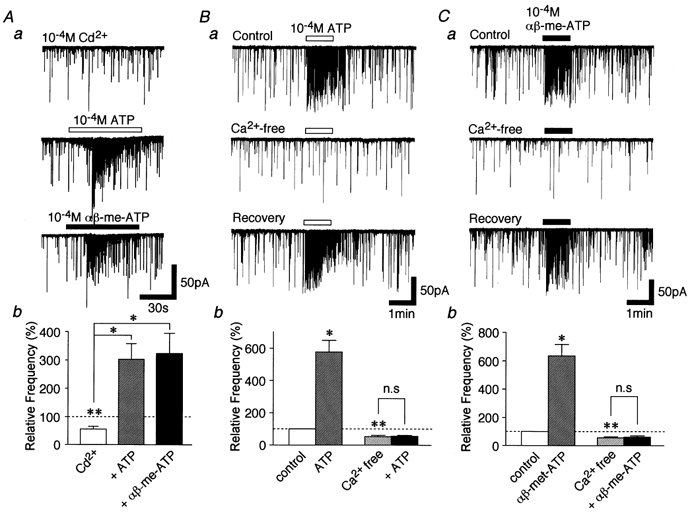
Aa, typical traces of mIPSCs observed from a P28–30 neurone after the application of 10−4m ATP or αβ-me-ATP in the presence of 10−4m Cd2+. Ab, each column is the mean of 5 neurones. All columns are normalized to the control. *P < 0.05, **P < 0.01. B and C, typical traces of mIPSCs observed from a P28–30 neurone in the Ca2+-free external solution after the application of 10−4m ATP (Ba) and αβ-me-ATP (Ca). Pooled data for ATP (Bb, n = 5) and αβ-me-ATP (Cb, n = 4) are presented. Each column is normalized to the control. *P < 0.05, **P < 0.01.
To confirm that αβ-me-ATP-elicited mIPSC frequency facilitation is mediated by ionotropic P2X but not metabotropic P2Y receptors, we tested the effect of thapsigargin, which blocks the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase and inhibits the Ca2+ refilling of intracellular stores. Thapsigargin (10−6m) itself increased mIPSC frequency to 179.6 ± 23.3 % of the control (P < 0.05, n = 5) without affecting mIPSC mean amplitude (Fig. 7C). This effect might be due to the thapsigargin-induced Ca2+ release from intracellular Ca2+ stores. However, αβ-me-ATP (10−4m) still facilitated mIPSC frequency to 571.0 ± 84.2 % of the thapsigargin condition (Fig. 7B and Cb).
Figure 7. Effect of thapsigargin on ATP- and αβ-me-ATP-elicited glycinergic mIPSC frequency facilitation.
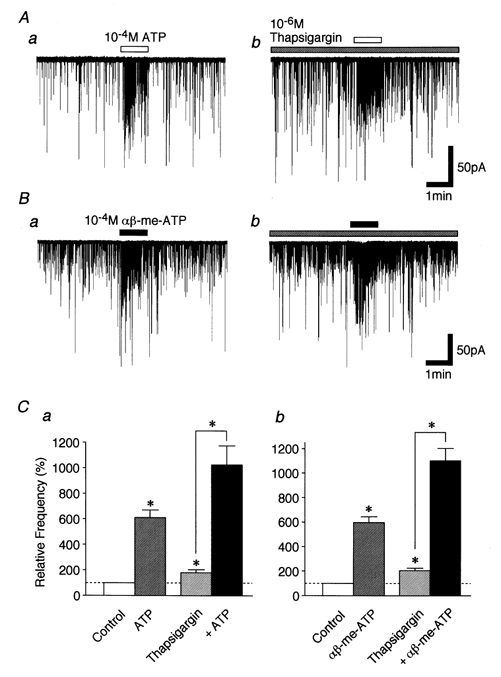
A and B, typical traces of mIPSCs recorded during the application of 10−4m ATP (A) and αβ-me-ATP (B) in the absence (a) and presence (b) of 10−6m thapsigargin. C, each column is the mean of 5 neurones. All columns are normalized to the control. *P < 0.05.
Pharmacological properties of ATP- and αβ-me-ATP-elicited postsynaptic and presynaptic responses
Firstly, we tested the effect of P2X receptor antagonists, such as TNP-ATP and PPADS, on ATP- and αβ-me-ATP-elicited postsynaptic currents in P28–30 neurones (Fig. 8). PPADS at low micromolar concentrations is insensitive to both P2X4 and P2X6 receptors, and TNP-ATP at nanomolar concentrations is a potent antagonist for P2X1, P2X3, heteromeric P2X2/3 and P2X1/5 receptors (Virginio et al. 1998). ATP-elicited postsynaptic currents (Fig. 8Aa) were partially blocked by adding 10−7m TNP-ATP (n = 4, Fig. 8Ab) and completely occluded by the cumulative application of 10−5m PPADS in addition to TNP-ATP (n = 4, Fig. 8Ac). However, ATP-elicited postsynaptic currents were completely blocked by adding 10−5m PPADS alone (n = 3, not shown). On the other hand, αβ-me-ATP-elicited postsynaptic currents (Fig. 8Ba) were completely blocked by adding either TNP-ATP (n = 4, Fig. 8Bb) or PPADS (n = 3, not shown). All antagonisms were reversible (Fig. 8Ad and Bc).
Figure 8. Effects of P2X receptor antagonists on ATP- and αβ-me-ATP-elicited postsynaptic currents.
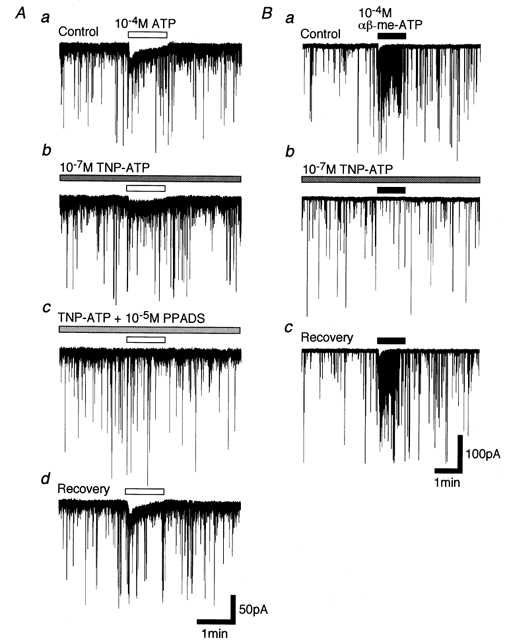
A, typical traces of 10−4m ATP-elicited postsynaptic currents in the absence of any antagonists (a), and in the presence of 10−7m TNP-ATP (b) and both 10−7m TNP-ATP and 10−5m PPADS (c). In d, ATP-elicited postsynaptic current was clearly recovered after washing out antagonists. B, typical traces of 10−4mαβ-me-ATP-elicited postsynaptic currents in the absence of any antagonists (a), and in the presence of 10−7m TNP-ATP (b). In c, αβ-me-ATP-elicited postsynaptic current was clearly recovered after washing out TNP-ATP.
Previous results indicated that the facilitatory effect of ATP on mIPSCs in P10–13 neurones is completely blocked by PPADS (Rhee et al. 2000). In the present study performed on P28–30 neurones, 10−5m PPADS blocked the ATP-elicited increase in glycinergic mIPSC frequency to 123.6 ± 31.9 % of the PPADS condition (P > 0.05, n = 6), but 10−7m TNP-ATP partially blocked this increase to 303.6 ± 40.8 % of the TNP-ATP condition (P < 0.05, n = 6) (Fig. 9A). Interestingly, PPADS and TNP-ATP reversibly blocked the αβ-me-ATP-elicited increase of glycinergic mIPSC frequency to 129.8 ± 28.4 and 130.7 ± 37.3 % of the respective drug conditions (P > 0.05, n = 6, Fig. 9B). Reactive blue 2 (RB-2, 10−5m) partially blocked ATP- and αβ-me-ATP-elicited presynaptic responses to 235.6 ± 46.8 % and 296.3 ± 62.5 % of the RB-2 condition, respectively (P < 0.05, n = 4, respectively, Fig. 9Ac and Bc). On the other hand, glycinergic mIPSC frequency was not changed by PPADS, TNP-ATP or RB-2 (data not shown).
Figure 9. Effects of P2X receptor antagonists on ATP- and αβ-me-ATP-elicited presynaptic responses.
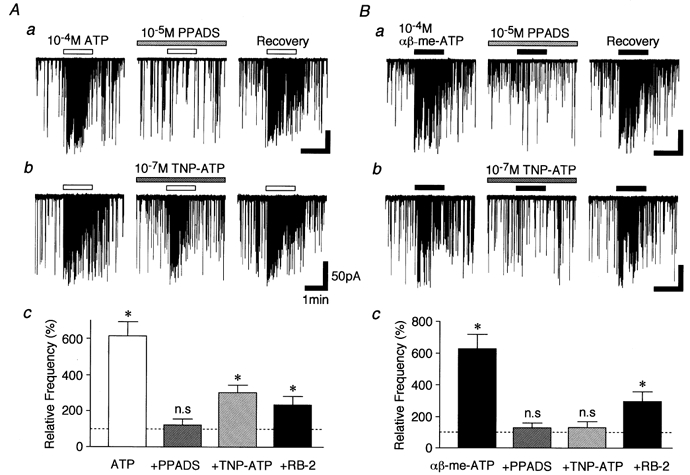
A, typical traces of mIPSCs observed after the application of 10−4m ATP in the presence of either 10−5m PPADS (a) or 10−7m TNP-ATP (b). Ac, each column is the mean of 5 neurones. All columns are normalized to their respective control. Note that ATP-induced facilitation of mIPSCs was completely blocked by PPADS, but partially blocked by TNP-ATP. *P < 0.05. B, typical traces of mIPSCs observed after the application of 10−4mαβ-me-ATP in the presence of PPADS (a) or TNP-ATP (b). Bc, each column is the mean of 4 neurones and normalized to its respective control. Note that αβ-me-ATP-induced facilitation of mIPSCs was completely blocked by either TNP-ATP or PPADS. *P < 0.05.
To elucidate the physiological significance of the present findings, the effect of αβ-me-ATP on evoked glycinergic IPSCs (eIPSCs) was tested on the slice preparation using the whole cell patch recording technique at VH of −60 mV (Fig. 10). Since lamina II interneurones are known to make local inhibitory circuits within the dorsal horn area (Todd, 1988; Li et al. 1999), a glass pipette for electrical stimulation was placed near to the lamina II area. In P28–30 neurones, αβ-me-ATP (10−5m) significantly reduced eIPSC amplitude to 68.8 ± 10.6 % of control (P < 0.05, n = 6, Fig. 10A and B), although it facilitated spontaneous glycine release (Fig. 10Ca and b). This inhibitory action was probably not due to the postsynaptic mechanism, since the distribution of spontaneous IPSC amplitude did not change during the application of αβ-me-ATP (Fig. 10Cb). On the contrary, αβ-me-ATP had no effect on glycinergic eIPSCs in P10–12 neurones (n = 4, Fig. 10A and B).
Figure 10. Effect of αβ-me-ATP on glycinergic eIPSCs.
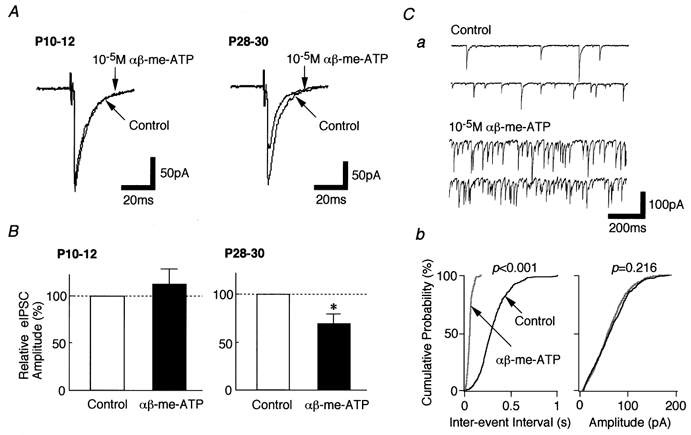
All recordings were performed in the slice preparation using the whole cell patch clamp technique. The incubation medium contained 10−5m CNQX, 10−5m AP5 and 10−5m bicuculline, but not TTX. A, typical recordings of eIPSCs obtained from P10–12 neurones (left) and P28–30 neurones (right). B, each column is the mean of 4 (P10–12) and 6 (P28–30) neurones and was normalized to its respective control. *P < 0.05. Ca, typical traces of mIPSCs observed before and during the application of 10−5mαβ-me-ATP. Cb, cumulative probability plots for inter-event interval (left) and amplitude (right) of spontaneous IPSCs obtained from the same neurone. P values indicate the results of K-S test for frequency and amplitude (908 events for control and 1518 events for αβ-me-ATP).
DISCUSSION
Developmental changes of ATP- and αβ-me-ATP-elicited responses
In the present study, we have investigated whether other P2X receptor subtypes can modulate spontaneous glycinergic transmission during postnatal development. We have previously revealed that αβ-me-ATP did not elicit any presynaptic or postsynaptic effects in P10–13 dorsal horn neurones (Rhee et al. 2000). These results are consistent with our present results performed on P10–12 neurones. With maturation, however, while the number of ATP-sensitive neurones did not change, αβ-me-ATP could modulate the glycine release from P16–18 neurones and had nearly the same ability to facilitate glycinergic mIPSCs for ATP and αβ-me-ATP in P28–30 neurones. The results indicate that the glycinergic presynaptic terminals might express αβ-me-ATP-sensitive P2X receptors in the later developmental stages. Since αβ-me-ATP-elicited presynaptic responses were greater in P28–30 neurones than in P16–18 neurones, there might be a progressive expression of αβ-me-ATP-sensitive components on glycinergic nerve terminals.
In a small number of P28–30 neurones tested, αβ-me-ATP elicited quickly desensitizing postsynaptic inward currents, whose pharmacological properties were nearly consistent with those of presynaptic response (Fig. 8 and Fig. 9). These results strongly suggest that a subset of dorsal horn neurones express αβ-me-ATP-sensitive P2X receptors. Since previous morphological studies suggest that a subset of lamina II inhibitory interneurones make local inhibitory circuits within the dorsal horn (Todd, 1988; Li et al. 1999), most, but not all, αβ-me-ATP-sensitive glycinergic nerve terminals might originate from dorsal horn interneurones. In addition, the present results (Fig. 10) partly support the previous findings. However, it should be noted that neurones sensitive to αβ-me-ATP were also sensitive to ATP, but some of the ATP-sensitive neurones tested (11/76, 14 %) were insensitive to αβ-me-ATP. Since ATP is a common agonist for all P2X receptors, but αβ-me-ATP is for only P2X1, P2X3, P2X1/5, P2X2/3 and P2X4/6 receptors (for review see Khakh et al. 2001), some of the SG neurones are likely to express αβ-me-ATP-sensitive P2X receptors, while others express αβ-me-ATP-insensitive P2X receptors in later developmental stages.
αβ-me-ATP activates ionotropic P2X receptors
The αβ-me-ATP-sensitive ATP receptor responsible for facilitating the mIPSCs required extracellular Ca2+ but did not require the voltage-gated Ca2+ channels in P28–30 neurons (Fig. 6), suggesting that the enhancement of mIPSC frequency results from Ca2+ influxes through αβ-me-ATP-sensitive receptors. In dorsal horn astrocytes, thapsigargin blocks ATP-elicited increase of intracellular Ca2+ concentration mediated by P2Y receptor activation (Salter & Hicks, 1995). The present results showing that thapsigargin had no effect on αβ-me-ATP-elicited presynaptic responses support the absence of a relationship with P2Y receptor activation. Accordingly, αβ-me-ATP may activate ionotropic P2X receptors on the glycinergic presynaptic nerve terminals, thus facilitating spontaneous glycine release at these synapses.
Pharmacological properties of αβ-me-ATP-sensitive P2X receptors
Although seven P2X receptor subunits have been cloned, it is hard to distinguish pharmacologically each subtype because of limited subunit-specific agonists and antagonists (for review see North & Barnard, 1997; Khakh et al. 2001). In the present study, the αβ-me-ATP-elicited increase of mIPSC frequency was completely blocked by adding either PPADS or nanomolar TNP-ATP (Fig. 8). Such pharmacological properties are almost consistent with the those of P2X1, P2X3, P2X2/3 and P2X1/5 receptors (Virginio et al. 1998; Torres et al. 1998). The immunohistochemical study of adult rats using subtype-specific antisera has suggested the existence of P2X1 and P2X3 immunoreactivity localized in the sensory ganglia and the superficial laminae of the dorsal horn. However, it is likely that P2X1 and P2X3 receptor subunits are in the central terminals of sensory afferent processes (Vulchanova et al. 1996, 1997, 1998). In addition, in situ hybridization study has revealed that both P2X1 and P2X3 subunit RNAs are not found in the spinal cord dorsal horn region of adult rat (Collo et al. 1996). Therefore, it might be reasonable to assume that P2X1, P2X3, P2X2/3 or P2X1/5 receptors are not αβ-me-ATP-sensitive P2X receptors. The density of innervation of dorsal horn neurones by primary afferent fibres increases during the postnatal development, particularly between P10 and P21 in the case of C-fibre inputs (Fitzgerald & Jennings, 1999). Therefore, it could be suggested that αβ-me-ATP activates P2X3 receptors on the primary afferent terminals to release glutamate and neuropeptides such as SP or CGRP. Such transmitters, through their metabotropic receptors, would eventually act on glycinergic nerve terminals to increase the probability of glycine release. However, in another set of experiments to elucidate such a possibility, t-ACPD (mGluR agonist), SP and CGRP had no effect on mIPSC frequency (data not shown). Additionally, the present results using thapsigargin (Fig. 7) also partly support the absence of a relationship with any metabotropic receptors.
P2X2, P2X4 and P2X6, particularly P2X4 and P2X6 subunit RNAs closely overlap in the dorsal horn of the spinal cord as well as the brain of adult rat. In addition, [3H]αβ-me-ATP binding sites are widely distributed in the rat CNS and are closely correlated with in situ hybridization data on P2X4 and P2X6 mRNA distributions (Bo & Burnstock, 1994; Collo et al. 1996). Such findings suggest the possibility that these subunits can coassemble into heteromeric P2X receptors (Collo et al. 1996; Le et al. 1998b). In fact, when coexpressed, heteromeric P2X4/6 receptors have unique properties, such as increased sensitivities to αβ-me-ATP, 2MeS-ATP, suramin and PPADS, as compared with homomeric P2X4 or P2X6 receptors (Le et al. 1998a). These pharmacological profiles of P2X4/6 receptors are also consistent with the present findings, although it was not determined whether P2X4/6 receptors are sensitive to nanomolar TNP-ATP. In addition, RB-2, which potentiates P2X4 receptor-mediated currents but antagonizes P2X4/6 receptor-mediated currents, significantly blocked αβ-me-ATP-elicited presynaptic responses. Therefore, the most plausible conclusion is that heteromeric P2X4/6 receptors might be expressed in the spinal dorsal horn and are responsible for the αβ-me-ATP-elicited facilitation of spontaneous glycinergic transmission. Such a conclusion is consistent with recent findings suggesting that P2X4/6 receptors play a significant role in the regulation of excitatory transmitter release in central synapses (Le et al. 1998a; Kato & Shigetomi, 2001).
Effect of αβ-me-ATP on glycinergic eIPSCs
Our observation that αβ-me-ATP concentration-dependently inhibits glycinergic eIPSCs in P28–30 neurones is rather unexpected, as P2X receptors are normally Ca2+ permeable. Indeed, P2X2 receptors have been suggested to potentiate GABAergic eIPSCs in cultured dorsal horn neurones (Hugel & Schlichter, 2000). One possibility which would explain the present finding is that αβ-me-ATP also acts postsynaptically to affect glycinergic eIPSC amplitude. However, this was not the case because the cumulative distribution in the amplitude of spontaneous events was not changed during the application of αβ-me-ATP. Alternatively, αβ-me-ATP might greatly depolarize the glycinergic nerve terminals and block the invasion of action potentials. Indeed, a recent study performed on rat hippocampus suggested that kainate, via ionotropic kainate receptors, inhibits evoked glutamate release by inactivation of Na+ channels secondary to depolarization (Schmitz et al. 2000). Further studies are needed to determine the mechanism of the present P2X receptor-mediated presynaptic inhibition.
Physiological significance of αβ-me-ATP-sensitive P2X receptors
P2X receptors, especially P2X2 and P2X3, play an important role in the transduction of pain signalling within the dorsal horn area as well as the nerve terminals of primary afferent neurones (Kennedy & Leff, 1995; Burnstock & Wood, 1996; Cook et al. 1997). Surprisingly, P2X3 receptors have only been found in sensory neurones including trigeminal, dorsal root, nodose ganglia and their central terminals (Collo et al. 1996; Vulchanova et al. 1996, 1997). This suggests that P2X3 receptors mainly participate in the modulation of neurotransmitter release from the primary afferent fibres as well as in the transduction of pain signals in the peripheral sensory neurones. On the other hand, P2X2 receptors are likely to act as negative feedback modulator in the inhibitory presynaptic nerve terminals of the dorsal horn neurones (Hugel & Schlichter, 2000; Rhee et al. 2000). Our present results strongly suggest that αβ-me-ATP-sensitive P2X receptors can modulate neurotransmitter release from the glycinergic inhibitory presynaptic terminals of the adult spinal cord neurones.
In general, receptors responsible for fast synaptic transmission, such as AMPA, NMDA, GABAA and glycine receptors, reduce their decay time constants by switching their receptor subunits during postnatal development (Singer et al. 1998; Bellingham et al. 1998; Hutcheon et al. 2000). This suggests a functional role for synaptic transmission in the promotion of precise timing operations in the nervous system. Our results also present an example of a change in the presynaptic nerve terminals, that is, from non-desensitizing P2X2 receptors to moderately desensitizing αβ-me-ATP-sensitive P2X receptors during postnatal development. Therefore, the developmental changes in P2X receptors in the presynaptic nerve terminals may contribute to synaptic plasticity such as the regulation of neuronal excitability and the fine controlling of pain signals in the spinal cord dorsal horn neurones.
Acknowledgments
We greatly appreciate the valuable comments of Dr P. Illes on the manuscript. This work was supported by Grants-in-Aid for Scientific Research (No. 13307003) from The Ministry of Education, Science and Culture, Japan, The Japan Health Sciences Foundation (No. 21279, Research on Brain Science), and Kyushu University Interdisciplinary Programs in Education and Projects in Research Development to N. Akaike.
References
- Akaike N, Harata N. Nystatin perforated patch recording and its application to analysis of intracellular mechanism. Japanese Journal of Physiology. 1994;44:433–473. doi: 10.2170/jjphysiol.44.433. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Goldstein PA, Lee CJ, Gu JG, MacDermott AB. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. Journal of Neuroscience. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, Lim R, Walmsley B. Developmental changes in EPCS quantal size and quantal content at a central glutamatergic synapse in rat. Journal of Physiology. 1998;511:861–869. doi: 10.1111/j.1469-7793.1998.861bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo X, Burnstock G. Distribution of [3H]α,β-methylene ATP binding sites in rat brain and spinal cord. NeuroReport. 1994;5:1601–1604. doi: 10.1097/00001756-199408150-00015. [DOI] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO Journal. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Current Opinion in Neurobiology. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct P2X receptors mediate ATP actions on nociceptive and non-nociceptive neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. Journal of Neuroscience. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Jennings E. The postnatal development of spinal sensory processing. Proceedings of the National Academy of Sciences of the USA. 1999;96:7719–7722. doi: 10.1073/pnas.96.14.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. Journal of Neuroscience. 1994;12:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Guzman M, Soto F, Gomez-Hernandez JM, Lund PE, Stuhmer W. Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Molecular Pharmacology. 1997;51:109–118. doi: 10.1124/mol.51.1.109. [DOI] [PubMed] [Google Scholar]
- Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- Hugel S, Schlichter R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. Journal of Neuroscience. 2000;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon B, Morley P, Poulter MO. Developmental change in GABAA receptor desensitization kinetics and its role in synapse function in rat cortical neurons. Journal of Physiology. 2000;522:3–17. doi: 10.1111/j.1469-7793.2000.t01-5-00003.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato F, Shigetomi E. Distinct modulation of evoked and spontaneous EPSCs by purinoceptors in the nucleus tractus solitarii of the rat. Journal of Physiology. 2001;530:469–486. doi: 10.1111/j.1469-7793.2001.0469k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Leff P. Painful connection for ATP. Nature. 1995;377:385–386. doi: 10.1038/377385a0. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PPA. International union of pharmacology. XXIV. Current status of the nomenculature and properties of P2X receptors and their subunits. Pharmacological Reviews. 2001;53:107–118. [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X4 receptor channels. Journal of Neuroscience. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le K-T, Babinski K, Seguela P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. Journal of Neuroscience. 1998a;18:7152–7159. doi: 10.1523/JNEUROSCI.18-18-07152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le K-T, Villeneuve P, Ramjaun AR, Mapherson PS, Beaudet A, Seguela P. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptor. Neuroscience. 1998b;83:177–190. doi: 10.1016/s0306-4522(97)00365-5. [DOI] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Li P, Calejesan AA, Zhuo M. ATP P2X receptors and sensory synaptic transmission between primary afferent fibers and spinal dorsal horn neurons in rats. Journal of Neurophysiology. 1998;80:3356–3360. doi: 10.1152/jn.1998.80.6.3356. [DOI] [PubMed] [Google Scholar]
- Li Y-Q, Li H, Kaneko T, Mizuno N. Local circuit neurons showing calbindin D28K-immunoreactivity in the substantia gelatinosa of the medullary dorsal horn of the rat. An immunohistochemical study combined with intracellular staining in slice preparation. Brain Research. 1999;840:179–183. doi: 10.1016/s0006-8993(99)01794-1. [DOI] [PubMed] [Google Scholar]
- North RA, Barnard EA. Nucleotide receptors. Current Opinion in Neurobiology. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Ishibashi H, Akaike N. Calcium channels in the GABAergic presynaptic nerve terminals projecting to Meynert neurons of the rat. Journal of Neurochemistry. 1999;72:800–807. doi: 10.1046/j.1471-4159.1999.0720800.x. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Wang ZM, Nabekura J, Inoue K, Akaike N. ATP facilitates spontaneous glycinergic IPSC frequency at dissociated rat dorsal horn interneuron synapses. Journal of Physiology. 2000;524:471–483. doi: 10.1111/j.1469-7793.2000.t01-1-00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Hicks JL. ATP causes release of intracellular Ca2+ via the phospholipase C beta/IP3 pathway in astrocytes from the dorsal spinal cord. Journal of Neuroscience. 1995;15:2961–2971. doi: 10.1523/JNEUROSCI.15-04-02961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Frerking M, Nicoll RA. Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron. 2000;27:327–338. doi: 10.1016/s0896-6273(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic transmission to rat brain stem motoneurons. Journal of Neurophysiology. 1998;80:2608–2620. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Juttner R, Grantyn R. Ca2+-permeable P2X receptor channels in cultured rat retinal ganglion cells. Journal of Neuroscience. 1999;19:3353–3366. doi: 10.1523/JNEUROSCI.19-09-03353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ. Electron microscope study of Golgi-stained cells in lamina II of the rat spinal dorsal horn. Journal of Comparative Neurology. 1988;275:145–157. doi: 10.1002/cne.902750112. [DOI] [PubMed] [Google Scholar]
- Torres GE, Egan TM, Voigt MM. Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible parters. Journal of Biological Chemistry. 1999;274:6653–6659. doi: 10.1074/jbc.274.10.6653. [DOI] [PubMed] [Google Scholar]
- Torres GE, Haines WR, Egan TM, Voigt MM. Co-expression of P2X1 and P2X5 receptor subunits reveals a novel ATP-gated ion channel. Molecular Pharmacology. 1998;54:989–993. doi: 10.1124/mol.54.6.989. [DOI] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, Adami N, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Virginio C, Robertson G, Surprenant A, North RA. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3 and heteromeric P2X2/3 receptors. Molecular Pharmacology. 1998;53:969–973. [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proceedings of the National Academy of Sciences of the USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. European Journal of Neuroscience. 1998;11:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]


