In this issue of The Journal of Physiology, Dempsey and colleagues (Sheel et al. 2001) contribute another key chapter in their ongoing series of elegant investigations on novel interactions involving the respiratory muscles, autonomic nervous system and cardiovascular regulation in humans. Earlier, they demonstrated that manipulation of the work of breathing during maximal exercise resulted in marked changes in locomotor muscle blood flow, cardiac output and both whole-body and active limb oxygen uptake (Harms et al. 1997, 1998). They also established the remarkable metabolic costs of supporting respiratory muscle function during maximal exercise, requiring up to 16 % of the cardiac output (Harms et al. 1998). Importantly, the reduced locomotor muscle blood flow and vascular conductance in the elevated work of breathing condition was associated with augmented noradrenaline (norepinephrine) spillover from the active limbs, suggesting enhanced sympathetic vasoconstriction (Harms et al. 1997). These physiological effects of the work of breathing have important functional consequences, as demonstrated by an ∼15 % improvement in endurance performance with respiratory muscle unloading (Harms et al. 2000).
The next generation of experiments attempted to establish the mechanisms underlying these fascinating physiological connections. In a paper recently published in this journal (St Croix et al. 2000), high-resistance, prolonged duty cycle breathing at rest, resulting in respiratory muscle fatigue, evoked an increase in leg muscle sympathetic nerve activity (MSNA) that was independent of central respiratory motor output, indicating a reflex origin. Moreover, the temporal nature of the response (MSNA was unchanged during the initial 1–2 min of the fatiguing task but increased progressively thereafter) was characteristic of a slower-developing muscle metaboreflex (chemoreflex), rather than a mechanoreflex stimulated by force development (which would be expected to evoke sympathoexcitation at the start of contractions).
The present article by Sheel et al. (2001) represents a critical extension of this work by establishing that this presumed respiratory muscle-limb reflex has the ability, at least under resting conditions, to reduce significantly limb blood flow and vascular conductance. Thus, together with previous observations (St Croix et al. 2000), the present contribution provides compelling evidence for the existence of a metaboreflex, with its origin in the respiratory muscles, that can modulate limb perfusion via stimulation of sympathetic nervous system vasoconstrictor neurones (Fig. 1).
Figure 1.
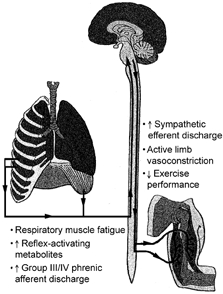
Respiratory muscle ‘metaboreflex’
Teleologically, this reflex may have as its fundamental goal the protection of oxygen delivery to the respiratory muscles, thus ensuring the ability to maintain pulmonary ventilation, proper regulation of arterial blood gases and pH and overall organismic homeostasis. Presumably, as the ‘vital organ’ responsible for supporting pulmonary function, perfusion of the respiratory muscles, particularly during physiological states in which there is competition for cardiac output such as heavy submaximal and maximal exercise, has priority over the locomotor muscles. This subservience of active limb blood flow may be similar to that previously established for the arterial baroreflex during large-muscle dynamic exercise (Rowell, 1997). Specifically, under conditions in which wide-spread vasodilatation has occurred, thus threatening the maintenance of systemic vascular resistance and arterial blood pressure, arterial baroreflex deactivation (unloading) will produce a strong reflex sympathetic vasoconstriction targeted, at least in part, at the active limbs. This vasoconstrictor drive can be sufficiently strong as to produce vasoconstriction in working locomotor muscles, thus ensuring the maintenance of arterial perfusion pressure.
As with any developing drama, several unanswered questions remain. For example what is the influence, if any, of this reflex during normal in vivo exercise? Can the reflex explain the physiological consequences of the work of breathing in limiting maximal aerobic capacity (maximal oxygen uptake) and human performance? Is the reflex active during moderate, submaximal aerobic exercise performed for health and fitness purposes in non-athletic adults? If so, at what intensity of exercise, level of pulmonary ventilation, etc., is the reflex engaged? Perhaps the reflex is tonically active in patients with clinical disorders associated with chronic elevations in the work of breathing (e.g. congestive heart failure or chronic obstructive lung disease)? Can the conditions under which this metaboreflex is triggered be modified by training of the respiratory muscles? Finally, does the ‘stealing’ work both ways? That is, can active limb metaboreflexes act to redirect blood flow away from respiratory muscles to the locomotor muscles, thus potentially compromising pulmonary function during heavy submaximal and maximal exercise?
As with our favourite page-turning suspense novel, we look forward to the answers to these and other questions in the next intriguing instalment of this series.
References
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Journal of Applied Physiology. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Journal of Applied Physiology. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, St Croix CM, Pegelow DF, Dempsey JA. Journal of Applied Physiology. 2000;89:131–138. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Clinical and Experimental Pharmacology and Physiology. 1997;24:117–125. doi: 10.1111/j.1440-1681.1997.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Journal of Physiology. 2001;537:277–289. doi: 10.1111/j.1469-7793.2001.0277k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Croix C, Morgan B, Wetter T, Dempsey J. Journal of Physiology. 2000;529:493–504. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


