Abstract
The respiratory centre within the brainstem is one of the most active neuronal networks that generates ongoing rhythmic activity. Stabilization of such vital activity requires efficient processes for activity-correlated adjustment of neuronal excitability. Recent investigations have shown that a regulatory factor coupling electrical activity with cell metabolism comprises ATP-dependent K+ channels (KATP channels), which continuously adjust the excitability of respiratory neurons during normoxia and increasingly during hypoxia.
We used the single-cell antisense RNA amplification-polymerase chain reaction (PCR) technique to demonstrate that respiratory neurons co-express the sulphonylurea receptor SUR1 with the Kir6.2 potassium channel protein.
Single channel measurements on rhythmically active inspiratory neurons of the brainstem slice preparation of newborn mice revealed that KATP channels are periodically activated in synchrony with each respiratory cycle.
The Na+-K+-ATPase was inhibited with ouabain to demonstrate that oscillations of the channel open probability disappear, although respiratory activity persists for a longer time. Such findings indicate that KATP channel open probability reflects activity-dependent fluctuations in the ATP concentration within submembrane domains.
We also examined the effects of extracellular [K+] and hypoxia. All changes in the respiratory rhythm (i.e. changes in cycle length and burst durations) affected the periodic fluctuations of KATP channel activity.
The data indicate that KATP channels continuously modulate central respiratory neurons and contribute to periodic adjustment of neuronal excitability. Such dynamic adjustment of channel activity operates over a high range of metabolic demands, starting below physiological conditions and extending into pathological situations of energy depletion.
KATP channels are hetero-octamers consisting of two different subunits, i.e. four inwardly rectifying K+ channel subunits, either Kir6.1 or Kir6.2, and four sulphonylurea receptors (SUR), which belong to the family of ATP-binding cassette (ABC) transporters (Aguilar-Bryan & Bryan, 1999; Baukrowitz & Fakler, 2000). They are characterized by strong inhibition by intracellular ATP and activation by MgADP. Such ATP/ADP dependence of KATP channels enables a metabolic control of neuronal excitability. Besides ATP/ADP ratios, KATP channel activity is also modulated by various intra- and extracellular factors such as low pH, adenosine, various G protein-coupled processes and the cytoskeleton (Cason et al. 1995; Hilgemann, 1997; Hiraoka, 1997; Aguilar-Bryan & Bryan, 1999; Baukrowitz & Fakler, 2000).
KATP channels are incorporated in several tissues including smooth muscle, cardiac myocytes, skeletal muscle and pancreatic β-cells. In the brain, KATP channels were reported in neurons of the hippocampus (Zawar et al. 1999), cortex (Jiang & Haddad, 1997) and the brainstem (Pierrefiche et al. 1996; Karschin et al. 1998). The functional significance of KATP channels is best described for the pancreas and the heart. In the pancreas, KATP channels act as a major regulator of insulin secretion, hyperglycaemia leading to an increase in ATP, which closes KATP channels, thereby causing membrane depolarization, Ca2+ influx and stimulation of insulin secretion (Miki et al. 1998). In cardiac cells, a fall of ATP levels during metabolic impairment activates KATP channels and thus modulates cardiac contractions (Terzic et al. 1995). Both pancreatic β-cells (Larsson et al. 1996) and cardiomyocytes (O'Rourke et al. 1994) revealed oscillatory variations of KATP conductances (gKATP), which were attributed to oscillations in the glycolytic pathway (Prentki et al. 1997). In the pancreas, periodic activation of gKATP was reported to cause oscillations of electrical activity and [Ca2+]i fluctuations during glucose stimulation (Larsson et al. 1996; Kinard et al. 1999). A similar rhythmic behaviour was observed in cardiomyocytes (O'Rourke et al. 1994), where metabolic stress initiates cyclic activation of KATP channels and suppression of depolarization-evoked [Ca2+]i transients.
Studies on the functional significance of gKATP in the brain have focused mostly on its contribution to hypoxic responses of neurons. Here we describe for the first time oscillatory activation of neuronal KATP channels in synchrony with normal rhythmic neuronal activity. Our test model is the respiratory network, which generates a very robust rhythm of alternating burst activities. The rhythm originates from a morphologically well-defined region located in the lower brainstem - the pre-Bötzinger complex (Smith et al. 1991). This respiratory centre can be in vitro isolated in a rhythmic slice preparation to directly investigate respiratory neurons for periodic modulation of excitability through KATP channels. Respiratory neurons are adequate models, because they receive periodic inhibitory and excitatory inputs that induce spontaneous, 1–2 s long-lasting bursts of action potentials every 3–4 s. Such intense burst discharges induce significant changes in transcellular ion concentrations, which demand high metabolic efforts for restoration. KATP channels are expected to be involved in this regulation.
Previously, we have analysed single KATP channels in respiratory neurons, determined a conductance of 75 pS and verified effective blockade by increased [ATP]i, glibenclamide or tolbutamide and modulation by adenosine, diazoxide and hypoxia (Mironov et al. 1998). Blockade of KATP channels was observed to induce membrane depolarization and increase of neuronal excitability (Pierrefiche et al. 1996).
In this study, we analysed the molecular structure of the channel using the single-cell antisense RNA amplification-polymerase chain reaction (PCR) technique and determined the fluctuations of KATP channel activity during ongoing respiratory activity. We also tested for the effects of the Na+-K+ pump blocker ouabain, changes in the extracellular potassium concentration ([K+]o) and hypoxic stimuli. Such measurements indicate a Na+-K+ pump-correlated mechanism for such oscillations.
METHODS
Slice preparation
Experiments were performed on a medullary slice preparation from neonatal NMRI mice (P5-P8) containing the kernel of the respiratory centre, which is spontaneously active (Smith et al. 1991). The technique of tissue isolation followed the approach developed for mice (Mironov et al. 1998). All animals were housed, cared for and killed in accordance with the recommendations of the European Commission (no. L358, ISSN 0378–6978), and protocols were approved by the Committee for Animal Research, Göttingen University.
The brainstem-spinal cord was isolated in ice-cold artificial cerebrospinal fluid (ACSF) and a single 700 μm thick transverse slice containing the pre-Bötzinger complex was cut from the brainstem, transferred to the recording chamber and mounted on the stage of an upright microscope (Axioscope, Zeiss, Oberkochen, Germany). The slice was fully submerged in continuously flowing ACSF (28 °C, 40–50 ml min−1) that was gassed with carbogen (95 % O2 and 5 % CO2). The perfusing solution was pumped to the experimental chamber through stainless-steel tubing to prevent leakage of dissolved gases. The ACSF solution was composed of (mm): 128 NaCl, 3 KCl, 1.5 CaCl2, 1.0 MgSO4, 21 NaHCO3, 0.5 NaH2PO4 and 30 d-glucose. Solutions of various K+ concentrations were obtained by replacing NaCl with KCl. The respiratory rhythm was recorded with suction electrodes from a rootlet of the hypoglossal nerve.
All drugs were applied directly to the bath and arrived at the experimental chamber after a delay of 8–12 s. Recovery was obtained by perfusing 400–500 ml fresh ACSF solution. Distinct activity levels of the respiratory network were attained by varying [K+]o levels between 3 and 12 mm. In order to induce hypoxic conditions, bath gases were changed from carbogen to 95 % N2 and 5 % CO2. Tissue oxygen pressure (PO2) was measured occasionally with oxygen-sensitive electrodes (Diamond Electro-Tech Inc., Ann Arbor, MI, USA) as described previously (Ballanyi et al. 1996; Mironov et al. 1998). A PO2 electrode was placed 100 ± 25 μm below the slice surface into the region where respiratory neurons were located. Fifteen to twenty seconds after O2 was replaced by N2 in the bath solution, extracellular tissue PO2 fell from 232 ± 39 to 6 ± 4 mmHg and remained constant (n = 15, P < 0.05) thereafter.
Electrophysiological recordings
Patch electrodes were manufactured from borosilicate glass (Clarke Electromedical, Pangbourne, UK), had tip openings of 1–2 μm and a DC resistance of 2–4 MΩ. Signals were fed to an EPC-7 patch-clamp amplifier (ESF, Friedland, Germany). Cells were either patched under visual control or ‘blindly’. Electrodes with an imposed internal hyperbaric pressure of 10–30 mmHg were inserted into tissue with a micromanipulator (Eppendorf-Netheler-Hinz GmbH, Hamburg, Germany) and slowly advanced through the tissue until an increase in pipette series resistance indicated contact with a cell. Following the formation of a gigaseal, channel activity and spike discharges were recorded in the cell-attached mode (usually with an applied potential of +40 mV that corresponds to a membrane potential of −100 mV, assuming a resting potential of −60 mV). KATP channels were identified by their conductance and gating characteristics as detailed previously (Mironov et al. 1998). Channel identity was verified by application of specific KATP channel blockers (glibenclamide, tolbutamide) or activators (diazoxide).
Hypoglossal (XII) rootlets were recorded with a blunt suction electrode to monitor the central respiratory rhythm. XII activity was amplified 5000–10 000 times, bandpass filtered (0.25–1.5 kHz), rectified and integrated (Paynter filter with a time constant of 50–100 ms).
All recordings were stored on a MacLab acquisition system (WissTech GmbH, Spechbach, Germany) for off-line analysis.
Data analysis
Defined periods of cell-attached channel recordings of 3 s duration containing inspiratory bursts were exported for analysis to Igor Pro (WaveMetrics, Inc., Lake Oswego, USA). Cell-attached recordings from inspiratory neurons displayed capacitive responses to action potential (AP) discharges (Lynch & Barry, 1989; Rajendra et al. 1992). The number of these APs ranged between 3 and 15 per inspiratory burst. In order to avoid interference with the recordings of channel activity, AP discharges were removed. Each AP was replaced by the mean level of channel activity averaged over 5 ms before and after the AP. KATP channel openings occurring simultaneously with AP discharge were thus deleted and the open probability was probably slightly underestimated during the periods of respiratory bursts. However, it is important to note that such a subtraction protocol did not significantly change or induce oscillations of channel open probability (see Discussion). Following the removal of APs the channel activity was isolated by setting the background noise ( ± 5 pA) to baseline levels.
The open probability (Po) of KATP channels was determined by dividing the mean of channel currents collected during up to 200 traces, by unitary current amplitudes and the number of identifiable channels. Finally the traces were smoothed with a ‘box-smoothing algorithm’.
Statistica software (StatSoft GmbH, Tulsa, USA) was used to assess the statistical significance (P < 0.05) of fluctuation parameters based on the Mann-Whitney U test.
Molecular biology
In order to harvest cytosol for RT-PCR analysis, cells were patched and identified as inspiratory neurons in the cell-attached configuration. After the whole-cell configuration was established, constant suction was applied for 1–2 min to aspirate the cytoplasm, while the access resistance was monitored to verify persistence of gigasealing. Amplification of the antisense RNA (aRNA) from single respiratory neurons was performed according to the procedure described in detail by Eberwine et al. (1992). In brief, 2.5 mm dideoxynucleotides (dNTPs), 2 ng μl−1 T7-oligo-d(T)24 primer and 0.5 U μl−1 avian myeloblastosis virus (AMV) reverse transcriptase (Roche Diagnostics, Mannheim, Germany) were mixed with the cytosol and incubated for 60–90 min at 37 °C for reverse transcription. Second-strand synthesis was performed with T4 DNA polymerase and Klenow fragment (1 U μl−1 each) followed by treatment with S1 nuclease (1 U μl−1) and subsequently Klenow enzyme (1 U μl−1) to remove hairpin loops and to produce blunt ends of the cDNA. After aRNA amplification with 100 U μl−1 T7 RNA polymerase, a second amplification, including the final synthesis of double-stranded cDNA, was conducted to yield adequate amounts of template for the expression profiling of different mRNAs from a single respiratory neuron. All amplification steps were conducted under RNase-free conditions. In the mouse both SUR1 and SUR2 genes contain multiple introns, but the Kir6 isoforms are intronless. To exclude contaminations by genomic DNA, control experiments were performed without AMV reverse transcriptase, or amplified aRNA was incubated after each amplification with RNase-free DNase I at 37 °C for 1 h. Polymerase chain reaction analysis was performed using as template (i) 1/50 of the total volume of the final aRNA amplification product, and (ii) alternatively, mRNA from single neurons that had been reverse transcribed for 60 min using 2.5 mm dNTPs, 25 μm random hexamer primers, 10 mm dithiothreitol, 2 U μl−1 RNase inhibitor and 10 U μl−1 M-MuLV reverse transcriptase (Roche Diagnistics). Kir6 and SUR core fragments were amplified with primers based on the cDNA sequences of Kir6.1 (Yamada et al. 1997), Kir6.2 (Sakura et al. 1995), SUR1 (Aguilar-Bryan et al. 1995) and SUR2 (Isomoto et al. 1996). Sense and antisense primers were chosen to specifically amplify fragments of 539–865 bp in length: mKir6.1 (Genbank accession number D88159, 865 bp), sense primer 5′-GAAGATGCTGGCCAGGAAGAG-3′, antisense primer 5′-CAGCCACTGACCTTGTCAACC-3′; mKir6.2 (MMU73626; 553 bp), sense primer 5′-GGAGAGGAGGGCCCGCTTCGTGTC-3′, antisense primer 5′-GGCGCTAATGATCATGCTTTTTCGGAGGTC-3′; rSUR1 (L40624, 539 bp), sense primer 5′-GCAGCCGAGAGCGAGGAAGATGA-3′, antisense primer 5′-ACAGCCAGGGCGGAGACACAGAGTA-3′; mSUR2 (D86037, 603 bp), sense primer 5′-CGCGGCGGTCATCGTGCTC-3′, antisense primer 5′-CGCCGCGCCTGCTCGTAGTT-3′ (conserved for SUR2A and SUR2B).
PCR amplifications were run with Taq polymerase (Qiagen, Hilden, Germany) for 35 cycles at 95 °C denaturing, 52–58 °C annealing, and 72 °C extension for 1.5 min each, with a final extension of 15 min at 72 °C. Amplified fragments were purified from agarose gels, digested at terminal restriction sites or blunt ends produced, and ligated into pBluescriptSKII vector (Stratagene, La Jolla, CA, USA). Double-stranded sequencing of the PCR products was performed on both strands using the Prism Sequenase dye terminator kit on an automatic sequencer (Perkin Elmer, Weiterstadt, Germany).
All Kir6 and SUR primers were tested for functionality and sensitivity with 0.05–0.1 ng of original cloned cDNA as template (and 50 ng of mouse tissue DNA). Amplified fragments of the neurofilament middle protein (NF-M) confirmed that the cytosol had been aspirated from neurons and not from glial cells. Moreover, adequate H2O controls performed for all primer combinations were negative.
RESULTS
Molecular biology of the KATP channel
For identification of the molecular composition of KATP channels, the harvested cytosol was processed either for aRNA/cDNA amplification (n = 2) or directly reverse-transcribed for subsequent PCR analysis (n = 4). Figure 1 shows the Kir6/SUR expression profiling after aRNA processing for two inspiratory neurons. The primer pair that was designed to amplify a Kir6.1 fragment revealed a strong 865 bp band from the vector template, but not from the cDNAs of the single respiratory neurons (using 1/50 of the total aRNA amplification product). Likewise, the primer combination that successfully amplified a 603 bp SUR2 fragment from atrial cDNA (50 ng) failed to produce detectable amplification products from the single cell source. In contrast, clearly detectable fragments were observed using Kir6.2 (552 bp) and SUR1 (539 bp) primers with cDNA from the two nerve cells. The same expression pattern, i.e. strong expression of Kir6.2/SUR1 and absence of Kir6.1/SUR2 signals, was detected in all four inspiratory neurons directly processed for RT-PCR after recording. When subcloned, grown to a large scale and sequenced on both strands, the amplified fragments were always identical to the published cDNA sequences. Thus PCR analysis of unprocessed and T7 RNApolymeraseamplified RNA with specific primers revealed that SUR1 and Kir6.2 isoforms translate to form the major subtype of KATP channel in inspiratory neurons.
Figure 1. PCR analysis of amplified aRNA from single respiratory brainstem neurons.
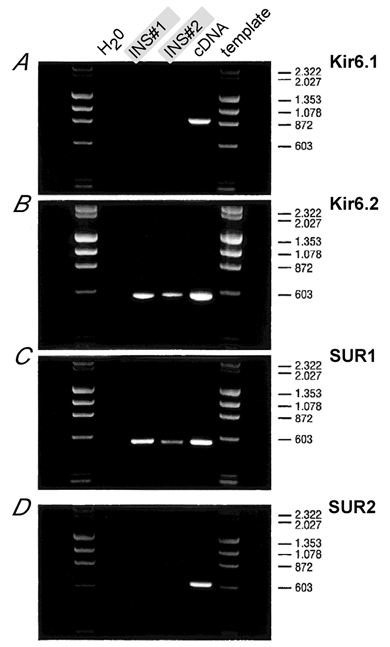
DNA fragments are amplified with primer pairs specific to Kir6.1 (A), Kir6.2 (B), SUR1 (C) and SUR2 (D). The first lanes next to the molecular weight marker (λHindIII-ΦXHaeIII digest) are H2O controls, templates in lanes 2 and 3 were of two different inspiratory neurons (INS#1, INS#2), and lane 4 used 0.1 ng of plasmid cDNA as positive control (except in D where 50 ng of atrial cDNA was used). Primers were sensitivity-tested to amplify fragments of 865 bp (Kir6.1), 553 bp (Kir6.2), 539 bp (SUR1) and 603 bp (SUR2). Fragment sizes of the molecular marker are indicated on the right. Note that fragments amplified from single respiratory neurons can only be detected for Kir6.2 and SUR1.
Functional modulation of ongoing KATP channel activity
Normoxic activities of respiratory neurons are effectively modulated by KATP channels (Pierrefiche et al. 1996). This was verified by the changes of ongoing respiratory activity after application of the KATP channel blockers glibenclamide (30–50 μm, n = 4) and tolbutamide (300–500 μm, n = 6). As shown in Fig. 2A such blockade of KATP channels resulted in an increase of the amplitude of integrated hypoglossal (XII) bursts of 9.6 ± 7.1 % (mean ± s.d., n = 7). The frequency of bursts was not affected. The changes of hypoglossal bursts corresponded with an increase in the amplitude of synaptic drives by 4.9 ± 2.7 % (mean ± s.d., n = 4) in individual respiratory neurons (Fig. 2B).
Figure 2. Respiratory rhythm and synaptic drive amplitude after glibenclamide application.
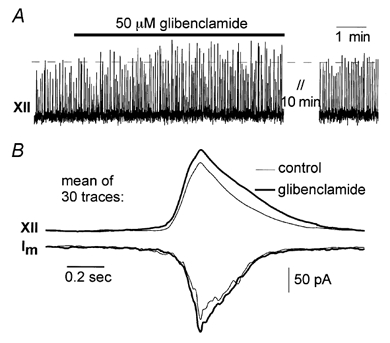
A, the amplitude of the integrated hypoglossal nerve (XII) activity increases after glibenclamide application. B, modulation of synaptic drives (membrane currents, Im, recorded in whole-cell mode at holding potential −60 mV) could only be discerned when 30 traces were averaged and smoothed. Synaptic drive amplitude increased with glibenclamide in parallel with hypoglossal activity.
Because of ongoing rhythmic bursting of respiratory neurons, we analysed whether channel activity also varies periodically. Figure 3A shows recordings from a neuron that was identified as inspiratory because of its discharge in synchrony with inspiratory hypoglossal (XII) activity. A time window of 3 s covering the duration of the inspiratory burst is depicted in Fig. 3B. Hypoglossal burst activity and the underlying grey area indicate the timing of inspiration. The trace below (grey) shows a sample of cell-attached recordings (Im). In order to selectively isolate channel activities, we eliminated action potentials and background noise (see Methods), as indicated by a superimposed black trace. A whole-cell recording - obtained after ‘breaking into’ the cell - indicates the timing of the synaptic drive.
Figure 3. Modulation of KATP channel activity during a respiratory cycle.
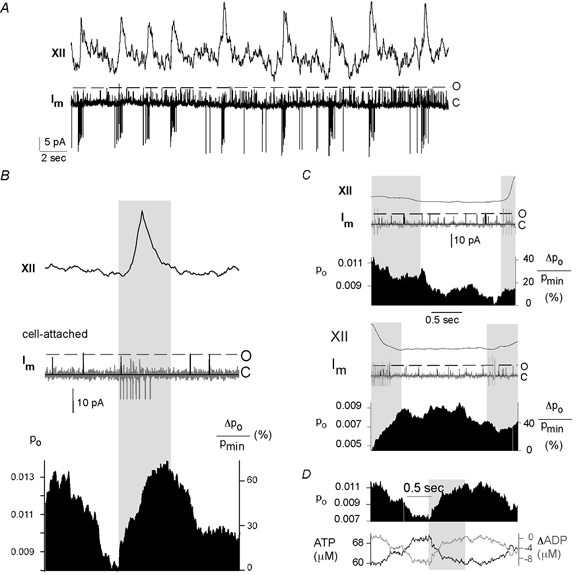
A, neurons were first identified as inspiratory by recording the bursting activity in the cell-attached mode with a pipette potential of +40 mV and correlating the membrane current, Im, with the integrated hypoglossal rootlet activity (XII). B, a time window selected around a respiratory burst, which is marked by the grey area. The integrated hypoglossal activity (XII) as averaged over 200 traces is depicted on top. The traces below show samples of cell-attached recordings exhibiting single-channel openings as well as whole-cell recordings (voltage clamp) from the same cell. Action potential discharges and background noise were removed from the cell-attached measurements as described in the text, yielding the superimposed black trace. Displayed below is the open probability (Po), obtained by averaging 200 such traces and smoothing with a box procedure. Also depicted is the relative change in Po given by ΔPo/Pmin× 100 (%), where Pmin is the minimum value of Po. Alternatively, time windows were selected to contain the time period before (C, top panel) and after (C, bottom panel) the respiratory burst. D, [ATP]i values (lower panel) were estimated from Po ([ATP]i = Kd(1/Po1/4 - 1)) with Kd = 29 μm (Ashcroft & Gribble, 1998) for a sample Po trace. Changes in ADP concentration were derived from [ATP]i, assuming that all ATP is converted to ADP.
An accurate estimate of channel open probability (Po) was then obtained by averaging 200 cell-attached traces and treating this average with a box-smoothing algorithm. Direct comparison of Po fluctuations is complicated by the large variations between the open probabilities of individual cells. Thus in addition to the open probability (Po), Po changes were computed as ΔPo/Pmin× 100 (%) (Fig. 3B, lowest trace). The pattern of Po fluctuations was consistent for all inspiratory neurons analysed (n = 13 cells). Such analyses revealed that Po rises throughout inspiration to attain maximal levels at the end or shortly after the inspiratory burst and then declines to minimal levels before the onset of the next inspiratory phase.
A similar procedure of data acquisition performed for separate time windows set before (Fig. 3C, upper panel) and after (Fig. 3C, lower panel) inspiratory bursts resulted in similar fluctuations. Due to the spontaneous variations of cycle lengths, successive bursts sometimes fell into the same time frame as indicated by the second grey area.
Quantitative description of Po modulations
The rise and fall of Po did not follow single exponential functions, and therefore were defined by the parameters cycle time (Ttot), rise time 10–90 % (t10–90 %), decay time 90–10 % (t90–10 %), and the delay between onset of XII peaks and the 10 % point of Po peaks (tdelay) (Fig. 4A). In addition, maximal Po fluctuations (ΔPpeak/Pmin), were computed as peak-to-peak amplitudes divided by the minimum value of Po.
Figure 4. Parameters of Po fluctuations.
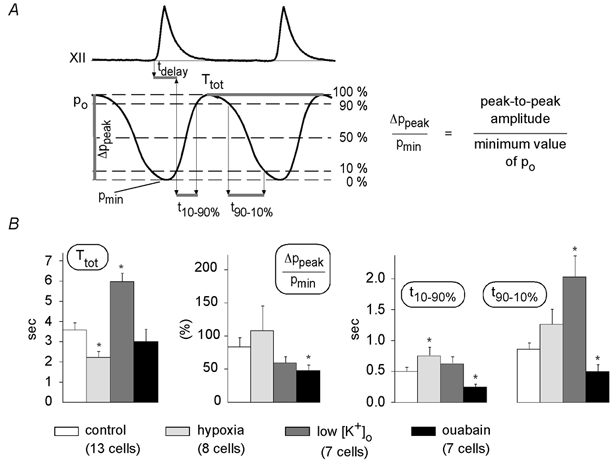
A, for a quantitative description of the changes in Po fluctuations, the following parameters were analysed: tdelay, the delay between onset of the rise of hypoglossal nerve activity and the 10 % point of Po; Ttot, the total duration of respiratory cycle; t10–90 %, the rise time from 10 to 90 % of peak amplitude; t90–10 %, the decay time from 90 to 10 % of peak amplitude; and ΔPpeak/Pmin, the maximum amplitude of Po fluctuations, which is always given as the percentage increase in Po. B, parameter ( ± s.e.m.) values were (at control conditions, hypoxia, low [K+]o and after ouabain application, respectively): Ttot 3.61 ± 0.33, 2.24 ± 0.28, 6.00 ± 0.40, 3.04 ± 0.59; t10–90 % 0.51 ± 0.06, 0.76 ± 0.14, 0.63 ± 0.11, 0.26 ± 0.04; t90–10 % 0.87 ± 0.11, 1.27 ± 0.26, 2.04 ± 0.34, 0.51 ± 0.11; ΔPo/Pmin 85 ± 14 %, 109 ± 37 %, 61 ± 9 %, 49 ± 8 %. A Mann-Whitney U test was performed to test for significant differences between control conditions and hypoxia, low [K+]o or ouabain. * Significant changes (P < 0.05). Interpretations of parameter values are discussed in the text.
Mean values ( ± s.e.m.) of these parameters obtained under control conditions, hypoxia, low [K+]o and ouabain application are depicted in Fig. 4B. Statistically significant effects (Mann-Whitney U test, P < 0.05) were observed for Ttot (hypoxia, low [K+]o), t10–90 % (hypoxia, ouabain), t90–10 % (low [K+]o, ouabain) and ΔPpeak/Pmin (ouabain). An average tdelay of 0.08 ± 0.12 s revealed that Po fluctuations display relatively fast kinetics, allowing quick reactivity to each respiratory burst. This delay time did not change significantly with varying PO2, [K+]o or ouabain application.
In order to verify activity-correlated fluctuation of Po, we also analysed spontaneously active KATP channels in neurons that were either tonically active or silent (Fig. 5B and C, respectively). Such comparison showed that only inspiratory neurons exhibited distinct fluctuations with a mean ΔPpeak/Pmin value of 85 ± 14 % (see Fig. 4B). In the other neurons, Po fluctuations were absent or exhibited incomparably small amplitudes (< 10 %).
Figure 5. Control experiments.
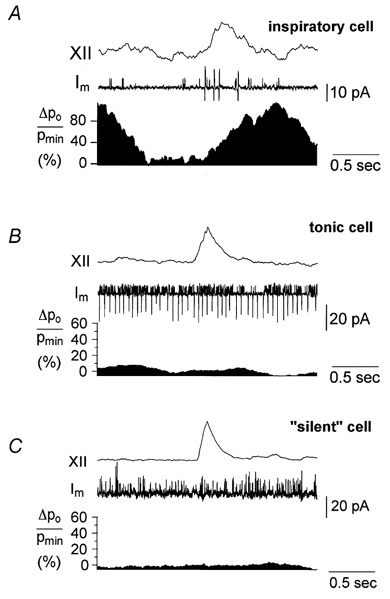
For controls, cell-attached recordings from tonic cells (2 cells) and ‘silent’ cells without action potential discharge (2 cells) were used (B and C, respectively). The analysis was performed as described for Fig. 3. A direct comparison of Po fluctuations is difficult because of large differences in open probabilities. Thus Po was not compared directly, but rather in terms of the relative change of Po, ΔPo/Pmin, expressed in %. It can be seen that neither the cell in B nor that in C displayed the same pattern of channel modulation as a control inspiratory neuron (A). Also, amplitudes of fluctuations of Po were much lower than those observed in A.
[K+]o-induced variation of cycle length
The respiratory rhythm can be changed by activating the respiratory network through elevation of [K+]o (Smith et al. 1991). Figure 6A shows traces of hypoglossal recordings, Po and ΔPo/Pmin fluctuations for elevated [K+]o of 8 or 11 mm. Under control conditions the respiratory rhythm was stabilized at high [K+]o values of 9–11 mm. When Po fluctuations during controls (13 cells) were compared with traces taken at lower [K+]o (7–9 mm, 7 cells), the most sensitive parameter was t90–10 %, which rose from 0.87 ± 0.11 to 2.04 ± 0.34 s, but Ttot also increased significantly (Fig. 4B), although the number of APs per burst did not change significantly when [K+]o was varied. The maximal fluctuation, ΔPpeak/Pmin, decreased from 85 ± 14 % (control) to 61 ± 9 % ([K+]o = 7–9 mm). As the respiratory network was activated by increased levels of [K+]o, the cycle length shortened and the averaged Po value increased. A plot of mean Po values against cycle length is shown in Fig. 6B. Each point represents the mean ( ± s.e.m.) of ten cycles of similar duration. Linear regression revealed on average a slope of −0.0012 ± 0.0011 s−1 for ten cells with a negative correlation coefficient of r = −0.83 (P < 0.01).
Figure 6. Changes in cycle lengths as induced by [K+]o variation.
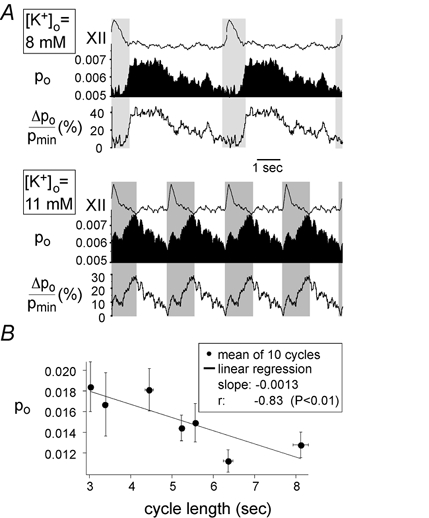
A, fluctuations of Po and ΔPo/Pmin for 8 mm (upper panel) or 11 mm[K+]o (lower panel). One hundred traces of duration 5 s and 2.5 s were selected for a [K+]o of 8 mm and 11 mm, respectively, and analysed as described in the text. Hypoglossal rootlet activity (XII), Po and ΔPo/Pmin were reproduced and repeated twice (above) or four times (below) for better visualization of periodic activities. It can be seen that the cycle length decreases and the mean open probability increases as [K+]o is elevated. However, the basic pattern of fluctuation remains the same: Po rises during the inspiratory bursts and falls after the inspiratory burst. Changes in fluctuation parameters tdelay, Ttot, t10–90 %, t90–10 % and ΔPpeak/Pmin are discussed in the text. B, plot of mean Po against cycle length. Mean values ( ± s.e.m.) of 10 cycles of similar lengths are represented by filled circles; the linear regression is given by a straight line (slope = −0.0013). Correlation analysis gave a correlation coefficient of −0.83 (P < 0.01).
Effect of hypoxia on KATP channel modulation
Ongoing rhythmic activity requires a high energy turnover in respiratory neurons, and a functional way to manipulate energy supply is hypoxia. Figure 7A shows the hypoxic response in the hypoglossal nerve consisting of an initial augmentation with an increase in respiratory burst frequency that is followed by a secondary depression with a decline in burst frequency. Likewise, the amplitudes of respiratory bursts rose initially and then fell with a similar time course. Besides enhancement of respiratory activity, XII activity developed a characteristic tonic activation (Haddad & Donnelly, 1990). The effect of hypoxia on KATP channel Po fluctuations was analysed during the period shortly before onset of hypoxic depression (indicated by the light grey area in Fig. 7B). Quantitative analyses of Po could not be performed during later periods because of the low number of inspiratory bursts.
Figure 7. Changes in cycle length induced by hypoxia.
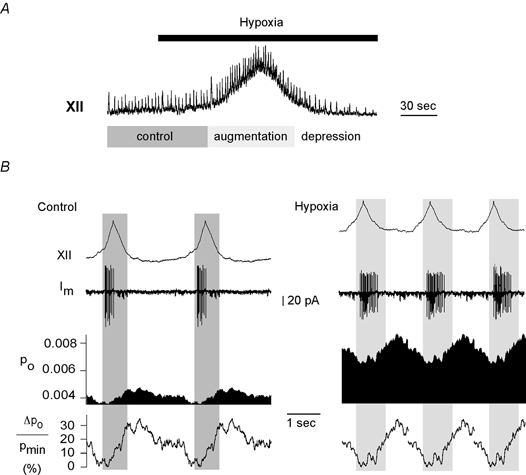
A, the response of hypoglossal activity to hypoxia. Po and ΔPo/Pmin were analysed separately for control sweeps (dark grey area) and for the augmentation phase of hypoxia (light grey area). Traces were reproduced twice during control (B, left panel, n = 34) and three times during hypoxia (B, right panel, n = 21). The open probability shows a marked increase during hypoxia (8 cells). The effect on fluctuation parameters is discussed in the text (see Fig. 4B).
Figure 7B shows Po and ΔPo/Pmin for traces obtained under control conditions (left panel, n = 34) as well as during hypoxic augmentation (right panel, n = 21). The duration of respiratory bursts is shown by the grey areas. Hypoxia led to a decrease in respiratory cycle length, Ttot, and an increase in Po fluctuation, ΔPpeak/Pmin, to 109 ± 37 % (Fig. 4B). Also, the value for t10–90 % rose significantly in parallel with the hypoxic augmentation of AP discharge, which increases on average from 3.9 to 12.8 APs per burst (8 cells). As previously reported (Mironov et al. 1998), the mean open probability increased synchronously with these changes of neuronal activity.
Effect of the Na+-K+ pump blocker ouabain
The close correlation between burst discharges and Po fluctuations indicates that Po fluctuations originate from periodic variations of ATP consumption due to activation of the Na+-K+ pump. We therefore determined the changes in Po shortly after application of the Na+-K+-ATPase blocker ouabain (20–100 μm). As previously described (Brockhaus et al. 1993), application of ouabain causes a slow blockade of the respiratory rhythm, as shown in Fig. 8A (insets). Analysis in 6/7 cells revealed a significant decline of Po measured during and between respiratory bursts (Fig. 8A). For comparison of respiratory Po fluctuations before and after ouabain application, typically less than 100 sweeps were available as the respiratory activity declined progressively. We found that the amplitudes of Po fluctuations, ΔPpeak/Pmin, declined to 49 ± 8 % (7 cells), as shown in the lowest panel of Fig. 8B. Ouabain also resulted in an increase in respiratory frequency and Po peaks as shown by the decrease of the parameters t10–90 % and t90–10 % (Fig. 4B).
Figure 8. Effect of ouabain on Po fluctuations.
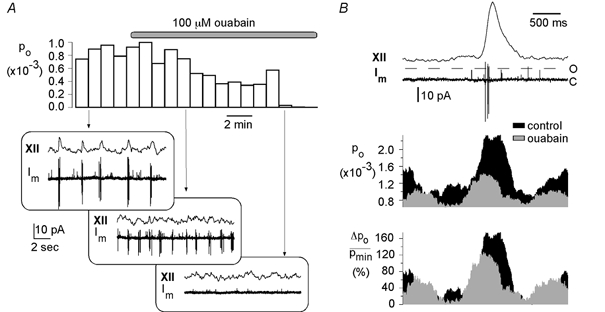
A, after application of 100 μm ouabain, Po decreased from 0.88 ± 0.10 × 10−3 to 0.21 ± 0.22 × 10−3 (mean ± s.d.). Depicted are Po values derived from consecutive data traces of 60 s duration. The boxes below show hypoglossal activity (XII) and membrane current (Im) recorded at different phases of ouabain application. They clearly illustrate that the amplitudes of both respiratory rhythm and action potentials decrease until they are blocked completely approximately 7 min after ouabain application. A 3 s time window surrounding the inspiratory burst is shown in B. The two upper curves display sample traces of hypoglossal recordings (XII) and membrane current, Im. Mean Po curves were obtained by averaging over 100 traces (control) and 90 traces (ouabain) containing an inspiratory burst. The relative change in Po, ΔPo/Pmin, is depicted below.
DISCUSSION
We showed that medullary inspiratory neurons of the NMRI mouse express KATP channels composed of Kir6.2 and SUR1 subunits. This corresponds with the channel population typically found in pancreatic β-cells (Inagaki et al. 1995) and in neurons of the dorsal nucleus of the vagus nerve of the rat (Karschin et al. 1998).
KATP channels were revealed to be active even during normoxia and to contribute to the modulation of synaptic drives and hypoglossal rhythmic activity. Analysis of KATP channels in inspiratory neurons showed that Po fluctuates in synchrony with rhythmic bursting of respiratory neurons. The functional aspect is that KATP channels are activity regulated and contribute to the modulation of ongoing spontaneous activity.
For quantitative description of such activity-dependent control, it is important to mention that the technique for calculating Po fluctuations produced an unavoidable systematic error. Firstly, Po could not be accurately computed during action potential discharges (APs). APs had to be subtracted from the trace and therefore some channel openings occurring during such APs and their afterpotentials have been missed. Consequently, Po was underestimated during these discharge periods, i.e. in reality, peak Po values are probably larger than described here. Secondly, the durations of spontaneous respiratory cycles were never constant. For computation of Po, however, many sweeps of variable cycle lengths had to be averaged. Consequently, estimates of respiration-related Po changes were less accurate the further the Po measurements were apart from the peak of the respiratory bursts, which was used to trigger the averages. This can be seen in Fig. 3C and D, where the selected time window preceded or respectively followed a respiratory burst. Thirdly, the membrane depolarization originating from the synaptic drive might lead to minor changes in single-channel currents and might thus affect Po. It is unlikely, however, that the observed Po fluctuations reflect such oscillations in membrane potential, as there was a clear time delay between the Po minimum and peak membrane depolarization during the synaptic drive (Fig. 3B). At the time of minimal Po, the cell had returned to its resting level. The contribution of voltage-dependent changes in Po, therefore, appears to be negligible.
In contrast to pancreatic β-cells and cardiomyocytes, where variation of KATP channel openings originates from oscillations in the glycolytic pathway, our data in inspiratory neurons suggest a novel regulatory mechanism. Our findings clearly reveal activity-correlated KATP channel fluctuations, which probably originate from oscillations of ATP/ADP concentration ratios generated by periodic ATP consumption of Na+-K+-ATPase activity. This assumption follows the rationale that intracellular [Na+] accumulates during each respiratory burst of discharges, resulting in a periodic acceleration of Na+-K+ pumping. Such variations in Na+-K+-ATPase activity would lead to periodic fluctuations in submembrane [ATP]i, directly inducing oscillations in Po of local KATP channels. This hypothesis is consistent with the observed reduction of Po oscillations after ouabain-induced blockade of Na+-K+-ATPase activity. Similar to other reports (Urbach et al. 1996; Kabakov, 1998; Abe et al. 1999), where coupling between KATP channels and Na+-K+ pumping was attributed to changes in the ATP/ADP ratio, we observed a steady decline of KATP channel activity after ouabain application. Two separate mechanisms may contribute to this effect. Firstly, blockade of the Na+-K+-ATPase will lead to a reduction in ATP consumption leading to elevation of intracellular ATP concentrations and ATP/ADP ratios, and thus to a decline of KATP channel activity. Secondly, ouabain slowly depolarizes neurons (Cousin et al. 1995; Balestrino et al. 1999) and finally blocks AP discharges, resulting in a decreased Na+ influx and lowered Na+-K+ pumping.
For a quantitative description of Po fluctuations, t10–90 %, t90–10 %, ΔPpeak/Pmin and Ttot were analysed. These parameters reflected a close similarity between Po fluctuations and respiratory parameters, such as cycle frequency and burst durations. Manipulations of neuronal excitability induced by changes in [K+]o, ouabain or hypoxia led to alterations of these Po parameters, which paralleled all changes in respiratory cycle length and cellular bursting behaviour: when respiratory frequency was lowered during reduction of [K+]o, Ttot and t90–10 % were prolonged. The surprising increase in ‘decay time’, t90–10 %, indicates that ATP depletion starts with the onset of neuronal activity and accumulates during prolonged respiratory bursts, while ATP replenishment recovers slowly thereafter. This reveals that full ATP replenishment may take longer than the duration of interburst intervals. Enhancement of t10–90 % during hypoxia, which reflects a prolonged rise time of Po, can be attributed to an increasing number of APs discharged per burst and consequently a stronger depletion of ATP. After ouabain application, the number and amplitude of APs were reduced, leading to a shorter delay of Po maximum and accordingly a significant decrease in t10–90 % and t90–10 %.
For all experimental conditions, the delay between onset of inspiratory burst activity and rise of Po amounted to approximately 100 ms. Much faster activation rates were reported for the activation of the Na+-K+-ATPase (Munakata et al. 1998). Therefore, the longer delays in KATP channel reactivity seen in the present experiments seem to originate from other processes, for example from the voltage-clamp technique. We speculate that the propagation of action potentials to the membrane patch, where KATP channels were analysed, was probably blocked due to the forced holding potential of approximately −100 mV (pipette potential: +40 mV). Such lack of local Na+ fluxes might provoke decreased ATP consumption. Secondly, the membrane patch that is sealed with the patch electrode probably forms an omega-shape of variable dimension (Hamill et al. 1981), thus creating an artificial compartment that is functionally separated from the remainding cytosol and cell metabolism. We conclude that low local Na+-K+- ATPase activity and sustained ATP concentrations in the pipette-attached membrane patch explain a slower reactivity of partially isolated KATP channels. The high variability of delays measured in individual neurons might also reflect such differences in diffusion distances of ATP molecules from the patch membrane carrying the KATP channels to the electrically activated peri-patch membrane segments.
Assuming that a change in ATP concentration is a dominant factor for inducing fluctuations in KATP channel activity, Po can be used to get a rough estimation of the changes in local free [ATP]i. For such estimation we used the equation [ATP] = Kd/(1/Po1/4 - 1), postulating four ATP-binding sites with equal affinity as described by Ashcroft & Gribble (1998) and blockade of channels, when one of these binding sites is occupied. We assumed a Kd value of 29 μm (Ashcroft & Gribble, 1998). Figure 3D illustrates the temporal fluctuations of estimated [ATP]i values as well as changes in the ADP concentration derived from [ATP]i. For all thirteen neurons analysed, such calculations indicate periodic changes in free [ATP]i ranging in the order of 5–40 μm. However, it should be emphasized that factors other than ATP, most noticeably ADP and pH, might contribute to the activation of Po (Cason et al. 1995; Hilgemann, 1997; Hiraoka, 1997; Aguilar-Bryan & Bryan, 1999; Baukrowitz & Fakler, 2000). Indeed, the intracellular pH falls periodically by up to 0.2 pH units during periods of synaptic inhibition that follow respiratory bursts. The pH fluctuations originate from a HCO3− efflux through transmitter-regulated Cl− channels (Ballanyi et al. 1994). Inhibition-dependent pH fluctuations could thus contribute to the activity- and ATP-induced Po fluctuations. The conclusion is that estimated fluctuations of ATP concentrations derived from Po variations were probably underestimated.
In conclusion, our results indicate that KATP channels are natural sensors of the instantaneous demand of metabolic energy. Their functional task appears to be a permanent and fast-acting adjustment of neuronal activity and cell metabolism. Therefore, KATP channels are endogenous regulators that damp rhythmic activity of neurons and contribute to membrane repolarization during and after each burst of action potentials. We conclude that functional neuronal networks demand a continuous [ATP]i supply and fast replenishment, and use KATP channels to accurately adjust the excitability of neurons to stabilize their activity levels and to protect network integrity.
Acknowledgments
This work was supported by the DFG. We thank Nicole Hartelt for excellent technical assistance.
References
- Abe T, Sato T, Kiyosue T, Saikawa T, Sakata T, Arita M. Inhibition of Na+-K+ pump alleviates the shortening of action potential duration caused by metabolic inhibition via blockade of KATP channels in coronary perfused ventricular muscles of guinea-pigs. Journal of Molecular and Cellular Cardiology. 1999;31:533–542. doi: 10.1006/jmcc.1998.0889. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocrine Reviews. 1999;20:101–135. doi: 10.1210/edrv.20.2.0361. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JPT, Boyd AE, III, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the β cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends in Neurosciences. 1998;21:288–294. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- Balestrino M, Young J, Aitken P. Block of (Na+,K+)ATPase with ouabain induces spreading depression-like depolarization in hippocampal slices. Brain Research. 1999;838:37–44. doi: 10.1016/s0006-8993(99)01674-1. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Doutheil J, Brockhaus J. Membrane potentials and microenvironment of rat dorsal vagal cells in vitro during energy depletion. Journal of Physiology. 1996;495:769–784. doi: 10.1113/jphysiol.1996.sp021632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K, Muckenhoff K, Bellingham MC, Okada Y, Scheid P, Richter DW. Activity-related pH changes in respiratory neurones and glial cells of cats. NeuroReport. 1994;6:33–36. doi: 10.1097/00001756-199412300-00010. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Fakler B. KATP channels gated by intracellular nucleotides and phospholipids. European Journal of Biochemistry. 2000;267:5842–5848. doi: 10.1046/j.1432-1327.2000.01672.x. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K, Smith JC, Richter DW. Microenvironment of respiratory neurons in the in vitro brainstem-spinal cord of neonatal rats. Journal of Physiology. 1993;462:421–445. doi: 10.1113/jphysiol.1993.sp019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason BA, Gordon HJ, Avery EGT, Hickey RF. The role of ATP sensitive potassium channels in myocardial protection. Journal of Cardiac Surgery. 1995;10:441–444. doi: 10.1111/j.1540-8191.1995.tb00675.x. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Nicholls DG, Pocock JM. Modulation of ion gradients and glutamate release in cultured cerebellar granule cells by ouabain. Journal of Neurochemistry. 1995;64:2097–2104. doi: 10.1046/j.1471-4159.1995.64052097.x. [DOI] [PubMed] [Google Scholar]
- Eberwine J, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R, Zettel M, Coleman P. Analysis of gene expression in single live neurons. Proceedings of the National Academy of Sciences of the USA. 1992;89:3010–3014. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad GG, Donnelly DF. O2 deprivation induces a major depolarization in brainstem neurons in the adult but not in the neonatal rat. Journal of Physiology. 1990;429:411–428. doi: 10.1113/jphysiol.1990.sp018265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW. Cytoplasmic ATP-dependent regulation of ion transporters and channels: mechanisms and messengers. Annual Review of Physiology. 1997;59:193–220. doi: 10.1146/annurev.physiol.59.1.193. [DOI] [PubMed] [Google Scholar]
- Hiraoka M. Pathophysiological functions of ATP-sensitive K+ channels in myocardial ischemia. Japanese Heart Journal. 1997;38:297–315. doi: 10.1536/ihj.38.297. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JPT, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6. 2) a smooth muscle type ATP-sensitive K+ channel. Journal of Biological Chemistry. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- Jiang C, Haddad GG. Modulation of K+ channels by intracellular ATP in human neocortical neurons. Journal of Neurophysiology. 1997;77:93–102. doi: 10.1152/jn.1997.77.1.93. [DOI] [PubMed] [Google Scholar]
- Kabakov AY. Activation of KATP channels by Na/K pump in isolated cardiac myocytes and giant membrane patches. Biophysical Journal. 1998;75:2858–2867. doi: 10.1016/S0006-3495(98)77728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin A, Brockhaus J, Ballanyi K. KATP channel formation by the sulphonylurea receptors SUR1 with Kir6. 2 subunits in rat dorsal vagal neurons in situ. Journal of Physiology. 1998;509:339–346. doi: 10.1111/j.1469-7793.1998.339bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinard TA, de Vries G, Sherman A, Satin LS. Modulation of the bursting properties of single mouse pancreatic β-cells by artificial conductances. Biophysical Journal. 1999;76:1423–1435. doi: 10.1016/S0006-3495(99)77303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson O, Kindmark H, Brandstrom R, Fredholm B, Berggren PO. Oscillations in KATP channel activity promote oscillations in cytoplasmic free Ca2+ concentration in the pancreatic β cell. Proceedings of the National Academy of Sciences of the USA. 1996;93:5161–5165. doi: 10.1073/pnas.93.10.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW, Barry PH. Action potentials initiated by single channels opening in a small neuron (rat olfactory receptor) Biophysical Journal. 1989;55:755–768. doi: 10.1016/S0006-3495(89)82874-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proceedings of the National Academy of Sciences of the USA. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov SL, Langohr K, Haller M, Richter DW. Hypoxia activates ATP-dependent potassium channels in inspiratory neurones of neonatal mice. Journal of Physiology. 1998;509:755–766. doi: 10.1111/j.1469-7793.1998.755bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata M, Fujimoto M, Jin YH, Akaike N. Characterization of electrogenic Na/K pump in rat neostriatal neurons. Brain Research. 1998;800:282–293. doi: 10.1016/s0006-8993(98)00533-2. [DOI] [PubMed] [Google Scholar]
- O'Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994;265:962–966. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Bischoff AM, Richter DW. ATP-sensitive K+ channels are functional in expiratory neurones of normoxic cats. Journal of Physiology. 1996;494:399–409. doi: 10.1113/jphysiol.1996.sp021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M, Tornheim K, Corkey BE. Signal transduction mechanisms in nutrient-induced insulin secretion. Diabetologia. 1997;40(suppl. 2):S32–41. doi: 10.1007/s001250051395. [DOI] [PubMed] [Google Scholar]
- Rajendra S, Lynch JW, Barry PH. An analysis of Na+ currents in rat olfactory receptor neurons. Pflügers Archiv. 1992;420:342–346. doi: 10.1007/BF00374468. [DOI] [PubMed] [Google Scholar]
- Sakura H, Ammala C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP- sensitive potassium channel subunit expressed in pancreatic β-cells, brain, heart and skeletal muscle. FEBS Letters. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzic A, Jahangir A, Kurachi Y. Cardiac ATP-sensitive K+ channels: regulation by intracellular nucleotides and K+ channel-opening drugs. American Journal of Physiology. 1995;269:C525–545. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- Urbach V, Van Kerkhove E, Maguire D, Harvey BJ. Cross-talk between ATP-regulated K+ channels and Na+ transport via cellular metabolism in frog skin principal cells. Journal of Physiology. 1996;491:99–109. doi: 10.1113/jphysiol.1996.sp021199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6. 1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. Journal of Physiology. 1997;499:715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawar C, Plant TD, Schirra C, Konnerth A, Neumcke B. Cell-type specific expression of ATP-sensitive potassium channels in the rat hippocampus. Journal of Physiology. 1999;514:327–341. doi: 10.1111/j.1469-7793.1999.315ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


