Abstract
Acidosis in cardiac muscle is associated with a decrease in developed force. We hypothesized that slow skeletal troponin I (ssTnI), which is expressed in neonatal hearts, is responsible for the observed decreased response to acidic conditions. To test this hypothesis directly, we used adult transgenic (TG) mice that express ssTnI in the heart. Cardiac TnI (cTnI) was completely replaced by ssTnI either with a FLAG epitope introduced into the N-terminus (TG-ssTnI*) or without the epitope (TG-ssTnI) in these mice. TG mice that express cTnI were also generated as a control TG line (TG-cTnI). Non-transgenic (NTG) littermates were used as controls.
We measured the force-calcium relationship in all four groups at pH 7.0 and pH 6.5 in detergent-extracted fibre bundles prepared from left ventricular papillary muscles. The force-calcium relationship was identical in fibre bundles from NTG and TG-cTnI mouse hearts, therefore NTG mice served as controls for TG-ssTnI* and TG-ssTnI mice. Compared to NTG controls, the force generated by fibre bundles from TG mice expressing ssTnI was more sensitive to Ca2+. The shift in EC50 (the concentration of Ca2+ at which half-maximal force is generated) caused by acidic pH was significantly smaller in fibre bundles isolated from TG hearts compared to those from NTG hearts. However, there was no difference in the force-calcium relationship between hearts from the TG-ssTnI* and TG-ssTnI groups.
We also isolated papillary muscles from the right ventricle of NTG and TG mouse hearts expressing ssTnI and measured isometric force at extracellular pH 7.33 and pH 6.75. At acidic pH, after an initial decline, twitch force recovered to 60 ± 3 % (n = 7) in NTG papillary muscles, 98 ± 2 % (n = 5) in muscles from TG-ssTnI* and 96 ± 3 % (n = 7) in muscles from TG-ssTnI hearts. Our results indicate that TnI isoform composition plays a crucial role in the determination of myocardial force sensitivity to acidosis.
Neonatal heart muscle is less sensitive to deactivation by acidic pH than is adult muscle (Solaro et al. 1986, 1988; Martin et al. 1991; Palmer & Kentish, 1996). The lower sensitivity of the neonatal heart muscle to deactivation by acidic pH during development may be due to different effects of extracellular pH on: (1) Ca2+ dynamics such as Ca2+ currents and the uptake and release of Ca2+ from the sarcoplasmic reticulum (SR), (2) intracellular pH and (3) the myofilament force-calcium relationship. Chen et al. (1996) have shown that acidosis causes a smaller inhibition of Ca2+ currents in neonatal rabbit myocytes compared to adult rabbit myocytes. However, Hulme & Orchard (1998) reported no effect of acidosis on Ca2+ currents in adult rat ventricular myocytes and an inhibitory effect of acidosis on SR Ca2+ uptake and release. Data reported by Nakanishi et al. (1990) suggest that during respiratory and metabolic acidosis intracellular pH decreases less in cells in the newborn compared to those in adults, and this contributes to the smaller pH sensitivity observed in neonatal preparations.
These differences in Ca2+ sensitivity and response to acidic pH may also be due, in part, to developmental differences in myofilament structure and function. Although differences may be due to the expression of embryonic and neonatal isoforms of myofilament proteins such as myosin heavy chain, tropomyosin and troponin T (TnT; Murphy, 1996), isoform shifts in troponin I (TnI) correlate well with shifts in the force-calcium relationship and altered sensitivity to acidic pH (Martin et al. 1991; Reiser et al. 1994). Heart muscle expresses two isoforms of TnI, which are encoded by two different genes (Hunkeler et al. 1991; Murphy et al. 1991). Slow skeletal TnI (ssTnI) is expressed during embryonic and early postnatal life and cardiac TnI (cTnI) is expressed in the adult heart. We (Martin et al. 1991) and others (Ding et al. 1995; Morimoto et al. 1999; Westfall et al. 1999, 2000, 2001) have shown that myofilament preparations containing ssTnI are less sensitive to deactivation by acidic pH than are preparations containing cTnI.
In the experiments reported here we tested the hypothesis that replacement of cTnI with ssTnI is sufficient to prevent the reduction in twitch force that occurs during acidic conditions in adult muscle. It is difficult to test this hypothesis directly using neonatal and adult preparations because there are additional variations between these muscles in both the expression of myofilament protein isoforms and the extent of SR development (Vetter et al. 1995; Vornanen 1996). Our approach was to investigate the role of different TnI isoforms directly using transgenic (TG) mice that express ssTnI in the heart. We have studied the effect of acidic pH on the isometric force developed by skinned fibre bundles and intact papillary muscles from TG and NTG mice. Although acidic pH decreased myofilament sensitivity to Ca2+ in both groups, deactivation by acidic pH was significantly blunted in the TG preparations. Moreover, in contrast to controls, papillary muscles containing ssTnI demonstrated unaltered steady-state tension when exposed to hypercapnic acidosis.
METHODS
TG animals
Adult mice that express ssTnI in the heart with (TG-ssTnI*) and without a FLAG epitope tag (TG-ssTnI) were generated as described previously (Fentzke et al. 1999; Arteaga et al. 2000). The epitope tag permitted the tracking of ssTnI expression in individual cells, which confirmed the complete replacement of cTnI with ssTnI (Fentzke et al. 1999). TG mice that express cTnI were used as a control TG line (James et al. 2000). The copy number of the transgene was similar in all groups of TG animals. All experiments were conducted and care of animals provided in compliance with animal care policies of the Animal Care Committee of the University of Illinois at Chicago.
Force measurements of skinned fibre bundles
Measurements of the force-calcium relationship were carried out on fibre bundles prepared from the left ventricular papillary muscles of adult mice (Wolska et al. 1999). Mice (4–6 months old) were anaesthetized with pentobarbital sodium (50 mg kg−1 body weight, i.p.), and hearts were quickly removed and put into a cold high relaxing (HR) solution of the following composition (mm): KCl 53, EGTA 10, Mops 20, free Mg2+ 1, MgATP2- 5, creatine phosphate 12, and 10 i.u. ml−1 creatine phosphokinase. The pH of the solution was adjusted to 7.0 or 6.5 with KOH. The ionic strength of all solutions was 150 mm. Papillary muscles from the left ventricle were dissected out, and small fibre bundles approximately 150–200 μm in width and 4–5 mm long were prepared. Fibre bundles were mounted between a micromanipulator and a force transducer with cellulose- acetate glue. Fibres were skinned in HR solution containing 1 % Triton X-100 for 30 min. A resting sarcomere length was established from laser diffraction patterns and set at 2.0 μm, or as indicated. Isometric tension was recorded at room temperature on a chart recorder. After skinning, the fibres were initially washed in HR solution and their thickness was measured. Thereafter, the fibres were exposed to solutions of varying Ca2+ concentration ([Ca2+]o range from 10−8 to 10−4.5m). All solutions also contained the protease inhibitors pepstatin A (2.5 μg ml−1), leupeptin (1 μg ml−1) and phenylmethylsulphonyl fluoride (PMSF, 50 μm). To determine the maximum stress developed by fibre bundles we measured the maximum calcium-activated isometric force (at free [Ca2+] of 10−4.5m) and divided by cross-sectional area, as described previously (Evans et al. 2000). The thickness of the fibre bundles was measured in two perpendicular planes at three points (the centre and two distal ends) using a mirror and a graticule fitted to the eyepiece of a microscope. The mean radius from three points was calculated and used to determine the cross-sectional area based on an elliptical shape of the fibre in cross-section.
Tension measurement in mouse isolated papillary muscles
Mice were anaesthetized with methoxyflurane and the heart was rapidly excised and perfused with a modified Krebs-Henseleit solution. Thin, unbranched and uniform papillary muscles with tricuspid valve and a small part of ventricle were carefully dissected from the right ventricle. The muscle preparation was mounted in an experimental chamber and perfused with a modified Krebs-Henseleit solution (0.2 mm Ca2+) at a flow of about 2.5 ml min−1. The muscle preparations were stimulated via platinum electrodes mounted along the sides of the muscle chamber. Stimulus strength was adjusted to 50 % above the stimulus threshold. The standard solution that was used had the following composition (mm): NaCl 118.5, KCl 5.0, MgSO4 1.2, NaH2PO4 2.0, NaHCO3 26, d-glucose 10.0, and Ca2+ as indicated. During dissection of the papillary muscles [Ca2+]o was 0.2 mm and [K+]o was raised to 15 mm to stop spontaneous beating of the heart. The solutions were equilibrated with a 95 % O2-5 % CO2 (pH 7.33 ± 0.03) or 85 % O2-15 % CO2 (pH 6.75 ± 0.02) gas mixture by continuous bubbling at 25 °C. The temperature in the muscle chamber was kept constant at 25.0 ± 0.1 °C by a heat exchanger at the inflow line and a circulating water bath. The remnant of the tricuspid valve served as a mounting point to the hook of a servo-controlled motor (Cambridge Technology), which was used to control the length of the muscle preparation. The basket connected to a force transducer was used as a second mounting point. Tension was measured by a force transducer (Ford 10; World Precision Instruments). After the initial equilibration [Ca2+]o was gradually increased to 1 mm and the muscle was stretched to generate 90 % of maximum developed force.
SR vesicle Ca2+ uptake
SR Ca2+ uptake was determined using a method described by Evans et al. (2000). Hearts were dissected from mice that had been anaesthetized with 50 mg kg−1 body weight sodium pentobarbital and were immediately placed in ice-cold saline and trimmed free of the atria. The hearts were then transferred to homogenizing buffer (HB; 2 ml (100 mg wet heart)−1), chopped into small pieces with scissors and homogenized. The HB contained (mm): KH2PO4 50, NaF 10, EDTA 1.0, sucrose 300, PMSF 0.3, DTT 0.5; pH 7.0.
Ca2+ uptake was measured over a range of varying [Ca2+]o (10−8 to 10−5m); in addition to the prevailing [Ca2+]o, the reaction mixture contained (mm): MgATP2- 5, free Mg2+ 0.5, imidazole 40, creatine phosphate 10, EGTA 0.5, potassium oxalate 5, sodium azide 5, procaine 10 and ruthenium red 0.03. The ionic strength was adjusted to 175 mm with KCl and the pH was adjusted to 7.1 with KOH. Ruthenium red and procaine were added to inhibit Ca2+ release from the SR, whereas sodium azide was added to inhibit Ca2+ uptake into mitochondria (Solaro & Briggs, 1974; Wimsatt et al. 1990). After 2 min of pre-incubation, the reaction was started by adding ventricular homogenate to the reaction mixture at a concentration of 0.20–0.25 mg protein ml−1 and proceeded at 37 °C for 2 min with constant stirring. Protein concentration was determined using the method of Lowry et al. (1951). The reaction was stopped by filtration through a 0.45 μm Millipore filter that was washed with ice-cold buffer containing 20 mm Tris and 2 mm EGTA, pH 7.0. Total SR vesicle Ca2+ uptake was calculated from the amount of 45Ca2+ bound to the filters, as determined by liquid scintillation spectroscopy.
Data computation and statistical analysis
All results are presented as means ±s.e.m. The significance of differences between the means was evaluated by one-way ANOVA. A value of P < 0.05 was the criterion for significance.
RESULTS
In the first series of experiments we compared the force-calcium relationship in skinned fibre bundles from NTG and TG-cTnI mice at pH 7.0 and pH 6.5. The force-calcium relationship was identical in the two groups (data not shown), therefore we used NTG mice as controls for TG animals. Next we compared the force- calcium relationship in NTG, TG-ssTnI and TG-ssTnI* mouse hearts. Figure 1 shows that compared to NTG controls, the force generated by myofilaments from TG-ssTnI* and TG-ssTnI hearts was more sensitive to Ca2+ at both pH values. At pH 7.0 the EC50 was 2.84 ± 0.10 μm (n = 6) for NTG preparations, 1.41 ± 0.12 μm (n = 6) for TG-ssTnI* preparations and 1.62 ± 0.14 μm (n = 6) for TG-ssTnI preparations. There was no difference in Hill coefficient between NTG (3.27 ± 0.17), TG-ssTnI* (2.63 ± 0.20) and TG-ssTnI (2.78 ± 0.20) preparations. Acidosis (pH 6.5) caused a desensitization of myofilaments in all groups that resulted in a rightward shift in the force-calcium relationship. However, the shift in EC50 was significantly smaller in the case of TG-ssTnI* and TG-ssTnI mice, suggesting a protective effect of ssTnI. At pH 6.5 the EC50 was 12.15 ± 0.72 μm for NTG preparations, 4.26 ± 0.25 μm for TG-ssTnI* preparations and 4.89 ± 0.49 μm for TG-ssTnI preparations (Fig. 1). The Hill coefficient decreased significantly only in preparations from TG animals, being 1.87 ± 0.06 in TG-ssTnI* and 2.07 ± 0.07 in TG-ssTnI preparations.
Figure 1.
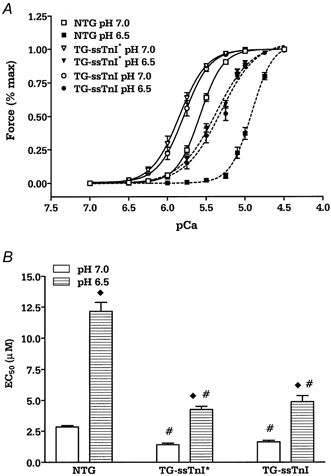
The effect of low pH on the normalized force-pCa relationship (where pCa = -log[Ca2+]; A) and the EC50 of NTG, TG-ssTnI* and TG-ssTnI skinned fibre bundles (B). Data are presented as means ±s.e.m. In NTG, TG-ssTnI* and TG-ssTnI fibre bundle preparations, n = 6 from 3–4 different hearts. # Significantly different from NTG; ⋄ significant difference in the group between data obtained at pH 6.5 and at pH 7.0.
Figure 2 summarizes the inhibition of maximum force at pH 6.5 compared to pH 7.0 in NTG, TG-ssTnI* and TG-ssTnI preparations. At pH 6.5, the maximum force developed by NTG fibre bundles was depressed to 75.4 ± 2.8 % of control values obtained at pH 7.0. This decrease in maximum developed force induced by acidic pH was significantly less in both TG-ssTnI* (83.2 ± 1.3 %) and TG-ssTnI (83.4 ± 1.3 %) preparations. In a separate series of experiments we tested, at two sarcomere lengths, whether maximum stress differs between NTG and TG-ssTnI* preparations. We did not find any significant changes. At a sarcomere length of 1.9 μm the maximum stress was 38.4 ± 5.3 mN mm−2 (n = 7) for NTG fibre bundles and 44.2 ± 5.3 mN mm−2 (n = 8) for TG-ssTnI* fibre bundles. At a sarcomere length of 2.3 μm the maximum stress was 65.3 ± 7.8 mN mm−2 for NTG fibre bundles and 69.2 ± 8.5 mN mm−2 for TG-ssTnI* fibre bundles.
Figure 2.
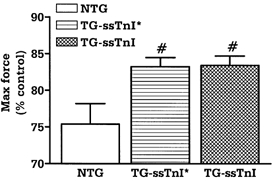
The effect of low pH on the maximum force developed by NTG, TG-ssTnI* and TG-ssTnI skinned fibre bundles at pCa 4.5. Data are presented as means ±s.e.m. In NTG, TG-ssTnI* and TG-ssTnI fibre bundle preparations, n = 6 from 3–4 different hearts. # Significantly different from NTG.
To determine whether expression of ssTnI can prevent the drop in force generation observed in acidic conditions in intact preparations, we performed experiments on right ventricular papillary muscles. Figure 3 shows original recordings of force in control and acidic conditions (1 mm ) in papillary muscles from control (A) and TG-ssTnI hearts (B). After the initial fall in developed force (transient force decline), force recovered to a steady level (steady force decline). Both the transient force decline and steady force decline were significantly greater in NTG compared to TG muscles. Figure 4 summarizes the transient (A) and steady (B) force decline in NTG, TG-ssTnI* and TG-ssTnI papillary muscles as a function of [Ca2+]o at acidic pH. The transient and steady force decline are presented as a percentage of the force developed in control pH conditions (pH 7.3). We also compared the kinetics of contraction and relaxation of papillary muscles from all three groups. There was no change in time to peak tension between groups (data not shown); however, the time to 90 % of twitch force relaxation was prolonged in muscles expressing ssTnI in control (pH 7.3; Fig. 5A) and acidic conditions (pH 6.8; Fig. 5B). To test whether prolongation of relaxation is mostly due to enhanced myofilament sensitivity to Ca2+ and not due to altered Ca2+ uptake by the SR, we measured SR Ca2+ uptake at pH 7.1 and pH 6.5 in SR vesicles from NTG and TG-ssTnI* mouse hearts. At pH 7.1, the EC50 was 0.41 ± 0.03 μm (n = 5) in NTG preparations and 0.44 ± 0.32 μm (n = 6) in TG-ssTnI* preparations; Vmax was 482 ± 15 nmol mg−1 min−1 in NTG preparations and 484 ± 21 nmol mg−1 min−1 in TG-ssTnI* preparations. At pH 6.5, the EC50 increased to 2.16 ± 0.14 μm (n = 5) in NTG and 1.99 ± 0.16 μm (n = 6) in TG-ssTnI* preparations; Vmax was reduced to 327 ± 11 nmol mg−1 min−1 in NTG preparations and 318 ± 10 nmol mg−1 min−1 in TG-ssTnI* preparations. The lack of a difference in SR Ca2+ uptake at both pH values indicates that the prolonged relaxation time is not due to altered SR function, supporting a role for an alteration in the sensitivity of myofilaments to Ca2+.
Figure 3.
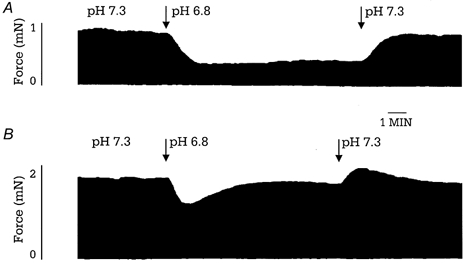
Representative example of the effect of hypercapnic acidosis on the isometric force of intact papillary muscles isolated from NTG mice (A) and TG-ssTnI mice (B) at 1 mm .
Figure 4.
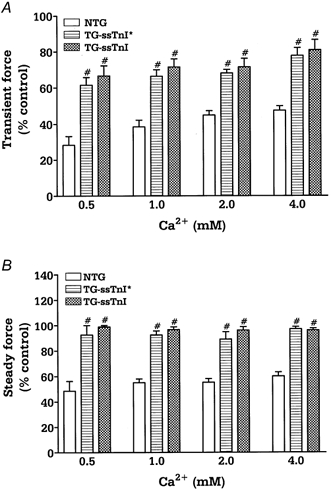
The effect of acidic conditions on transient (A) and steady-state (B) force decline in NTG (n = 7), TG-ssTnI* (n = 5) and TG-ssTnI (n = 7) intact papillary muscles at different values of [Ca2+]o. The force developed by each muscle at control pH was taken as 100 %. Data are presented as means ±s.e.m.# Significantly different from NTG.
Figure 5.
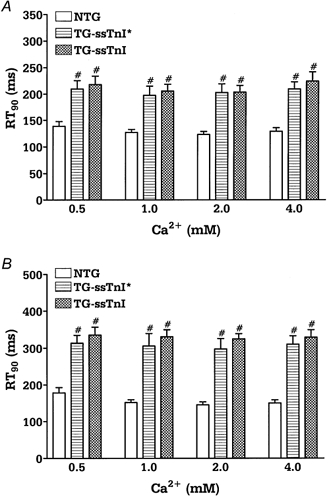
Time at 90 % of relaxation (RT90) in control (A) and acidic conditions (B) in intact NTG (n = 7), TG-ssTnI* (n = 5) and TG-ssTnI (n = 7) papillary muscles. Data are presented as means ±s.e.m.# Significantly different from NTG.
DISCUSSION
The results presented in this paper provide the first direct evidence that replacing cTnI with ssTnI, the isoform that is expressed at embryonic and early neonatal ages, is sufficient to reduce the steady-state force decline drop in intact papillary muscles subjected to acidic conditions. The lower sensitivity of neonatal heart muscle to acidic conditions has been observed previously and studied by us (Solaro et al. 1986, 1988) and others (Jarmakani et al. 1978; Nakanishi et al. 1985, 1990). The mechanisms responsible for the inhibition of myofilament force generation by acidosis include the following: (1) reduction in the affinity of troponin C (TnC) for Ca2+, (2) alterations in thin filament activation, especially the TnI-TnC interaction, and (3) inhibition of the actin-myosin interaction. Studies by ourselves (Blanchard & Solaro, 1984; El-Saleh & Solaro, 1988; Wattanapermpool et al. 1995) and others (Parsons et al. 1997) have shown that the affinity of both skeletal and cardiac TnC (cTnC) for Ca2+ is decreased as pH is reduced. Moreover, acidosis decreases the affinity of TnI for TnC, and the affinity of TnC for Ca2+ is decreased to a greater extent in the presence of TnI, suggesting an important role for TnI (El-Saleh & Solaro, 1988). The importance of the TnI-TnC interaction in the differential response of cardiac and skeletal myofilaments to acidosis was confirmed by Ball et al. (1994). Using fast skeletal myofibrils from which the whole troponin complex was extracted, Ball et al. (1994) reported that the substitution of both cTnC and cTnI is required to produce a change in EC50 with acidic pH, similar to the case for native cardiac myofibrils. Kawashima et al. (1995), using the extraction- reconstitution technique, confirmed the importance of the troponin isoforms in the determination of the calcium-sensitive ATPase activity in response to acidosis. Morimoto et al. (1999) reported that TnI is a determinant of the differential pH sensitivity in slow skeletal muscle, whereas TnC is responsible for the presence of the differential responses to acidic pH in fast skeletal and cardiac muscles.
It appears likely that the C-terminal regions of TnI play an important role in the effects of acidic pH in striated myofilaments. There are three major domains of TnI: a C-terminal domain, an inhibitory domain and an N-terminal domain. It is likely that differences between cTnI and ssTnI in the C-terminal region are important determinants of the differences in the response to acidic pH in myofilaments from TG and NTG animals. Rarick et al. (1997) proposed that both the inhibitory domain and a large portion of the C-terminus of cTnI are essential for full inhibitory activity and Ca2+ sensitivity. To test which region of TnI influences myofilament Ca2+ sensitivity, Westfall et al. (1999, 2000) used adenoviral infection of heart cells to switch cTnI with two different TnI chimeras - one with a cardiac N-terminus, a slow skeletal inhibitory domain and a slow skeletal C-terminus, and another with a slow skeletal N-terminus, a cardiac inhibitory domain and a cardiac C-terminus. They reported that two regions in TnI influence Ca2+ sensitivity, with the C-terminal domain mostly responsible for the Ca2+ threshold and cooperativity and the N-terminal domain having a smaller effect on Ca2+ sensitivity. Westfall et al. (1999, 2000, 2001) also suggested that pH sensitivity is restricted to the C-terminal domain of TnI. Using chimeras consisting of a fast skeletal TnI N-terminus plus a cardiac inhibitory domain and a cardiac C-terminus, Li et al. (2001) identified the C-terminus of TnI as being critical in the deactivation of striated myofilaments by acidosis. There are regions of charged amino acids that are common to all isoforms of TnI in the C-terminal regions. For example, amino acids 187–199 of cTnI are REVGDWRKNIDAL, and amino acids 155–168 in ssTnI are PVEVGDWRKNVEAM. Moreover, there are histidines at position 173 in cTnI and at position 164 in ssTnI that are likely to be influenced by acidosis.
Despite data presented by Westfall et al. (1999, 2000, 2001), the role of the N-terminal extension of cTnI in influencing pH sensitivity in cardiac muscle cannot be excluded. The N-terminal extension is present only in cTnI, and contains two serines at positions 22 and 23, which are phosphorylated by protein kinase A (PKA). Phosphorylation of these sites results in an increased ‘off’ rate for Ca2+ binding to TnC, a reduction in myofilament sensitivity to Ca2+ and an increased rate of cross-bridge cycling (Robertson et al. 1982; Zhang et al. 1995). Furthermore, Mundina-Weilenmann et al. (1996) reported that acidosis leads to increased phosphorylation of cTnI in Langendorff-perfused hearts, even in the absence of β-adrenergic stimulation. Under our experimental conditions this increased phosphorylation of cTnI would result in a reduced myofilament sensitivity to Ca2+, and would contribute to the reduction in developed force only in papillary muscles expressing cTnI. This could explain, at least in part, our observation that the preparations from NTG animals were more sensitive to acidic pH. Further experiments are required to test whether the phosphorylation of cTnI contributes to the desensitization caused by acidosis. These experiments will require the generation of a new line of TG mice expressing cTnI with mutated sites for PKA-dependent phosphorylation.
One of the striking results of our experiments was our finding that the steady-state force of papillary muscles was unaffected by acidic pH. A straightforward explanation of this observation is that the increased Ca2+ transient observed during acidic conditions compensates for the reduced myofilament sensitivity to Ca2+ in TG preparations (Harrison et al. 1992; Hulme & Orchard, 1998). Metzger & Moss (1990) have reported that force per cross-bridge in fast- and slow-twitch fibres is similarly reduced at acidic pH. However at maximal Ca2+ activation, stiffness was depressed only in fast-twitch fibres and was unchanged in slow-twitch fibres. These data indicate that at acidic pH there is no significant change in the number of force-generating cross-bridges in slow fibres. However, the force generated per cross-bridge could be reduced. It is possible that in muscles expressing ssTnI only the force generated by each cross-bridge is reduced and the total number of cross-bridges is unchanged, therefore the small increase in Ca2+ transient is able to compensate for the decrease in force generated by each single cross-bridge.
Our study provides further evidence for the key role of TnI properties in the determination of cardiac function. For example, the state of TnI plays an important role in the recovery of heart function after an ischaemic episode. Depending on the duration of ischaemia, TnI can be degraded at specific sites by proteolysis; this modification correlates well with so-called cardiac stunning (Van Eyk et al. 1998; Solaro, 1999; Murphy et al. 2000). The properties of TnI can also be modified during hypertrophy and heart failure by altering the levels of cTnI phosphorylation (Wolff et al. 1996; Bodor et al. 1997). The present study provides further evidence for the key role of ssTnI in the cardiac protective effect observed during hypercapnic acidosis. This protective effect of ssTnI may be particularly important for fetal hearts, which are exposed to hypoxic conditions.
Acknowledgments
This research was supported by NIH research grants PO1 HL-62426 Project 1 (R.J.S. and A.F.M.) and Project 4 (P.P.deT), R37 HL-22231 (R.J.S.), R29 HL-58591 (B.M.W.), HL-52322 (P.P.deT.) and a Schweppe Career Development Award (B.M.W). A Minority Individual in Postdoctoral Training Supplement to HL22231 supported G.M.A, and NHLBI Training Grant (T32-HL07692) supported R.M.P. and J.K. P.P.deT. is an Established Investigator of the American Heart Association. The authors are grateful to Dr Jeffrey Robbins for generously supplying transgenic mice expressing cTnI.
References
- Arteaga GM, Palmiter KA, Leiden JM, Solaro RJ. Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I. Journal of Physiology. 2000;526:541–549. doi: 10.1111/j.1469-7793.2000.t01-1-00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KL, Johnson MD, Solaro RJ. Isoform specific interactions of troponin I and troponin C determine pH sensitivity of myofibrillar Ca2+ activation. Biochemistry. 1994;33:8464–8471. doi: 10.1021/bi00194a010. [DOI] [PubMed] [Google Scholar]
- Blanchard EM, Solaro RJ. Inhibition of the activation and troponin calcium binding of dog cardiac myofibrils by acidic pH. Circulation Research. 1984;55:382–391. doi: 10.1161/01.res.55.3.382. [DOI] [PubMed] [Google Scholar]
- Bodor GS, Oakeley AE, Allen PD, Crimmins DL, Ladenson JH, Anderson PA. Troponin I phosphorylation in the normal and failing adult human heart. Circulation. 1997;96:1495–1500. doi: 10.1161/01.cir.96.5.1495. [DOI] [PubMed] [Google Scholar]
- Chen F, Wetzel GT, Friedman WF, Klitzner TS. Developmental changes in the effects of pH on contraction and Ca2+ current in rabbit heart. Journal of Molecular and Cellular Cardiology. 1996;28:635–642. doi: 10.1006/jmcc.1996.0059. [DOI] [PubMed] [Google Scholar]
- Ding XL, Akella AB, Gulati J. Contributions of troponin I and troponin C to the acidic pH-induced depression of contractile Ca2+ sensitivity in cardiotrabeculae. Biochemistry. 1995;34:2309–2316. doi: 10.1021/bi00007a027. [DOI] [PubMed] [Google Scholar]
- El Saleh SC, Solaro RJ. Troponin I enhances acidic pH-induced depression of Ca2+ binding to the regulatory sites in skeletal troponin C. Journal of Biological Chemistry. 1988;263:3274–3278. [PubMed] [Google Scholar]
- Evans CC, Pena JR, Phillips RM, Muthuchamy M, Wieczorek DF, Solaro RJ, Wolska BM. Altered hemodynamics in transgenic mice harboring mutant tropomyosin linked to hypertrophic cardiomyopathy. American Journal of Physiology — Heart and Circulatory Physiology. 2000;279:H2414–2423. doi: 10.1152/ajpheart.2000.279.5.H2414. [DOI] [PubMed] [Google Scholar]
- Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, Martin AF, Solaro RJ, Moss RL, Leiden JM. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. Journal of Physiology. 1999;517:143–157. doi: 10.1111/j.1469-7793.1999.0143z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, Frampton JE, McCall E, Boyett MR, Orchard CH. Contraction and intracellular Ca2+, Na+, and H+ during acidosis in rat ventricular myocytes. American Journal of Physiology. 1992;262:C348–357. doi: 10.1152/ajpcell.1992.262.2.C348. [DOI] [PubMed] [Google Scholar]
- Hulme JT, Orchard CH. Effect of acidosis on Ca2+ uptake and release by sarcoplasmic reticulum of intact rat ventricular myocytes. American Journal of Physiology. 1998;275:H977–987. doi: 10.1152/ajpheart.1998.275.3.H977. [DOI] [PubMed] [Google Scholar]
- Hunkeler NM, Kullman J, Murphy AM. Troponin I isoform expression in human heart. Circulation Research. 1991;69:1409–1414. doi: 10.1161/01.res.69.5.1409. [DOI] [PubMed] [Google Scholar]
- James J, Zhang Y, Osinska H, Sanbe A, Klevitsky R, Hewett TE, Robbins J. Transgenic modeling of a cardiac troponin I mutation linked to familial hypertrophic cardiomyopathy. Circulation Research. 2000;87:805–811. doi: 10.1161/01.res.87.9.805. [DOI] [PubMed] [Google Scholar]
- Jarmakani JM, Nakazawa M, Nagatomo T, Langer GA. Effect of hypoxia on mechanical function in the neonatal mammalian heart. American Journal of Physiology. 1978;235:H469–474. doi: 10.1152/ajpheart.1978.235.5.H469. [DOI] [PubMed] [Google Scholar]
- Kawashima A, Morimoto S, Suzuki A, Shiraishi F, Ohtsuki I. Troponin isoform dependent pH dependence of the Ca2+-activated myofibrillar ATPase activity of avian slow and fast skeletal muscles. Biochemical and Biophysical Research Communications. 1995;207:585–592. doi: 10.1006/bbrc.1995.1228. [DOI] [PubMed] [Google Scholar]
- Li G, Martin AF, Solaro RJ. Localization of regions of troponin I important in deactivation of cardiac myofilaments by acidic pH. Journal of Molecular and Cellular Cardiology. 2001;33:1309–1320. doi: 10.1006/jmcc.2000.1392. [DOI] [PubMed] [Google Scholar]
- Lowry DH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry. 1951;193:165–175. [PubMed] [Google Scholar]
- Martin AF, Ball K, Gao L, Kumar P, Solaro RJ. Identification and functional significance of troponin I isoforms in neonatal rat heart myofibrils. Circulation Research. 1991;69:1244–1252. doi: 10.1161/01.res.69.5.1244. [DOI] [PubMed] [Google Scholar]
- Metzger JM, Moss RL. Effects of tension and stiffness due to reduced pH in mammalian fast- and slow-twitch skinned skeletal muscle fibres. Journal of Physiology. 1990;428:737–750. doi: 10.1113/jphysiol.1990.sp018238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S, Harada K, Ohtsuki I. Roles of troponin isoforms in pH dependence of contraction in rabbit fast and slow skeletal and cardiac muscles. Journal of Biochemistry. 1999;126:121–129. doi: 10.1093/oxfordjournals.jbchem.a022412. [DOI] [PubMed] [Google Scholar]
- Mundina-Weilenmann C, Vittone L, Cingolani HE, Orchard CH. Effects of acidosis on phosphorylation of phospholamban and troponin I in rat cardiac muscle. American Journal of Physiology. 1996;270:C107–114. doi: 10.1152/ajpcell.1996.270.1.C107. [DOI] [PubMed] [Google Scholar]
- Murphy AM. Contractile protein phenotypic variation during development. Cardiovascular Research. 1996;31:E25–33. [PubMed] [Google Scholar]
- Murphy AM, Jones L, Sims HF, Strauss AW. Molecular cloning of rat cardiac troponin I and analysis of troponin I isoform expression in developing rat heart. Biochemistry. 1991;30:707–712. doi: 10.1021/bi00217a018. [DOI] [PubMed] [Google Scholar]
- Murphy AM, Kogler H, Georgakopoulos D, McDonough JL, Kass DA, Van Eyk JE, Marban E. Transgenic mouse model of stunned myocardium. Science. 2000;287:488–491. doi: 10.1126/science.287.5452.488. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Okuda H, Nakazawa M, Takao A. Effect of acidosis on contractile function in the newborn rabbit heart. Pediatric Research. 1985;19:482–488. doi: 10.1203/00006450-198505000-00015. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Seguchi M, Tsuchiya T, Yasukouchi S, Takao A. Effect of acidosis on intracellular pH and calcium concentration in the newborn and adult rabbit myocardium. Circulation Research. 1990;67:111–123. doi: 10.1161/01.res.67.1.111. [DOI] [PubMed] [Google Scholar]
- Palmer S, Kentish JC. Developmental differences and regional similarities in the responses of rat cardiac skinned muscles to acidosis, inorganic phosphate and caffeine. Journal of Molecular and Cellular Cardiology. 1996;28:797–805. doi: 10.1006/jmcc.1996.0074. [DOI] [PubMed] [Google Scholar]
- Parsons B, Szczesna D, Zhao J, Van Slooten G, Kerrick WG, Putkey JA, Potter JD. The effect of pH on the Ca2+ affinity of the Ca2+ regulatory sites of skeletal and cardiac troponin C in skinned muscle fibres. Journal of Muscle Research and Cell Motility. 1997;18:599–609. doi: 10.1023/a:1018623604365. [DOI] [PubMed] [Google Scholar]
- Rarick HM, Tu XH, Solaro RJ, Martin AF. The C terminus of cardiac troponin I is essential for full inhibitory activity and Ca2+ sensitivity of rat myofibrils. Journal of Biological Chemistry. 1997;272:26887–26892. doi: 10.1074/jbc.272.43.26887. [DOI] [PubMed] [Google Scholar]
- Reiser PJ, Westfall MV, Schiaffino S, Solaro RJ. Tension production and thin-filament protein isoforms in developing rat myocardium. American Journal of Physiology. 1994;36:H1589–1596. doi: 10.1152/ajpheart.1994.267.4.H1589. [DOI] [PubMed] [Google Scholar]
- Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. Journal of Biological Chemistry. 1982;257:260–263. [PubMed] [Google Scholar]
- Solaro RJ. Troponin I, stunning, hypertrophy, and failure of the heart. Circulation Research. 1999;84:122–124. doi: 10.1161/01.res.84.1.122. [DOI] [PubMed] [Google Scholar]
- Solaro RJ, Briggs FN. Estimating the functional capabilities of sarcoplasmic reticulum in cardiac muscle. Calcium binding. Circulation Research. 1974;34:531–540. doi: 10.1161/01.res.34.4.531. [DOI] [PubMed] [Google Scholar]
- Solaro RJ, Kumar P, Blanchard EM, Martin AF. Differential effects of pH on calcium activation of myofilaments of adult and perinatal dog hearts. Evidence for developmental differences in thin filament regulation. Circulation Research. 1986;58:721–729. doi: 10.1161/01.res.58.5.721. [DOI] [PubMed] [Google Scholar]
- Solaro RJ, Lee JA, Kentish JC, Allen DG. Effects of acidosis on ventricular muscle from adult and neonatal rats. Circulation Research. 1988;63:779–787. doi: 10.1161/01.res.63.4.779. [DOI] [PubMed] [Google Scholar]
- Van Eyk JE, Powers F, Law W, Larue C, Hodges RS, Solaro RJ. Breakdown and release of myofilament proteins during ischemia and ischemia/reperfusion in rat hearts: identification of degradation products and effects on the pCa-force relation. Circulation Research. 1998;82:261–271. doi: 10.1161/01.res.82.2.261. [DOI] [PubMed] [Google Scholar]
- Vetter R, Studer R, Reinecke H, Kolar F, Ostadalova I, Drexler H. Reciprocal changes in the postnatal expression of the sarcolemmal Na+-Ca2+-exchanger and SERCA2 in rat heart. Journal of Molecular and Cellular Cardiology. 1995;27:1689–1701. doi: 10.1016/s0022-2828(95)90788-2. [DOI] [PubMed] [Google Scholar]
- Vornanen M. Excitation-contraction coupling of the developing rat heart. Molecular and Cellular Biochemistry. 1996:163–164. 5–11. doi: 10.1007/BF00408635. [DOI] [PubMed] [Google Scholar]
- Wattanapermpool J, Reiser PJ, Solaro RJ. Troponin I isoforms and differential effects of acidic pH on soleus and cardiac myofilaments. American Journal of Physiology. 1995;37:C323–330. doi: 10.1152/ajpcell.1995.268.2.C323. [DOI] [PubMed] [Google Scholar]
- Westfall MV, Albayya FP, Metzger JM. Functional analysis of troponin I regulatory domains in the intact myofilament of adult single cardiac myocytes. Journal of Biological Chemistry. 1999;274:22508–22516. doi: 10.1074/jbc.274.32.22508. [DOI] [PubMed] [Google Scholar]
- Westfall MV, Albayya FP, Turner II, Metzger JM. Chimera analysis of troponin I domains that influence Ca2+-activated myofilament tension in adult cardiac myocytes. Circulation Research. 2000;86:470–477. doi: 10.1161/01.res.86.4.470. [DOI] [PubMed] [Google Scholar]
- Westfall MV, Turner I, Albayya FP, Metzger JM. Troponin I chimera analysis of the cardiac myofilament tension response to protein kinase A. American Journal of Physiology — Cell Physiology. 2001;280:C324–332. doi: 10.1152/ajpcell.2001.280.2.C324. [DOI] [PubMed] [Google Scholar]
- Wimsatt DK, Hohl CM, Brierley GP, Altschuld RA. Calcium accumulation and release by the sarcoplasmic reticulum of digitonin-lysed adult mammalian ventricular cardiomyocytes. Journal of Biological Chemistry. 1990;265:14849–14857. [PubMed] [Google Scholar]
- Wolff MR, Buck SH, Stoker SW, Greaser ML, Mentzer RM. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: Role of altered β-adrenergically mediated protein phosphorylation. Journal of Clinical Investigation. 1996;98:167–176. doi: 10.1172/JCI118762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolska BM, Keller RS, Evans CC, Palmiter KA, Phillips RM, Muthuchamy M, Oehlenschlager J, Wieczorek DF, de Tombe PP, Solaro RJ. Correlation between myofilament response to Ca2+ and altered dynamics of contraction and relaxation in transgenic cardiac cells that express β-tropomyosin. Circulation Research. 1999;84:745–751. doi: 10.1161/01.res.84.7.745. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhao JJ, Mandveno A, Potter JD. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circulation Research. 1995;76:1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]


