Abstract
Experiments were performed to determine whether 5-HT1A receptors (a) modulate the activity of cardiac and bronchoconstrictor vagal preganglionic neurones (CVPNs and BVPNs) in the nucleus ambiguus (NA) and (b) are involved in pulmonary C-fibre afferent-evoked excitation of CVPNs, by right-atrial injections of phenylbiguanide (PBG). These experiments were carried out on α-chloralose-anaesthetized, artificially ventilated and atenolol (1 mg kg−1)-pretreated cats.
The ionophoretic application of 8-OH-DPAT (a selective 5-HT1A receptor agonist) influenced the activity of 16 of the 19 CVPNs tested. 8-OH-DPAT tended to cause inhibition at low currents (40 nA) and excitation at high currents (120 nA). The activity of 15 of these neurones increased in response to the application of 8-OH-DPAT. In six of the CVPNs tested, this excitatory action of 8-OH-DPAT was attenuated by co-application of the selective 5-HT1A receptor antagonist WAY-100635.
The pulmonary C-fibre afferent-evoked excitation of eight CVPNs was attenuated by ionophoretic application of WAY-100635.
In three out of four CVPNs, the ionophoretic application of PBG caused excitation.
In five out of the nine identified BVPNs that were tested with ionophoretic application of 8-OH-DPAT, excitation was observed that was attenuated by WAY-100635.
WAY-100635 (i.v. or intra-cisternally) also reversed bradycardia, hypotension and the decrease in phrenic nerve activity evoked by the i.v. application of 8-OH-DPAT (42 μg kg−1).
In conclusion, the data indicate that 5-HT1A receptors located in the NA play an important role in the reflex activation of CVPNs and BVPNs, and support the view that overall, these receptors play a fundamental role in the reflex regulation of parasympathetic outflow.
Blockade of central 5-HT1A receptors attenuates the reflex activation of cardiac vagal preganglionic neurones (CVPNs) by cardiopulmonary afferents in rat (Bogle et al. 1990) and rabbit (Skinner et al. 1998), and upper-airway receptors and aortic nerve stimulation in rabbit (Dando et al. 1998; Skinner et al. 1998). The reflex activation of bronchoconstrictor vagal preganglionic neurones (BVPNs) is also attenuated by blockade of central 5-HT1A receptors in cat (Bootle et al. 1996) and guinea-pig (Bootle et al. 1998). In addition, activation of central 5-HT1A receptors by 8-hydroxy-2-(di-N-propylamino)tetralin (8-OH-DPAT) administered i.v., into the IVth ventricle and by microinjection into brain nuclei containing these neurones, the nucleus ambiguus (NA) and dorsal vagal nucleus (DVN), causes a vagally mediated bradycardia in cats (Ramage & Fozard, 1987; Izzo et al. 1988; McCall et al. 1994; Shepheard et al. 1994) and rats (Sporton et al. 1991; Chitravanshi & Calaresu, 1992). There has also been an incidental report that a novel selective 5-HT1A receptor agonist, U-93385E, causes a profound bradycardia in man (see McCall et al. 1994). Binding sites for 5-HT1A receptors have also been localized in both the NA and the DVN in cats (Dashwood et al. 1988), rats (Pazos & Palacios, 1985; Thor et al. 1992) and humans (Pazos et al. 1987). In addition, both regions are densely innervated by 5-HT-immunoreactive terminals (Steinbusch, 1981; Sykes et al. 1994), and 5-HT-containing terminal boutons have been shown to make synaptic contact with vagal preganglionic neurones (Izzo et al. 1988, 1993). The present study was carried out to determine whether the discharge of CVPNs or BVPNs located in the NA is modulated by the activation of 5-HT1A receptors, and whether the pulmonary C-fibre afferent-evoked excitation of CVPNs induced by right-atrial injections of the selective 5-HT3 receptor agonist phenylbiguanide (PBG) also involves these receptors. In addition, the opportunity was taken to determine whether these preganglionic vagal neurones located in the NA are also excited by the ionophoretic application of PBG, as was found for those located in the rat DVN (Wang et al. 1996, 1998). Preliminary reports of some of these observations have already been made (Wang, 2000; Wang & Ramage, 2001). Furthermore, data on the effects of right-atrial injections of PBG alone on five of the CVPNs identified has been published previously (Wang et al. 2000).
METHODS
The experiments were carried out under the Animals (Scientific Procedures) Act, 1986. At the end of the experiments, animals were killed by an overdose of anaesthetic and exsanguination.
General preparation
Experiments were carried out on 17 anaesthetized (70 mg kg−1α-chloralose and 6 mg kg−1 pentobarbitone sodium, i.v.) male adult cats (2.5–3.4 kg). The level of anaesthesia, before and after neuromuscular blockade, was assessed by the absence of a withdrawal reflex and/or the cardiovascular response to paw-pinch and by the stability of resting cardiovascular and respiratory variables and pupil size; if and when required, additional anaesthetic was administered (α-chloralose, 10–15 mg kg−1, i.v.).
Rectal temperature was monitored and maintained between 38 and 39 °C with a Harvard homoeothermic blanket. When surgical anaesthesia was established, the brachial veins and arteries on both sides and one femoral vein were cannulated for administration or withdrawal of drugs/fluids and for recording blood pressure (BP) using a pressure transducer (Gould) connected to a Grass Model 7D Polygraph (Grass Medical Instruments, Quincy, MA, USA). The bladder was cannulated to prevent undue filling during the period of the experiment, thus avoiding the reflex effects associated with bladder distension. A cervical tracheotomy was performed and the trachea was cannulated just below the larynx. Tracheal pressure (TP) was monitored by a pressure transducer (Gould) connected to a side arm of the tracheal cannula. A silicone cannula, prefilled with phenylbiguanide (PBG, 400 μg ml−1), was advanced into the right atrium via the right external jugular vein. ECG was recorded by leads attached to each of the forepaws of the animal, from which heart rate (HR) was derived. The animals were placed in a stereotaxic frame and ventilated artificially (Harvard Ventilator model 551) with oxygen-enriched air, maintaining a small positive end-expiratory pressure (1–2 cmH2O). As soon as ventilation had started, the neuromuscular blocker vecuronium bromide (200 μg kg−1, i.v.) was administered, supplemented with an i.v. infusion of 480 μg kg−1 h−1 vecuronium bromide. This infusion (6 ml kg−1 h−1) comprised 500 ml of the plasma substitute Gelofusine, 500 ml water, 8.4 g NaHCO3, 2 g glucose and 80 mg vecuronium bromide, and was given to maintain blood volume, counteract the development of non-respiratory acidosis and maintain neuromuscular blockade. Arterial blood gas variables were measured using a Corning blood gas analyser (Model 238). The blood gases and pH were monitored regularly and maintained at a PO2 of 100–180 mmHg, a PCO2 of 35–45 mmHg, and pH 7.3–7.4 by i.v. injection of sodium bicarbonate (1 m) and/or adjusting the volume and frequency of ventilation. In all experiments, animals were pretreated with the β1-adrenoceptor antagonist atenolol (1 mg kg−1, i.v.) to block sympathetic drive to the heart (see Bogle et al. 1990). Thus, changes in HR could be presumed to be due to changes in activity in the cardiac vagal efferent fibres.
The right phrenic nerve was dissected by a dorsolateral approach, cut peripherally and desheathed. The cut central end of the nerve was placed on bipolar silver wire recording electrodes. Clamps applied to the vertebral process at C7 and L2 or L3 were used to elevate and stabilize the animal. To expose the brainstem, the nuchal muscles were removed, the occipital bone opened and the dura overlying the brainstem and cerebellum cut and reflected laterally. In some experiments the cerebellum was displaced rostrally with a small retractor to allow access to the NA.
Preparation of cardiac and pulmonary vagal branches
A right thoracotomy was performed between the fourth and sixth ribs to gain access to the right cranial, caudal cardiac and pulmonary branches of the vagus nerve, as described previously (McAllen & Spyer, 1976). The intact cardiac and pulmonary branches and the whole vagus nerve between the cranial and caudal cardiac branches were placed on fine silver wire (0.125 mm in diameter) bipolar electrodes with a 2 mm gap. The wires were insulated from one another with wax and sealed round the nerves with President light body dental polyvinylsiloxane (Coltene). These silver wires had been soldered onto insulated copper wires, which were secured to the thorax. The electrodes were connected to an isolated stimulator (DS2A, Digitimer, Welwyn Garden City, UK) that was triggered by a Digitimer D4030 programmer. The vagal branches were left intact and, typically, stimulation of the main cardiac branch (1 ms pulses at 100 μA, 50 Hz) evoked ‘cardiac arrest’ without a change in TP, whilst stimulation of the pulmonary branches evoked changes in TP but not HR.
Single-unit recording and identification of CVPNs and BVPNs
Extracellular recordings were made from neurones in the region of the NA using ‘piggy-back’ electrodes, which were assembled from a single glass recording electrode and a multi-barrelled glass electrode (Wang et al. 1998). The recording barrel contained 4 m sodium chloride. One of the barrels contained pontamine sky blue dye (2 % dissolved in 0.5 m sodium acetate) for automatic current balancing and marking the recording sites; one other barrel was filled with the glutamate receptor agonist dl-homocysteic acid (DLH, 100 mm, pH 8.5), and the other barrels contained a selection of the following drugs: 8-OH-DPAT (20 mm, pH 4), N-(2-(1-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridyl)cyclohexane carboxamide (WAY-100635; 10 mm, pH 4) and PBG (10 mm, pH 10.6). CVPNs or BVPNs were identified by their antidromic activation following electrical stimulation of the thoracic cardiac or pulmonary branches of the vagus nerve (100–500 μA, 1 ms pulses, 0.2–1.0 Hz), as described previously (McAllen & Spyer, 1976, 1978). The criteria used to determine antidromic activation were the constant latency of the evoked response and its collision with appropriately timed ongoing activity (Fig. 1A and Fig. 7A). Furthermore, the failure of stimulation of the whole vagus nerve below the pulmonary nerve branching point to antidromically activate these neurones eliminated the possibility that current spread from the cardiac or pulmonary branch to the whole vagus nerve was causing antidromic activation of these neurones. Pulmonary C-fibre afferents were stimulated by injection of a bolus of PBG (14–32 μg kg−1 in 100–200 μl) into the right atrium. To prevent tachyphylaxis, the minimum interval between two PBG injections was 5 min, and the volume for a single injection was restricted to less than 200 μl to avoid stimulation of receptors in the atrium wall by volume expansion.
Figure 1. Identification of a B-fibre cardiac vagal preganglionic neurone (CVPN) in the nucleus ambiguus (NA).
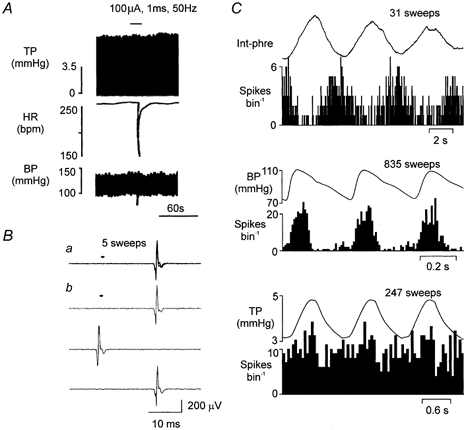
A, traces showing the effect of right cardiac vagal branch stimulation on, from top to bottom, tracheal pressure (TP), heart rate (HR; beats per minute, bpm) and blood pressure (BP). B, traces showing a CVPN activated antidromically (latency, 18.5 ms) by stimulating the right cardiac branch of the vagus nerve (400 μA, 1 ms, 0.5 Hz). a, five consecutive sweeps superimposed to show the constant latency of the evoked spike; b, three consecutive sweeps showing that the evoked spike (see top and bottom trace) was cancelled by the spontaneous spike (see middle trace). The small horizontal bar in a and b indicates the point of stimulation. C, event-triggered histograms of the activity of the same CVPN as in A triggered by integrated phrenic nerve activity (PNA; 100 ms bin width; top panel), the R-wave of the ECG (10 ms bin width; middle panel) and by TP (50 ms bin width; lower panel). Above the histograms is an average of the integrated phrenic nerve activity (Int-phre), ECG-triggered arterial BP and the TP waves, respectively. The number of sweeps above each panel refers to both the average and the histogram.
Figure 7. Identification of a B-fibre bronchoconstrictor vagal preganglionic neurone (BVPN) in the NA.
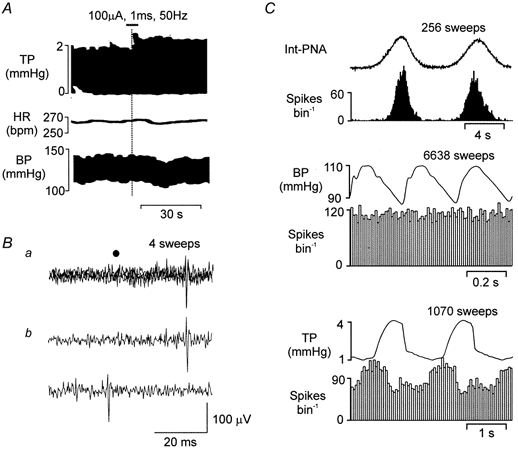
A, traces showing the effect of right pulmonary vagal branch stimulation on, from top to bottom, TP, HR and BP. The horizontal bar at the top of the graph shows the duration of the stimulus, while the vertical line shows the time point when TP began to increase. B, traces showing a BVPN activated (latency, 26.5 ms) by stimulating antidromically the right pulmonary branch of the vagus nerve (200 μA, 1 ms, 0.83 Hz). a, four consecutive sweeps superimposed to show the constant latency of the evoked spike; b, two consecutive sweeps showing that the evoked spikes (top), were cancelled by the spontaneous spike (bottom). The filled circle indicates stimulus artefacts. C, event-triggered rate histograms of the activity of another BVPN triggered by integrated PNA (Int-PNA; 100 ms bin width; upper panel), the R-wave of the ECG (10 ms bin width; middle panel) and TP (50 ms bin width; lower panel). Above the histograms is an average of integrated PNA, ECG-triggered arterial BP and the TP waves, respectively. The number of sweeps above each panel refers to both the average and the histogram.
Data capture and analysis
Neuronal activity, phrenic nerve activity (PNA) and ECG signals were amplified (× 2000, × 20 000 and × 5, respectively) and filtered (0.5–5 kHz; Neurolog, AC preamplifier NL104 and filter NL125; Neurolog System, Digitimer). PNA was then integrated using an EMG integrator (NL 703, Neurolog System). Arterial BP, HR, TP, ECG, raw and integrated PNA and neuronal activity were displayed on a computer using a 1401 interface (CED 1401 Plus, Cambridge Electronic Design) and Spike2 software (CED) and stored on video tape using a digital data recorder (VR100B, Instrutech, Great Neck, NY, USA). Off-line analysis of the recorded data (phrenic nerve-triggered, TP-triggered, and ECG-triggered correlations) was made using Spike2 software. Baseline values for mean arterial pressure (MAP) and HR were taken as the mean over the 40 s before the administration of PBG. The maximal overall changes evoked by PBG were compared to baseline. For the analysis of neuronal firing properties, the mean baseline and mean burst firing rate (number of spikes in a burst) were measured and averaged over four consecutive respiratory cycles. To assess of the effect of ionophoretic application of 5-HT1A receptor ligands on neuronal firing, the mean burst firing rate was compared before, during and after drug application. The control baseline burst firing rate was normalized to 100 % and all of the changes are expressed as a percentage of this control. The excitation or inhibition evoked by drug application was only considered if the change in the baseline burst firing rate exceeded 20 %. In addition, the mean burst firing rate of the four respiratory cycles before PBG injection was taken as the baseline burst firing rate. The number of spikes in the first burst after the PBG injection was compared with that of baseline, and if the change in the number of spikes in a burst was greater than 20 %, this was considered to be excitation. This excitation was then re-analysed to determine whether it occurred within 5 s, since neuronal responses to PBG occurring within this latency can be taken to be the result of pulmonary C-fibre stimulation alone (5 s window, see Daly & Kirkman, 1988; Jones et al. 1998; Wang et al. 2000). Beyond this duration, changes in activity could be attributed to PBG-induced activation of other afferents that are downstream of the pulmonary circulation (Daly & Kirkman, 1988). However, since the B-fibre CVPNs were firing in the postinspiratory and stage 2 expiratory (PI-E2) phases of the respiratory cycle (Gilbey et al. 1984), it was difficult to analyse the mean change in firing rate after PBG injection, as in most cases the burst of firing after PBG injection overlapped the ‘5 s window’. Therefore, the 1st second of the PBG-evoked response that fell within the 5 s window was analysed and compared to the mean of the 1st second of the previous four bursts. The effects of ionophoretic application of WAY-100635 on those CVPNs excited by PBG injection were analysed by comparing the excitation evoked by PBG in the whole burst and the 1st second of the burst excitation that fell within the 5 s window before and during ionophoretic application of WAY-100635. All comparisons of the means were made using Student's paired or unpaired t test. Differences between means were taken as significant when P < 0.05, and all data are presented as means ±s.e.m., except where indicated.
Localization of recording sites
Recording sites were marked by ionophoretic ejection of pontamine sky blue. Following the experiments, brainstems were removed and fixed in 10 % formal saline, and serial frozen sections (80 μm) were cut and stained with neutral red. The marked recording sites were visualized and displayed on standard sections of brainstem taken from the stereotaxic atlas of the cat (Berman, 1968).
Systemic pharmacology study
Since the effect of WAY-100635 on the cardiovascular effects of 8-OH-DPAT have not previously been determined in the cat, at the end of the central recording study, the effect of this antagonist on the i.v. 8-OH-DPAT-evoked changes in HR and arterial BP was studied in six of the animals. Arterial BP, ECG and PNA were recorded for 10 min prior to injections. Slow i.v. injection of 0.5 ml normal saline was followed after 1–2 min by an injection of 8-OH-DPAT (42 μg kg−1) in 0.5 ml saline. WAY-100635 was then injected either i.v. (0.5 or 1 mg kg−1 in 0.2 ml, n = 3) or intra-cisternally (i.c.; 200 μg kg−1 in 20 μl, n = 3) 2–3 min after the bradycardia evoked by 8-OH-DPAT had reached a maximum.
Drugs
Drugs were obtained from the following sources: α-chloralose, DLH, atenolol, 8-OH-DPAT, WAY-100635 and PBG from Sigma Aldrich Chemicals (Poole, Dorset, UK); pentobarbitone sodium from Rhône Mérieux (Harlow, Essex, UK); pontamine sky blue dye from BDH (Poole, Dorset, UK); Gelofusine from Braun Medical (Aylesbury, Buckinghamshire, UK); vecuronium bromide from Organon Technika (Cambridge, UK).
RESULTS
B-fibre CVPNs
A total of 31 antidromically identified vagal preganglionic neurones with axons in the cardiac branches (Fig. 1A, n = 22) and pulmonary branches (Fig. 7A, n = 9) of the vagus nerve were recorded in this study. The calculated axon conduction velocities for cardiac and pulmonary axons were all in the B-fibre range: 11.3 ± 1.1 and 9.0 ± 0.9 m s−1, respectively. The recording sites of these vagal preganglionic neurones were located similarly to those reported previously (Wang et al. 2000), within or ventrolateral to the NA. Baseline values (means ±s.d.) for the animal (n = 17) during data collection were MAP 86 ± 2 mmHg, HR 146 ± 3 beats min−1, TP inflation 5.6 ± 0.4 mmHg and deflation 1.8 ± 0.2 mmHg, PO2 148 ± 5 mmHg, PCO2 37 ± 2 mmHg and pH 7.36 ± 0.01.
Effect of ionophoretic application of 8-OH-DPAT
Twenty-two neurones were found to be only antidromically activated from the cardiac branches of the vagus and had the same characteristics as those recorded previously from this area (see McAllen & Spyer, 1976, 1978; Gilbey et al. 1984; Wang et al. 2000); they were, therefore, classified as CVNPs. They fired during the PI-E2 phase of the respiratory cycle and their activity was also positively correlated to the arterial BP wave (Fig. 1B). The mean firing frequency and the mean burst firing rate of these neurones, with (n = 15) or without (n = 7) ionophoretic application of DLH (8–45 nA), was 2.9 ± 0.7 spikes s−1 and 18.6 ± 4.8 spikes burst−1, respectively.
Ionophoretic application of 8-OH-DPAT for 19 of these CVPNs (current range 20–120 nA) tended to cause inhibition at low currents and excitation at high currents (Fig. 2A and Fig. 6). At the highest current used, 15 CVPNs were found to be excited, one inhibited and three unaffected. When 8-OH-DPAT was applied to eight CVPNs at a current of 120 nA, their activity increased by 125 ± 40 %. When 8-OH-DPAT was applied at a low current (40 nA), activity was found to be inhibited in seven out of the 13 CVPNs tested (from 20 % to complete inhibition of baseline firing; Figs 2B, 3B and 6), having no effect on the other six. At the middle-current range (80 nA), 8-OH-DPAT evoked excitation (n = 7), inhibition (n = 3) or had no effect (n = 5) in 15 CVPNs tested (Fig. 2B and Fig. 3B). In those eight neurones in which the high current (120 nA) evoked excitation (Figs 2, 3A and B, and 6), the low current of 8-OH-DPAT (40 nA) caused inhibition in five (Fig. 2B and Fig. 6) and had no effect on the other three (Fig. 2A and Fig. 6). Mean data for these 19 CVPNs for the high (120 nA) and low (40 nA) current application of 8-OH-DPAT were +168 ± 60 % (P < 0.05, n = 8) and −27 ± 13 % (P < 0.05, n = 13) from baseline, respectively (Fig. 3B, filled circles and continuous line).
Figure 2. Traces showing the effects of ionophoretic application of 8-OH-DPAT onto two different CVPNs with B-fibre axons.
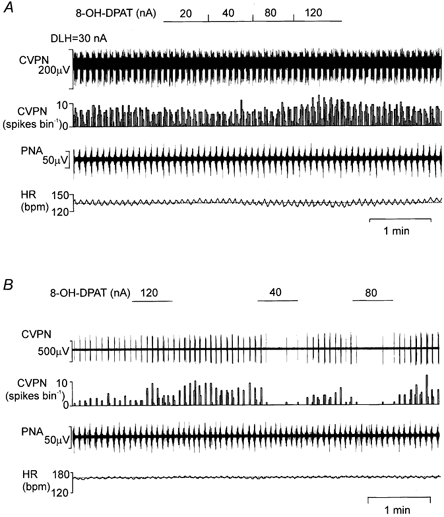
Records from top to bottom: raw activity of B-fibre CVPNs, rate histogram (1 s bin), PNA and HR. A, there was no effect of 8-OH-DPAT on the CVPN at low currents (20–80 nA), while at the high current (120 nA) excitation was observed. B shows the biphasic effect of 8-OH-DPAT (on a different CVPN to that in A), at the lower current (40–80 nA) evoking inhibition, while at the higher current (120 nA) evoking excitation. Note that the dotted lines on the HR traces represent the baseline mean HR. DLH, dl-homocysteic acid.
Figure 6. Effects of ionophoretic application of PBG (20–120 nA) on the ongoing activity of a CVPN.
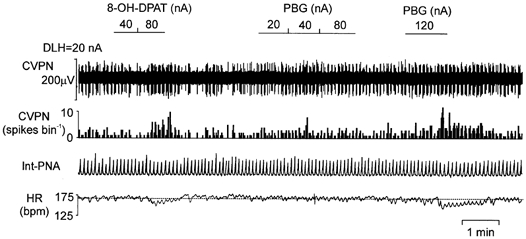
Records from top to bottom: raw activity of a B-fibre CVPN, rate histogram (1 s bin), integrated PNA (Int-PNA) and HR. Ionophoretic application of PBG dose-dependently increased the ongoing activity of the CVPN, while 8-OH-DPAT, on the same neurone, also evoked excitation. Note that the dotted lines on the HR traces represent the mean baseline HR, and both 8-OH-DPAT and PBG, ionophoretically applied, evoked a drop in HR, while the CVPN activity was increased.
Figure 3. Effects of ionophoretic application of 8-OH-DPAT on CVPNs and effects of WAY-100635 on 8-OH-DPAT-evoked excitation.
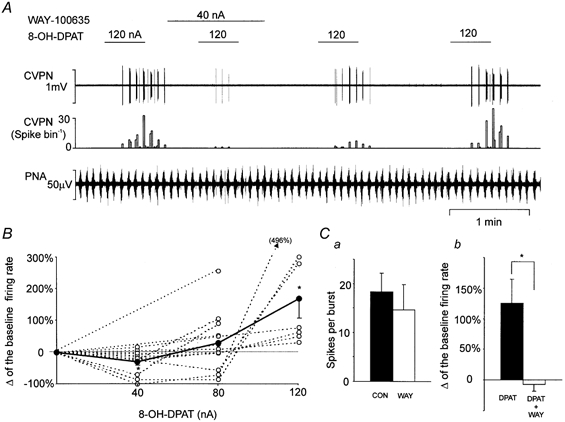
A, records from top to bottom: raw activity of a B-fibre CVPN, rate histogram (1 s bin) and PNA. (Same CVPN as in Fig. 2B.) B, dose-response curves showing the biphasic dose-response action of 8-OH-DPAT on CVPNs. The continuous line with filled circles represents the mean change in the firing rate, while the dotted lines with open circles represent data from individual CVPNs; *P < 0.05 compared with baseline. It should be noted that many lines and symbols overlap. C, bar histogram (n = 6) showing the effects of ionophoretic application of WAY-100635 (WAY; 40–80 nA) on baseline ongoing activity (CON; a) and 8-OH-DPAT (DPAT)-evoked excitation (b). Filled bars represent baseline and open bars represent the effect of application of WAY-100635; *P < 0.05.
Effects of WAY-100635 on 8-OH-DPAT-evoked excitation
In six CVPNs, ionophoretic application of 8-OH-DPAT (80–120 nA) evoked increases in baseline burst firing rate by 125 ± 40 % of the control value (P < 0.05). However, in the presence of WAY-100635 (40–80 nA, 1–4 min), 8-OH-DPAT failed to evoke any effect (−7 ± 11 %; Fig. 3Cb). The 8-OH-DPAT-evoked excitatory response subsequently recovered between 2 and 5 min after the cessation of WAY-100635 application (Fig. 3A). WAY-100635 alone had no effect on the baseline burst firing rate (18 ± 4 vs. 15 ± 5 spikes burst−1; Fig. 3Ca).
Effect of WAY-100635 on pulmonary C-fibre afferent-evoked excitation
The ionophoretic application of WAY-100635, at the same current that inhibited 8-OH-DPAT-evoked excitation of CVPNs, significantly attenuated the excitation of CVPNs evoked by right-atrial injection of PBG, when analysed either as a whole burst or as the amount of activity that occurred in the 1st second of the 5 s window after PBG injection (Fig. 4 and Fig. 5). In detail, right-atrial injection of PBG (10.7–19.2 μg kg−1) evoked excitation in eight B-fibre CVPNs, increasing the whole burst firing rate from 10 ± 4 to 49 ± 14 spikes burst−1 or by 2079 ± 900 % (P < 0.001) from baseline. Within the 1st second of the burst (of the 5 s window) the firing rate was also significantly increased from 5 ± 2 to 14 ± 4 spikes burst−1 or by 587 ± 210 % (P < 0.001) from baseline. This increase in CVPN activity was accompanied by a significant bradycardia of 51 ± 8 beats min−1 (P < 0.001) and a fall in MAP of 21 mmHg (P < 0.001) from baseline values.
Figure 4. Effects of ionophoretic application of WAY-100635 on the excitation evoked in a CVPN by right-atrial injection of phenylbiguanide (PBG).
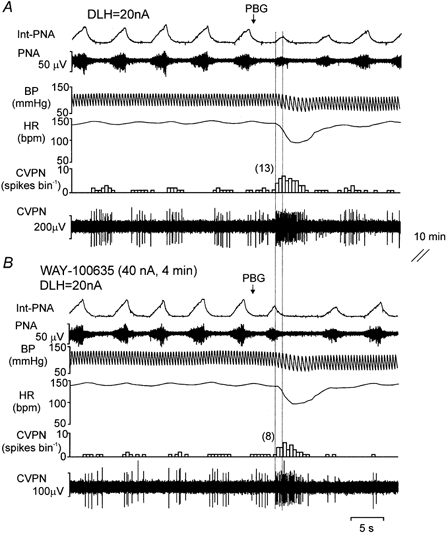
Records from top to bottom: integrated PNA (Int-PNA), raw PNA, arterial BP, HR and CVPN rate histogram (0.5 s bin) and raw ongoing activity showing the effect of intra-arterial PBG (15 μg kg−1; see arrow) in the absence (A) and presence (B) of WAY-100635 applied ionophoretically to the vicinity of this CVPN. The 1st second of the burst excitation within the 5 s window following the PBG injection (see Methods) is shown by the two vertical dotted lines, and the numbers in parentheses represent the number of spikes evoked by PBG within this window.
Figure 5. Bar histogram to show the effect of ionophoretic application of WAY-100635 on the excitation of CVPNs evoked by a right-atrial injection of PBG.
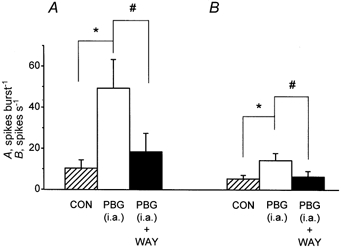
Histograms of the mean data (n = 8) of CVPN activity with vertical bars showing s.e. From left to right: background activity ( CON), activity evoked by right-atrial (i.a.) injections of PBG (□) and the effect of WAY-100635 (WAY) on the activity evoked by a right-atrial injection of PBG (▪). A, the whole burst. B, the 1st second of excitation (in spikes s−1) within a 5 s window after a right-atrial injection of PBG. This activity was compared using Student's paired t test. *#P < 0.05.
CON), activity evoked by right-atrial (i.a.) injections of PBG (□) and the effect of WAY-100635 (WAY) on the activity evoked by a right-atrial injection of PBG (▪). A, the whole burst. B, the 1st second of excitation (in spikes s−1) within a 5 s window after a right-atrial injection of PBG. This activity was compared using Student's paired t test. *#P < 0.05.
Ionophoretic application of WAY-100635 (40–80 nA for 2–5 min) had no significant effect on the baseline burst firing rate (10 ± 4 vs. 11 ± 5 spikes burst−1), but significantly attenuated the excitation evoked by PBG (administered into the right atrium), the whole burst being decreased from 49 ± 14 to 18 ± 9 spikes burst−1 (P < 0.05, Fig. 5A) in all eight neurones tested. Further analysis of the 1st second of the PBG-evoked excitation revealed that WAY-100635 also attenuated this part of the PBG-evoked excitation in seven out of the eight neurones tested. As a group (n = 8), WAY-100635 significantly attenuated the 1st second of the increased burst evoked by PBG from 14 ± 4 to 6 ± 3 spikes burst−1 (P < 0.02, n = 8; Fig. 5B). In three neurones, subsequent right-atrial injection of PBG, approximately 5 min after the termination of the application of WAY-100635, evoked a similar excitation to that observed in the control condition (not shown). In the other five neurones the recording was lost before it could be determined whether their response to an injection PBG had recovered.
Effects of ionophoretic application of PBG on CVPNs
Ionophoretic application of PBG increased the firing rate in three out four of CVPNs tested, in a dose-dependent manner (Fig. 6). PBG (40–120 nA) increased the burst firing rate by between 180 and 338 % in these three CVPNs and only increased the firing by 12 % (40 nA PBG) in the remaining one. As a group, PBG significantly excited the CVPNs by 239 ± 50 % (n = 4). In all four of these CVPNs, 8-OH-DPAT also evoked excitation when applied ionophoretically at a high current (80–120 nA, Fig. 6).
Effects of 8-OH-DPAT on BVPNs
Nine neurones were identified by antidromic activation of the pulmonary branches of the vagus nerve, which, when electrically stimulated (100 μA, 1 ms, 50 Hz; Fig. 7A) evoked only an increase in TP. They exhibited ongoing activity during the inspiratory phase of the respiratory cycle and a lack of cardiac rhythm (Fig. 7B). In four of these neurones, the activity was also found to be positively correlated to lung inflation, while in another three it was correlated to lung deflation (Fig. 7B); in the remaining two there was no correlation between lung inflation and neuronal firing. These pulmonary vagal preganglionic neurones had the same characteristics as reported previously for pulmonary vagal efferent activity with bronchoconstrictor function (see Widdicombe, 1966; McAllen & Spyer, 1978; Mitchell et al. 1987) and were therefore classified as bronchoconstrictor neurones. The mean firing frequency and the mean burst firing rate of the nine identified BVPNs, with (n = 1) and without ionophoretic application of DLH (30 nA), was 5.0 ± 1.5 spikes s−1 and 30 ± 9 spikes burst−1, respectively.
Ionophoretic application of 8-OH-DPAT (20–80 nA) evoked excitation in five out of nine BVPNs tested (Fig. 8). The burst firing rate was significantly increased by 8-OH-DPAT by 61 ± 18 % (n = 5). In one neurone, ionophoretic application of 8-OH-DPAT caused a 62 % inhibition at 40 nA and an excitation of 56 % at the ejection current of 80 nA. In three BVPNs, ionophoretic application of WAY-100635 (40–80 nA) attenuated the 8-OH-DPAT-evoked excitation (Fig. 8B). As a group, WAY-100635 significantly (P < 0.05) inhibited 8-OH-DPAT evoked excitation from 61 ± 18 % (n = 5) to 2 ± 8 % (n = 3) above baseline values. 8-OH-DPAT had no effect on the remaining four neurones, although in two of them application of DLH did evoke excitation at a current lower than that at which 8-OH-DPAT was tested. There was no obvious relationship between the response of these BVPNs to lung inflation and their response to 8-OH-DPAT.
Figure 8. Effects of ionophoretic application of 8-OH-DPAT and WAY-100635 on a BVPN with a B-fibre axon.
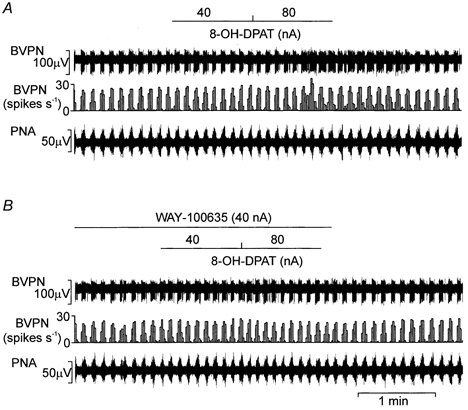
Ionophoretic application of 8-OH-DPAT (40–80 nA) excited this BVPN (A), and co-application of WAY-100635 (40 nA) attenuated the 8-OH-DPAT-evoked excitation (B). Records from top to bottom: raw activity of a B-fibre BVPN, rate histogram (1 s bin) and PNA.
Effect of WAY-100635 (i.v. or i.c.) on i.v. 8-OH-DPAT-evoked reductions in HR and BP
In six cats, i.v. injection of 8-OH-DPAT (42 μg kg−1) evoked a significant fall in HR from 142 ± 5 to 107 ± 9 beats min−1 (P < 0.01), and a significant reducion in MAP from 84 ± 3 to 53 ± 4 mmHg (P < 0.001, Fig. 9). 8-OH-DPAT also inhibited PNA. The effect of 8-OH-DPAT was rapid in onset, in that HR, MBP and PNA started to decline immediately following the injection, with the decline reaching a maximum within 1 min after 8-OH-DPAT injection. Subsequent application of WAY-100635 either i.v. (0.5–1 mg kg−1 in 0.2 ml, n = 3) or i.c. (200 μg kg−1 in 20 μl, n = 3), 2–3 min after the bradycardia and hypotension had reached a maximum, reversed all changes in these variables back to baseline levels within 1 min (Fig. 9). The expected duration of the cardiovascular effects of this i.v. dose of 8-OH-DPAT would be at least 30 min in cats (see Ramage & Mirtsou-Fidani, 1995). In three cats, a subsequent dose of 8-OH-DPAT (42 μg kg−1) between 5 and 10 min after WAY-100635 (i.v., n = 1; i.c., n = 2) evoked either no effect or had a much smaller effect on HR, MBP and PNA than the initial dose (Fig. 9).
Figure 9. Effects of i.v. application of 8-OH-DPAT and, subsequently, WAY-100635 (i.c. or i.v.) on arterial BP, HR and respiration.
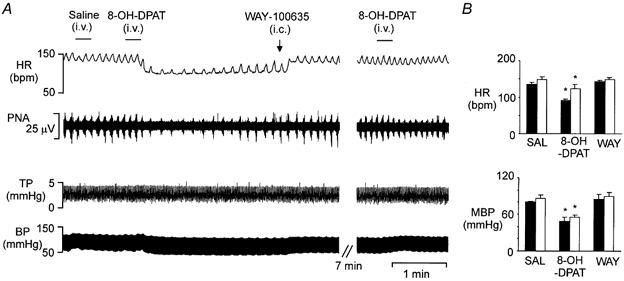
A, traces showing the bradycardia, hypotension and decreased respiration evoked by i.v. application of 8-OH-DPAT (42 μg kg−1). Records from top to bottom: HR, raw PNA, TP and arterial BP. B, histograms of the mean data (n = 3), with vertical bars showing s.e. From left to right: the effect of saline (SAL), followed by 8-OH-DPAT and then WAY-100635 on absolute values of HR and arterial BP (MBP). □, group (n = 3) in which WAY-100635 was administered i.c. (200 μg kg−1 in a volume of 20 μl), as in A; ▪, group in which WAY-100635 was administered i.v. (0.5–1.0 mg kg−1 in a volume of 0.2 ml). *P < 0.05 compared to baseline.
DISCUSSION
Presynaptic location of the inhibitory and excitatory effects of 8-OH-DPAT and verification that the excitatory effect is mediated by 5-HT1A receptors
The present experiments demonstrate that the activity of both putative CVPNs and BVPNs, with myelinated axons located in the vicinity of the NA, is reduced by low, and increased by high ionophoretic currents of the archetypical 5-HT1A receptor agonist 8-OH-DPAT (Middlemiss & Fozard, 1983; see Hoyer et al. 1994). The excitatory effect of 8-OH-DPAT is attenuated in the presence of the selective 5-HT1A receptor antagonist WAY-100635 (Forster et al. 1995), which was also applied ionophoretically in the vicinity of these neurones. This attenuation was found to be reversible. The failure of WAY-100635 alone to have any effect on these neurones suggests that the 5-HT1A receptors are not under tonic activation. Furthermore, the bradycardia and hypotension evoked by i.v. 8-OH-DPAT could be reversed by i.v. or i.c. application of WAY-100635. Since this bradycardia occurred in animals that had been pretreated with the selective β1-adrenoceptor antagonist atenolol, it can be assumed that it is due primarily to an increase in vagal tone to the heart. Thus, this is the first report to demonstrate that the cardiovascular effects of 8-OH-DPAT in the cat can be reversed by WAY-100635, and that the excitatory effects of 8-OH-DPAT, applied ionophoretically to vagal preganglionic neurones, can be antagonized by WAY-100635.
In an earlier report (Wang et al. 1996), the excitatory effect of 8-OH-DPAT, when applied ionophoretically to rat dorsal preganglionic vagal neurones, was found to be inhibited by WAY-100082, another 5-HT1A receptor antagonist. However, in approximately 80 % of these rat dorsal vagal preganglionic neurones, 8-OH-DPAT evoked depression, with no excitatory effect being observed when the current was increased (Wang et al. 1995). It should be noted that these dorsal vagal neurones differed from those examined in the present study in that they had unmyelinated axons, and no attempt was made to determine whether their efferents travelled in the cardiac or pulmonary branches of the vagus nerve. Furthermore, it is considered that the majority of these dorsal vagal neurones innervate the gut (Leslie et al. 1982; Norman et al. 1985). In the study of Wang et al. (1995) and in the present study, the inhibitory action of 8-OH-DPAT was not tested against WAY-100635.
Since the activation of 5-HT1A receptors is generally understood to cause hyperpolarization by opening K+ channels (Colino & Halliwell, 1987), inhibition would be the expected response. However, data from rat brainstem slice experiments indicate that 8-OH-DPAT has no effect on dorsal vagal preganglionic neurones (Browning & Travagli, 1999). This observation implies that 5-HT1A receptors are not located postsynaptically on vagal preganglionic neurones. Thus, in the present study and in that of Wang et al. (1995) the inhibitory action of 8-OH-DPAT, as well as its excitatory action, are probably due to the activation of receptors located presynaptically to these neurones. In this latter respect, inhibitory postsynaptic currents, recorded from rat dorsal vagal preganglionic neurones in vitro, evoked by stimulating the nucleus tractus solitarii (NTS), were attenuated by activation of 5-HT1A receptors and blocked by bicuculline (Browning & Travagli, 1999). This indicates that the excitation caused by activating 5-HT1A receptors is due to the disinhibition of a tonic GABAergic input. Furthermore, in anaesthetized cats, microinjection of bicuculline into the NA has been shown to cause a profound increase in vagal drive to the heart (Dimicco et al. 1979). Whether these 5-HT1A receptors are located on GABAergic nerve terminals, axons and/or soma, and whether these GABAergic neurones are local interneurones, remains to be determined. It can only be concluded from the present study that they are located in the NA. Thus, the 5-HT1A receptors that mediate excitation can be considered to be heteroreceptors rather than autoreceptors. The putative inhibitory 5-HT1A receptors, however, could be autoreceptors located on the 5-HT-containing terminals that innervate this GABAergic pathway (see Fig. 10). Since lower currents are required to activate these inhibitory receptors, this may suggest that they are located nearer to the recording site than the excitatory 5-HT1A receptors, or may just reflect differences in function. As the recordings were carried out using ‘piggy-back’ electrodes, a condition in which the ionophoretic barrels are some distance from the recording electrode (approximately 10 μm), the excitatory 5-HT1A receptors could be closer to the recording site than the inhibitory receptors. However, the ability of the high-current excitatory response to overcome the low-current inhibitory response favours the view that both types of this receptor are located presynaptically (see Fig. 10).
Figure 10. Diagrammatic representation of the involvement of 5-HT receptors in the control of the activity of BVPNs and CVPNs.
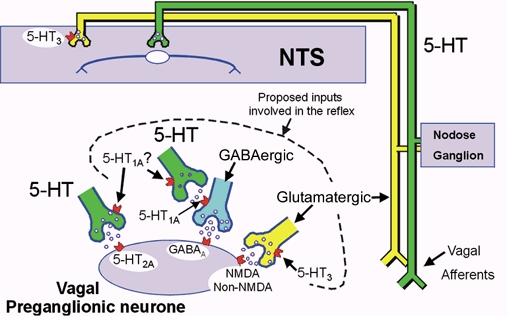
5-HT-containing neurones are shown in green, whereas glutamate-containing neurones are shown in yellow. These vagal preganglionic neurones can be activated reflexly via the nucleus tractus solitarii (NTS) by the cardiopulmonary afferents that run in the vagus nerve. It is proposed that the NTS neurones activate both a 5-HT-containing and a glutamatergic pathway. The 5-HT pathway inhibits the GABA-mediated (blue) ‘brake’, allowing the glutamatergic pathway to fully excite the preganglionic vagal neurones. This results in a bradycardia or bronchoconstriction. It should be noted that the 5-HT1A receptors are located presynaptically, not postsynaptically. In addition, the diagram shows a 5-HT-containing pathway directly innervating the vagal preganglionic neurones, which activates 5-HT2A receptors to cause excitation. This pathway is not believed to be involved in the reflex activation of vagal preganglionic neurones; however, it is speculated that the putative 5-HT1A receptors that mediate the inhibition of vagal preganglionic neuronal activity could be located on the nerve terminals of this pathway and, as such, would function as autoreceptors. It should be noted that these inhibitory 5-HT1A receptors could also be located on the terminals of the 5-HT-containing pathway, which inhibits the putative GABAergic ‘brake’. This figure and legend is a modification and update of that published by Ramage (2000).
Indirect evidence to support the view that these 5-HT1A receptors are located presynaptically comes from the observation that the effect of 5-HT on rat slice dorsal vagal preganglionic neurones is one of excitation that is mediated by 5-HT2A receptors (Browning & Travagli, 1999). This would also imply that the 5-hydroxytryptaminergic innervation of these neurones (Izzo et al. 1988, 1993) is excitatory, activating 5-HT2A receptors. However, none of the reflexes that have been investigated so far that activate CVPNs (Bogle et al. 1990) and BVPNs (Bootle et al. 1998) could be blocked by 5-HT2 receptor antagonists. This would imply that this pathway is probably not involved in the reflex activation of CVPNs and BVPNs. This is also another site at which the putative inhibitory 5-HT1A receptor, if an autoreceptor, could be located (see Fig. 10), and would imply that in the present experiments this pathway is tonically active. Finally, the ability of the selective 5-HT3 receptor agonist PBG to cause excitation of CVPNs indicates that 5-HT3 receptors are also located in the NA. However, these receptors can be considered to be located presynaptically, as demonstrated for the DVN, in which these 5-HT3 receptors have been shown to cause excitation of preganglionic vagal neurones by causing the release of glutamate (Wang et al. 1998).
5-HT1A receptors and reflex activation of CVPNs and BVPNs
The question arises, do the 5-HT1A receptors, identified in the present study in the vicinity of the NA, play a physiological role in the control of the excitability of CVPNs and BVPNs? The blockade of central 5-HT1A receptors is known to attenuate the excitation of these neurones by cardiopulmonary afferents, stimulation of upper-airway receptors and aortic nerve stimulation (see Introduction). In the present experiments, right-atrial injections of PBG to activate pulmonary C-fibre afferents (Paintal, 1969; see Coleridge & Coleridge, 1984) caused excitation of CVPNs, and this excitation could be significantly attenuated by the ionophoretic application of WAY-100635 at a current that attenuates the excitatory effect of 8-OH-DPAT on that neurone. In addition, the effects of WAY-100635 on this reflex-evoked excitation were found to be reversible. These observations indicate that this excitation is mediated by 5-HT1A receptors located in the NA, and that this receptor subtype, and therefore a 5-hydroxytryptaminergic pathway at this level, is involved in the reflex activation of these CVPNs. Right-atrial injections of PBG, as well as activating pulmonary C-fibres, also excite afferents downstream from the pulmonary capillaries, such as cardiac C-fibres (see Coleridge & Coleridge, 1979). It has been demonstrated in the cat that the onset latency for the activation of pulmonary C-fibres alone ranges between 2 and 5 s, depending upon the level of cardiac output (Daly & Kirkman, 1988), hence the use of a 5 s window for analysis (see Methods). The ability of WAY-100635 to significantly inhibit the 1st second of evoked activity indicates that pulmonary C-fibre afferent stimulation of CVPNs involves the activation of 5-HT1A receptors. Indeed, as the whole burst evoked by the intra-atrial application of PBG is significantly attenuated by WAY-100635, this indicates that cardiac C-fibre-evoked stimulation of CVPNs also involves the activation of 5-HT1A receptors. Thus, the present data suggest that the major site at which central 5-HT1A receptors are involved in the reflex activation of CVPNs and BVPNs is within the local vicinity of these preganglionic vagal neurones (i.e. the NA and presumably the DVN). Another possible site of this reflex arc is the NTS, the site of termination of most cardiovascular and lung afferents (see Lawrence & Jarrott, 1996). However, preliminary data from vagal afferent activation of these neurones does not support such a possibility (Ramage & Mifflin, 1998), although these experiments, along with more recent experiments (Jeggo et al. 2000), suggest that at the level of the NTS, 5-HT3 receptors are being activated by vagal afferents. Indeed, central blockade of 5-HT3 receptors has been reported to attenuate the reflex bradycardia caused by upper-airway stimulation (Dando et al. 1995) and by cardiopulmonary afferent activation, the latter when granisetron, a 5-HT3 receptor antagonist, is microinjected bilaterally into the NTS (Pires et al. 1998). Thus, a role for 5-HT3 receptors in the reflex activation of vagal preganglionic neurones at the level of the vagal preganglionic neurones themselves would also seem to be highly likely (see above).
5-Hydroxytryptaminergic pathways and their involvement in reflex activation of CVPNs and BVPNs
Taken together, the data suggest that 5-HT1A receptors play an important role in the reflex activation of CVPNs and BVPNs at the level of the preganglionic neurones themselves, as well as providing evidence for a role for 5-HT3 receptors at this level and at the level of the NTS. In fact, it would seem that 5-hydroxytryptaminergic pathways are controlling the reflex activation of these vagal preganglionic neurones through at least two different receptors and at different brainstem levels. It should also be pointed out that the activation of brainstem 5-HT1B/1D receptors has been shown to inhibit the reflex excitation of CVPNs (Dando et al. 1998) and BVPNs (Bootle et al. 1998), although antagonist data suggest that they are not being activated during a reflex. Furthermore, the role of the 5-hydroxytryptaminergic pathway that innervates CVPNs (Izzo et al. 1988, 1993) remains to be determined, as do the sites of cell bodies for all of these pathways. 5-HT innervation of the dorsal medulla is known to arise in part from neurones in the mid-line raphé nuclei (Schaffar et al. 1988) and from vagal sensory afferents (Gaudin-Chazel et al. 1982; Nosjean et al. 1990; Sykes et al. 1994), and probably from within the NTS (Calza et al. 1985). Furthermore, the question arises as to whether this disinhibition of the putative tonic GABAergic inhibitory input to these neurones (see above) is the only pathway by which NTS neurones that have been activated by vagal afferents cause excitation of CVPNs and BVPNs; for instance, glutamatergic pathways could also be directly involved. Indeed, at the level of the NTS, a glutamatergic pathway has been shown to be important for the reflex activation of CVPNs (Chianca & Machado, 1996). However, no data exist on whether glutamate antagonists microinjected into the nuclei containing vagal preganglionic neurones attenuate vagal afferent-evoked bradycardias, or whether depletion of 5-HT in these nuclei blocks reflex-evoked bradycardias. Nevertheless, data from brainstem slice preparations have shown that identified CVPNs in the NA receive a monosynaptic glutamatergic input from the NTS (Neff et al. 1998). The hypothesis has therefore been put forward that this 5-HT1A receptor-mediated pathway ‘switches off’ a GABAergic ‘brake’ onto the vagal preganglionic neurones, allowing the glutamatergic pathway to fully excite the CPVNs, resulting in a bradycardia (Ramage, 2000; see Fig. 10). Interestingly, it has been reported recently that central 5-HT1A receptors also play a role in the reflex regulation of parasympathetic outflow to the bladder (Testa et al. 1999; Conley et al. 2001). Thus, a 5-hydroxytryptaminergic pathway (via 5-HT1A receptors) is likely to be a fundamental mechanism by which the reflex activation of parasympathetic outflow is regulated.
Acknowledgments
This work was supported by the Wellcome Trust (grant 050894/Z). We are also grateful for the technical assistance of Mr S. Wilkinson.
References
- Berman AL. The Brainstem of the Cat. Madison, WI, USA: University of Wisconsin; 1968. [Google Scholar]
- Bogle RG, Pires JGP, Ramage AG. Evidence that central 5-HT1A receptors play a role in the von Bezold-Jarisch reflex in the rat. British Journal of Pharmacology. 1990;100:757–760. doi: 10.1111/j.1476-5381.1990.tb14088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootle DJ, Adcock JJ, Ramage AG. Involvement of central 5-HT1A receptors in the reflex activation of pulmonary vagal motoneurones by inhaled capsaicin in anaesthetized cats. British Journal of Pharmacology. 1996;117:724–728. doi: 10.1111/j.1476-5381.1996.tb15250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootle DJ, Adcock JJ, Ramage AG. The role of central 5-HT receptors in the bronchoconstriction evoked by inhaled capsaicin in anaesthetised guinea-pigs. Neuropharmacology. 1998;37:243–250. doi: 10.1016/s0028-3908(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Characterization of the in vitro effects of 5-hydroxytryptamine (5-HT) on identified neurones of the rat dorsal motor nucleus of the vagus (DMV) British Journal of Pharmacology. 1999;128:1307–1315. doi: 10.1038/sj.bjp.0702908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza L, Giardino L, Grimaldi R, Rigoli M, Steinbusch HMW, Tiengo M. Presence of 5-HT-positive neurons in the medial nuclei of the solitary tract. Brain Research. 1985;347:135–139. doi: 10.1016/0006-8993(85)90900-x. [DOI] [PubMed] [Google Scholar]
- Chianca DA, Jr, Machado BH. Microinjection of NMDA antagonist into the NTS of conscious rats blocks the Bezold-Jarisch reflex. Brain Research. 1996;718:185–188. doi: 10.1016/0006-8993(96)00010-8. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Calaresu FR. Additive effects of dopamine and 8-OH-DPAT microinjected into the nucleus ambiguus in eliciting vagal bradycardia in rats. Journal of the Autonomic Nervous System. 1992;41:121–128. doi: 10.1016/0165-1838(92)90134-3. [DOI] [PubMed] [Google Scholar]
- Coleridge JCG, Coleridge HM. Chemoreflex regulation of the heart. In: Berne RM, Sperelakis N, editors. Handbook of Physiology, The Cardiovascular System, The Heart. Vol. 1. Bethesda, MD, USA: American Physiological Society; 1979. pp. 653–676. section 2. [Google Scholar]
- Coleridge JCG, Coleridge HM. Afferent vagal C-fibre innervation of the lungs and airways and its functional significance. Reviews of Physiology, Biochemistry and Pharmacology. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Colino A, Halliwell JV. Differential modulation of three separate K-conductances in hippocampal CA1 neurons by serotonin. Nature. 1987;328:73–77. doi: 10.1038/328073a0. [DOI] [PubMed] [Google Scholar]
- Conley RK, Williams TJ, Ford APDW, Ramage AG. The role of α1-adrenoceptors and 5-HT1A receptors in the control of the micturition reflex in male anaesthetized rats. British Journal of Pharmacology. 2001;133:61–72. doi: 10.1038/sj.bjp.0704043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MdeB, Kirkman E. Cardiovascular responses to stimulation of pulmonary C fibres in the cat: their modulation by changes in respiration. Journal of Physiology. 1988;402:43–63. doi: 10.1113/jphysiol.1988.sp017193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando SB, Jordan D, Ramage AG. The role of central 5-HT3 receptors in the modulation of the response to upper airway stimulation in the anaesthetized rabbit. Journal of Physiology. 1995;489.P:156P. [Google Scholar]
- Dando SB, Skinner MR, Jordan D, Ramage AG. Modulation of the vagal bradycardia evoked by stimulation of upper airway receptors by central 5-HT1 receptors in anaesthetized rabbits. British Journal of Pharmacology. 1998;125:409–417. doi: 10.1038/sj.bjp.0702085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashwood MR, Gilbey MP, Jordan D, Ramage AG. Autoradiographic localisation of 5-HT1A binding sites in the brainstem of the cat. British Journal of Pharmacology. 1988;94:386P. [Google Scholar]
- Dimicco JA, Gale K, Hamilton B, Gillis RA. GABA receptor control of parasympathetic outflow to heart: characterization and brainstem localization. Science. 1979;204:1106–1109. doi: 10.1126/science.451556. [DOI] [PubMed] [Google Scholar]
- Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y, Fletcher A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. European Journal of Pharmacology. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- Gaudin-Chazal G, Portalier P, Barrit MC, Puizillout JJ. Serotonin-like immunoreactivity in paraffin-sections of the nodose ganglia of the cat. Neuroscience Letters. 1982;33:169–172. doi: 10.1016/0304-3940(82)90246-4. [DOI] [PubMed] [Google Scholar]
- Gilbey MP, Jordan D, Richter DW, Spyer KM. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. Journal of Physiology. 1984;356:65–78. doi: 10.1113/jphysiol.1984.sp015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. VII international union of pharmacology classification of receptors for 5-hydroxtryptamine (serotonin) Pharmacological Reviews. 1994;46:157–203. [PubMed] [Google Scholar]
- Izzo PN, Deuchars J, Spyer KM. Localization of cardiac vagal preganglionic motoneurones in the rat: Immunocytochemical evidence of synaptic inputs containing 5-hydroxytryptamine. Journal of Comparative Neurology. 1993;327:572–583. doi: 10.1002/cne.903270408. [DOI] [PubMed] [Google Scholar]
- Izzo PN, Jordan D, Ramage AG. Anatomical and pharmacological evidence supporting the involvement of serotonin in the central control of cardiac vagal motoneurones in the anaesthetized cat. Journal of Physiology. 1988;406:19P. [Google Scholar]
- Jeggo RD, Wang Y, Jordan D, Ramage AG. The role of 5-HT3 receptors in the cardiopulmonary afferent-evoked response of NTS neurones in the anaesthetized rat. Journal of Physiology. 2000;523.P:258P. [Google Scholar]
- Jones JFX, Wang Y, Jordan D. Activity of C fibre cardiac vagal efferents in anaesthetized cats and rats. Journal of Physiology. 1998;507:869–880. doi: 10.1111/j.1469-7793.1998.869bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Progress in Neurobiology. 1996;48:21–53. doi: 10.1016/0301-0082(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Leslie RA, Gwyn DG, Hopkins DA. The central distribution of the cervical vagus nerve and gastric afferent and efferent projections in the rat. Brain Research Bulletin. 1982;8:37–43. doi: 10.1016/0361-9230(82)90025-9. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Spyer KM. The location of cardiac vagal preganglionic motoneurones in the medulla of the cat. Journal of Physiology. 1976;258:187–204. doi: 10.1113/jphysiol.1976.sp011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen RM, Spyer KM. Two types of vagal preganglionic motoneurones projecting to the heart and lungs. Journal of Physiology. 1978;282:353–364. doi: 10.1113/jphysiol.1978.sp012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall RB, Escandon NA, Harris LT, Clement ME. Tolerance development to the vagal-mediated bradycardia produced by 5-HT1A receptor agonists. Journal of Pharmacology and Experimental Therapeutics. 1994;271:777–781. [PubMed] [Google Scholar]
- Middlemiss DN, Fozard JR. 8-Hydroxy-2-(di-n-proplyamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. European Journal of Pharmacology. 1983;90:151–153. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Herbert DA, Baker DG, Basbaum CB. In vivo activity of tracheal parasympathetic ganglion cells innervating tracheal smooth muscle. Brain Research. 1987;37:157–160. doi: 10.1016/0006-8993(87)91537-x. [DOI] [PubMed] [Google Scholar]
- Neff RA, Mihalevich M, Mendelowitz D. Stimulation of the NTS activates NMDA and non-NMDA receptors in rat cardiac vagal neurons in the nucleus ambiguous. Brain Research. 1998;792:277–282. doi: 10.1016/s0006-8993(98)00149-8. [DOI] [PubMed] [Google Scholar]
- Norman WP, Pagani FD, Ormsbee HS, Kasbekar DK, Gillis RA. Use of horseradish peroxidase to identify hindbrain sites that influence gastric motility in the cat. Gastroenterology. 1985;88:701–705. doi: 10.1016/0016-5085(85)90140-4. [DOI] [PubMed] [Google Scholar]
- Nosjean A, Compoint C, Buisseret-Delmas C, Orer HS, Merahi N, Puizillout JJ, Laguzzi R. Serotonergic projections from the nodose ganglia to the nucleus tractus solitarius: an immunohistochemical and double labeling study in the rat. Neuroscience Letters. 1990;114:22–26. doi: 10.1016/0304-3940(90)90422-6. [DOI] [PubMed] [Google Scholar]
- Paintal AS. Mechanism of stimulation of type J pulmonary receptors. Journal of Physiology. 1969;203:511–532. doi: 10.1113/jphysiol.1969.sp008877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Research. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain. III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience. 1987;21:97–122. doi: 10.1016/0306-4522(87)90326-5. [DOI] [PubMed] [Google Scholar]
- Pires JGP, Silva SR, Ramage AG, Futuro-Neto HA. Evidence that 5-HT3 receptors in the nucleus tractus solitarius and other brainstem areas modulate the vagal bradycardia evoked by activation of the von Bezold-Jarisch reflex in the anesthetized rat. Brain Research. 1998;791:229–234. doi: 10.1016/s0006-8993(98)00109-7. [DOI] [PubMed] [Google Scholar]
- Ramage AG. Central 5-HT1A receptors and vagal tone to the airways. Trends in Pharmacological Sciences. 2000;21:201–202. doi: 10.1016/s0165-6147(00)01481-4. [DOI] [PubMed] [Google Scholar]
- Ramage AG, Fozard JR. Evidence that the putative 5-HT1A receptor agonists, 8-OH-DPAT and ipsapirone, have a central hypotensive action that differs from that of clonidine in anaesthetised cats. European Journal of Pharmacology. 1987;138:179–191. doi: 10.1016/0014-2999(87)90431-6. [DOI] [PubMed] [Google Scholar]
- Ramage AG, Mifflin SW. Vagal-evoked excitation of a sub-population of neurones in the nucleus of the solitary tract (NTS) involves 5-HT3 receptors in the anaesthetized rat. Journal of Physiology. 1998;509.P:129P. [Google Scholar]
- Ramage AG, Mirtsou-Fidani V. Examination of the cardiovascular effects of WAY-100802 a selective 5-HT1A antagonist in anaesthetized cats. British Journal Pharmacology. 1995;116:289P. [Google Scholar]
- Schaffar N, Kessler JP, Bosler O, Jean A. Central serotonergic projections to the nucleus tractus solitarii: evidence from a double labelling study in the rat. Neuroscience. 1988;26:951–958. doi: 10.1016/0306-4522(88)90111-x. [DOI] [PubMed] [Google Scholar]
- Shepheard SL, Jordan D, Ramage AG. Comparison of the effects of IVth ventricular administration of some tryptamine analogues with those of 8-OH-DPAT on autonomic outflow in the anaesthetized cat. British Journal of Pharmacology. 1994;111:616–624. doi: 10.1111/j.1476-5381.1994.tb14781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MR, Ramage AG, Jordan D. Effect of antagonism of central 5-HT1A receptors on baroreceptor and cardiopulmonary receptor reflexes in anaesthetized rabbits. Journal of Physiology. 1998;513.P:85P. [Google Scholar]
- Sporton SCE, Shepheard SL, Jordan D, Ramage AG. Microinjections of 5-HT1A agonists into the dorsal motor vagal nucleus produce a bradycardia in the atenolol-pretreated anaesthetized rat. British Journal of Pharmacology. 1991;104:467–470. doi: 10.1111/j.1476-5381.1991.tb12452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous system of the rat — cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Sykes RM, Spyer KM, Izzo PN. Central distribution of substance P, calcitonin gene-related peptide and 5-hydroxytryptamine in vagal sensory afferents in the rat dorsal medulla. Neuroscience. 1994;59:195–210. doi: 10.1016/0306-4522(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Testa R, Guarneri L, Poggesi E, Angelico P, Velasco C, Ibba M, Cilia A, Motta G, Riva C, Leonardi A. Effect of several 5-hydroxytryptamine1A receptor ligands on the micturition reflex in rats: comparison with WAY 100635. Journal of Pharmacology and Experimental Therapeutics. 1999;290:1258–1269. [PubMed] [Google Scholar]
- Thor KB, Blitz-Siebert A, Helke CJ. Autoradiographic localization of 5HT1 binding sites in autonomic areas of the rat dorsomedial medulla oblongata. Synapse. 1992;10:217–227. doi: 10.1002/syn.890100305. [DOI] [PubMed] [Google Scholar]
- Wang Y. Evidence that 5-HT1A receptors are involved in the excitation of cardiac vagal preganglionic neurones at the level of the nucleus ambiguous during cardiopulmonary afferent activation in anaesthetized cats. Journal of Physiology. 2000;523.P:257P. [Google Scholar]
- Wang Y, Jones JFX, Jeggo RD, Daly MdeB, Jordan D, Ramage AG. Effect of pulmonary C-fibre afferent stimulation on cardiac vagal neurones in the nucleus ambiguus in anaesthetized cats. Journal of Physiology. 2000;526:157–165. doi: 10.1111/j.1469-7793.2000.t01-1-00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jones JFX, Ramage AG, Jordan D. Effects of 5-HT and 5-HT1A receptor agonists and antagonists on dorsal vagal preganglionic neurones in anaesthetized rats: An ionophoretic study. British Journal of Pharmacology. 1995;116:2291–2297. doi: 10.1111/j.1476-5381.1995.tb15067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ramage AG. Activation of 5-HT1A receptors in the nucleus ambiguus excites bronchoconstrictor vagal preganglionic neurones in anaesthetized cats. Journal of Physiology. 2001;533.P:93P. doi: 10.1111/j.1469-7793.2001.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ramage AG, Jordan D. Mediation by 5-HT3 receptors of an excitatory effect of 5-HT on dorsal vagal preganglionic neurones in anaesthetized rats: an ionophoretic study. British Journal of Pharmacology. 1996;118:1697–1704. doi: 10.1111/j.1476-5381.1996.tb15594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ramage AG, Jordan D. Presynaptic 5-HT3 receptors evoke an excitatory response in dorsal vagal preganglionic neurones in anaesthetized rats. Journal of Physiology. 1998;509:683–694. doi: 10.1111/j.1469-7793.1998.683bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JG. Action potentials in parasympathetic and sympathetic efferent fibres to the trachea and lungs of dogs and cats. Journal of Physiology. 1966;186:56–88. doi: 10.1113/jphysiol.1966.sp008020. [DOI] [PMC free article] [PubMed] [Google Scholar]


