Abstract
The effects of external Cs+ on magnocellular neurosecretory cells were studied during intracellular recordings from 93 supraoptic nucleus neurones in superfused explants of rat hypothalamus.
Bath application of 3–5 mm Cs+ provoked reversible membrane depolarisation and increased firing rate in all of the neurones tested. Voltage-current analysis revealed an increase in membrane resistance between −120 and −55 mV. The increase in resistance was greater below −85 mV than at more positive potentials.
Voltage-clamp analysis showed that external Cs+ blocked the hyperpolarisation-activated inward current, IH. Under current clamp, application of ZD 7288, a selective blocker of IH, caused an increase in membrane resistance at voltages ≤−65 mV. Voltage-current analysis further revealed that blockade of IH caused hyperpolarisation when the initial voltage was < −60 mV but had no effect at more positive values.
Current- and voltage-clamp analysis of the effects of Cs+ in the presence of ZD 7288, or ZD 7288 and tetraethyl ammonium (TEA), revealed an increase in membrane resistance throughout the range of voltages tested (−120 to −45 mV). The current blocked by Cs+ in the absence of IH was essentially voltage independent and reversed at −100 mV. The reversal potential shifted by +22.7 mV when external [K+] was increased from 3 to 9 mm. We conclude that, in addition to blocking IH, external Cs+ blocks a leakage K+ current that contributes significantly to the resting potential of rat magnocellular neurosecretory cells.
Hypothalamic magnocellular neurosecretory cells (MNCs) are responsible for the release of either vasopressin or oxytocin into the blood (Poulain & Wakerley, 1982). Following their synthesis in MNC somata, these peptides are packaged in vesicles and transported to axon terminals in the neurohypophysis (Brownstein et al. 1980) where secretion is triggered by the arrival of action potentials (Dreifuss et al. 1971). Previous work has established that different rates and patterns of firing evoked by stimuli affecting MNCs have a profound impact on excitation-secretion coupling (e.g. Dutton & Dyball, 1979; Bicknell & Leng, 1981; Bicknell, 1988). Changes in firing rate and pattern, therefore, are both important features of the response of these neurones to physiological and pathological conditions. In both types of MNC, action potentials are initiated at the soma as a result of interactions between afferent synaptic signals and intrinsic membrane properties (Renaud & Bourque, 1991). Since the integrative properties of the membrane are governed by the complement, density and distribution of ion channels within the somato-dendritic compartment, increasing attention is being placed on the identification and characterisation of the membrane channels expressed in MNCs (for review see Hatton & Li, 1998).
Experiments on hypothalamic slices have previously shown the presence of the hyperpolarisation-activated inward current (IH) in guinea-pig MNCs (Erickson et al. 1993). Although this study revealed an involvement of IH in the control of burst firing, the presence and significance of IH in rat MNCs remained somewhat controversial for two primary reasons. First, experiments using rat hypothalamic explants showed that application of external Cs+, a well-known blocker of IH (Halliwell & Adams, 1982; Pape, 1996), depolarises MNCs (Stern & Armstrong, 1997; Ghamari-Langroudi & Bourque, 1998). Since blockade of active IH normally leads to hyperpolarisation (e.g. Maccaferri & McBain, 1996), the occurrence of a depolarising effect of Cs+ in rat MNCs seemed inconsistent with a presumed presence of IH. Second, the hallmark features of IH are (i) that it activates slowly during hyperpolarising steps (hundreds of milliseconds) and (ii) that its amplitude increases during steps to more negative potentials (Halliwell & Adams, 1982). Thus, in current-clamped cells expressing IH, voltage responses to hyperpolarising current pulses typically feature slow depolarising sags whose amplitudes increase with hyperpolarisation (McCormick & Pape, 1990). However, experiments on rat MNCs held at voltages near threshold have revealed depolarising sags whose amplitudes first increase and then decrease in response to hyperpolarising pulses of increasing amplitude (e.g. Stern & Armstrong, 1996, 1997). Despite these seemingly paradoxical observations, we revealed recently that rat MNCs do express a significant density of IH and that this current plays an excitatory role in the regulation of electrical activity (Ghamari-Langroudi & Bourque, 2000). Our analysis further suggested that the unusual behaviour of depolarising sags in rat MNCs held near threshold is probably due to the fact that the sags reflect not only the progressive activation of IH, but also the deactivation of the K+ current responsible for sustained outward rectification (Stern & Armstrong, 1997).
Although these observations have highlighted the complex interactions that result from the presence of overlapping voltage-sensitive currents at sub- and near-threshold potentials, the basis for the depolarising effects of Cs+ in rat MNCs remains unknown. Moreover, the robust depolarising effect of external Cs+ suggests that the presence of a conductance distinct from IH can also significantly modulate the electrical activity of these neurosecretory neurones. In this study, therefore, we investigated the ionic basis for the depolarising effects of external Cs+ in rat MNCs. Our results indicate that Cs+ blocks both IH and a leakage K+ current that contributes significantly to the resting potential.
METHODS
Preparation of superfused explants
Hypothalamic explants were prepared as described previously (Ghamari-Langroudi & Bourque, 1998, 2000). Briefly, male Long-Evans rats (150–300 g) were briefly (5–10 s) restrained in a soft, disposable, plastic cone (Harvard Apparatus Canada, Saint-Laurent, QC, Canada) and killed by decapitation using a small rodent guillotine (model 51330; Stoelting Company, Wood Dale, IL, USA). This tissue-harvesting protocol was approved by the Animal Care Committee of McGill University. The brain was then rapidly removed from the cranial vault. A block of tissue (∼8 mm × 8 mm × 2 mm) comprising the basal hypothalamus was excised using razor blades and pinned, ventral side up, to the slanted (∼30 deg) Sylgard base of a temperature-controlled (32–34 °C) superfusion chamber. Within 2–3 min of decapitation, explants were being superfused (0.5–1 ml min−1) with an oxygenated (95 % O2-5 % CO2) artificial cerebrospinal fluid (ACSF; see below) delivered via a Tygon tube placed over the medial tuberal region. The arachnoid membranes covering the ventral surface of the supraoptic nucleus were removed using fine forceps and a cotton wick was placed at the rostral tip of the explant to facilitate drainage of ACSF.
Solutions and drugs
The ACSF (pH 7.4; 295 ± 1 mosmol kg−1) was composed of (mm): NaCl, 121; MgCl2, 1.3; KCl, 3; NaHCO3, 26; glucose, 10; CaCl2, 2.5 (all from Fisher Scientific Company, Pittsburgh, PA, USA). The ACSF was supplemented, where indicated in the text, with 0.3–0.6 μm tetrodotoxin (TTX; Sigma Chemical Co., St Louis, MO, USA). The effects of Cs+ were examined by diluting a 1 m stock of CsCl (in H2O) into ACSF stored in a secondary reservoir (50 ml), and by switching the supply to the delivery tube between the control and secondary reservoir. The IH blocker ZD 7288 (from Tocris Cookson Inc., Ballwin, MO, USA) was prepared as a 30 mm stock solution (in H2O) and stored at 4 °C. The effects of ZD 7288 were examined by bath application of ACSF containing a dilution of the stock solution as described above. In experiments examining the effects of Cs+ in the presence of different concentrations of extracellular K+, several accessory reservoirs were used to allow the control and Cs+-containing solutions to carry the same concentration of K+.
Electrophysiology
Intracellular recordings were obtained using sharp micropipettes prepared from glass capillary tubes (1.2 mm o.d.; A. M. Systems Inc., Everett, WA, USA) pulled on a P87 Flaming-Brown puller (Sutter Instruments Co., Novato, CA, USA). Pipettes were filled with 2 m potassium acetate, yielding a DC resistance of 70–150 MΩ relative to a Ag-AgCl wire electrode immersed in ACSF. Recordings of membrane voltage (DC to 5 kHz) and current (DC to 0.3 kHz) were obtained through an Axoclamp 2A amplifier (Axon Instruments Inc., Foster City, CA, USA). Voltage recordings were performed in continuous current-clamp (‘bridge’) mode whereas current recordings were performed using the discontinuous single-electrode voltage-clamp (dSEVC) mode. Switching frequencies in dSEVC mode were adjusted (2–3.5 kHz) to ensure that a complete decay of the electrode potential was achieved between periods of current injection. Signals acquired during each experiment were displayed on a chart recorder and digitised (44 kHz; Neurodata Instruments Co., Delaware Water Gap, PA, USA) for back-up storage onto videotape. Current and voltage pulses were delivered through an external pulse generator, or via a Digidata 1200-B interface driven by Clampex 8.0 software (Axon Instruments Inc.) running on a Pentium III computer. All signals were digitised online at 10 kHz and stored on the computer's hard drive. Averaging of current traces and digital subtraction were performed offline using Clampfit 8.0 software.
Statistics
Throughout the paper, group data are reported as means plus or minus the standard error of the mean (±s.e.m.). Differences between mean values recorded under control and test conditions were evaluated using Student's paired t test and differences were considered significant when P < 0.05.
Analysis of IH and GH (hyperpolarisation-activated conductance)
The properties of IH evoked by steps to different voltages were derived from an analysis of the Cs+-sensitive, time-dependent currents revealed by subtracting current traces recorded in the presence of Cs+ from traces recorded in control conditions. The time-dependent current was fitted using a monoexponential function using Clampfit 8 software. The amplitude of the evoked IH was defined as the difference between starting and steady-state current values and the time constant of activation was derived directly from the best fit through the data points.
The voltage dependency of GH can be assessed experimentally by plotting the relative amplitude of current tails evoked at a fixed potential following pulses delivered to different conditioning potentials (e.g. McCormick & Pape, 1990). In MNCs, however, relatively large currents are activated or deactivated upon the termination of voltage steps to negative potentials. In particular, the amplitude of the transient K+ current measured at −50 mV following a prepulse to −120 mV can exceed 1.5 nA (Bourque, 1988), a value about one hundred times greater than that of the IH tail expected to result from the same protocol. We therefore estimated GH as done previously (Ghamari-Langroudi & Bourque, 2000), from the amplitude of IH measured at various test voltages (V) divided by the driving force (V - EH), where EH is the reversal potential of IH. Moreover, since we could not measure EH directly, the value was arbitrarily set at −35 mV, a value reflecting the median EH reported during sharp electrode voltage-clamp studies in a variety of cell types (Pape, 1996). Normalised values of GH (%GH) were calculated as: %GH= 100 ×GH(V)/GH(max), where GH(V) is the value of GH at voltage V, and where GH(max) is defined as the value of GH at −120 mV. The data were fitted using a Boltzmann equation:
using Sigmaplot 5 software (Jandel Scientific, San Rafael, CA, USA), where V1/2 is the half-maximal voltage and k is the slope factor characterising the relationship.
RESULTS
The data presented below were obtained during intracellular recordings made from 93 supraoptic nucleus neurones impaled with sharp microelectrodes in superfused explants of rat hypothalamus. These cells had resting membrane potentials more negative than −50 mV, input resistances exceeding 150 MΩ, and fired action potentials whose amplitudes were greater than 60 mV when measured from baseline. Each of these cells also displayed frequency-dependent spike broadening (Andrew & Dudek, 1985; Bourque & Renaud, 1985) and transient outward rectification (Bourque, 1988) when examined from initial membrane potentials below, i.e. negative to, −75 mV. These combined characteristics have been shown to be specific to hypothalamic magnocellular neurosecretory neurones, but not to neighbouring non-neuroendocrine cells, during intracellular recordings in vitro (Renaud & Bourque, 1991; Tasker & Dudek, 1991) and in vivo (Bourque & Renaud, 1991; Dyball et al. 1991).
Effects of external Cs+ on membrane potential and spike discharge
Previous studies have shown that during current-clamp recordings from MNCs held at membrane potentials below the threshold for action potential discharge, bath application of 2–5 mm Cs+ causes a steady-state membrane depolarisation (Stern & Armstrong, 1997; Ghamari-Langroudi & Bourque, 1998). We therefore investigated whether external Cs+ could significantly affect the firing of action potentials. When tested on 15 spontaneously active MNCs, bath application of 3–5 mm external Cs+ caused a consistent and reversible membrane depolarisation with a mean amplitude of 5.6 ± 0.6 mV (e.g. Fig. 1A). This effect was accompanied by a 5.4-fold increase in the mean (±s.e.m..) frequency of firing from 0.34 ± 0.07 Hz in the control to 1.84 ± 0.35 Hz in the presence of Cs+ (P < 0.05). As illustrated in Fig. 1B, bath application of Cs+ (3–5 mm) to silent MNCs (membrane potential (Vm) between −55 and −70 mV) also consistently and reversibly depolarised all of the cells tested (6.1 ± 0.4 mV, n = 39). In 27 (69 %) of these cells the Cs+-induced membrane depolarisation reached the threshold for action potential discharge and the mean peak firing rate achieved in the presence of Cs+ was 1.3 ± 0.2 Hz. In six MNCs the depolarising effects of Cs+ were examined both in the control solution and in ACSF containing TTX (0.3–0.6 μm) to block Na+-dependent action potentials. As illustrated in Fig. 1B, the depolarising effects of Cs+ persisted in the presence of TTX. Moreover, response amplitudes were not significantly different in the two conditions (P < 0.05; paired t test), indicating that the Cs+-evoked depolarisation resulted from an effect on the postsynaptic cell membrane rather than from a presynaptic action.
Figure 1. Effects of extracellular Cs+ on membrane potential and firing rate.
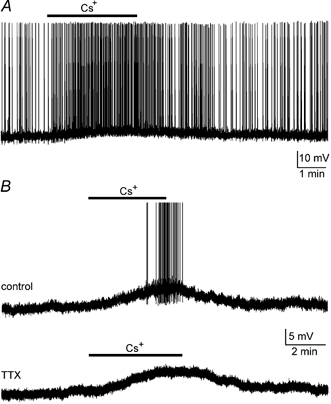
A, chart recording of a spontaneously active supraoptic MNC impaled in a superfused explant of rat hypothalamus. Bath application of 3 mm Cs+ (bar) provoked a reversible membrane depolarisation accompanied by an increase in firing rate. B, chart recordings from another supraoptic neurone in which the initial membrane potential was adjusted to −61 mV by continuous injection of hyperpolarising current (-30 pA). Bath application of 3 mm Cs+ in ACSF (bar; upper trace) provoked a reversible membrane depolarisation and the appearance of action potential discharge. Action potentials are truncated in this panel. The lower trace shows the response of the same cell in ACSF containing 0.6 μm tetrodotoxin (TTX) to block Na+-dependent action potentials.
Effects of Cs+ on steady-state voltage-current properties
As suggested, the intrinsic membrane properties of MNCs appear to be the target of action of Cs+. The possible effects of Cs+ on membrane conductance, therefore, were examined by studying voltage-current (V–I) relationships in control and in the presence of 3–5 mm Cs+ in five MNCs. For this purpose, the initial membrane potential of each cell was current clamped 5–15 mV below the threshold for spike discharge and steady-state V–I relationships were obtained by injecting square current pulses (1–3 s) of varying amplitude (+50 to −400 pA) at intervals ≤ 8 s (e.g. Fig. 2A). The absolute voltage at the end of each pulse was then measured and plotted as a function of the corresponding absolute current. As illustrated in Fig. 2B, steady-state V–I relationships recorded in the presence of Cs+ exhibited an increase in slope compared to the control, and a reversal potential near −85 mV. The increase in slope (i.e. membrane resistance) suggested that the main effect of Cs+ was to inhibit one or more of the conductances that were active under steady-state conditions. Since changes in steady-state voltage provoked by Cs+ (ΔVCs) at any initial voltage (Vi) partly reflected the magnitude of the Cs+-sensitive conductance at that particular voltage, a qualitative description of the voltage dependency of the affected currents was derived by examining the relationship between ΔVCs and Vi. As shown in Fig. 2C, the mean (±s.e.m..) values of ΔVCs were not linearly related to Vi but were more pronounced at voltages below −85 mV. The depolarising effects of Cs+, therefore, were due either to the inhibition of an inwardly rectifying current reversing near −85 mV, or to the suppression of multiple currents whose different voltage sensitivities and reversal potentials combined to generate the plot shown in Fig. 2C. When examined below −100 mV, where the relationship between ΔVCs and Vi was essentially linear, the slope of the steady-state V–I relationships obtained from MNCs showed a 71 % increase from 177 ± 29 MΩ in control to 304 ± 49 MΩ in the presence of Cs+ (P < 0.05; n = 5).
Figure 2. Voltage-current analysis of the Cs+-mediated depolarisation.
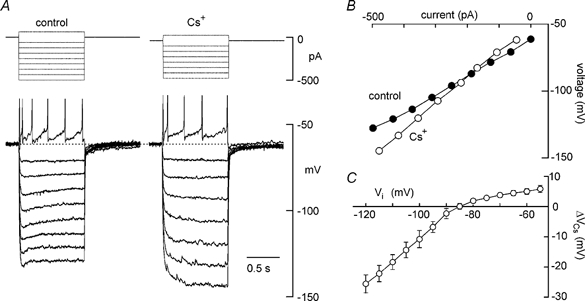
A, voltage responses (lower traces) to current pulses (upper) applied in the absence (control) and presence of 3 mm Cs+. All samples digitised in the last 100 ms of the voltage responses to each current pulse were averaged to determine steady-state voltage. B, plot of the steady-state voltage, measured in the absence and presence of Cs+, as a function of the absolute current being injected into the cell. Using graphs such as these, changes in voltage provoked by Cs+ (ΔVCs) at different initial voltages (Vi) were measured as the vertical difference (in mV) between the control and Cs+ curves, at 5 mV increments. C, a plot of mean (±s.e.m.) ΔVCs-Vi data measured in 5 different MNCs. Note that the plot shows strong inward rectification and a reversal of polarity near −85 mV.
Cs+ blocks IH
One of the inwardly rectifying currents that is known to be blocked by external Cs+ is the hyperpolarisation-activated inward current, IH (Halliwell & Adams, 1982; Pape, 1996). However, as explained earlier, blockade of the inward current mediated by IH normally leads to hyperpolarisation (e.g. Maccaferri & McBain, 1996), not depolarisation. Moreover, the graphs in Fig. 2B and C implied that the depolarising effect of Cs+ above −85 mV was due to the blockade of an active outward current, rather than to the recruitment of an additional inward current. We therefore hypothesised that Cs+ was blocking a current other than IH to cause depolarisation and that its effects on steady-state V–I relationships reflected actions on both conductances. An alternative hypothesis was that Cs+ did not block IH in rat MNCs and that the graph in Fig. 2C entirely reflected the properties of the conductance that was blocked by Cs+ to provoke depolarisation. We thus sought to determine whether Cs+ effectively blocked IH in rat MNCs using voltage-clamp analysis. Current-voltage (I–V) relationhips were obtained by delivering a series of prolonged (2–3 s) voltage steps to values between −115 and −40 mV at sufficiently low frequency (≤ 0.05 Hz) to allow complete inter-pulse deactivation of voltage-sensitive currents. Such trials were performed at constant intervals before, during and after application of Cs+ (Fig. 3A). Digital subtraction of current traces recorded in the presence of Cs+ from those obtained in control yielded a family of current traces reflecting the time-dependent, Cs+-sensitive current (i.e. putative IH) recorded at each voltage (Fig. 3B). These traces were fitted with a single exponential function and the time constant of activation (τH) and steady-state amplitudes were measured at each of the voltages tested. These data were averaged across the population of cells tested (n = 5) and plotted as a function of voltage. The mean current-voltage (I–V) relationship obtained indicated that the time- and voltage-dependent inward current blocked by Cs+ had an apparent activation threshold near −60 mV (Fig. 3C). For each cell the conductance-voltage (G–V) relationship was obtained and the average G–V data were fitted with a Boltzmann equation (see Methods for details). As shown in Fig. 3D, the mean G–V relationship revealed a half-activation voltage (V1/2) of −76 mV, and a slope factor (k) of 12. These values are in excellent agreement with results obtained in a previous study of MNCs (V1/2=−78 mV, k = 12; Ghamari-Langroudi & Bourque, 2000) using ZD 7288, a selective blocker of IH (Harris & Constanti, 1995). Moreover, when examined between −120 and −80 mV, τH of the time-dependent, Cs+-sensitive current varied from 125 ± 17 to 470 ± 123 ms, respectively (Fig. 3E). These values are also similar to those reported for IH blocked by ZD 7288 (123 ± 25 to 563 ± 87 ms; Ghamari-Langroudi & Bourque, 2000). External Cs+, therefore, is an effective blocker of IH in rat MNCs.
Figure 3. Cs+ blocks IH in rat MNCs.
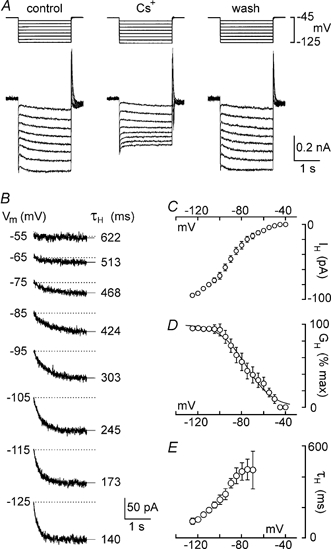
Voltage-clamp recordings were made of the membrane currents evoked by voltage steps lasting 2.5 s to a variety of potentials (Vm) between −50 and −125 mV. Multiple trials were evoked in the absence and presence of Cs+, and current traces recorded in each condition were averaged to reduce noise. A, superimposed current traces (lower) resulting from voltage steps (upper) applied before (control), during (Cs+) and after (wash) application of 5 mm Cs+. All traces were obtained by averaging 3 individual trials in each condition. B, the Cs+-sensitive, time-dependent currents recorded at each Vm (noted at the left of each trace) obtained by digitally subtracting traces recorded in the presence of Cs+ from control traces. Superimposed on each trace is a monoexponential fit of the data points (thin line extending beyond the trace). The amplitude (difference between the end of the fit and the dotted line) and time constant of activation of the time-dependent current (τH; noted at the right of each trace) were derived from the fits. C, mean (±s.e.m.) current-voltage relationship of the Cs+-sensitive, time-dependent current (IH) recorded from 4 MNCs. D, mean conductance (GH)-voltage relationship derived from the data shown in C. The points are superimposed by a Boltzmann distribution (see Methods for details). E, plot of the mean (±s.e.m.) values of τH as a function of voltage. Note that activation kinetics accelerate with hyperpolarisation.
Contribution Of ih to the Cs+-induced inward rectification
Since Cs+ effectively blocked IH in rat MNCs, then part or all of the apparent inward rectification observed in Fig. 2C could be due to the blockade of IH. We therefore examined the specific effects of blocking IH on the V–I properties of five MNCs. As illustrated in Fig. 4A, bath application of the selective blocker of IH ZD 7288 (30–70 μm; Harris & Constanti, 1995) caused a significant increase in the amplitude of voltage responses to prolonged (2–3 s) hyperpolarising current pulses. When examined below −100 mV, the slope of the V–I relationship obtained from MNCs showed a 28 % increase from 172 ± 19 MΩ in control to 221 ± 27 MΩ in the presence of ZD 7288 (P = 0.024; n = 5; e.g. Fig. 4B. An examination of the mean voltage changes evoked by ZD 7288 (ΔVZD) at each Vi led to the plot shown in Fig. 4C. The graph suggests that the blockade of IH provoked a hyperpolarising effect of increasing magnitude at values of Vi below −60 mV, but that it had no effect above −60 mV. These effects were consistent with the voltage dependency of IH shown in Fig. 3C, and they revealed that the inhibition of IH by Cs+ could have contributed part or all of the apparent inward rectification observed in Fig. 2C.
Figure 4. Voltage-current analysis of IH blockade.
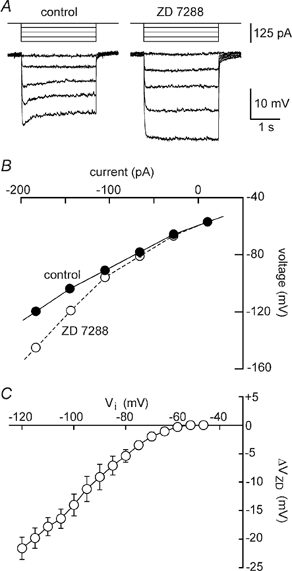
A, voltage responses (lower) to 2.5 s current pulses (upper) were recorded from a MNC in the absence (control) and presence of 66 μm ZD 7288. Holding potential was −65 mV. B, the plots show the absolute steady-state voltages achieved in response to current pulses delivered in each of the conditions. C, the graph shows the mean (±s.e.m.) amplitude of voltage changes evoked by ZD 7288 (ΔVZD) as a function of control steady-state voltage (Vi) in 5 MNCs. Note the strong inward rectification at negative voltages and that application of ZD 7288 has no effect on Vi at potentials ≤−60 mV.
Effects of Cs+ on V–I properties in the absence of IH
The voltage dependency of the Cs+-sensitive current responsible for membrane depolarisation was examined by performing steady-state V–I analysis in the absence of IH. When tested on nine MNCs pre-exposed to 30–70 μm ZD 7288, application of 3–5 mm Cs+ caused a mean depolarisation of 6.3 ± 0.9 mV. Under these conditions, steady-state V–I relationships measured in the absence and presence of Cs+ revealed an increase in slope resistance that appeared to prevail across the entire range of voltages tested (Fig. 5A and B). Indeed, in the presence of ZD 7288 mean values of ΔVCs varied as a more linear function of Vi than in the absence of the drug (Fig. 5C). When measured at values negative to −100 mV and in the continuous presence of ZD 7288, the slope resistance of MNCs increased from 204 ± 11 to 281 ± 20 MΩ upon applying Cs+ (P < 0.05; n = 9). Finally, the polarity of the voltage changes evoked by Cs+ in the absence of IH reversed around −99 mV, a value approximating the equilibrium potential for K+ ions observed previously under similar recording conditions (Kirkpatrick & Bourque, 1996).
Figure 5. Effects of Cs+ on membrane potential in the absence of IH.
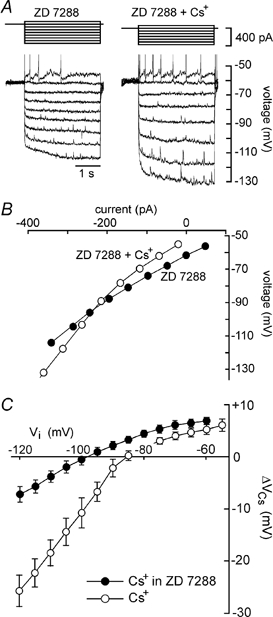
A, superimposed voltage responses (lower) to 3 s current pulses (upper) applied in the absence (control) and presence of 3 mm Cs+ (66 μm ZD 7288 was present throughout). B, the plots show the absolute steady-state voltages achieved in response to current pulses delivered in each of the conditions shown in A. C, filled circles plot the mean (±s.e.m.) amplitude of the voltage changes evoked by Cs+ (ΔVCs), as a function of control voltage (Vi), in the presence of ZD 7288. The data were measured in 9 different MNCs as described in Fig. 2B, from plots such as that shown in B. Note that the plot shows much less rectification in the presence than in the absence of ZD 7288 (open circles, data from Fig. 2C), and that the reversal potential of the effect lies near −100 mV.
Analysis of the Cs+-sensitive current in the absence of IH
The results described previously suggested that the depolarising effects of extracellular Cs+ in MNCs might be due to the inhibition of a steady-state K+ current lacking significant voltage dependency. We therefore examined the properties of the Cs+-sensitive, ZD 7288-resistant current under voltage clamp. As illustrated in Fig. 6A, bath application of 3–5 mm Cs+ to MNCs pre-exposed to ZD 7288 and TTX evoked a reversible inward current when cells were clamped at a voltage near −50 mV. Steady-state I–V analysis was performed in the absence and presence of 3–5 mm Cs+ by examining averaged current responses to clamp steps (1–2 s long; every 8–16 s) to a variety of voltages (e.g. Fig. 6B). The amplitude of the current responses evoked in each condition was plotted as a function of command potential (Fig. 6C) and for each cell a graph of the difference current amplitude was constructed. As illustrated in Fig. 6D, the mean (n = 4) Cs+-sensitive (i.e. difference) current underlying the depolarisation of MNCs showed weak inward rectification and a reversal potential near −100 mV. In three cells, measurements of Cs+-evoked responses were repeated while explants were superfused with ACSF containing alternately 3 and 9 mm[K+]o (as well as 35 μm ZD 7288). In the presence of 9 mm[K+]o the reversal potential of the Cs+-evoked depolarisation was shifted by +22.7 ± 2.6 mV compared to values recorded in 3 mm[K+]o (P < 0.05; data not shown). This value was reasonably close to the shift predicted by the Nernst equation (+28.7 mV) for a K+-selective membrane at our recording temperature, confirming that the Cs+-evoked depolarisation is mediated by the suppression of a K+ conductance.
Figure 6. I–V analysis of the ZD 7288-resistant, Cs+-sensitive current.
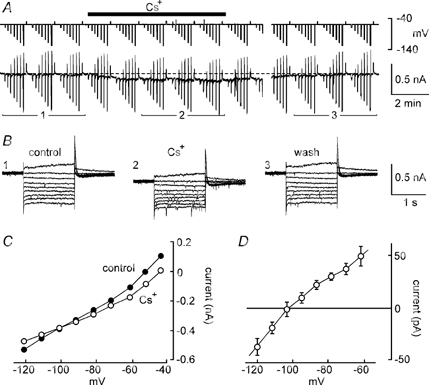
A, chart recording of membrane current (lower) and voltage (upper) in a voltage-clamped MNC. Vertical deflections are changes in voltage and current associated with repeated series of computer-generated steps for I–V analysis. The cell was continuously exposed to 66 μm ZD 7288 and the effects of bath application of 3 mm Cs+ (bar) were examined. Note the steady-state inward current evoked in the presence of Cs+, and that a gap (5 min) is inserted in the recording. B, superimposed current responses to 1.5 s steps to voltages between −120 and −45 mV (holding potential, −50 mV) before (1, control), during (2, Cs+) and after (3, wash) the application of 3 mm Cs+. The traces shown represent the digitally averaged (n = 3 trials) responses recorded during the corresponding periods numerically identified in A. C, steady-state I–V relationships obtained from the data in B. D, graph showing the mean (±s.e.m.) difference current plots measured in 4 MNCs. The points represent the voltage dependency of the steady-state current blocked by Cs+ in the presence of ZD 7288.
Externally applied TEA is well known to block the outward currents flowing through a variety of K+ channels. We therefore examined whether the depolarising effects of Cs+ could be occluded by TEA. Intracellular recordings were obtained from MNCs in explants superfused with solutions containing 3–5 mm TEA. In each of three MNCs tested under current clamp, bath application of 3 mm Cs+ still provoked reversible membrane depolarisations (e.g. Fig. 7A). When tested under voltage clamp (n = 5), application of Cs+ in the presence of 3–5 mm TEA and 60–70 μm ZD 7288 induced an inward current at voltages near rest, and a decrease in slope conductance (Fig. 7B and C). In the presence of TEA and in absence of IH, the mean decrease in slope conductance observed in five MNCs was 0.80 ± 0.16 nS, a value not significantly different from that observed without TEA (P > 0.05). The K+ channels responsible for the depolarising effects of Cs+, therefore, are not sensitive to 3–5 mm TEA.
Figure 7. The Cs+-sensitive leak current in MNCs is not blocked by TEA.
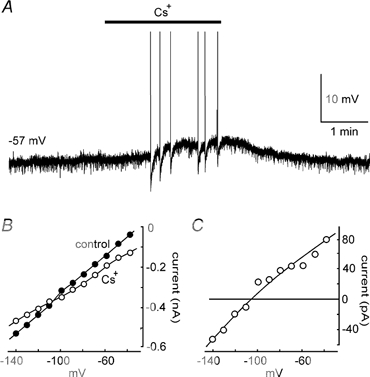
A, the effect of 3 mm Cs+ on a MNC recorded in the presence of 5 mm TEA. B, current-voltage plots recorded from another cell in the absence (control) and presence of 3 mm Cs+. ZD 7288 (70 μm), TTX (0.5 μm) and TEA (5 mm) were present throughout. The graph in C shows the voltage dependency of the Cs+-sensitive current obtained by subtraction of the Cs+ from the control plot in B.
DISCUSSION
It has been known for some time that hyperpolarising steps activate a slow inward current in MNCs (Bourque, 1987). Although this behaviour might have been surmised to reflect the presence of IH, recently published observations appeared to contradict the possible presence of this current in rat MNCs. Indeed, current-clamp experiments in hypothalamic explants (Stern & Armstrong, 1997; Ghamari-Langroudi & Bourque, 1998) revealed that application of external Cs+, a well-known blocker of IH (Halliwell & Adams, 1982; Mayer & Westbrook, 1983), causes a membrane depolarisation instead of the hyperpolarisation expected to result from blockade of IH (e.g. Maccaferri & McBain, 1996). Moreover, in MNCs held at voltages near threshold, depolarising sags associated with voltage responses to hyperpolarising current pulses of increasing magnitude become progressively smaller as they approach −100 mV (e.g. Stern & Armstrong, 1997). This behaviour is opposite to that normally observed in cells expressing IH (e.g. McCormick & Pape, 1990). Despite these observations, however, the existence of a functionally significant IH in rat MNCs was recently established through the combined use of voltage-clamp analysis and bath application of ZD 7288, a selective blocker of IH (Ghamari-Langroudi & Bourque, 2000). The analysis further suggested that the progressive reduction in sag amplitude observed during hyperpolarising responses in control conditions might be due to the deactivation of a steady-state voltage-dependent K+ current; perhaps that which underlies slow outward rectification in rat MNCs (Stern & Armstrong, 1997). Although these studies have begun to clarify our understanding of the membrane currents expressed at near- and subthreshold voltages in rat MNCs, the ionic basis for the Cs+-induced depolarisation remained unexplained.
Cs+ blocks superimposed inward and outward currents in MNCs
Our voltage-current analysis indicated that the depolarising effects of bath-applied Cs+ were associated with an increase in slope resistance, implying that ion channels are blocked, or otherwise closed, in the presence of the cation. Since membrane depolarisation results necessarily from the generation of a relative inward current, the active current being blocked by Cs+ must be flowing in the outward direction under steady-state conditions. Analysis of the effects of Cs+ at different steady-state voltages initially suggested the involvement of an inwardly rectifying current reversing near −85 mV. The curve plotted in Fig. 2C, however, reflected not only the properties of the Cs+-sensitive conductance responsible for depolarisation, but also the effects of blocking the superimposed IH. Indeed, our experiments showed that Cs+ effectively blocks IH in rat MNCs (Fig. 3) and that suppression of IH alone imparts a strong inward rectification to the apparent voltage-dependent properties of the total Cs+-sensitive conductance derived from V–I analysis (Fig. 2C and Fig. 4).
The depolarising effects of Cs+ are due to blockade of a leakage K+ current
Having established that IH blockade could confound the analysis of the conductance responsible for the production of depolarising responses, we proceeded to examine the effects of Cs+ in the absence of IH, by recording from MNCs in the continuous presence of 30–70 μm ZD 7288. Our results indicated that the depolarising effects of Cs+ recorded in the absence of IH were due to the suppression of a K+-selective current showing only weak inward rectification, and which reversed polarity near −100 mV (Fig. 6D and Fig. 7C). These observations confirm that much of the inward rectification, and the relatively positive reversal potential (−85 mV), characterising the effects of Cs+ in control solutions is due to the simultaneous blockade of IH (which reverses near −35 mV; Pape, 1996) and of a relatively linear leakage K+ current (IKL). Although previous studies have shown that externally applied Cs+ can cause membrane depolarisation through the suppression of inwardly rectifying K+ currents (Williams et al. 1988; Jarolimek et al. 1994), the present results suggest that the Cs+-sensitive K+ conductance in MNCs is relatively insensitive to membrane potential over the range of voltages examined (−40 to −120 mV).
Relative contributions of IKL and IH at subthreshold voltages
Our results thus show that two distinct Cs+-sensitive conductances are present in MNCs of the rat supraoptic nucleus. Since the conductance underlying IKL (GKL) does not show significant voltage dependency, and since GH becomes fully active at voltages below about −100 mV (Fig. 3D), the relative maximal contributions of these conductances can be approximated by an inspection of voltage-current relationships below −100 mV. In control solutions, application of Cs+ increased membrane resistance below −100 mV from 177 to 304 MΩ. This represents the suppression of 2.36 nS of the membrane conductance active at this range of voltages. Over the same range of potentials, application of ZD 7288 was found to increase resistance from 172 to 221 MΩ. This suggests that GH alone accounts for 1.32 nS of the steady-state membrane conductance below −100 mV. This value is similar to the maximal value of GH derived from voltage-clamp experiments using ZD 7288 (1.1 nS; Ghamari-Langroudi & Bourque, 2000) and from an analysis of the time-dependent current blocked by Cs+ under voltage clamp (1.2 nS; Fig. 3). The Cs+-sensitive conductance responsible for membrane depolarisation therefore represents about 1 nS of the total membrane conductance. This estimate is supported by the finding that in the presence of ZD 7288, Cs+ increased membrane resistance from 204 to 281 MΩ, a change corresponding to the blockade of 1.34 nS. One can surmise, therefore, that GKL represents about 20 % of the input conductance of rat MNCs impaled in hypothalamic explants.
Physiological role of the Cs+-sensitive GKL
Although the MNCs from which we recorded in this study were not specifically identified as either vasopressin or oxytocin containing, it is worth emphasising that all 93 of the cells tested in our experiments displayed a Cs+-induced response consistent with the expression of GKL at rest. Since quantitative immunocytochemical studies have shown that approximately 69 % of the MNCs present in the supraoptic nucleus of Long-Evans rats (Rhodes et al. 1981) synthesise vasopressin while the remainder express oxytocin, it is likely that both types of neurone were sampled in our study. We can surmise, therefore, that both types of MNC express GKL at rest and that modulation of this conductance could ultimately regulate the secretion of both hormones from the neurohypophysis.
The most obvious physiological role for GKL is a contribution to the resting potential. The analysis presented in the previous paragraph suggests that approximately 20 % of the input conductance of MNCs at rest is provided by steady-state Cs+-sensitive GKL. The inhibition of this resting GKL by 2–5 mm Cs+ provoked a mean membrane depolarisation of about 6 mV. Given that MNCs typically rest at voltages within 10 mV of the threshold for action potential discharge (e.g. Mason, 1983; Stern & Armstrong, 1996), the outward current normally flowing through GKL channels evidently provides an important source of tonic intrinsic inhibition. Interestingly, a previous study has shown that the depolarising effects of noradrenaline are in part due to the suppression of a K+ current that is active at the resting potential (Randle et al. 1985). Although it is not yet known whether these effects can be occluded by external Cs+, the observation suggests that modulation of the inhibitory effect of GKL by neurotransmitters might represent an effective mechanism for the regulation of firing and hormone release. It should also be noted that Li & Hatton (1996) previously showed that activation of histamine receptors can depolarise MNCs through the G-protein-mediated suppression of a resting voltage-independent leakage conductance. Whether histamine modulates a residual Cs+-sensitive GKL or a distinct K+ conductance remains to be determined.
Involvement of GKL in depolarising after-potentials
Another possible function for GKL might be a contribution to the generation of depolarising after-potentials (DAPs). Previous studies in MNCs have shown that action potentials are followed by DAPs (Andrew & Dudek, 1983; Bourque, 1986) and that the summation of consecutive DAPs contributes to the establishment of the plateau potential that sustains firing during phasic bursts (Bourque et al. 1998; Ghamari-Langroudi & Bourque, 1998). Modulation of the DAP, therefore, represents a powerful mechanism for the control of firing pattern by neurotransmitters in MNCs (e.g. Papas & Bourque, 1997; Brown et al. 1999). A previous study has provided evidence that the DAP may result from the transient suppression of a baseline K+ current following each action potential (Li & Hatton, 1997b). Although Ca2+ influx appears to be involved in the induction of DAPs (Bourque, 1986; Li & Hatton, 1997a), the nature of the putative K+ conductance being modulated is unknown. In a previous study (Ghamari-Langroudi & Bourque, 1998) we reported that external Cs+ effectively blocks DAPs at low millimolar concentrations. Interestingly, the blockade of DAPs during exposure to Cs+ is accompanied by membrane depolarisation and the time course of these two effects is similar during both onset and recovery (e.g. Fig. 1 in Ghamari-Langroudi & Bourque, 1998). Since the results of the present study indicate that the Cs+-evoked depolarisation is due to the blockade of GKL, DAPs may in fact be due to a transient, action potential-evoked suppression of GKL and the loss of DAPs in the presence of Cs+ may be due to the occlusion of the basal K+ current required for its expression. Additional studies will be required to test this hypothesis.
Molecular nature of the channels underlying GKL
The participation of specialised ‘leakage’ K+ channels in the regulation of resting potential has long been inferred from the observation that many transmitters regulate membrane potential through the modulation of voltage-insensitive, as opposed to voltage-activated or inwardly rectifying, K+ conductances (Brown, 2000; North, 2000). The molecular nature of putative leakage K+ channels has recently emerged from the cloning of several members of the two-pore K+ channel family, such as TASK-1 (Duprat et al. 1997), TWIK-1 (Lesage et al. 1996), TREK-1 (Fink et al. 1996) and TRAAK (Fink et al. 1998). Could any of these channels underlie the GKL of MNCs? Although many additional experiments will be required to provide an answer to this question, it is interesting to note that channels encoded by TASK are insensitive to TEA, but are blocked by external Cs+ (Czirjak et al. 2000), as was the GKL recorded in supraoptic MNCs. Moreover, TASK-1 mRNA is present in a variety of central neurones (Talley et al. 2000), including those in the supraoptic nucleus (E. M. Talley & D. A. Bayliss, personal communication). Whether TASK-1 or analogous channels mediate the depolarising effects of Cs+ in MNCs remains to be determined.
Acknowledgments
This work was supported by an operating grant from the Canadian Institutes of Health Research and by an MRC Senior Scientist Award to C.W.B.
References
- Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–1052. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- Andrew RD, Dudek FE. Spike broadening in magnocellular neuroendocrine cells of rat hypothalamic slices. Brain Research. 1985;334:176–179. doi: 10.1016/0006-8993(85)90583-9. [DOI] [PubMed] [Google Scholar]
- Bicknell RJ. Optimizing release from peptide hormone secretory nerve terminals. Journal of Experimental Biology. 1988;139:51–65. doi: 10.1242/jeb.139.1.51. [DOI] [PubMed] [Google Scholar]
- Bicknell RJ, Leng G. Relative efficiency of neural firing patterns for vasopressin release in vitro. Neuroendocrinology. 1981;33:295–299. doi: 10.1159/000123248. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Calcium-dependent spike after-current induces burst firing in magnocellular neurosecretory cells. Neuroscience Letters. 1986;70:204–209. doi: 10.1016/0304-3940(86)90464-7. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Intrinsic features and control of phasic burst onset in magnocellular neurosecretory cells. In: Ciriello J, Calaresu FR, Renaud LP, Polosa C, editors. Organization of the Autonomic Nervous System: Central and Peripheral Mechanisms. New York: Alan R. Liss Inc.; 1987. pp. 387–396. [Google Scholar]
- Bourque CW. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. Journal of Physiology. 1988;97:331–347. doi: 10.1113/jphysiol.1988.sp017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Kirkpatrick K, Jarvis CR. Extrinsic modulation of spike afterpotentials in rat hypothalamoneurohypophysial neurons. Cellular and Molecular Neurobiology. 1998;18:3–12. doi: 10.1023/a:1022566924921. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Renaud LP. Activity dependence of action potential duration in rat supraoptic neurosecretory neurons recorded in vitro. Journal of Physiology. 1985;363:429–439. doi: 10.1113/jphysiol.1985.sp015720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Renaud LP. Membrane properties of rat magnocellular neuroendocrine cells in vivo. Brain Research. 1991;540:349–352. doi: 10.1016/0006-8993(91)90535-4. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Ghamari-Langroudi M, Leng G, Bourque CW. κ-Opioid receptor activation inhibits post-spike depolarizing after-potentials in rat supraoptic nucleus neurones in vitro. Journal of Neuroendocrinology. 1999;11:825–828. doi: 10.1046/j.1365-2826.1999.00419.x. [DOI] [PubMed] [Google Scholar]
- Brown DA. Neurobiology: the acid test for resting potassium channels. Current Biology. 2000;10:R456–459. doi: 10.1016/s0960-9822(00)00532-7. [DOI] [PubMed] [Google Scholar]
- Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207:373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Molecular Endocrinology. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- Dreifuss JJ, Kalnins I, Kelly JS, Ruf KB. Action potentials and release of neurohypophysial hormones in vitro. Journal of Physiology. 1971;215:805–817. doi: 10.1113/jphysiol.1971.sp009499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Yeyes R, Neurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO Journal. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton DA, Dyball REJ. Phasic firing enhances vasopressin release from the rat neurohypophysis. Journal of Physiology. 1979;290:433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball REJ, Tasker J-G, Wuarin J-P, Dudek FE. In vivo intracellular recording of neurons in the supraoptic nucleus of the rat hypothalamus. Journal of Neuroendocrinology. 1991;3:383–386. doi: 10.1111/j.1365-2826.1991.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Erickson KR, Ronnekliev OK, Kelly MJ. Electrophysiology of guinea-pig supraoptic neurones: role of a hyperpolarization-activated cation current in phasic firing. Journal of Physiology. 1993;460:407–425. doi: 10.1113/jphysiol.1993.sp019478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Romay G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO Journal. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO Journal. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Caesium blocks depolarizing after-potentials and phasic firing in rat supraoptic neurones. Journal of Physiology. 1998;510:165–175. doi: 10.1111/j.1469-7793.1998.165bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Excitatory role of the hyperpolarization-activated inward current in phasic and tonic firing of rat supraoptic neurons. Journal of Neuroscience. 2000;20:4855–4863. doi: 10.1523/JNEUROSCI.20-13-04855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Research. 1982;250:71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Harris NC, Constanti A. Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea-pig substantia nigra neurons in vitro. Journal of Neurophysiology. 1995;74:2366–2378. doi: 10.1152/jn.1995.74.6.2366. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Li Z. Mechanisms of neuroendocrine cell excitability. In: Zingg HH, Bourque CW, Bichet DG, editors. Vasopressin and Oxytocin, Molecular, Cellular and Clinical Advances. New York: Plenum Press; 1998. pp. 79–95. [Google Scholar]
- Jarolimek W, Bijak M, Misgeld U. Differences in the Cs block of baclofen and 4-aminopyridine induced potassium currents of guinea-pig CA3 neurons in vitro. Synapse. 1994;18:169–177. doi: 10.1002/syn.890180302. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K, Bourque CW. Activity dependence and functional role of the apamin-sensitive K+ current in rat supraoptic neurones in vitro. Journal of Physiology. 1996;494:389–398. doi: 10.1113/jphysiol.1996.sp021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romay G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO Journal. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hatton GI. Histamine-induced prolonged depolarization in rat supraoptic neurons: G-protein-mediated, Ca2+-independent suppression of K+ leakage conductance. Neuroscience. 1996;70:145–158. doi: 10.1016/0306-4522(95)00373-q. [DOI] [PubMed] [Google Scholar]
- Li Z, Hatton GI. Ca2+ release from internal stores: role in generating depolarizing after-potentials in rat supraoptic neurones. Journal of Physiology. 1997a;498:339–350. doi: 10.1113/jphysiol.1997.sp021862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hatton GI. Reduced outward K+ conductances generate depolarizing after-potentials in rat supraoptic nucleus neurones. Journal of Physiology. 1997b;505:95–106. doi: 10.1111/j.1469-7793.1997.095bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. The hyperpolarization-activated current (IH) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. Journal of Physiology. 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay cells. Journal of Physiology. 1990;431:319–342. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WT. Electrical properties of neurons recorded from the rat supraoptic nucleus in vitro. Proceedings of the Royal Society B. 1983;217:141–161. doi: 10.1098/rspb.1983.0003. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. Journal of Physiology. 1983;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Potassium-channel closure taken to TASK. Trends in Neurosciences. 2000;23:234–235. doi: 10.1016/s0166-2236(00)01592-7. [DOI] [PubMed] [Google Scholar]
- Papas S, Bourque CW. Galanin inhibits continuous and phasic firing in rat hypothalamic magnocellular neurosecretory cells. Journal of Neuroscience. 1997;17:6048–6056. doi: 10.1523/JNEUROSCI.17-16-06048.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annual Review of Physiology. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7:773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Randle JCR, Bourque CW, Renaud LP. α1-Adrenergic receptor activation depolarizes rat supraoptic neurosecretory neurons in vitro. American Journal of Physiology. 1985;251:R569–574. doi: 10.1152/ajpregu.1986.251.3.R569. [DOI] [PubMed] [Google Scholar]
- Renaud LP, Bourque CW. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Progress in Neurobiology. 1991;36:131–169. doi: 10.1016/0301-0082(91)90020-2. [DOI] [PubMed] [Google Scholar]
- Rhodes CH, Morrell JI, Pfaff DW. Immunohistochemical analysis of magnocellular elements in rat hypothalamus: distribution and numbers of cells containing neurophysin, oxytocin and vasopressin. Journal of Comparative Neurology. 1981;198:45–64. doi: 10.1002/cne.901980106. [DOI] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Changes in the electrical properties of supraoptic oxytocin and vasopressin neurons during lactation. Journal of Neuroscience. 1996;16:4861–4871. doi: 10.1523/JNEUROSCI.16-16-04861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Sustained outward rectification of oxytocinergic neurones in the rat supraoptic nucleus: ionic dependence and pharmacology. Journal of Physiology. 1997;500:497–508. doi: 10.1113/jphysiol.1997.sp022036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Dudek FE. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. Journal of Physiology. 1991;434:271–293. doi: 10.1113/jphysiol.1991.sp018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Colmers WF, Pan ZZ. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurones in vitro. Journal of Neuroscience. 1988;8:3499–3506. doi: 10.1523/JNEUROSCI.08-09-03499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


