Abstract
The role of ghrelin in the regulation of pancreatic protein secretion was investigated in vivo using anaesthetized rats with pancreatic ductal cannulas, and in isolated pancreatic acinar cells and pancreatic lobules in vitro.
In vivo, pancreatic protein output stimulated by CCK-8 (400 pmol kg−1 h−1) was dose-dependently inhibited by continuous ghrelin infusion (1.2 and 12 nmol kg−1 h−1) by 45 ± 8 and 84 ± 7 %, respectively.
In rats with acute subdiaphragmatic vagotomy, ghrelin (12 nmol kg−1 h−1) significantly inhibited CCK-stimulated pancreatic protein secretion by 75 ± 18 %.
Infusion of ghrelin (12 nmol kg−1 h−1) abolished pancreatic protein secretion caused by the central vagal stimulant 2-deoxy-d-glucose (75 mg kg−1), whereas bethanechol-stimulated pancreatic protein output was inhibited by only 59 ± 7 %.
In vitro, ghrelin (10−11–10−7m) produced no change in basal amylase release from dispersed, purified acinar cells. Co-incubation of ghrelin (10−11−10−7m) with CCK−8 (10−10m) demonstrated no inhibition of CCK-stimulated amylase release from dispersed acini. In contrast, ghrelin (10−9−10−7m) dose-dependently inhibited amylase release from pancreatic lobules exposed to 75 mm potassium.
Our results show that (1) ghrelin is a potent inhibitor of pancreatic exocrine secretion in anaesthetized rats in vivo and in pancreatic lobules in vitro; and (2) the actions of ghrelin are indirect and may be exerted at the level of intrapancreatic neurons.
Ghrelin, a novel 28 amino acid peptide, was initially purified from rat stomach (Kojima et al. 1999) and localized to gastric oxyntic glands (Date et al. 2000a), the hypothalamic arcuate nucleus (Kojima et al. 1999) and kidney (Mori et al. 2000). In common with synthetic growth hormone secretagogues (GHSs), ghrelin causes growth hormone release in humans and rats following either peripheral or central administration, and from primary cultured pituitary cells (Kojima et al. 1999; Takaya et al. 2000; Wren et al. 2000; Date et al. 2000b). In rats, ghrelin has been found to stimulate food intake (Wren et al. 2000; Nakazato et al. 2001), to induce adiposity (Tschop et al. 2000) and to increase body weight (Tschop et al. 2000; Nakazato et al. 2001), suggesting a possible role in regulation of feeding behaviour and energy metabolism. In addition, ghrelin has been recently reported to stimulate gastric acid secretion and gastric motility in anaesthetized rats (Masuda et al. 2000; Date et al. 2001). The latter observation suggests that ghrelin may regulate gastrointestinal function.
Pancreatic exocrine secretion is controlled physiologically by both the autonomic nervous system and a number of brain-gut peptides. Among these peptides, cholecystokinin (CCK), secretin and cocaine- and amphetamine-regulated transcript peptide (CART) have been reported to stimulate pancreatic secretion (Chey, 1993; Cowles et al. 2001), whereas neuropeptide Y and somatostatin serve as inhibitors (Mulholland et al. 1991; Chey, 1993). The present study was performed to examine the effect of ghrelin on pancreatic exocrine function in anaesthetized rats. The present authors sought to (1) demonstrate an in vivo inhibitory effect of ghrelin on exocrine pancreatic secretion stimulated by CCK, 2-deoxy-d-glucose (2-DG), or bethanechol; and (2) determine the in vitro effects of ghrelin on amylase release from purified pancreatic acini and pancreatic lobules.
METHODS
Materials
Rat ghrelin peptide was obtained from Phoenix Pharmaceuticals (Mountain View, CA, USA). CCK-8 was purchased from Research Plus, Inc. (Bayonne, NJ, USA). Bethanechol, 2-deoxy-d-glucose, potassium chloride, N-2-hydroxyethylpiperazine-N‘-2-ethanesulfonic acid (Hepes), bovine serum albumin (BSA), soy bean trypsin inhibitor (SBTI), ketamine and xylazine were obtained from Sigma (St Louis, MO, USA). PE10 and PE50 polyethylene tubing was purchased from Becton Dickinson (Sparks, MD, USA). Infusion pumps and thermal barriers were obtained from Harvard Apparatus, Inc. (South Natic, MA, USA). Collagenase was obtained from Boehringer Mannheim (Mannheim, Germany). Nitex nylon mesh (150 μm) was obtained from Tetko, Inc. (Kansas City, MO, USA). The amylase assay kit was purchased from Pharmacia Upjohn (Kalamazoo, MI, USA). The protein assay kit was obtained from Bio-Rad laboratories (Hercules, CA, USA).
In vivo studies
Animal preparation
Male Sprague-Dawley rats (200–250 g) obtained from Harlan (Indianapolis, IN, USA) were used for all experiments. Experimental protocols were reviewed and approved by the University Committee on Use and Care of Animals of the University of Michigan. Animals were cared for under pathogen-free guidelines and housed at constant ambient temperature with alternating light-dark cycles of 12 h. They were permitted free access to water and standard rodent laboratory chow. They were fasted overnight, with continued access to water, prior to experiments.
On the morning of the experiment, rats were anaesthetized with intramuscular injection of a mixture of xylazine and ketamine (13 and 87 mg (kg body weight)−1, respectively). Supplemental doses were used every 2 h as needed to maintain adequate anaesthesia. An intravenous cannula was placed into the right external jugular vein for infusion of 0.9 % NaCl (1 ml h−1) and other chemicals. Through an upper midline laparotomy, the duodenum was elevated and the bile-pancreatic duct isolated as it entered the posterior duodenum. Through a small incision, a polyethylene cannula (PE10) was advanced into the bile-pancreatic common duct and was secured in place with fine silk suture. A second polyethylene cannula (PE50) was placed into the duodenum and its tip secured proximal to the ampulla for infusion of previously harvested bile-pancreatic juice. The abdominal wound was covered with a saline-moist gauze and the rats were maintained at 37 °C with a thermal barrier. At the end of experiments, animals were killed by CO2 inhalation and cervical dislocation.
Pancreatic secretion study
Secreted bile-pancreatic juice was collected for 45 min prior to starting the experiments to allow for stabilization of flow following surgical manipulation. Bile- pancreatic secretions were collected over 15 min periods. Volume was recorded and aliquots assayed for protein content. Previously collected bile-pancreatic juice was re-infused via the duodenal cannula at the rate of 1.2 ml h−1. Protein measurements were made using the Bio-Rad protein assay kit.
CCK-8 stimulation
CCK-8 was infused continuously at a rate of 400 pmol kg−1 h−1 for 90 min. Preliminary studies determined that this produced submaximal stimulation of pancreatic secretion. Infusions of synthetic rat ghrelin (1.2 or 12 nmol kg−1 h−1) were begun 15 min before CCK-8 administration and continued for the remainder of the experiment.
2-DG stimulation
2-DG was dissolved in saline and administered as a bolus intravenous injection at the end of the basal collection period. Ghrelin infusion (12 nmol kg−1 h−1) was started 15 min before 2-DG injection and continued for the remaining time of the experiment.
Bethanechol stimulation
Bethanechol was dissolved in saline and administered as a continuous intravenous infusion beginning at the end of the basal collection period. Ghrelin infusion (12 nmol kg−1 h−1) was begun 15 min before the bethanechol infusion and continued for the rest of the experiment.
Subdiaphragmatic vagotomy
Through a midline laparotomy, the oesophagus was exposed. Subdiaphragmatic vagal trunks were exposed halfway between the diaphragm and the gastric cardia and both anterior and posterior trunks were transected.
Dispersed acini studies
Preparation of acinar cells
Preparation of dispersed rat pancreatic acini was performed according to Williams et al. (1978). Briefly, rats that had been fasted overnight were killed by CO2 inhalation, and the pancreata were removed and placed in a Petri dish containing fresh Krebs-Ringer buffer (KRB) solution consisting of (mm): 110 NaCl, 0.5 CaCl2, 1.1 MgCl2, 4.7 KCl, 0.55 Na2HPO4, 32.5 NaHCO3, 22 glucose, 2 glutamine, 0.1 mg ml−1 soybean trypsin inhibitor and 1 % (v/v) Eagle's minimal essential amino acids, equilibrated with 5 % CO2-95 % O2, adjusted to pH 7.4. After fat and lymphatic tissue were trimmed away, each pancreas was digested with collagenase, mechanically dispersed, and passed through a 150 μm mesh nylon cloth. Acini were purified by centrifugation at 50 g for 3 min in KRB solution containing 4 % BSA, and resuspended in incubation buffer that consisted of Hepes buffered Ringer (HRB) solution containing (mm): 127 NaCl, 1.28 CaCl2, 0.55 MgCl2, 4.7 KCl, 0.55 Na2HPO4, 10 Hepes, 11 glucose, 2 glutamine, 1 % (v/v) Eagle's minimal essential amino acids, 0.1 mg ml−1 soybean trypsin inhibitor and 10 mg ml−1 BSA, oxygenated with 100 % O2, and adjusted to pH 7.4.
Amylase assay
Acini were pre-incubated at 37 °C with minimal shaking for 60 min, followed by treatment with different agonists in 1 ml aliquots in polystyrene vials for the indicated times. Acini were then pelleted. The acinar cells contained within the pellet were lysed with a sonicator for 15 s. Amylase content in both the pellet and the supernatants was determined using the Phadebas reagent and expressed as a percentage of total cellular amylase content.
Pancreatic lobule studies
Pancreatic lobules, containing intrapancreatic nerve terminals and islets in addition to exocrine cells, were prepared by the method of Scheele & Palade (1975). Fasted rats were killed by CO2 inhalation and pancreata were removed. After trimming away peripancreatic fat and lymph nodes, the pancreatic tissue was divided into 4 mm × 5 mm lobular units; lobules were placed in Krebs- Henseleit-bicarbonate (KHB) solution containing (mm): 110 NaCl, 1.8 CaCl2, 1.1 MgCl2, 4.7 KCl, 1.1 Na2HPO4, 25 NaHCO3, 25 Hepes, 11 glucose, 2 glutamine, 0.1 mg ml−1 soybean trypsin inhibitor, and 1 % (v/v) Eagle's minimal essential amino acids, equilibrated with 5 % CO2-95 % O2, and adjusted to pH 7.4. Lobules were allowed to recover for 30 min at 37 °C. Lobular tissues were then exposed to the agonist with or without the presence of graded concentrations of ghrelin for 30 min. The medium was then aspirated, diluted 1:10 and assayed for amylase. To the remaining tissue, 1 ml KHB was added and the tissue was homogenized. Tissue homogenates were then assayed for amylase. Amylase released was expressed as the percentage of total tissue content.
Statistical analysis
Data were expressed as multiples of basal secretion. The results are expressed as means ± s.e.m. A two-way analysis of variance (ANOVA) was used to analyse differences between treatment groups. Integrated protein output was calculated by cumulative protein secretion during the time in which both ghrelin and agonists were infused. Student's unpaired t test was used to analyse data from integrated protein secretion, volume and amylase output, and from dispersed acinar cell and pancreatic lobule preparations. Differences were considered significant when P values were less than 0.05.
RESULTS
Effects of ghrelin on basal pancreatic secretion
Basal secretion was averaged from three 15 min collections of bile-pancreatic juice prior to the application of tested agents. The volume of basal secretion averaged 0.33 ± 0.02 ml per 15 min (n = 6). Basal pancreatic protein secretion averaged 3.2 ± 0.8 mg h−1. Basal amylase release was 3.7 ± 0.5 U min−1. Ghrelin infusion (12 nmol kg−1 h−1) had no significant effect upon basal secretory volume, protein or amylase outputs.
Effects of ghrelin on CCK-stimulated pancreatic secretion
CCK-8 was infused at a rate of 400 pmol kg−1 h−1, which was chosen to stimulate submaximal pancreatic secretion (Schonfeld et al. 1989). Pancreatic protein output increased significantly above basal levels by 15 min, reaching peak values at 45 min, and remained significantly elevated for the duration of the study (Fig. 1). The average volume of bile-pancreatic juice increased from 0.32 ± 0.03 to 0.38 ± 0.04 ml per 15 min (n = 6, P < 0.05). Ghrelin was infused at two doses (1.2 and 12 nmol kg−1 h−1), beginning 15 min before initiation of CCK-8 infusion. These doses of ghrelin were chosen based on the studies in which ghrelin significantly increased food intake in rats (Tschop et al. 2000; Wren et al. 2000) or stimulated gastric acid secretion (Masuda et al. 2000). Significant and dose-related inhibition of CCK-8-stimulated protein secretion was observed (Fig. 1). Ghrelin at 1.2 nmol kg−1 h−1 produced a 45 ± 8 % inhibition of the integrated CCK-stimulated pancreatic protein secretion (P < 0.05), while a dose of 12 nmol kg−1 h−1 inhibited integrated pancreatic protein output by 84 ± 7 % (P < 0.01). CCK at a dose of 400 pmol kg−1 h−1 significantly increased amylase secretion from the basal of 3.9 ± 0.6 U min−1 to the peak rate of 23.3 ± U min−1 (P < 0.05 vs. basal). CCK-stimulated amylase release was dose-dependently inhibited by ghrelin. Infusion of ghrelin at doses of 1.2 and 12 nmol kg−1 h−1 significantly reduced CCK-stimulated amylase output to 15.6 ± 1.4 U min−1 (P < 0.05 vs. CCK alone) and 8.7 ± U min−1 (P < 0.05 vs. CCK alone), respectively. CCK-induced increases in secretory volume were also inhibited by ghrelin infusion. In rats administered 1.2 and 12 nmol kg−1 h−1 ghrelin, secretion volume remained unchanged after CCK infusion, averaging 0.35 ± 0.03 and 0.34 ± 0.01 ml per 15 min, respectively.
Figure 1. Effect of ghrelin on CCK-stimulated pancreatic protein secretion.
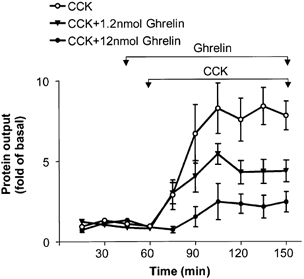
Ghrelin (1.2 and 12 nmol kg−1 h−1) was infused from 45 to 150 min and CCK-8 (400 pmol kg−1 h−1) from 60 to 150 min. The protein content of pancreatic secretion was measured at 15 min intervals. Data are expressed as means ± s.e.m. For each group, n = 6 (two-way ANOVA, P < 0.01).
Effects of ghrelin on CCK-stimulated pancreatic secretion in rats with acute vagotomy
When subdiaphragmatic vagotomy was performed acutely, ghrelin (12 nmol kg−1 h−1) retained the ability to inhibit CCK (400 pmol kg−1 h−1)-induced increase in pancreatic protein secretion by 75 ± 18 % (P < 0.05, n = 6; Fig. 2).
Figure 2. Effect of ghrelin on pancreatic protein secretion stimulated by CCK-8 (400 pmol kg−1 h−1) in acutely vagotomized rats.
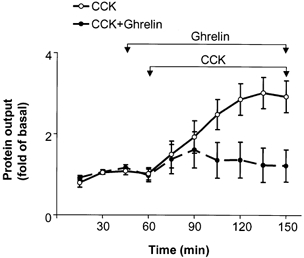
Subdiaphragmatic vagotomy was performed prior to beginning measurements. Ghrelin (12 nmol kg−1 h−1) was infused continuously from 45 to 150 min. CCK infusion was begun at 60 min in both groups. For both groups, n = 6 (two-way ANOVA, P < 0.05).
Effects of ghrelin on 2-DG-stimulated pancreatic secretion
2-DG is a well-characterized centrally acting vagal stimulant (Becker et al. 1988; Putnam et al. 1989). Infusion of ghrelin was begun 15 min before 2-DG administration (75 mg kg−1) (Fig. 3). Ghrelin at 12 nmol kg−1 h−1 abolished 2-DG-stimulated pancreatic secretion.
Figure 3. Inhibition of 2-DG-stimulated pancreatic protein secretion by ghrelin.
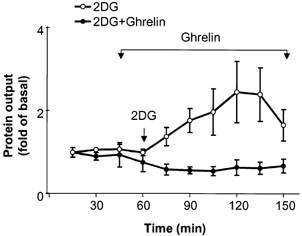
Ghrelin (12 nmol kg−1 h−1) was infused from 45 to 150 min and 2-DG (75 mg kg−1) was given by intravenous bolus injection at 60 min. The results are expressed as means ± s.e.m. For each group, n = 4 (two-way ANOVA, P < 0.05).
Effects of ghrelin on bethanechol-stimulated pancreatic secretion
The effects of ghrelin on cholinergically stimulated pancreatic secretion were studied by using bethanechol, a muscarinic receptor agonist without significant nicotinic receptor effect (Putnam et al. 1989). Bethanechol at an infusion rate of 3 mg kg−1 h−1 stimulated pancreatic protein secretions by 5 ± 0.9-fold at 45 min (n = 6, P < 0.05; Fig. 4). Infusion of ghrelin at a rate of 12 nmol kg−1 h−1 decreased integrated bethanechol-stimulated pancreatic protein secretion by 59 ± 7 % (P < 0.05).
Figure 4. Effect of ghrelin on bethanechol-stimulated pancreatic protein secretion.
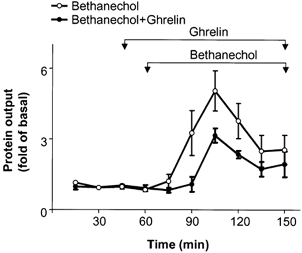
Ghrelin (12 nmol kg−1 h−1) was infused from 45 to 150 min and bethanechol (3 mg kg−1 h−1) from 60 to 150 min. Protein output was measured in 15 min intervals. The results are expressed as means ± s.e.m. Each group contained six animals (two-way ANOVA, P < 0.05).
In vitro effects of ghrelin
To test for a direct inhibitory effect of ghrelin on pancreatic exocrine function, dispersed acinar cells were incubated with various doses of the peptide in vitro. Ghrelin alone produced no significant change in basal amylase release (Fig. 5). Co-incubation of CCK-8 (10−10m) with various concentrations of ghrelin produced no alteration in amylase release compared to that observed with CCK-8 alone (Fig. 5).
Figure 5. In vitro effects of ghrelin on CCK-8-stimulated amylase release from dispersed rat acinar cells.
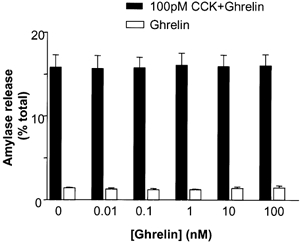
Acinar cells were incubated with varying concentrations of ghrelin (10−11–10−7m) in the presence or absence of CCK-8 (10−10m). The results for amylase are calculated as a percentage of total amylase content. The results are expressed as means ± s.e.m. from four separate experiments.
Incubation of pancreatic lobules in a medium containing elevated potassium (75 mm) significantly increased amylase release. Ghrelin, at concentrations from 10−9 to 10−7m, dose-dependently decreased amylase release from pancreatic lobules that were exposed to potassium (Fig. 6).
Figure 6. Effect of ghrelin co-incubation on potassium chloride (75 mm)-stimulated amylase release (multiple of control) from pancreatic lobules.
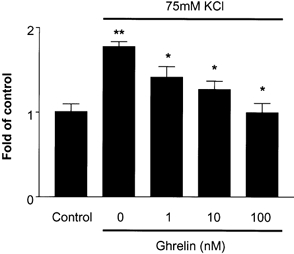
The results are expressed as means ± s.e.m. for five separate experiments. *P < 0.05 vs. KCl; **P < 0.01 vs. control.
DISCUSSION
The present study supports the concept that ghrelin inhibits pancreatic secretion via a mechanism involving the intrapancreatic nervous system. This contention is supported by the following six pieces of evidence: (1) ghrelin significantly inhibited CCK-stimulated pancreatic secretion in vivo; (2) ghrelin inhibited CCK-stimulated pancreatic secretion in rats with acute vagotomy; (3) continuous infusion of ghrelin abolished pancreatic secretion stimulated by 2-DG; (4) pancreatic secretion stimulated by bethanechol was only partially attenuated by infusion of ghrelin; (5) ghrelin demonstrated no direct effect on the basal and CCK-stimulated amylase release in dispersed acinar cells; and (6) ghrelin blocked potassium-induced amylase release from pancreatic lobules in vitro.
Ghrelin is a novel 28 amino acid peptide secreted from the endocrine cells of gastric mucosa (Kojima et al. 1999). It is an endogenous ligand for the growth hormone secretagogue (GHS) receptor, which has been demonstrated in both the central nervous system and peripheral tissues (Howard et al. 1996; Guan et al. 1997; Papotti et al. 2000). Whereas ghrelin was initially identified as a growth-hormone-releasing peptide, a broader array of actions have recently been reported. Expression of ghrelin and its related receptor, GHS receptor, in kidney indicates that ghrelin may play an important role in the regulation of renal function (Mori et al. 2000). Stimulation of food intake and subsequent body weight gain have been demonstrated in rats administered ghrelin either centrally or peripherally (Tschop et al. 2000; Wren et al. 2000; Nakazato et al. 2001). In the gastrointestinal system, ghrelin has been demonstrated to regulate gastric acid secretion and gastric motility (Masuda et al. 2000; Date et al. 2001).
That ghrelin may be involved in regulation of digestive processes is supported by the observation that ghrelin-producing cells correspond to X/A-like endocrine cells in the gastrointestinal tracts of rats and humans identified by light and electron microscopic immunohistochemistry and by in situ hybridization (Date et al. 2000a). Ghrelin receptor (GHS receptor) mRNA has also been identified in the gastrointestinal tract including stomach, small intestine and pancreas (Guan et al. 1997; Date et al. 2000a). While the pancreas has been demonstrated to express GHS receptor by RT-PCR (Guan et al. 1997), the functional response to ghrelin has not previously been reported. The present studies demonstrate that ghrelin is a potent inhibitor for stimulated pancreatic protein secretion in vivo. As a result of rapid degradation in serum, relatively high doses are required during peripheral administration of ghrelin to achieve concentrations equivalent to postprandial blood levels (Tschop et al. 2000). The doses used in our studies correspond to those reported by Tschop et al. (2000) and Wren et al. (2000) to increase food intake in rats.
Regulation of pancreatic exocrine secretion occurs at multiple levels, including the central nervous system, pancreatic innervation, intrapancreatic neurons and via receptors on pancreatic acinar cells. While ghrelin inhibited pancreatic protein output in vivo, the effect was indirect because ghrelin did not have an effect when dispersed pancreatic acini were exposed to the peptide in vitro. These in vitro studies strongly suggest that the inhibitory actions of ghrelin are not mediated by direct actions on the acinar cells. Similar indirect inhibitory action on pancreatic secretions has been reported for neuropeptide Y (NPY; Mulholland et al. 1991). Similar to NPY, ghrelin appears to act on the intrapancreatic neurons to inhibit pancreatic protein secretion.
Several recent reports suggest that ghrelin acts within the central nervous system. Ghrelin up-regulates the expression of c-fos, NPY and agouti-related protein (AGRP) genes in hypothalamus (Hewson & Dickson, 2000; Kamegai et al. 2000; Nakazato et al. 2001) and c-fos mRNA expression in dorsomotor nucleus of the vagus (Date et al. 2001). Stimulation of gastric secretion by ghrelin administered by intracerebroventricular injection is mediated by direct central activation of a central vagal mechanism (Date et al. 2001). Three observations from the present study support the idea that the actions of ghrelin on pancreatic secretion are directed at the level of intrapancreatic neurons. First, ghrelin abolished pancreatic protein secretion stimulated by 2-DG, a centrally acting vagal stimulant, but only partially inhibited pancreatic protein secretion stimulated by CCK or bethanechol. Secondly, vagotomy failed to reverse the inhibitory effects of ghrelin on CCK stimulation of pancreatic secretion. Thirdly, ghrelin dose-dependently decreased amylase release stimulated by depolarizing concentration of KCl in pancreatic lobules.
In summary, the present study has demonstrated that ghrelin is a potent inhibitor of pancreatic exocrine secretion in anaesthetized rats. The study supports the conclusions that (1) the actions of ghrelin are indirect; and (2) ghrelin may affect intrapancreatic neurotransmission as a mechanism of action.
Acknowledgments
This study was supported by National Institutes of Health Grant RO1DK43225 (M.W.M).
References
- Becker S, Biebel W, Sender MV. Nervous control of gastric and pancreatic secretory response to 2-deoxy-D-glucose in the dog. Digestion. 1988;39:187–196. doi: 10.1159/000199624. [DOI] [PubMed] [Google Scholar]
- Chey WY. Hormonal control of pancreatic exocrine secretion. In: Go VLW, editor. The Pancreas: Biology Pathobiology and Disease. 2. New York: Raven Press; 1993. pp. 403–424. [Google Scholar]
- Cowles RA, Segura BJ, Mulholland MW. Stimulation of rat pancreatic exocrine secretion by cocaine- and amphetamine-regulated transcript peptide. Regulatory Peptides. 2001;99:61–68. doi: 10.1016/s0167-0115(01)00226-9. [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000a;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- Date Y, Murakami N, Kojima M, Kuroiwa T, Matsukura S, Kangawa K, Nakazato M. Central effects of a novel acylated peptide, ghrelin, on growth hormone release in rats. Biochemical and Biophysical Research Communications. 2000b;275:477–480. doi: 10.1006/bbrc.2000.3342. [DOI] [PubMed] [Google Scholar]
- Date Y, Nakazato M, Kojima M, Kuroiwa T, Matsukura S, Kangawa K, Nakazato M. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochemical and Biophysical Research Communications. 2001;280:904–907. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Research. Molecular Brain Research. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. Journal of Neuroendocrinology. 2000;12:1047–1049. doi: 10.1046/j.1365-2826.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, Demartino JA, Gupta SK, Schaeffer SK, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000;141:4797–4800. doi: 10.1210/endo.141.12.7920. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochemical and Biophysical Research Communications. 2000;276:905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- Mori K, Yoshimoto A, Takaya K, Hosoda K, Ariyasu H, Yahata K, Mukoyama M, Sugawara A, Hosoda H, Kojima M, Kangawa K, Nakao K. Kidney produces a novel acylated peptide, ghrelin. FEBS Letters. 2000;486:213–216. doi: 10.1016/s0014-5793(00)02308-5. [DOI] [PubMed] [Google Scholar]
- Mulholland MW, Lally K, Taborsky GJ., Jr Inhibition of rat pancreatic exocrine secretion by neuropeptide Y: studies in vivo and in vitro. Pancreas. 1991;6:433–440. doi: 10.1097/00006676-199107000-00010. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matrukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Papotti M, Ghe C, Cassoni P, Catapano F, Deghenghi R, Ghigo E, Muccioli G. Growth hormone secretagogue binding sites in peripheral human tissues. Journal of Clinical Endocrinology and Metabolism. 2000;85:3803–3807. doi: 10.1210/jcem.85.10.6846. [DOI] [PubMed] [Google Scholar]
- Putnam WS, Liddle RA, Williams JA. Inhibitory regulation of rat exocrine pancreas by peptide YY and pancreatic polypeptide. American Journal of Physiology. 1989;256:G698–703. doi: 10.1152/ajpgi.1989.256.4.G698. [DOI] [PubMed] [Google Scholar]
- Scheele GA, Palade GE. Studies on the guinea pig pancreas: parallel discharge of exocrine enzyme activities. Journal of Biological Chemistry. 1975;250:2660–2670. [PubMed] [Google Scholar]
- Schonfeld JV, Muller MK, Demirtas B, Soukup J, Runzi M, Goebell H. Effect of neural blockade on somatostatin-induced inhibition of exocrine pancreatic secretion. Digestion. 1989;43:81–86. doi: 10.1159/000199865. [DOI] [PubMed] [Google Scholar]
- Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K. Ghrelin strongly stimulates growth hormone release in humans. Journal of Clinical Endocrinology and Metabolism. 2000;85:4908–4911. doi: 10.1210/jcem.85.12.7167. [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Williams JA, Korc M, Dormer RL. Action of secretagogues on a new preparation of functionally intact, isolated pancreatic acini. American Journal of Physiology. 1978;235:517–524. doi: 10.1152/ajpendo.1978.235.5.E517. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]


