Abstract
One to five weeks of chronic exposure to hypoxia has been shown to reduce peak blood lactate concentration compared to acute exposure to hypoxia during exercise, the high altitude ‘lactate paradox’. However, we hypothesize that a sufficiently long exposure to hypoxia would result in a blood lactate and net lactate release from the active leg to an extent similar to that observed in acute hypoxia, independent of work intensity.
Six Danish lowlanders (25–26 years) were studied during graded incremental bicycle exercise under four conditions: at sea level breathing either ambient air (0 m normoxia) or a low-oxygen gas mixture (10 % O2 in N2, 0 m acute hypoxia) and after 9 weeks of acclimatization to 5260 m breathing either ambient air (5260 m chronic hypoxia) or a normoxic gas mixture (47 % O2 in N2, 5260 m acute normoxia). In addition, one-leg knee-extensor exercise was performed during 5260 m chronic hypoxia and 5260 m acute normoxia.
During incremental bicycle exercise, the arterial lactate concentrations were similar at sub-maximal work at 0 m acute hypoxia and 5260 m chronic hypoxia but higher compared to both 0 m normoxia and 5260 m acute normoxia. However, peak lactate concentration was similar under all conditions (10.0 ± 1.3, 10.7 ± 2.0, 10.9 ± 2.3 and 11.0 ± 1.0 mmol l−1) at 0 m normoxia, 0 m acute hypoxia, 5260 m chronic hypoxia and 5260 m acute normoxia, respectively. Despite a similar lactate concentration at sub-maximal and maximal workload, the net lactate release from the leg was lower during 0 m acute hypoxia (peak 8.4 ± 1.6 mmol min−1) than at 5260 m chronic hypoxia (peak 12.8 ± 2.2 mmol min−1). The same was observed for 0 m normoxia (peak 8.9 ± 2.0 mmol min−1) compared to 5260 m acute normoxia (peak 12.6 ± 3.6 mmol min−1). Exercise after acclimatization with a small muscle mass (one-leg knee-extensor) elicited similar lactate concentrations (peak 4.4 ± 0.2 vs. 3.9 ± 0.3 mmol l−1) and net lactate release (peak 16.4 ± 1.8 vs. 14.3 mmol l−1) from the active leg at 5260 m chronic hypoxia and 5260 m acute normoxia.
In conclusion, in lowlanders acclimatized for 9 weeks to an altitude of 5260 m, the arterial lactate concentration was similar at 0 m acute hypoxia and 5260 m chronic hypoxia. The net lactate release from the active leg was higher at 5260 m chronic hypoxia compared to 0 m acute hypoxia, implying an enhanced lactate utilization with prolonged acclimatization to altitude. The present study clearly shows the absence of a lactate paradox in lowlanders sufficiently acclimatized to altitude.
Upon acute ascent to altitude, blood lactate concentrations are elevated at a given sub-maximal work level but peak values are essentially unaltered. During more prolonged exposure to hypoxia, a reduction in blood lactate concentration has been reported at both sub-maximal and maximal exercise intensities. The earliest observations were made in the 1930s (Dill et al. 1931; Edwards, 1936) and subsequently confirmed (Dill et al. 1967; Hansen et al. 1967; Cerretelli, 1980; West et al. 1983; Bender et al. 1989; Young et al. 1992; Kayser et al. 1993; Beidleman et al. 1997). In 1986, West summarized the literature in the field and termed the phenomenon the ‘lactate paradox’, i.e. in spite of prevailing hypoxia, lactate accumulation in blood during exercise becomes reduced towards sea level values during sub-maximal exercise. Later detailed studies have demonstrated that the low blood lactate concentration is primarily a function of a reduced net lactate release from the exercising legs (Bender et al. 1989; Brooks et al. 1992, 1998).
Based on findings obtained in a field study in the Himalayas, at an altitude of 5400 m, it was suggested that the lower blood lactate concentration during exercise was a transient phenomenon of acclimatization (Lundby et al. 2000). Peak lactate concentration at exhaustion was lower after 1 and 4 weeks at altitude than at sea level or acute hypoxia. However, blood lactate accumulation was significantly higher after 4 weeks compared to 1 week of acclimatization, and after more than 6 weeks of acclimatization, peak lactate concentration was similar to those at sea level and during acute hypoxia. Indeed, there are similar findings in the literature when lactate responses have been studied after prolonged exposure to altitude. In people living at sea level, Dempsey et al. (1972) observed the same blood lactate concentration in chronic hypoxia (∼45 days) as that during acute hypoxia at both sub-maximal and maximal exercise intensities, and the concentration tended to be higher than that at sea level. Moreover, Grassi et al. (1995) observed that peak lactate concentration was higher during maximal exercise after 5 weeks compared to 1 week of acclimatization at 5050 m. In addition, of their six subjects, two had a higher peak lactate concentration after 5 weeks of acclimatization compared to that at sea level. With this background, we hypothesized that after a prolonged period of acclimatization to altitude, blood lactate accumulation and net lactate release from active muscle are similar to those during acute hypoxia and higher than at sea level during sub-maximal and maximal exercise, in accordance with the proposal that the lactate paradox is a transient phenomenon. A corollary was that blood acid-base balance is, at this phase of acclimatization, comparable to that at sea level at rest and during exercise.
The lactate paradox has only been observed during bicycle and treadmill exercise where a dominant fraction of the muscle mass was engaged in physical activity. In small muscle group exercise, blood lactate concentrations are the same in acute and chronic hypoxia (Kayser et al. 1995; Savard et al. 1995). Therefore, to examine further whether skeletal muscle maintains its normal capacity to produce lactate at altitude, during exercise engaging a large muscle mass, blood lactate responses during two-leg cycling were compared to those during one-leg knee extension.
METHODS
Subjects
Six healthy, physically active subjects (5 males and 1 female, aged 25–26 years (range, 21–35 years)), born and residing at sea level in the Copenhagen area, participated in the study. The subjects were informed about the possible risks and discomfort involved before giving their written consent to participate. The study was performed according to the Declaration of Helsinki and was approved by the Ethical Committee of the Copenhagen and Frederiksberg Communities, Denmark.
Study periods
Each subject underwent four incremental bicycle exercise tests on a cycle ergometer: two tests in the field laboratory on Mt Chacaltaya, Bolivia (5260 m) after ∼9 weeks of acclimatization (breathing ambient air, 5260 m chronic hypoxia, and breathing a normoxic air mixture, 5260 m acute normoxia); and two tests at sea level (breathing ambient air, 0 m normoxia, and breathing a low-oxygen gas mixture, 0 m acute hypoxia), carried out in Copenhagen (∼0 m). In addition, each subject underwent two one-leg knee extensor exercise tests at 5260 m after ∼9 weeks of acclimatization. The sea level trials were performed 10 months after the subjects returned from Bolivia.
Prior to the sojourn at altitude, it was uncertain whether all subjects could withstand prolonged high altitude exposure in primitive living conditions. In fact, not all subjects who left for Bolivia were studied. In order to minimize the number of invasive procedures, yet ensure that all measurements obtained in such cases were used, the decision was made to perform sea level experiments after de-acclimatization, normalization of physiological responses and return to daily life at sea level. Therefore, sea level trials were performed in the same month, but a year after the subjects departed for Bolivia. Physical activity level, body weight, haemoglobin and haematocrit were similarly compared to sea level values before the altitude studies. Thus, peak power output (345 ± 23 vs. 339 ± 15 W), maximal oxygen uptake (4.5 ± 0.6 vs. 4.3 ± 0.3 l min−1) and forearm venous lactate concentration (8.3 ± 0.5 vs. 7.9 ± 0.3 mmol l−1) were similar during the incremental bicycle exercise before compared to 10 months after the altitude studies. In addition, maximal oxygen uptake in acute hypoxia was 2.8 l min−1 before and 2.7 l min−1 after the altitude studies, showing that there was no effect of the acclimatization period on either normoxic or hypoxic conditions. Moreover, as the number of invasive procedures was limited to three per subject, we did not perform additional altitude studies.
Acclimatization
Upon arrival in Bolivia, the subjects spent ∼5 days in La Paz (∼3700 m). Subsequently, they moved to the laboratory at Chacaltaya (5260 m) for 1 day and returned to La Paz for the night, followed by ∼2 days at a base camp (4700 m) before climbing Mt Potosi (6088 m). The subjects then moved to Chacaltaya and, except for a 4 day climb of Mt Illimani (6462 m), they resided there (5260 m) until week 9 when the studies were performed. All subjects were given oral iron supplementation during the first 5–6 weeks following arrival in La Paz.
Bicycle exercise
Each subject underwent four incremental bicycle exercise tests on a cycle ergometer, two at 5260 m after ∼9 weeks of acclimatization (5260 m chronic hypoxia and 5260 m acute normoxia) and two at sea level (0 m normoxia and 0 m acute hypoxia). The bicycle exercise tests were performed on a mechanically braked cycle ergometer (Monark 818, Varberg, Sweden). On the study days (beginning at 08.00 h) catheters were placed in a femoral artery and femoral vein under local anaesthesia (lidocaine (lignocaine), 20 mg ml−1) for blood sampling. The tip of the arterial catheter (20 gauge, Ohmeda, UK) was advanced to 6 cm proximal to the inguinal ligament using the Seldinger technique. The venous catheter was a radiopack Teflon catheter with side holes (Cook, Denmark). A thermistor was inserted through the end hole of the venous catheter for blood flow measurements by the constant infusion thermodilution technique (Andersen et al. 1985). After placement of the catheters, the subjects remained supine for at least 30 min before sitting on the cycle ergometer. The two tests at altitude or at sea level were performed on the same day separated by at least 1 h, or when the arterial lactate concentration had dropped below 1 mmol l−1. At altitude, the first exercise bout was performed by breathing either ambient air (5260 m chronic hypoxia) or a high-oxygen gas mixture (47 % O2 in N2, 5260 m acute normoxia). At sea level, the exercise bout was performed by breathing either ambient air (0 m normoxia) or a low-oxygen gas mixture (10 % O2 in N2, 0 m acute hypoxia). The exercise started at a workload of 80 W for 2.5 min followed every 2.5 min with an increase in workload of 80 W. The workload increments were slightly different towards exhaustion for the individual subjects in order to complete at least three work intensities in all four conditions. The exercise duration ranged from approximately 7 min during 0 m acute hypoxia and 5260 m chronic hypoxia to 12 min during 0 m normoxia and 5260 m acute normoxia. During 0 m acute hypoxia and 5260 m acute normoxia, baseline measurements were obtained after subjects breathed the respective gas mixtures for 5 min from a Douglas bag. Subjects then began to exercise, during which oxygen uptake was measured continuously. Femoral venous blood flow was measured just before the blood samples were taken at the end of each workload (increment of 80 W each 2.5 min), and at the moment of exhaustion. Arterial and femoral venous blood samples were drawn anaerobically prior to the start of exercise and at the end of each workload and placed on ice. The blood samples were analysed within 15 min for plasma lactate (EML105, Radiometer, Copenhagen, Denmark). Haematocrit, haemoglobin and oxygen saturation (OSM3 hemoxymeter, Radiometer), and blood pH, O2 and CO2 tension (ABL5, Radiometer) were also measured. The remaining blood was centrifuged and the plasma stored at −50 °C.
One-leg knee-extensor exercise
Each subject underwent two graded incremental one-leg knee-extensor exercise tests at 5260 m after ∼9 weeks of acclimatization. The two tests were performed on the same day with at least 1 h between the exercise tests or when the arterial lactate concentration had dropped below 1.0 mmol l−1. Placement of catheters and procedures were as described for the bicycle exercise. The one-leg knee-extensor exercise was performed while breathing ambient air (5260 m chronic hypoxia) or 47 % O2 in N2 (5260 m acute normoxia) in random order. The exercise began at 20 W for 2.5 min followed each 2.5 min with an increase in workload of 20 W until exhaustion. Baseline measurements in the 5260 m acute normoxia test were obtained after breathing the 47 % O2 in N2 from a Douglas bag for 5 min. Femoral venous blood flow was measured just before the blood samples were taken. Arterial and femoral venous blood samples were drawn anaerobically prior to the start of exercise and at the end of each workload.
Calculations
The measured pH, PO2 and PCO2 were corrected for temperature using the blood temperature as measured in the femoral vein. Plasma bicarbonate was calculated according to Siggaard-Andersen (1977) and blood base deficit was calculated based on the Van Slyke equation of Siggaard-Andersen (1977) and corrected for hypercarbia and oxygen desaturation according to Schlichtig (1997).
Statistical analysis
All data are expressed as means ±s.e.m. for n = 6 subjects. The non-parametric Wilcoxon-signed rank test was applied to determine differences between data obtained at each time point for the four conditions. Statistical significance was set at P < 0.05.
RESULTS
Body mass, haemoglobin, and cycle ergometry (Table 1)
Table 1.
Whole-body measurements and oxygen uptake and power output during bicycle exercise and one-leg knee-extensor exercise
| Copenhagen | Mt Chacaltaya | |||
|---|---|---|---|---|
| 0 m normoxia | 0 m acute hypoxia | 5260 m chronic hypoxia | 5260 m acute normoxia | |
| Weight (kg) | 76 ± 2.9 | — | 69 ± 1.8§ | — |
| Haemoglobin (g dl−1) | 14.3 ± 0.5 | — | 18.7 ± 0.5§ | — |
| Haematocrit (%) | 45.3 ± 0.6 | — | 52.1 ± 0.5§ | — |
| (l min−1) | 4.3 ± 0.3 | 2.7 ± 0.2§ | 2.8 ± 0.2§ | — |
| (ml min−1 (kg body mass)−1) | 56 ± 2 | 35 ± 2§ | 40 ± 2*§ | — |
| Wmax, bicycle (W) | 339 ± 15 | 233 ± 10§ | 245 ± 5§ | 332 ± 5# |
| Wmax, bicycle (W (kg body mass)−1) | 4.6 ± 0.2 | 3.1 ± 0.1§ | 3.6 ± 0.1*§ | 4.8 ± 0.1# |
| Wmax, one-leg knee extensor (W) | — | — | 77 ± 2 | 75 ± 5 |
Values are means ±s.e.m. of six subjects.
Significant difference between 5260 m chronic hypoxia vs. 0 m acute hypoxia and 5260 m acute normoxia vs. 0 m normoxia.
Significant difference between 5260 m chronic hypoxia vs. 0 m normoxia and 0 m acute hypoxia vs. 0 m normoxia.
Significant difference between 5260 m acute normoxia vs. 5260 m chronic hypoxia.
Body mass of the subjects at sea level was 74 ± 3 kg before acclimatization and 76 ± 3 kg 10 months after acclimatization when the sea level trials were carried out. All subjects lost body mass (7.3 ± 1.0 kg) during their stay of ∼9 weeks at 5260 m despite an adequate food supply. Haemoglobin concentration increased from 14.2 ± 0.4 g dl−1 at sea level to 18.9 ± 0.3 g dl−1 after ∼9 weeks at 5260 m with a corresponding increase in haematocrit. No significant differences in absolute maximal oxygen uptake () and power output (Wmax) could be observed during exercise at 5260 m chronic hypoxia and 0 m acute hypoxia. Thus, adjusted for body mass both variables were higher at 5260 m chronic hypoxia.
Arterial lactate concentration and net leg lactate release (Fig. 1)
Figure 1. Arterial lactate concentration and net lactate release from the active leg during incremental bicycle exercise (A) and incremental one-leg knee-extensor exercise (B).
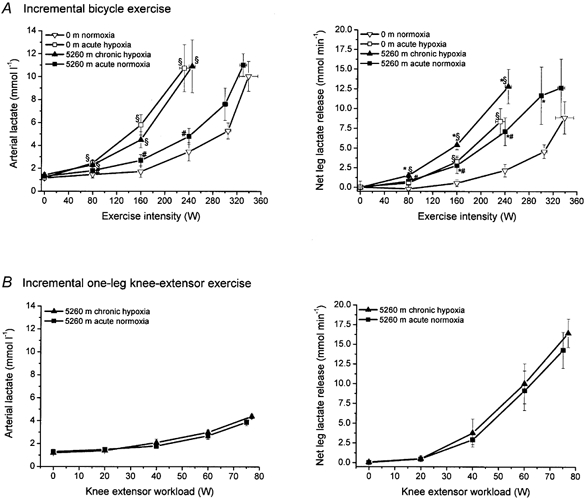
* Significant difference between 5260 m chronic hypoxia vs. 0 m acute hypoxia and 5260 m acute normoxia vs. 0 m normoxia. § Significant difference between 5260 m chronic hypoxia vs. 0 m normoxia and 0 m acute hypoxia vs. 0 m normoxia. # Significant difference between 5260 m acute normoxia vs. 5260 m chronic hypoxia. Values are means ±s.e.m. for six subjects with incremental bicycle exercise and five subjects with incremental one-leg knee-extensor exercise.
During incremental bicycle exercise, arterial blood lactate concentrations at each workload were similar during exercise at 5260 m chronic hypoxia and 0 m acute hypoxia, and, at the same workloads, were greater than those during exercise at 0 m normoxia and 5260 m acute normoxia (Fig. 1A). At exhaustion, peak arterial lactate concentrations were similar for all conditions. Despite this, net leg lactate release at 5260 m chronic hypoxia was higher during both sub-maximal and maximal work than it was at 0 m acute hypoxia. In addition, in the 5260 m acute normoxic condition, net leg lactate release was higher for sub-maximal work than it was at 0 m normoxia except towards peak work. Peak net lactate release from the exercising leg was approximately 13 mmol min−1 after acclimatization to hypoxia compared to about 9 mmol min−1 at sea level. Thus, after ∼9 weeks of acclimatization to 5260 m, peak arterial lactate concentration was similar to that at sea level, but net lactate release from the exercising leg was higher irrespective of oxygen tension.
During the incremental one-leg exercise, maximal work of the knee-extensor muscles was similar during exercise at 5260 m chronic hypoxia and 5260 m acute normoxia (Fig. 1B). Also, arterial lactate concentrations and net lactate release from the active leg were similar during exercise at 5260 m chronic hypoxia and 5260 m acute normoxia. Net leg lactate release at the moment of exhaustion was 14.5–16.5 mmol min−1, which implies that the small active muscle mass involved in knee-extensor exercise (2.6 ± 0.4 kg) elicited a peak net lactate release higher than that with cycle exercise, where a far larger muscle mass is involved in the exercise (∼7 kg). Thus peak net lactate release was ∼6 mmol min−1 (kg muscle)−1 during one-leg knee-extensor exercise and ∼2 mmol min−1 (kg muscle)−1 in the bicycle exercise, a 3-fold difference.
Arterial catecholamine concentration (Fig. 2)
Figure 2. Arterial noradrenaline and adrenaline concentrations at the moment of exhaustion for incremental bicycle exercise (A) and one-leg knee-extensor exercise (B).
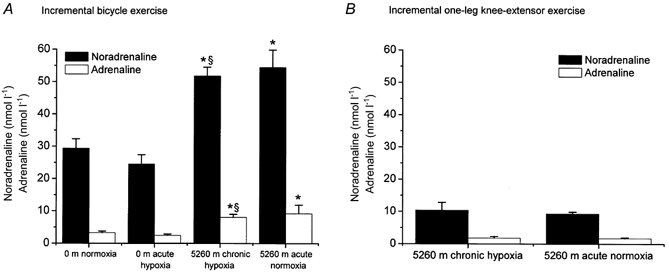
For details see Fig. 1.
At the moment of exhaustion from incremental bicycle exercise, noradrenaline and adrenaline levels were similar at 0 m normoxia and 0 m acute hypoxia, while the corresponding values for both 5260 m chronic hypoxia and 5260 m acute normoxia were nearly 2-fold greater. With one-leg knee-extensor exercise, peak catecholamine values, although substantially lower compared to incremental bicycle exercise, were similar during exercise at 5260 m chronic hypoxia and 5260 m acute normoxia.
Acid-base balance (Tables 2 and 3, and Figs 3–6)
Table 2.
Summary of variables at rest and the at moment of exhaustion for incremental bicycle exercise
| 0 m normoxia | 0 m acute hypoxia | 5260 m chronic hypoxia | 5260 m acute normoxia | |||||
|---|---|---|---|---|---|---|---|---|
| Rest | Exhaustion | Rest | Exhaustion | Rest | Exhaustion | Rest | Exhaustion | |
| Pa,CO2 (mmHg) | 37 ± 1 | 32 ± 2 | 27 ± 3§ | 25 ± 1 | 20 ± 1§ | 19 ± 1§ | 23 ± 1* | 25 ± 1 |
| Pv,CO2 (mmHg) | 45 ± 1 | 82 ± 4 | 41 ± 2 | 53 ± 3§ | 30 ± 2§ | 53 ± 3§ | 31 ± 1* | 74 ± 2# |
| Pa,O2 (mmHg) | 104 ± 4 | 98 ±3 | 48 ± 4§ | 30 ± 1§ | 52 ± 2§ | 50 ± 5*§ | 150 ± 3*# | 148 ± 8 *# |
| Pv,O2 (mmHg) | 35 ± 13 | 11 ± 2 | 23 ± 2 | 7 ± 1§ | 28 ± 3 | 10 ± 2 | 34 ± 7 | 19 ± 2# |
| Sa,O2 (%) | 98 ± 0.1 | 96 ± 0.3 | 89 ± 3§ | 62 ± 2§ | 87 ± 1§ | 71 ± 3*§ | 98 ± 0# | 97 ± 0# |
| Sv,O2 (%) | 48 ± 5 | 7 ± 1 | 47 ± 5 | 4 ± 1§ | 45 ± 7 | 5 ± 2 | 43 ± 3 | 10 ± 2# |
| Blood flow (l min−1) | 0.5 ± 0.1 | 9.4 ± 1 | 0.6 ± 0.1 | 8.3 ± 1.3 | 0.6 ± 0.1 | 7.5 ± 0.3 | 0.6 ± 0.1 | 8.3 ± 0.7 |
| Leg oxygen uptake (ml min−1) | 46 ± 10 | 1815 ± 214 | 46 ± 10 | 1035 ± 205§ | 61 ± 16 | 1308 ± 90§ | 79 ± 18 | 1978 ± 209# |
Values are means ±s.e.m. of six subjects. Pa,CO2, arterial PCO2; Pv,CO2, femoral venous PCO2; Pa,O2, arterial PO2; Pv,O2, femoral venous PO2; Sa,O2, arterial O2 saturation; Sv,O2, femoral venous O2 saturation.
Significant difference between 5260 m chronic hypoxia vs. 0 m acute hypoxia and 5260 m acute normoxia vs. 0 m normoxia.
Significant difference between 5260 m chronic hypoxia vs. 0 m normoxia and 0 m acute hypoxia vs. 0 m normoxia.
Significant difference between 5260 m acute normoxia vs. 5260 m chronic hypoxia.
Table 3.
Summary of variables at rest and at the moment of exhaustion for one-leg knee-extensor exercise
| 5260 m chronic hypoxia | 5260 m acute normoxia | |||
|---|---|---|---|---|
| Rest | Exhaustion | Rest | Exhaustion | |
| Pa,CO2 (mmHg) | 21 ± 2 | 20 ± 1 | 24 ± 2 | 22 ± 2 |
| Pv,CO2 (mmHg) | 30 ± 1 | 49 ± 1 | 33 ± 2 | 58 ± 2 |
| Pa,O2 (mmHg) | 50 ± 1 | 52 ± 1 | 148 ± 5# | 162 ± 5# |
| Pv,O2 (mmHg) | 25 ± 3 | 20 ± 1 | 30 ± 4 | 27 ± 1# |
| Sa,O2 (%) | 85 ± 1 | 83 ± 2 | 98 ± 0# | 98 ± 0# |
| Sv,O2 (%) | 39 ± 7 | 18 ± 3 | 52 ± 7# | 28 ± 3# |
| Blood flow (l min−1) | 0.6 ± 0.1 | 5.6 ± 0.4 | 0.4 ± 0.1 | 4.9 ± 0.2 |
| Leg oxygen uptake (ml min−1) | 63 ± 20 | 928 ± 50 | 42 ± 10 | 885 ± 50 |
Values are means ±s.e.m. of six subjects.
Significant difference between 5260 m acute normoxia and 5260 m chronic hypoxia. For explanation see Table 2 legend.
Figure 3. Arterial pH during incremental bicycle exercise (A) and one-leg knee-extensor exercise (B).
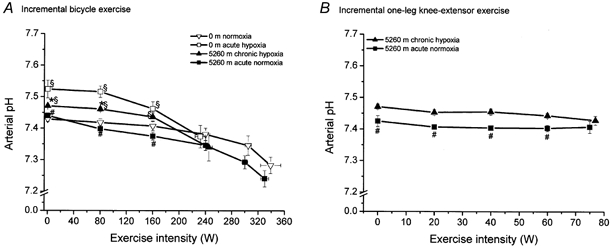
For details see Fig. 1.
Figure 6. Relationship between net lactate release and net proton release during incremental bicycle exercise (A) and incremental one leg-knee-extensor exercise (B).
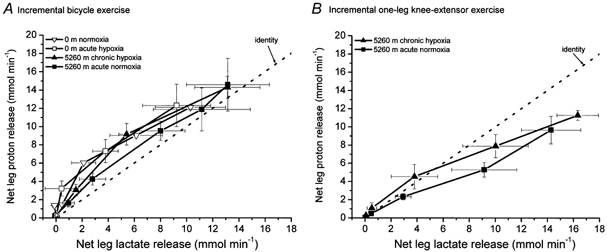
For details see Fig. 1.
At sea level under resting conditions, 5 min of breathing a low-oxygen mixture (0 m acute hypoxia) caused an increase in blood pH due to respiratory alkalosis (Fig. 3). After 9 weeks of acclimatization to altitude, blood pH was still elevated, averaging 7.47 compared to 7.43 at sea level (0 m normoxia). Breathing a high-oxygen air mixture for 5 min after acclimatization (5260 m acute normoxia) caused a lowering of blood pH to sea level values (0 m normoxia). At exhaustion during incremental bicycle exercise, blood pH was substantially higher at 0 m acute hypoxia and 5260 m chronic hypoxia compared to 0 m normoxia and 5260 m acute normoxia. However, the drop in arterial pH from rest to exhaustion was ∼0.15 units in all four conditions.
At rest, plasma bicarbonate concentration (HCO3−) was substantially lower at 5260 m chronic hypoxia (∼16 mmol l−1) compared to 0 m normoxia (∼26 mmol l−1) and 0 m acute hypoxia (∼23 mmol −1; Fig. 4). Although the HCO3− concentration was lower at the moment of exhaustion after acclimatization (5260 m chronic hypoxia) compared to that at 0 m normoxia and 5260 m acute normoxia, the drop in bicarbonate concentration from rest to exercise was substantially smaller. The HCO3− concentration is a reflection of pH and PCO2, which are substantially different under hypoxic and normoxic conditions. Thus the arterial HCO3− concentration does not necessarily reflect blood buffer capacity. The net leg HCO3− release was linear with exercise intensity for both bicycle exercise and one-leg knee-extensor exercise. No differences could be observed between hypoxic and normoxic conditions. The leg CO2 transported as free CO2 increased with work from about 5 % to 10 % while HCO3− decreased correspondingly from 95 % to 90 %. No differences between conditions were observed.
Figure 4. Arterial bicarbonate concentration and net bicarbonate release from the active leg during incremental bicycle exercise (A) and incremental one-leg knee-extensor exercise (B).
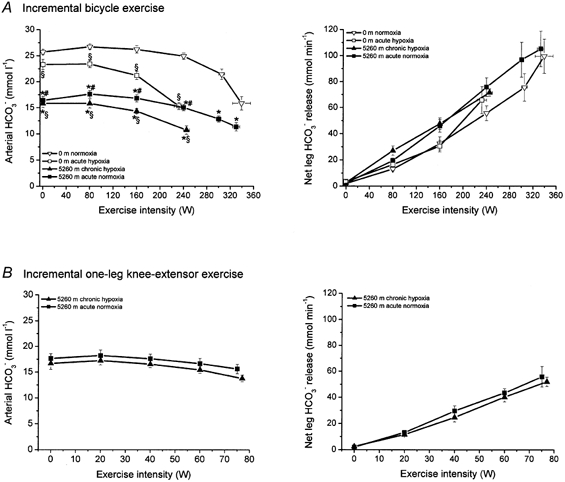
For details see Fig. 1.
The calculated base deficit (BD) was corrected for both hypercarbia and oxygen desaturation (adjusted for changes in buffering due to differences in haemoglobin). BD is the change in strong base needed to restore pH to 7.4. Thus, the difference between the femoral arterial and venous BD multiplied by blood flow represents the net amount of protons released by the leg (Fig. 5). This product had a similar pattern to the net lactate release, as shown in Fig. 1, although no significant differences could be observed between 0 m acute hypoxia and 5260 m chronic hypoxia as was the case for the net lactate release. The difference between net proton release and net lactate release from the active leg represents non-lactate-related proton release. During bicycle exercise, net proton release was always higher than net lactate release but the slope of the relationship between these parameters was 1.0 (Fig. 6). No differences were observed between conditions. During one-leg knee-extensor exercise, net proton release was similar to net lactate release up to 40 W, followed by a lower net proton release than lactate release, with a slope < 1.
Figure 5. Arterial base deficit and calculated leg net proton release during incremental bicycle exercise (A) and incremental one-leg knee-extensor exercise (B).
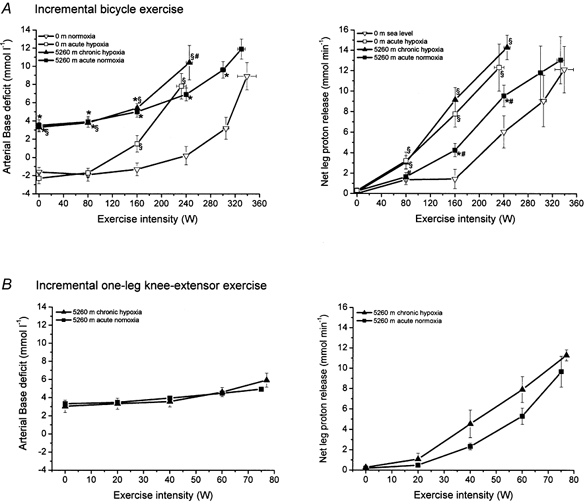
For details see Fig. 1.
DISCUSSION
The main finding of the present study was that there was no lactate paradox, as similar blood lactate concentrations were observed at the moment of exhaustion with 5260 m chronic hypoxia and 0 m acute hypoxia. At both sub-maximal and maximal exercise, net lactate release by the active skeletal muscle was somewhat higher after 9 weeks of chronic exposure to hypoxia (5260 m) compared to 0 m acute hypoxia. This was also the case when the comparison was made between 5260 m acute normoxia and 0 m normoxia. Similar differences were observed for the net proton release from the active leg. In spite of the elevated net lactate release, arterial blood lactate concentrations were not higher, indicating a larger lactate usage by the rest of the body other than the active muscle. In other altitude studies in which the lactate paradox has been reported (West et al. 1983; Kayser et al. 1993; Biedleman et al. 1997), a loss of muscle mass has been implied as a potential contributing factor. However, the findings in this study argue against such a rationale, since not only was leg net lactate release higher during acute hypoxia, but it was also, when loss of muscle mass was accounted for, even greater per unit muscle mass.
In the field study of Lundby et al. (2000) it was suggested that the lactate paradox is transient in the course of acclimatization. They showed that after 4 weeks of acclimatization the peak lactate concentration in blood was higher compared to that after 1 week of acclimatization to 5400 m. Indeed, after 6 weeks of acclimatization, peak lactate concentration was similar compared to acute hypoxic and sea level concentrations. The results of the present study support the observation of Lundby et al. (2000), since after 9 weeks of acclimatization (5260 m chronic hypoxia) arterial lactate concentration was similar to that for 0 m acute hypoxia at sub-maximal and maximal work. In addition, net lactate release from the exercising leg was higher after acclimatization to 5260 m chronic hypoxia compared to 0 m acute hypoxia. Moreover, all subjects lost body weight and magnetic resonance imaging revealed that the leg lean body area was reduced by an average of ∼9 %, whereas the fat area was unchanged (Zacho & Nowak, 2000). Thus, blood peak arterial concentration, when expressed per kilogram of body mass, would be somewhat higher during exercise at 5260 m chronic hypoxia compared to 0 m acute hypoxia and the higher leg net lactate release at 5260 m chronic hypoxia would be even more pronounced if expressed per kilogram of leg muscle. The subjects were rather active, and, in addition to daily outdoor activity including trekking, they participated in two climbs to peaks above 6000 m. Moreover, the food supply was ample throughout the time of acclimatization. Despite high physical activity and sufficient food supply, a substantial body mass and muscle loss was observed.
A difference between the present study together with that of Lundby et al. (2000) and the studies in which a reduced peak lactate concentration with acclimatization to severe hypoxia was observed is the duration of acclimatization; ∼7–9 weeks compared to ∼2–3 weeks (Dill et al. 1967; Hansen et al. 1967; Bender et al. 1989; Brooks et al. 1992, 1998; Young et al. 1992; Beidleman et al. 1997). However, studies more comparable to the present one are those of Dempsey et al. (1972) and Grassi et al. (1995, 1996). The latter group compared short supra-maximal (Grassi et al. 1995) and incremental (Grassi et al. 1996) cycle exercise at sea level and after 1 and 5 weeks of acclimatization to 5050 m. Peak lactate concentration with supra-maximal exercise was slightly higher at sea level compared to altitude. However, peak lactate was significantly higher after 5 weeks compared to 1 week of acclimatization. During the longer-lasting incremental bicycle exercise, lactate concentration was higher for a given workload after both 1 and 5 weeks of acclimatization compared to that at sea level, although peak lactate concentration was slightly reduced at altitude. Grassi and co-workers (1995, 1996) speculated that the reduced peak lactate concentration at altitude that they observed could, in part, be related to the exercise protocol. During short supra-maximal exercise the factor(s) responsible for the reduction in peak lactate are partially or completely offset by acclimatization, whereas this appeared not to be the case for exercise of longer duration. In the present study, the exercise time to exhaustion at altitude (5260 m chronic hypoxia) was about 7 min, which should be long enough for the presumed reduction in peak lactate to be observed. Furthermore, in an additional experiment with 20 min of exercise at moderate intensity the arterial lactate concentration was 4-fold and the leg net lactate release 6-fold higher at 5260 m chronic hypoxia compared to sea level (G. van Hall, J. A. L. Calbet, H. Søndergaard & B. Saltin, unpublished data). This not only underscores the absence of a lactate paradox in our subjects but also emphasizes the fact that duration and/or intensity increments do not obscure our findings that a lactate paradox does not exist after 9 weeks of acclimatization. Indeed, the present study together with the studies of Dempsey et al. (1972), Grassi et al. (1995, 1996) and Lundby et al. (2000) suggest that with a long period of acclimatization (5 weeks or more) blood lactate accumulation during exercise is no longer attenuated.
A striking observation was that leg net lactate release was substantially higher during exercise at sub-maximal and maximal work at 5260 m chronic hypoxia compared to 0 m acute hypoxia despite similar blood lactate concentrations. In addition, leg net lactate release during exercise was higher at 5260 m acute normoxia compared to 0 m normoxia. This implies that after prolonged acclimatization to hypoxia, blood lactate utilization was enhanced during exercise by liver, heart, and skeletal muscle other than the active leg muscles. Although speculative, skeletal muscles other than the active leg muscles could be the main tissue exhibiting the enhanced lactate utilization as it has the ability to increase its lactate usage severalfold at low activity levels (Richter et al. 1988; Brooks et al. 1992). Furthermore, we have data showing that during 20 min of moderate intensity exercise at 5260 m chronic hypoxia, lactate utilization by skeletal muscle is higher than during exercise at sea level (G. van Hall, J. A. L. Calbet, H. Søndergaard & B. Saltin, unpublished data). Our findings on the blood lactate concentration during exercise with a small muscle mass confirm those of others (Raynaud et al. 1986, 1988; Kayser et al. 1995; Savard et al. 1995), and provide additional details on leg net lactate release. During incremental one-leg knee-extensor exercise at 5260 m chronic hypoxia, the leg net lactate release was 3-fold higher at maximal work compared to bicycle exercise. A similar blood lactate concentration and leg net lactate release were observed during one-leg knee-extensor exercise at 5260 m chronic hypoxia and 5260 m acute normoxia. This implies that the ability of skeletal muscle to produce lactate is not impaired in chronic hypoxia, and that when muscle oxygenation can be maintained as during small muscle mass exercise, lactate release from active muscle increases with workload and is less affected by oxygen tension and saturation.
In earlier studies of chronic hypoxia, a lowering of noradrenaline and adrenaline levels was observed during exercise. Furthermore, it was suggested that, in particular, the adrenaline levels in the blood were important for the acceleration of glycolysis and pyruvate production above what would be decarboxylated and used in the mitochondria (Brooks et al. 1992). In the present knee-extensor exercise study, adrenaline levels at the moment of exhaustion were far lower compared to those with bicycle exercise yet it is with this type of exercise that the highest rate of lactate production is observed. This pattern argues against the concept that muscle lactate production is tightly regulated by plasma adrenaline. In addition, effective β-adrenergic blockade did not prevent the reduction in blood lactate after 3 weeks of acclimatization and, therefore, it was suggested that the β-adrenergic pathways are not responsible for the lactate paradox (Mazzeo et al. 1994). Hochachka and co-workers (Hochachka et al. 1986, 1992) have proposed that the rate of glycolysis is downregulated in chronic hypoxia to better match muscle mitochondrial respiratory capacity, i.e. there is a tighter coupling to oxidative phosphorylation. Earlier it was believed that the mitochondrial capacity became elevated as part of the acclimatization process. On this latter point, there is now a consensus that it does not occur. Mitochondrial volume density has even been reported to be decreased with acclimatization to severe hypoxia (Howald et al. 1990; Hoppeler et al. 1990). Andean natives are not superior in this respect. In fact their mitochondrial volume density and oxidative enzyme activity are lower than in sea level residents if they are sedentary (Saltin & Gollnick, 1983; Kayser et al. 1991; Desplanches et al. 1996). The key factor in the regulation of glycolysis is the redox state of the active muscle. Very little is known about it, as direct measurements cannot be performed. When interpreting the indirect data available in the literature it is not apparent whether there are any changes in skeletal muscle ATP, ADP, AMP, creatine phosphate and inorganic phosphate levels at a given workload at sea level or at altitude that would cause less activation of phosphofructokinase following acclimatization (Green et al. 1989, 2000). It has been suggested that anaerobic glycolysis is depressed after acclimatization due to a reduced ability to activate the contractile apparatus (Green et al. 1989). In a recent study, muscle biopsies were taken before and after 3–4 days upon return to sea level following a 3 week expedition to Mt Denali (6194 m) (Green et al. 2000). No differences were observed in ATP, ADP and AMP levels but creatine phosphate fell less with exercise, and IMP and lactate levels were attenuated after acclimatization. They concluded that 3 weeks of acclimatization resulted in an improved energy state in contracting muscle under normoxic conditions; however, this was not related to a higher oxidative potential or lower glycolytic potential.
At rest a small BD release, i.e. a net proton release across the leg, was observed under all conditions. With incremental bicycle exercise, the net leg proton release showed a similar pattern to net lactate release; no significant differences in net proton release were found between 0 m acute hypoxia and 5260 m chronic hypoxia. However, the net proton release calculated via BD, being independent of lactate measurements, support the absence of the lactate paradox after ∼9 weeks acclimatization. During incremental bicycle exercise, the net proton release was higher for each exercise work increment at 0 m normoxia and 0 m acute hypoxia. At lower workloads this was also the case with 5260 m chronic hypoxia and 5260 m acute normoxia. However, at the moment of exhaustion with the bicycle exercise at 5260 m chronic hypoxia and the last two increments during bicycle exercise at 5260 m acute normoxia, no difference between net lactate and proton release could be observed. With one-leg knee-extensor exercise a similar pattern for 5260 m chronic hypoxia and 5260 m acute normoxia was seen but shifted towards lower net proton release. At the two lowest workloads, net lactate and proton release were similar; however, at the two highest workloads net lactate release was found to be higher than net proton release. The tendency for a tighter relationship between lactate and proton release from active skeletal muscle after acclimatization compared to that at sea level may be caused by several changes in the course of acclimatization. It has been suggested that the decrease in lactate accumulation might be explained by the reduced alkali (bicarbonate) reserve at high altitude (Edwards, 1936; Cerretelli, 1967; West, 1986). However, Kayser et al. (1993) did not observe an increase in peak blood lactate concentration with bicarbonate ingestion in lowlanders acclimatized for 4 weeks, despite a lower blood and intra-muscular pH. This suggests that changes in buffer capacity are unlikely to play a major role in the reduced maximal lactate concentration and the gradual return to ‘normal’ concentrations with time of acclimatization. However, muscle acid-base balance regulation is poorly understood. For ingested or intravenously administered HCO3− to be effective in muscle pH regulation, HCO3− has to enter the muscle cell and after conversion to CO2 leave the cell in the form of dissolved CO2. Skeletal muscle has two known methods of HCO3− transport, Na+-dependent HCO3− cotransport and HCO3−-Cl− exchange. In cardiac myocytes it is well established that Na+-HCO3− cotransport mediates proton-equivalent efflux whereas Cl−-HCO3− exchange mediates proton-equivalent influx (Leem et al. 1999). Thus, bicarbonate influx may play a more important role than the muscle HCO3− pool in muscle pH regulation during exercise. However, during exercise, intracellular buffering via creatine phosphate breakdown and proteins seems to be more important. During exercise, intracellular pH is governed by the balance of proton production, intracellular buffering, HCO3− uptake via sarcolemmal Na+-HCO3− cotransport, sarcolemmal acid efflux via Na+-H+ exchange, and lactate-H+ cotransport (Fig. 7). This also implies that during high rates of glycolysis, i.e. proton production, proton efflux via Na+-H+ exchange and lactate-H+ cotransport become extremely important. Lactate-H+ cotransport in skeletal muscle is governed via H+-linked monocarboxylate transporters 1 and 4 (MCT-1 and MCT-4; Juel, 1997). Moreover, since MCT-1 and −4 are also present in intra-cellular membrane structures, they may also play a key role in maintaining proton homeostasis in different intracellular compartments (Bonen, 2000; Duboucaud et al. 2000). Training has been shown to increase MCT-1 and MCT-4 content and improve the ability to release lactate and protons from muscle during contraction in rat (Baker et al. 1998) and humans (Pilegaard et al. 1999). If during exercise proton efflux from the myocytes is important to maintain intracellular pH within limits, then protons may accumulate in the interstitial space. Thus buffering might be more important in the interstitial space in order to keep the proton concentration low and to maintain a gradient for lactate-H+ cotransport. Inhibition of extracellular carbonic anhydrase during high intensity exercise has been shown to decrease blood lactate concentration (Kowalchuk et al. 1992). The interstitial buffer capacity seems to be rather limited compared to the blood buffer capacity, and thus high rates of perfusion of skeletal muscle may also play a role in keeping the intramuscular pH balance. The early decrease in peak lactate concentration at altitude might indeed be a result of insufficient proton buffer capacity or transport of protons out of the cell. The gradual return to ‘normal’ maximal lactate concentration with time of acclimatization may be caused by up regulation of the Na+-H+ and lactate-H+ systems and modulation of carbonic anhydrase. Changes in transport systems and enzymes imply that new proteins have to be synthesized. Muscle protein synthesis rates are slow for contractile (∼1.3 % day−1), mitochondrial (∼1.9 % day−1) and sarcoplasmic (3.8 % day−1) proteins (Welle, 1999) at sea level and potentially even lower under hypoxic conditions (Hochachka et al. 1996). Therefore, adaptation at the protein level can be expected to proceed slowly. Therefore, an extended period of acclimatization to altitude might be needed for adaptive changes to occur in transporters and enzymes to re-establish sea level homeostasis.
Figure 7. Schematic presentation of muscle proton transport.
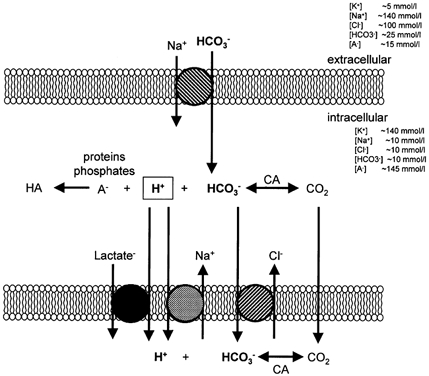
CA, carbonic anhydrase; A, weak acid.
That major changes to protein levels occur with prolonged exposure to hypoxia is best shown by morphological changes that have been reported to occur in fibre size and capillarization (Saltin & Gollnick, 1983; Hoppeler et al. 1990; Kayser et al. 1991).
The changes in fibre size and capillarization with prolonged exposure to hypoxia may also suggest that the absence of the lactate paradox phenomenon in the present study was due to better cardiovascular adaptations leading to improved oxygen transport and perfusion of skeletal muscle. In this respect, adaptation at the muscle microcirculatory level may be crucial. With chronic exposure to hypoxia, muscle capillary density is elevated. This is caused not by capillary proliferation but because skeletal muscle fibre cross-sectional area becomes reduced (Saltin & Gollnick, 1983; Hoppeler et al. 1990). This adaptation may be viewed in the light of a crucial role of diffusion distance in skeletal muscle. In this respect, loss of muscle mass with acclimatization may be a functional adaptation to enhance oxygen delivery by reducing diffusion distance from the capillary to the centre of the fibre.
A final point is that oxygen availability in the active muscles may be a decisive factor not only for the respiratory rate of muscle mitochondria but also for normal activation of glycogenolysis. In small muscle group exercise, oxygen delivery is never compromised at severe altitude and this also relates to lactate production. When a larger fraction of the muscle mass is engaged in the exercise, oxygen delivery is severely hampered in the early phases of acclimatization and so is lactate metabolism. With the gradual elevation in haemoglobin, arterial oxygen delivery is substantially improved, which coincides with the disappearance of the lactate paradox. Thus, we propose that the transient nature of the lactate paradox is a result of temporary, severe disturbance in muscle acid-base balance and mitochondrial oxygen availability.
Acknowledgments
We thank all the subjects who participated in the study, Birgitte Jessen and Harrieth Wagner for excellent technical assistance, and Göran Rådegran and Rob Roach for organising the study. We also thank the Bolivian Academy of Science and the Instituto Boliviano Biologia de Altura (Bolivia) for invaluable help. We are grateful to Radiometer (Copenhagen, Denmark) and Monark (Sweden) for placing valuable equipment at our disposal. This study was supported by a grant from the Carlsberg Foundation (Denmark). The Copenhagen Muscle Research Centre is in receipt of a grant from the Danish National Research Foundation (grant no. 504–14).
References
- Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as a model for study of isolated exercising muscle in humans. Journal of Applied Physiology. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Baker SK, McCullagh JA, Bonen A. Training intensity-dependent and tissue-specific increases in lactate uptake and MCT-a in heart and muscle. Journal of Applied Physiology. 1998;84:987–994. doi: 10.1152/jappl.1998.84.3.987. [DOI] [PubMed] [Google Scholar]
- Beidleman BA, Muza SR, Rock PB, Fulco CS, Lyons TP, Hoyt RW, Cymerman A. Exercise responses after altitude acclimatization are retained during reintroduction to altitude. Medicine and Science in Sports and Exercise. 1997;29:1588–1595. doi: 10.1097/00005768-199712000-00007. [DOI] [PubMed] [Google Scholar]
- Bender PR, Groves BM, McCullough RE, McCullough RG, Trad L, Young AJ, Cymerman A, Reeves JT. Decreased exercise muscle lactate release after high altitude acclimatization. Journal of Applied Physiology. 1989;67:1456–1462. doi: 10.1152/jappl.1989.67.4.1456. [DOI] [PubMed] [Google Scholar]
- Bonen A. Lactate transporters (MCT proteins) in heart and skeletal muscles. Medicine and Science in Sports and Exercise. 2000;32:778–789. doi: 10.1097/00005768-200004000-00010. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Wolfel EE, Butterfield GE, Cymerman A, Roberts AC, Mazzeo RS, Reeves JT. Poor relation between arterial [lactate] and leg net release during exercise at 4,300 m altitude. American Journal of Physiology. 1998;275:R1192–1201. doi: 10.1152/ajpregu.1998.275.4.R1192. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Wolfel EE, Groves BM, Bender PR, Butterfield GE, Cymerman A, Mazzeo RS, Sutton JR, Wolfe RR, Reeves JT. Muscle accounts for glucose disposal but not blood lactate appearance during exercise after acclimatization to 4,300 m. Journal of Applied Physiology. 1992;72:2435–2445. doi: 10.1152/jappl.1992.72.6.2435. [DOI] [PubMed] [Google Scholar]
- Cerretelli P. Lactacid O2 debt in chronic and acute hypoxia. In: Margaria R, editor. Exercise at Altitude. Amsterdam: Excerpta Medica; 1967. pp. 58–64. [Google Scholar]
- Cerretelli P. Gas exchange at high altitude. In: West JB, editor. Pulmonary Gas Exchange. New York: Academic Press; 1980. pp. 97–147. [Google Scholar]
- Dempsey JA, Forster HV, Birnbaum ML, Reddan WG, Thoden J, Grover RF, Rankin J. Control of exercise hypernea under varying durations of exposure to moderate hypoxia. Respiration Physiology. 1972;16:213–231. doi: 10.1016/0034-5687(72)90052-7. [DOI] [PubMed] [Google Scholar]
- Desplanches D, Hoppeler H, Tüscher L, Mayet MH, Spielvogel H, Ferretti G, Kayser B, Leunenberger M, Grünenfelder A, Favier R. Muscle tissue adaptations of high-altitude natives to training in chronic hypoxia and acute normoxia. Journal of Applied Physiology. 1996;81:1946–1951. doi: 10.1152/jappl.1996.81.5.1946. [DOI] [PubMed] [Google Scholar]
- Dill DB, Edwards HT, Fölling A, Oberg SA, Pappenheimer AM, Tabott JH. Adaptations of the organism to changes in oxygen pressure. Journal of Physiology. 1931;71:47–63. doi: 10.1113/jphysiol.1931.sp002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill DB, Myhre LG, Brown DK, Burrus K, Gehlsen G. Work capacity in chronic exposure to altitude. Journal of Applied Physiology. 1967;23:555–560. doi: 10.1152/jappl.1967.23.4.555. [DOI] [PubMed] [Google Scholar]
- Duboucaud H, Butterfield GE, Wolfel EE, Bergman B, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. American Journal of Physiology - Endocrinology and Metabolism. 2000;278:571–579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- Edwards HT. Lactic acid in rest and work at high altitude. American Journal of Physiology. 1936;116:367–375. [Google Scholar]
- Grassi B, Ferretti G, Kayser B, Marzorati M, Colombini A, Marconi C, Cerretelli P. Maximal rate of blood lactate accumulation during exercise at altitude in humans. Journal of Applied Physiology. 1995;79:331–339. doi: 10.1152/jappl.1995.79.1.331. [DOI] [PubMed] [Google Scholar]
- Grassi B, Marzorati M, Kayser B, Bordini M, Colombini A, Conti M, Marconi C, Cerretelli P. Peak blood lactate and blood lactate vs. workload during acclimatization to 5, 050 m and in deacclimatization. Journal of Applied Physiology. 1996;80:685–692. doi: 10.1152/jappl.1996.80.2.685. [DOI] [PubMed] [Google Scholar]
- Green H, Roy B, Grant S, Otto C, Pepe A, McKenzie D, Johnson M. Human skeletal muscle exercise metabolism following an expedition to Mount Denali. American Journal of Physiology — Regulatory, Integrative and Comparitive Physiology. 2000;279:R1872–1879. doi: 10.1152/ajpregu.2000.279.5.R1872. [DOI] [PubMed] [Google Scholar]
- Green HJ, Sutton J, Young P, Cymerman A, Houston CS. Operation Everest II: muscle energetics during maximal exhaustive exercise. Journal of Applied Physiology. 1989;66:142–150. doi: 10.1152/jappl.1989.66.1.142. [DOI] [PubMed] [Google Scholar]
- Hansen JE, Stelter GP, Vogel JA. Arterial pyruvate, lactate, pH and PCO2 during work at sea level and high altitude. Journal of Applied Physiology. 1967;23:523–530. doi: 10.1152/jappl.1967.23.4.523. [DOI] [PubMed] [Google Scholar]
- Hochachka PW. Defence strategies against hypoxia and hypothermia. Science. 1986;231:234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxia tolerance: molecular/metabolic defence and rescue mechanisms for surviving oxygen lack. Proceedings of the National Academy of Sciences of the USA. 1996;93:9493–9498. doi: 10.1073/pnas.93.18.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Stanley C, McKenzie DC, Villena A, Monge C. Enzyme mechanisms for pyruvate-to-lactate flux attenuation: a study of sherpas, Quenchuas, and hummingbirds. International Journal of Sports Medicine. 1992;13(suppl. 1):S119–S122. doi: 10.1055/s-2007-1024613. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Kleinert E, Schlegel C, Claassen H, Howald H, Kayar SR, Cerretelli P. II. Morphological adaptations of human skeletal muscle to chronic hypoxia. International Journal of Sports Medicine. 1990;11(suppl. 1):S3–S9. doi: 10.1055/s-2007-1024846. [DOI] [PubMed] [Google Scholar]
- Howald H, Pette D, Simoneau J-A, Uber A, Hoppeler H, Cerretelli P. III. Effects of chronic hypoxia and muscle enzyme activities. International Journal of Sports Medicine. 1990;11(suppl. 1):S10–S14. doi: 10.1055/s-2007-1024847. [DOI] [PubMed] [Google Scholar]
- Juel C. Lactate-proton co-transport in skeletal muscle. Physiological Reviews. 1997;77:321–358. doi: 10.1152/physrev.1997.77.2.321. [DOI] [PubMed] [Google Scholar]
- Kayser B, Ferretti G, Grassi B, Binzoni T, Cerretelli P. Maximal lactic capacity at altitude: effect of bicarbonate loading. Journal of Applied Physiology. 1993;75:1070–1074. doi: 10.1152/jappl.1993.75.3.1070. [DOI] [PubMed] [Google Scholar]
- Kayser B, Hoppeler H, Claassen H, Cerretelli P. Muscle structure and performance capacity of Himalayan Sherpas. Journal of Applied Physiology. 1991;70:1938–1942. doi: 10.1152/jappl.1991.70.5.1938. [DOI] [PubMed] [Google Scholar]
- Kayser B, Narici MV, Cibella F. Fatigue and performance at high altitude. In: Sutton JR, Houston CS, Coates G, editors. Hypoxia and Molecular Medicine. Burlington, VT, USA: Queen City Printers; 1995. pp. 222–234. [Google Scholar]
- Kowalchuk JM, Heigenhauser GJF, Sutton JR, Jones NL. Effects of acetazolamide on gas exchange and acid-base control after maximal exercise. Journal of Applied Physiology. 1992;72:278–287. doi: 10.1152/jappl.1992.72.1.278. [DOI] [PubMed] [Google Scholar]
- Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. Journal of Physiology. 1999;517:159–180. doi: 10.1111/j.1469-7793.1999.0159z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C, Saltin B, van Hall G. The ‘lactate paradox’, evidence for a transient change in the course of acclimatization to severe hypoxia in lowlanders. Acta Physiologica Scandinavica. 2000;170:265–269. doi: 10.1046/j.1365-201x.2000.00785.x. [DOI] [PubMed] [Google Scholar]
- Mazzeo RS, Brooks GA, Butterfield GE, Cymerman A, Roberts AC, Selland M, Wolfel EE, Reeves JT. β-adrenergic blockade does not prevent the lactate response to exercise after acclimatization to high altitude. Journal of Applied Physiology. 1994;76:610–615. doi: 10.1152/jappl.1994.76.2.610. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Terzis G, Halestrap A, Juel C. Distribution of the lactate/H+ transporter isoforms MCT1 and MCT4 in human skeletal muscle. American Journal of Physiology. 1999;276:E843–848. doi: 10.1152/ajpendo.1999.276.5.E843. [DOI] [PubMed] [Google Scholar]
- Raynaud J, Bailliart O, Duran JC, Marotte H, Durand J. PO2, PCO2, pH and lactates in muscular venous blood during rhythmic forearm exercise at sea level and high altitude (3,850 m) In: Sutton JR, Houston CS, Coates G, editors. Hypoxia. The Tolerable Limits. Indianapolis: Benchmark Press Inc.; 1988. [Google Scholar]
- Raynaud J, Douguet D, Legros P, Capderou A, Raffestin B, Durand J. Time course of muscular blood metabolites during forearm rhythmic exercise in hypoxia. Journal of Applied Physiology. 1986;60:1203–1208. doi: 10.1152/jappl.1986.60.4.1203. [DOI] [PubMed] [Google Scholar]
- Richter EA, Kiens B, Saltin B, Christensen NJ, Savard G. Skeletal muscle glucose uptake during dynamic exercise in humans: effect of muscle mass. American Journal of Physiology. 1988;254:E555–561. doi: 10.1152/ajpendo.1988.254.5.E555. [DOI] [PubMed] [Google Scholar]
- Saltin B, Gollnick PD. Skeletal muscle adaptability: significance for metabolism and performance. In: Peachey LD, Adrian B, editors. Handbook of Physiology, Skeletal Muscle. Bethesda, MD, USA: American Physiological Society; 1983. pp. 555–631. chap. 19. [Google Scholar]
- Savard GK, Areskog N-H, Saltin B. Cardiovascular response to exercise in humans following acclimatization to extreme altitude. Acta Physiologica Scandinavica. 1995;154:499–509. doi: 10.1111/j.1748-1716.1995.tb09935.x. [DOI] [PubMed] [Google Scholar]
- Schlichtig R. [Base excess] and [strong ion difference] during O2-Co2 exchange. In: Nemoto EM, Lamanna JC, editors. Oxygen Transport To Tissue XVIII. New York: Plenum Press; 1997. pp. 97–102. [DOI] [PubMed] [Google Scholar]
- Siggaard-Andersen O. The Van Slycke equation. Scandinavian Journal of Clinical and Laboratory Investigations. 1977;37(suppl. 146):15–20. doi: 10.3109/00365517709098927. [DOI] [PubMed] [Google Scholar]
- Welle S. Human Protein Metabolism. New York: Springer-Verlag; 1999. [Google Scholar]
- West JB. Lactate during exercise at extreme altitude. Federation Proceedings. 1986;45:2953–2957. [PubMed] [Google Scholar]
- West JB, Boyer SJ, Graber DJ, Hacket PH, Maret KH, Milledge JS, Peters RM, Pizzo CJ, Samaja M, Sarnquist FH, Schoene RB, Winslow RM. Maximal exercise at extreme altitudes on Mount Everest. Journal of Applied Physiology. 1983;55:688–698. doi: 10.1152/jappl.1983.55.3.688. [DOI] [PubMed] [Google Scholar]
- Young PM, Sutton JR, Green HJ, Reeves JT, Rock PB, Houston CS, Cynerman A. Operation Everest II: metabolic and hormonal responses to incremental exercise to exhaustion. Journal of Applied Physiology. 1992;73:2574–2579. doi: 10.1152/jappl.1992.73.6.2574. [DOI] [PubMed] [Google Scholar]
- Zacho M, Nowak M. Changes in lean mass and fat mass. In: Zacho M, Boushel R, Holm I, Saltin B, editors. The 1998 Chacaltaya Expedition. Vol. 46. Frederica, Denmark: E. Rasmussen Bogtrykkeri; 2000. [Google Scholar]


