Abstract
It has been proposed that the activation of NMDA receptors and upregulation of protein kinase C (PKC) underlie the exaggerated and persistent pain experienced in the inflammatory state. However, there is no direct evidence to show that inflammation alters the function of NMDA receptors.
We examined the voltage-dependent properties of NMDA receptor channels in rat dorsal horn neurones that receive sensory inputs from an inflamed hindpaw.
Peripheral inflammation was induced by injections of complete Freund's adjuvant (CFA). Membrane currents were measured using the perforated patch-clamp technique.
After CFA treatment, the current–voltage relationship of NMDA receptor channels was shifted in the hyperpolarized direction. This resulted in enhanced NMDA responses at negative potentials.
The change was mediated by PKC because the voltage shift was blocked by the selective PKC inhibitors chelerythrine and bisindolylmaleimide I.
Furthermore, the Mg2+ blockade of NMDA receptors was reduced. This reduction could account for the shift in the voltage dependence of NMDA receptor channels.
These results indicate that NMDA receptor channel characteristics in the dorsal horn are altered by inflammation, and that the changes observed could contribute to the hyperalgesia and allodynia associated with tissue injury.
Chronic tissue inflammation or nerve injuries often trigger exaggerated nociceptive (pain) responses to sensory stimuli. Innocuous stimuli become painful (allodynia) and mild noxious stimuli cause severe pain (hyperalgesia). These abnormal nociceptive behavioural responses have been shown to arise from hyperexcitability of dorsal horn neurones in the spinal cord and caudal medulla (Dubner & Ruda, 1992; Dickenson et al. 1997; Woolf & Costigan, 1999). Since sensitization of dorsal horn neurones cannot develop or be maintained in the presence of N-methyl-d-aspartate (NMDA) receptor antagonists (Woolf & Thompson, 1991; Coderre & Melzack, 1992; Dougherty et al. 1992), NMDA receptors are believed to play a critical role in the development of neuronal plasticity. It is proposed that prolonged and repeated stimulation of peripheral nerves tonically activates NMDA receptors. This results in a large increase in Ca2+ influx through NMDA channels and activation of various protein kinases, including protein kinase C (PKC; Dickenson et al. 1997; Woolf & Costigan, 1999). PKC in turn potentiates NMDA responses (Chen & Huang, 1992). This positive feedback would enhance PKC activity and intracellular Ca2+ excessively, leading to neuronal excitotoxicity and cell death (Mayer et al. 1999).
There is good evidence supporting the participation of PKC in the sensitization of dorsal horn neurones. Following inflammation, PKCγ immunoreactivity in the spinal dorsal horn is upregulated in parallel with the development of mechanical allodynia (Martin et al. 1999). Mutant mice lacking the PKCγ gene cannot develop full-blown allodynia after partial sciatic nerve injury (Malmberg et al. 1997). In addition, selective PKC inhibitiors have been found to block the development of behavioural hyperalgesia (Coderre, 1992; Sluka & Willis, 1997). These observations suggest that inflammation-induced upregulation of PKC could affect the function of NMDA receptors. However, there are no data to show that the properties of NMDA receptors in dorsal horn neurones are indeed changed by inflammation, or that such changes are mediated by PKC. We therefore examined the current–voltage (I–V)-dependent properties of NMDA receptors in dorsal horn neurones isolated from the spinal cord of rats with peripheral inflammation. Preliminary studies have been reported in an abstract (Guo et al. 1999).
METHODS
Induction of peripheral inflammation
The procedures for the induction of inflammation, tissue dissection and cell isolation were approved by the Institutional Animal Care and Use Committee at University of Texas Medical Branch.
Sprague-Dawley rats (15–30 days old) were lightly anaesthetized with methoxyflurane (dose, 0.2 mg in a 730 ml glass jar). Complete Freund's adjuvant (CFA; Mycobacterium butyricum; DIFCO, Detroit, MI, USA) emulsion (1:1 peanut oil/saline, 10 mg Mycobacterium ml−1) was injected into the ankle and plantar surface (50 μl each) of the left hindpaw of each rat. The CFA injections produced localized inflammation characterized by redness, oedema and hyperalgesia in the hindpaw and ankle.
Behavioural tests
Mechanical allodynia was assessed by paw withdrawals in response to von Frey filament stimulation. A series of successively stronger von Frey filaments (0.1–20.17 g) was applied to the plantar surface of the tested paw for 2–3 s or until paw withdrawal. Four attempts were made for each filament. The response to a filament was considered positive if three out of four trials produced positive responses. Thermal hyperalgesia was assessed by measuring paw withdrawal latencies to radiant heat generated by a heated bulb placed directly under the tested paw (Hargreaves et al. 1988). The maximum cut-off latency was set at 15 s to avoid tissue damage. Each rat was tested five to six times with a 5–10 min waiting period between tests. The withdrawal latencies of the last four trials were averaged. The paw circumference of each rat was recorded.
Cell recordings
Dorsal horn neurones were isolated from the L4-L6 segments of the spinal cord ipsilateral to the injection side, 2–7 days after CFA injection. The cell dissociation procedure was similar to that described by Gu & Huang (1998). In brief, rats were anaesthetized with sodium pentobarbital (50 mg kg−1, i.p.). The lumbar region of the spinal cord was rapidly removed from the animal and put into an ice-cold, oxygenated (95 % O2 and 5 % CO2) artificial cerebrospinal fluid (ACSF) solution that contained (mm): 125 NaCl, 3.5 KCl, 2 CaCl2, 1 MgCl2, 24 NaHCO3, 1.25 NaH2PO4, 15 glucose; pH 7.30–7.45, osmolarity 300–310 mosmol l−1. The animal was killed immediately afterwards by cutting the descending aorta. The tissue was then glued onto a vibratome slicer, cut into 250–300 μm horizontal slices and incubated in the ACSF solution at 34.5 °C for 1 h. Slices were then transferred to a dissecting solution containing 2.65 or 3.67 units ml−1 papain (Sigma P3250) and incubated at 34.5 °C for 40–65 min. The dissecting solution consisted of (mm): 135 NaCl, 5 KCl, 1.5 CaCl2, 6 MgSO4, 2 KH2PO4, 10 glucose, 10 Hepes; pH 7.20, 295–310 mosmol l−1. After incubation, the slices were washed with enzyme-free dissecting solution and stored at room temperature (20–23 °C). Prior to an experiment, dorsal horns including laminae I-II were isolated from tissue slices with a scalpel. Neurones were dissociated by triturating the tissue with a series of fire-polished Pasteur pipettes.
Whole-cell currents were recorded at room temperature using the perforated patch-clamp technique. Amphotericin B (240 μg ml−1, Calbiochem, La Jolla, CA, USA) was added to the pipette solution. The external recording solution contained (mm): 140 NaCl, 2 KH2PO4, 4 KCl, 2 CaCl2, 10 Hepes, 10 glucose, 2 μm glycine and varying concentration of MgCl2; pH 7.35–7.40, 300–310 mosmol l−1. The pipette solution contained (mm): 110 caesium methanesulphate, 23 CsCl, 10 Hepes, 10 glucose; pH 7.20, 295–310 mosmol l−1. Current recordings were made with a patch-clamp amplifier (Axopatch 200A, Axon Instruments, Foster City, CA, USA). Whole-cell currents were filtered at 2 kHz and sampled at 250 μs per point.
Drug application
NMDA was pressure-applied onto a neurone through an applicator consisting of two or more glass pipettes. The solution flow was controlled by solenoid valves. The concentrations of chemicals used were as follows: 100 μm NMDA, 2 μm glycine (Sigma, St Louis, MO, USA), 3.3 μm chelerythrine chloride (Chelery) and 2 μm bisindolylmaleimide I (BIS-I; CalBiochem).
Data analyses
All data are expressed as means ± s.e.m. Paw circumferences, mechanical thresholds and paw withdrawal latencies obtained from different rats were averaged. Changes among different testing days were analysed by one-way ANOVA. Post hoc comparisons between pre- and post-saline or CFA injection were made with Dunnett's method. Student's t test was used to assess the significance of changes in conductance. The level of statistical significance was set at P < 0.05.
The conductance (g) of each cell was obtained according to the equation: g = I/(V - Vrev), where I is the NMDA current, V the membrane potential and Vrev the reversal potential. The maximal conductance (gmax) was obtained by fitting the g–V curve of each cell with the Boltzmann equation: g = gmax/(1+eA), where A = (V0.5 - V)(ZδFV/RT), V0.5 is the potential at which g/gmax = 0.5, R is the gas constant, T is the absolute temperature, Z is the valency, δ is the electrical distance and F is the Faraday constant.
RESULTS
Hyperalgesic and allodynic behaviour in young rats
Since young (15- to 30-day-old) rats were used in our electrophysiological experiments, it was important to establish that the inflammation-induced hyperalgesic and allodynic behaviours in these rats are similar to that observed in adult rats. CFA was injected subcutaneously into the left plantar surface and the ankle area. The injected paw became swollen within 24 h and the animals exhibited limping and guarding behaviour. The paw swelling persisted for up to 14 days (Fig. 1). During the same period, the mean mechanical threshold and paw withdrawal latencies to radiant heat for the injected paw were reduced significantly. In control rats, saline was injected. As a result of normal growth of young rats, the paw circumference at 10 and 14 days after the saline injection was larger than that before the injection. There were no significant changes in the mechanical threshold or in paw withdrawal latencies in the saline-injected rat group. These results are similar to those observed in adult rats (Martin et al. 1999).
Figure 1. Complete Freund's adjuvant (CFA) produces paw swelling, mechanical allodynia and heat hyperalgesia.
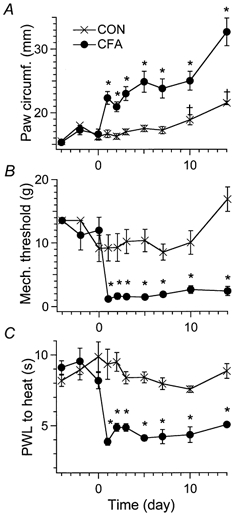
A, the circumference of the CFA-injected paw increased significantly 1 day after CFA treatment. The swelling remained for up to 14 days (*P < 0.05). The data points for day 1 to day 10 after CFA treatment represent the mean of data obtained from 6–10 rats. The data points for days −4, −2 and 14 are averages obtained from 3–4 rats. Standard errors of the mean (s.e.m.) are indicated by vertical bars. As a result of the normal growth of young rats, the paw circumferences of saline-injected rats were significantly larger at days 10 and 14 than at days −4 and −2 (†P < 0.05). B, mechanical thresholds. C, paw withdrawal latencies (PWLs) of the CFA-injected paw in response to heat stimuli. Both the mechanical thresholds and PWLs in the CFA-injected paw were greatly reduced 1 day after CFA injection (*P < 0.05). The mechanical allodynia and the thermal hyperalgesic conditions were maintained for at least 14 days.
CFA shifts the I–V relationship of NMDA responses
We compared the properties of NMDA-activated currents in dorsal horn neurones isolated from control and CFA-treated rats. To ensure that changes in NMDA responses were not the result of developmental changes in NMDA receptor expression or different cell populations, the same age of rats and the same cell isolation procedures were used in control and CFA groups. Since NMDA currents in physiological concentrations of Mg2+ (> 1.0 mm) at hyperpolarized potentials were small and difficult to study, most experiments were performed in an external solution containing 30 μm Mg2+. Membrane currents in response to NMDA applications were measured at different holding potentials. In control (CON) cells, peak NMDA responses at −80 mV were much smaller than those measured at −60 mV (Fig. 2A). The maximum peak current in the cell shown in Fig. 2 occurred between −50 and −40 mV. Thus, the I–V curve exhibited a prominent region of negative slope, a characteristic of voltage-dependent blockade of NMDA-receptor channels by Mg2+ (Fig. 2B). We then examined the properties of NMDA-activated currents in dorsal horn cells isolated from CFA-treated (CFA) rats. NMDA-activated currents also showed the voltage-dependent blockade as in CON cells. However, the maximum peak currents occurred at more hyperpolarized potentials (e.g. −60 mV). Thus, the I–V curve was shifted in the hyperpolarized direction. The reversal potentials of the currents in CON and CFA cells were similar (Fig. 2).
Figure 2. CFA treatment shifts the current–voltage (I–V) relationship of NMDA-activated currents in the hyperpolarized direction.

A, examples of NMDA-activated currents recorded at the indicated holding potentials obtained from three different cells isolated from either control (CON) or CFA-treated rats. In the CFA group, one cell was tested in the absence of a protein kinase C (PKC) inhibitor (CFA); the other was tested by pre-treating the cell with the PKC inhibitor chelerythrine (Chelery) for 2 min (CFA + Chelery). MgCl2 (30 μm) was present in all of the experiments. Note that the NMDA responses at −40 mV were larger than those at −60 mV in the CON cell. In contrast, the response at −60 mV was greater than that at −40 mV in the CFA cell. B, peak NMDA currents were plotted as a function of holding potential. The currents reversed at +4 mV for the CON cell shown, and at +5 mV for both the CFA and CFA + Chelery cells. The I–V curve of the CFA cell was shifted to the left with respect to the curves of the CON and CFA + Chelery cells. The peak currents for the CON and CFA + Chelery cells were maximal at ∼-50 mV, while the maximal peak current for the CFA cell occurred at −60 mV.
The shift in I–V curves is mediated by PKC
It has been shown that the levels of PKC, particularly the PKCγ subtype, increase substantially in the dorsal horn after inflammation (Martin et al. 1999). To determine whether our observed change in the voltage dependence of NMDA currents after CFA treatment resulted from elevated PKC activity, we examined the voltage-dependent properties of NMDA receptor channels of cells isolated from CFA-treated rats in the presence of PKC inhibitors. Two inhibitors (Chelery and BIS-I) were used. Chelery acts on the catalytic domain of PKC, whereas BIS-I is a competitive inhibitor of the ATP-binding site of PKC. When Chelery was included in the external solution, the maximum peak current of the I–V curve returned to ∼-50 mV (Fig. 2). Thus, the NMDA-activated currents obtained from CFA + Chelery cells had a similar voltage dependence to those obtained from CON cells. The same results were obtained from CFA + BIS-I cells (Fig. 3).
Figure 3. PKC inhibitors block the shift of the conductance-voltage (g–V) curves of NMDA-activated currents.

A, normalized conductance (g/gmax)-V curves of all tested cells. Continuous lines plotted according to the Boltzmann equation were generated using the averaged values of V0.5 = −62.06 ± 1.47 mV (n = 8) for CON cells and −70.82 ± 1.25 mV (n = 19) for CFA cells. Inflammation resulted in a significant shift in the g/gmax-V curve (P < 0.01) in the hyperpolarized direction. B, after treatment with the PKC inhibitor Chelery, or bisindolylmaleimide I (BIS-I), the g/gmax-V curves obtained from CFA cells were no longer shifted. The V0.5 of the g/gmax-V curves obtained from CFA + Chelery cells was −62.53 ± 1.42 mV (n = 11), and from CFA + BIS-I cells it was −65.05 ± 1.13 mV (n = 17). C, summary of the V0.5 values obtained from control (CON; open bars) and CFA-treated rats (hatched bars). Only CFA cells in the absence of the PKC inhibitor showed significant changes in the V0.5 from the CON cells (*P < 0.01). V0.5 = −62.55 ± 1.70 mV (n = 6) for CON + Chelery cells; V0.5 = −64.98 ± 0.62 mV (n = 4) for CON + BIS-I cells. Thus, Chelery and BIS-I had no effect on V0.5 in CON cells, but abolished the change in V0.5 in CFA-treated rats.
To analyse membrane conductance quantitatively, we calculated normalized conductances (g/gmax) of the NMDA responses at different membrane potentials using the procedure described in Methods, Data analyses. The conductance-voltage (g–V) curves for all cells tested under different conditions were compared (Fig. 3). The g–V curve was shifted to the left following inflammation. The half-maximal potential (V0.5) was −62.06 ± 1.47 mV (n = 8) for control rats and was −70.82 ± 1.25 mV (n = 19) for CFA-treated rats (P < 0.01). The gmax of NMDA currents was not changed by inflammation (CON: 1.94 ± 0.32 pS (n = 8); CFA: 2.10 ± 0.29 pS (n = 19), P > 0.05). The slope of the g–V curve (Zδ) was not altered by CFA treatment (CON: 1.99 ± 0.21 (n = 8), CFA: 2.10 ± 0.12 (n = 19), P > 0.05; Fig. 3A).
We then analysed the effects of the PKC inhibitors on the normalized g–V curves (Fig. 3B and C). To make sure that the PKC inhibitors by themselves did not change the voltage-dependent properties of NMDA-activated currents, the effects of Chelery or BIS-I on NMDA responses were also examined in control rats. The V0.5 values of the g–V curve obtained from CON + Chelery neurones (-62.55 ± 1.70 mV, n = 6) and from CON + BIS-I neurones (-64.98 ± 0.62 mV, n = 4) were not significantly different from that of the control group (Fig. 3C). Thus, the PKC inhibitors had no effect on the g–V properties of NMDA responses in CON neurones. In contrast, the leftward shifts of the g–V curves of CFA cells were blocked by Chelery and by BIS-I. The V0.5 value of the conductance curve was −62.53 ± 1.42 mV (n = 11) for CFA + Chelery neurones and was −65.05 ± 1.13 mV (n = 17) for CFA + BIS-I neurones (Fig. 3B). These results suggest strongly that PKC mediates the shift in the I–V relationship.
Inflammation alters the Mg2+ blockade of NMDA receptor channels
Since the Mg2+ blockade of NMDA receptor channels can be altered by elevation of PKC inside cells (Chen & Huang, 1992), we compared the Mg2+ blockade in CON and CFA neurones. The NMDA-activated currents were measured at −60 mV in external solutions containing various concentrations of Mg2+ (Fig. 4A). The blockade of NMDA currents in cells isolated from the CFA rat group was significantly less at all of the Mg2+ concentrations examined. Dose-response curves for Mg2+ blockade of NMDA-activated currents were obtained. The apparent dissociation constant for Mg2+ block (KMg) in control neurones was 27.22 ± 2.29 μm (n = 12), which is similar to that obtained in trigeminal neurones (Chen & Huang, 1992). In the CFA rat group, the KMg was 44.05 ± 2.43 μm (n = 11), which is significantly different from that in the CON group (P < 0.01).
Figure 4. The Mg2+-induced blockade of NMDA receptors is reduced in CFA cells.

The Mg2+ blockade of NMDA currents was also determined at the physiological Mg2+ concentration of 1 mm. Since the NMDA current at −60 mV was too small to be measured accurately under such conditions, experiments were performed at −40 and −20 mV, potentials close to the firing threshold of cells. In 1 mm Mg2+, the blockade of NMDA responses in CFA neurones was also significantly less than that in CON neurones (Fig. 4B).
We also determined whether the reduction in Mg2+ blockade of NMDA currents after CFA treatment (Fig. 4) could explain the shift in the g–V curve (Fig. 3). Assuming that the binding site for Mg2+ is located inside the channel, the KMg is then a function of voltage, i.e. KMg = KMgoe(ZδVF/RT), where KMgo is the apparent dissociation constant at a membrane potential of 0 mV; Z, δ, V, R, F and T are as defined as in Methods, Data analyses. Using the value of δ obtained from Fig. 3 (1.0) and the KMg measured at −60 mV (Fig. 4A; 27.22 μm for CON and 44.05 μm for CFA), KMgo was calculated to be 3.05 mm for CON and 4.94 mm for CFA animals. Interestingly, the value of KMgo calculated from control neurones is similar to that of the NMDA currents measured in trigeminal dorsal horn neurones (Chen & Huang, 1992) and to that of the NR2A-mediated synaptic currents in adult spinal dorsal horn measured in a normal Mg2+ (1 mm) solution (Momiyama, 2000). We then calculated the relative conductance (g/gmax) at various voltages according to the equation g/gmax = 1 - ([Mg2+]/(KMg+[Mg2+])). When [Mg2+] = 30 μm, the midpoint of the g–V curve (i.e. g/gmax = 0.5) shifted from −58.8 mV in the CON condition to −64.9 mV in the CFA condition. The magnitude of the shift was ∼-6 mV. If we instead used the parameter values obtained from the more detailed studies of KMg in trigeminal neurones (Chen & Huang, 1992; i.e. where δ = 0.73 for CON and 0.81 for PKC (CFA)), the magnitude of the shift was ∼-8 mV. These values are in general agreement with the shift observed in our experiments (Fig. 3A). Thus, the reduction in the Mg2+ blockade of NMDA receptors resulting from the high PKC activity in neurones after inflammation can account for most of the observed shift in the I–V or g–V curves (Fig. 2 and Fig. 3).
The theoretical g–V curves obtained using our estimated values of KMgo, δ (1.0) and [Mg2+] (1 mm) were also obtained. The calculated relative conductances at −40 and −20 mV are consistent with the experimental values measured in 1 mm Mg2+ (Fig. 4C). The g–V curve for CFA neurones is also shifted by −6 mV. The major difference between the g–V curves obtained in 30 μm and in 1 mm Mg2+ is that the midpoint of the curve in 1 mm Mg2+ occurs at a depolarized potential (in 1 mm Mg2+: −14.2 mV for CON and −20.3 mV for CFA; in 30 μm Mg2+: −58.8 mV for CON and −64.9 mV for CFA).
DISCUSSION
In this study, we induced inflammation by injections of CFA into the rat hindpaw and studied NMDA responses in dorsal horn neurones isolated from the spinal cord ipsilateral to the injection side. We showed that inflammation causes a hyperpolarized shift in the I–V and g–V relationships of NMDA-activated currents (Fig. 2 and Fig. 3). The changes are PKC mediated because the shift is prevented by the PKC inhibitors, Chelery and BIS-I. We further showed that the apparent affinity of NMDA receptors for Mg2+ is reduced in these neurones (Fig. 4). The change in the affinity for Mg2+ can account for the shift in g–V curves of NMDA currents both in 30 μm and in 1 mm Mg2+ (Fig. 3 and Fig. 4). These results suggest that the elevated endogenous levels of PKC observed following inflammation alter the Mg2+ blockade characteristics of NMDA receptor channels, and thus modulate the activity of the channels.
The change in the Mg2+ blockade observed in this study is smaller than that observed in experiments where PKC is introduced directly into trigeminal dorsal horn cells (Chen & Huang, 1992). The difference could be because PKC in CFA neurones is not uniformly upregulated and/or because the level of PKC upregulation in CFA cells is lower than the level of exogenous PKC used in trigeminal experiments. In addition, PKC activity could be dissipated somewhat during cell preparation. Nevertheless, the reduced Mg2+ blockade is significant in the solution containing either 30 μm or 1 mm Mg2+ (Fig. 4).
The activation of PKC has been shown to enhance NMDA-evoked currents in most preparations (reviewed by MacDonald et al. 1998). However, the effects of PKC on the Mg2+ blockade of NMDA currents vary. In hippocampal neurones, PKC does not change the affinity of NMDA receptors for Mg2+, nor does it shift the voltage dependence of NMDA currents (Xiong et al. 1998). In oocytes, phorbol ester, a PKC activator, reduces the Mg2+ affinity for the cloned 1/ε1 and 1/ε2 receptors by approximately twofold (Wagner & Leonard, 1996). We have shown that in trigeminal dorsal horn neurones, PKC causes both Mg2+-dependent and Mg2+-independent potentiation of NMDA currents (Chen & Huang, 1992). The Mg2+-dependent potentiation, which involves a reduction in the affinity of NMDA receptors for Mg2+, is responsible for most of the PKC effects observed (Chen & Huang, 1992). In the spinal dorsal horn neurones studied here, PKC also resulted in Mg2+-dependent potentiation (Fig. 2). One obvious factor that can contribute to the dissimilar PKC effects among various studies is the differential NMDA receptor subunit expression in the central nervous system (Cull-Candy et al. 2001). Functional studies of NMDA receptors have established that different NMDA receptor subtypes exhibit varying channel kinetics, Mg2+ blockade characteristics and PKC sensitivity (Wagner & Leonard, 1996; Cull-Candy et al. 2001). Compared to rat NR1/NR2C and NR1/NR2D receptors, or mouse 1/ε3 and 1/ε4 receptors, NR1/NR2A and NR1/NR2B receptors or 1/ε1 and 1/ε2 receptors produce larger channel conductances and faster NMDA responses, and are more sensitive to the Mg2+ block and PKC potentiation (Monyer et al. 1994; Wagner & Leonard, 1996; Wyllie et al. 1996; Rumbaugh & Vicini, 1999; Misra et al. 2000). Furthermore, rats of various ages have been used in different studies. Developmental changes in NMDA receptor subunit expression (Nabekura et al. 1994; Portera-Cailliau et al. 1996; Urch et al. 2001) may also contribute to the variance in the Mg2+ blockade and PKC modulation.
The NMDA receptor subtypes expressed in our isolated dorsal horn neurones have not been determined. NR1 mRNA and protein are expressed widely in the spinal cord. Among the NR2 subunits, the NR2A mRNA and protein are the most abundant and found throughout different laminae. In contrast, NR2B mRNA is weakly or moderately expressed in laminae 2 and 9 (Luque et al. 1994). The NR2B protein is found in laminae I and II (Yung, 1998) and is mostly expressed in nerve fibres (Boyce et al. 1999). NR2C mRNA and protein are seldom detected (Tolle et al. 1993; Luque et al. 1994; Laurie et al. 1997), whereas NR2D mRNA and protein are expressed at low levels in both the ventral and dorsal horn (Luque et al. 1994; Laurie et al. 1997; Dunah et al. 1998). The NR2A subunit is the major NR2 subunit participating in synaptic transmission in the superficial dorsal horn; NR2B and NR2D subunits mediate extrasynaptic responses (Momiyama, 2000). NR2B antagonists have been found to selectively block nociceptive behaviour in rats with inflammation or nerve injuries (Boyce et al. 1999). It is of interest to establish the significance of different subunit expression in NMDA responses under pathological conditions.
It remains unclear how PKC changes the Mg2+ blockade of NMDA receptors. One possibility is that PKC phosphorylates NMDA receptors, resulting in changes in their properties (Liao et al. 2001). Direct phosphorylation of NMDA receptors, however, may not be the only mechanism by which PKC regulates the function of NMDA receptors (Zheng et al. 1997). PKC has been shown to potentiate NMDA responses indirectly by activation of the tyrosine kinase (Src) signalling cascade (Lu et al. 1999). Increasing evidence also suggests that PKC modulates the distribution and function of NMDA receptors by participating in the interactions between NMDA receptors and postsynaptic density (PSD) and cytoskeletal proteins (Sheng & Pak, 2000). For example, phorbol ester rapidly disrupts the discrete distribution of NR1 receptors (Ehlers et al. 1995; Tingley et al. 1997). Agents that stabilize (e.g. phalloidin) and disrupt (e.g. cytochalasin-D) the polymerization of actin reduce PKC potentiation of the activity of mouse 1/ε2 receptors (Wagner & Leonard, 1999). Increasing the expression of PSD-95 in oocytes has been found to progressively suppress the phorbol ester potentiation of 1/ε2 or NR1/NR2A receptor-mediated responses (Yamada et al. 1999; Liao et al. 2000). Thus, phosphorylation of cytoskeletal or anchoring proteins by the elevated PKC levels in inflamed tissue may contribute to the observed reduction in the Mg2+ blockade of NMDA currents. In support for this, in a preliminary study, Wyneken et al. (1999) found that phosphorylation of PSD proteins in a giant liposomal preparation reduced the Mg2+ blockade of NMDA receptors. Using a stretched membrane matrix of cultured cortical neurones to simulate brain tissue deformation and traumatic brain injury, Zhang et al. (1996) found that NMDA responses were greatly potentiated at hyperpolarized potentials. The apparent affinity for Mg2+ blockade at −80 mV was reduced by as much as 20-fold. Since the reduction of the Mg2+ blockade was partially restored by the PKC inhibitor calphostin C, PKC activated by the injury was at least in part responsible for the increase in NMDA receptor activity (Zhang et al. 1996). Inflammation and nerve injuries are often accompanied by swelling, blebbing and morphological changes in neuronal and non-neuronal cells. These changes have been associated with abnormal cytoskeletal structures (Tanner et al. 1998). Thus, the inflammation-induced disruption of interactions between NMDA receptors and anchoring or cytoskeletal molecules could exaggerate the modulatory actions of PKC and alter the voltage-dependent properties of NMDA receptors.
Since NMDA-receptor-mediated responses are slow and long-lasting (Forsythe & Westbrook, 1988), one consequence of the enhanced NMDA responses in neurones of CFA-treated rats is larger and longer excitatory postsynaptic potentials (EPSPs) at the dorsal horn. These amplified synaptic responses would facilitate the bursting activity of neurones, a phenomenon often observed under inflammatory and nerve-injured conditions (Haley et al. 1990; Leem et al. 1996; Pitcher & Henry, 2000). Another consequence of the enhanced NMDA currents is the increased Ca2+ entry as a result of high Ca2+ permeability of NMDA receptors (MacDermott et al. 1986; Mayer & Westbrook, 1987). This increased Ca2+ influx would further enhance the effect of PKC potentiation of NMDA responses (Zheng et al. 1997). Indeed, PKC activation by phorbol ester has been shown to profoundly affect NMDA-receptor-mediated synaptic transmission in the spinal dorsal horn. It has been shown that phorbol ester doubles the amplitude and prolongs the duration of NMDA-induced depolarization under physiological conditions ([Mg2+] = 1.3 mm). This results in prolonged slow EPSPs and enhanced spike discharges in neurones responding to repetitive stimulations of the dorsal roots (Gerber et al. 1989). Our results show that a spontaneous increase in PKC activity in inflamed neurones reduces the Mg2+ blockade of NMDA receptors and shifts the I–V relationship of NMDA receptors, thus enhancing NMDA responses at hyperpolarized potentials. This enhancement could lead to an increase in synaptic transmission in the dorsal horn and contribute to the development of pathological nociceptive responses.
Acknowledgments
We thank Drs Y. Gu and W. D. Willis for comments and S. Y. Wong for technical assistance. This work was support by grants from National Institutes of Health (NS30045 and NS11255) and the Human Frontier Science Program (RG0073) to L.-Y.M.H.
References
- Boyce S, Wyatt A, Webb JK, O'Donnell R, Mason G, Rigby M, Sirinathsinghji D, Hill RG, Rupniak NM. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology. 1999;38:611–623. doi: 10.1016/s0028-3908(98)00218-4. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Coderre TJ. Contribution of protein kinase C to central sensitization and persistent pain following tissue injury. Neuroscience Letters. 1992;140:181–184. doi: 10.1016/0304-3940(92)90097-q. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. Journal of Neuroscience. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Current Opinion in Neurobiology. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Chapman V, Green GM. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. General Pharmacology. 1997;28:633–638. doi: 10.1016/s0306-3623(96)00359-x. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Palecek J, Paleckova V, Sorkin LS, Willis WD. The role of NMDA and non-NMDA excitatory amino acid receptors in the excitation of primate spinothalamic tract neurons by mechanical, chemical, thermal, and electrical stimuli. Journal of Neuroscience. 1992;12:3025–3041. doi: 10.1523/JNEUROSCI.12-08-03025.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends in Neurosciences. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Luo J, Wang YH, Yasuda RP, Wolfe BB. Subunit composition of N-methyl-d-aspartate receptors in the central nervous system that contain the NR2D subunit. Molecular Pharmacology. 1998;53:429–437. doi: 10.1124/mol.53.3.429. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Tingley WG, Huganir RL. Regulated subcellular distribution of the NR1 subunit of the NMDA receptor. Science. 1995;269:1734–1737. doi: 10.1126/science.7569904. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Westbrook GL. Slow excitatory postsynaptic currents mediated by N-methyl-d-aspartate receptors on cultured mouse central neurones. Journal of Physiology. 1988;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber G, Kangrga I, Ryu PD, Larew JS, Randic M. Multiple effects of phorbol esters in the rat spinal dorsal horn. Journal of Neuroscience. 1989;9:3606–3617. doi: 10.1523/JNEUROSCI.09-10-03606.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Huang LY. Cross-modulation of glycine-activated Cl− channels by protein kinase C and cAMP-dependent protein kinase in the rat. Journal of Physiology. 1998;506:331–339. doi: 10.1111/j.1469-7793.1998.331bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Haber B, Huang L-Y M. Peripheral inflammation modulates NMDA receptor-mediated responses in rat spinal dorsal horn neurons. Society for Neuroscience Abstracts. 1999;25:920. [Google Scholar]
- Haley JE, Sullivan AF, Dickenson AH. Evidence for spinal N-methyl-d-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Research. 1990;518:218–226. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Bartke I, Schoepfer R, Naujoks K, Seeburg PH. Regional, developmental and interspecies expression of the four NMDAR2 subunits, examined using monoclonal antibodies. Brain Research. Molecular Brain Research. 1997;51:23–32. doi: 10.1016/s0169-328x(97)00206-4. [DOI] [PubMed] [Google Scholar]
- Leem JW, Choi EJ, Park ES, Paik KS. N-methyl-d-aspartate (NMDA) and non-NMDA glutamate receptor antagonists differentially suppress dorsal horn neuron responses to mechanical stimuli in rats with peripheral nerve injury. Neuroscience Letters. 1996;211:37–40. doi: 10.1016/0304-3940(96)12714-2. [DOI] [PubMed] [Google Scholar]
- Liao GY, Kreitzer MA, Sweetman BJ, Leonard JP. The postsynaptic density protein PSD-95 differentially regulates insulin- and Src-mediated current modulation of mouse NMDA receptors expressed in Xenopus oocytes. Journal of Neurochemistry. 2000;75:282–287. doi: 10.1046/j.1471-4159.2000.0750282.x. [DOI] [PubMed] [Google Scholar]
- Liao GY, Wagner DA, Hsu MH, Leonard JP. Evidence for direct protein kinase-C mediated modulation of N-methyl-D- aspartate receptor current. Molecular Pharmacology. 2001;59:960–964. doi: 10.1124/mol.59.5.960. [DOI] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nature Neuroscience. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- Luque JM, Bleuel Z, Malherbe P, Richards JG. Alternatively spliced isoforms of the N-methyl-D-aspartate receptor subunit 1 are differentially distributed within the rat spinal cord. Neuroscience. 1994;63:629–635. doi: 10.1016/0306-4522(94)90510-x. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321:519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Xiong XG, Lu WY, Raouf R, Orser BA. Modulation of NMDA receptors. Progress in Brain Research. 1998;116:191–208. doi: 10.1016/s0079-6123(08)60438-0. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Liu H, Wang H, Malmberg AB, Basbaum AI. Inflammation-induced up-regulation of protein kinase Cγ immunoreactivity in rat spinal cord correlates with enhanced nociceptive processing. Neuroscience. 1999;88:1267–1274. doi: 10.1016/s0306-4522(98)00314-5. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proceedings of the National Academy of Sciences of the USA. 1999;96:7731–7736. doi: 10.1073/pnas.96.14.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-d-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. Journal of Physiology. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra C, Brickley SG, Farrant M, Cull-Candy SG. Identification of subunits contributing to synaptic and extrasynaptic NMDA receptors in Golgi cells of the rat cerebellum. Journal of Physiology. 2000;524:147–162. doi: 10.1111/j.1469-7793.2000.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama A. Distinct synaptic and extrasynaptic NMDA receptors identified in dorsal horn neurones of the adult rat spinal cord. Journal of Physiology. 2000;523:621–628. doi: 10.1111/j.1469-7793.2000.t01-1-00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nabekura J, Kawamoto I, Akaike N. Developmental change in voltage dependency of NMDA receptor-mediated response in nucleus tractus solitarii neurons. Brain Research. 1994;648:152–156. doi: 10.1016/0006-8993(94)91915-1. [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Henry JL. Cellular mechanisms of hyperalgesia and spontaneous pain in a spinalized rat model of peripheral neuropathy: changes in myelinated afferent inputs implicated. European Journal of Neuroscience. 2000;12:2006–2020. doi: 10.1046/j.1460-9568.2000.00087.x. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C, Price DL, Martin LJ. N-methyl-d-aspartate receptor proteins NR2A and NR2B are differentially distributed in the developing rat central nervous system as revealed by subunit-specific antibodies. Journal of Neurochemistry. 1996;66:692–700. doi: 10.1046/j.1471-4159.1996.66020692.x. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Vicini S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. Journal of Neuroscience. 1999;19:10603–10610. doi: 10.1523/JNEUROSCI.19-24-10603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Pak DT. Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Annual Review of Physiology. 2000;62:755–778. doi: 10.1146/annurev.physiol.62.1.755. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Willis WD. The effects of G-protein and protein kinase inhibitors on the behavioral responses of rats to intradermal injection of capsaicin. Pain. 1997;71:165–178. doi: 10.1016/s0304-3959(97)03371-x. [DOI] [PubMed] [Google Scholar]
- Tanner KD, Levine JD, Topp KS. Microtube disorientation and axonal swelling in unmyelinated sensory axons during vincristine-induced painful neuropathy in rat. Journal of Comparative Neurology. 1998;395:481–492. [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-d-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. Journal of Biological Chemistry. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Tolle TR, Berthele A, Zieglgansberger W, Seeburg PH, Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. Journal of Neuroscience. 1993;13:5009–5028. doi: 10.1523/JNEUROSCI.13-12-05009.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urch CE, Rahman W, Dickenson AH. Electrophysiological studies on the role of the NMDA receptor in nociception in the developing rat spinal cord. Brain Research. Developmental Brain Research. 2001;126:81–89. doi: 10.1016/s0165-3806(00)00141-3. [DOI] [PubMed] [Google Scholar]
- Wagner DA, Leonard JP. Effect of protein kinase-C activation on the Mg2+-sensitivity of cloned NMDA receptors. Neuropharmacology. 1996;35:29–36. doi: 10.1016/0028-3908(95)00177-8. [DOI] [PubMed] [Google Scholar]
- Wagner DA, Leonard JP. Protein kinase C potentiation of currents from mouse ξ1/ε2 NMDA receptors expressed in Xenopus oocytes depends on f-actin/g-actin cycling. Neuroscience Letters. 1999;272:187–190. doi: 10.1016/s0304-3940(99)00524-8. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proceedings of the National Academy of Sciences of the USA. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-d-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Wyllie DJ, Behe P, Nassar M, Schoepfer R, Colquhoun D. Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed in Xenopus oocytes. Proceedings of the Royal Society B. 1996;263:1079–1086. doi: 10.1098/rspb.1996.0159. [DOI] [PubMed] [Google Scholar]
- Wyneken U, Marengo JJ, Cheyre JE, Cardemil C, Orrego F. NMDA receptor channel regulation by endogenous modulator present in isolated PSDs: a patch clamp study. Society for Neuroscience Abstracts. 1999;26:734. [Google Scholar]
- Xiong ZG, Raouf R, Lu WY, Wang LY, Orser BA, Dudek EM, Browning MD, MacDonald JF. Regulation of N-methyl-d-aspartate receptor function by constitutively active protein kinase C. Molecular Pharmacology. 1998;54:1055–1063. [PubMed] [Google Scholar]
- Yamada Y, Chochi Y, Takamiya K, Sobue K, Inui M. Modulation of the channel activity of the ε2/ξ1-subtype N- methyl-d-aspartate receptor by PSD-95. Journal of Biological Chemistry. 1999;274:6647–6652. doi: 10.1074/jbc.274.10.6647. [DOI] [PubMed] [Google Scholar]
- Yung KK. Localization of glutamate receptors in dorsal horn of rat spinal cord. NeuroReport. 1998;9:1639–1644. doi: 10.1097/00001756-199805110-00069. [DOI] [PubMed] [Google Scholar]
- Zhang L, Rzigalinski BA, Ellis EF, Satin LS. Reduction of voltage-dependent Mg2+ blockade of NMDA current in mechanically injured neurons. Science. 1996;274:1921–1923. doi: 10.1126/science.274.5294.1921. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhang L, Wang AP, Bennett MV, Zukin RS. Ca2+ influx amplifies protein kinase C potentiation of recombinant NMDA receptors. Journal of Neuroscience. 1997;17:8676–8686. doi: 10.1523/JNEUROSCI.17-22-08676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


