Abstract
Spontaneous non-synaptic epileptiform activity was induced by bathing rat hippocampal slices in low-Ca2+ solution. Extracellular recordings from electrodes placed on both sides of a complete cut showed that non-synaptic activity was synchronized across the lesion.
Ion-selective electrode recordings showed that each event was accompanied by a transient increase in extracellular potassium that diffused across the lesion. The synchrony was destroyed when a thin film was inserted into the lesion site.
Local pressure ejection of KCl evoked an event that subsequently propagated across the lesion.
After a complete lesion was made, afterdischarges evoked on one half of a slice were not detected on the other half.
Voltage-sensitive dye imaging methods showed that epileptic activity propagated across the mechanical lesion without significant attenuation or additional delays. The velocity of the activity was consistent with that of the slow diffusion of a potassium wave.
Since field effects were significantly attenuated across the lesion and all gap junctions and cell processes across the lesion would be cut, these data show that extracellular diffusion, most probably potassium, is sufficient to synchronize populations of neurons and propagate slow frequency epileptiform activity.
The generation and spread of spontaneous epileptiform discharges are generally attributed to synaptic excitatory feedback. However, studies performed in situ using ion- selective electrodes (Heinemann et al. 1977; Pumain et al. 1985) have shown that during an epileptic seizure extracellular calcium concentration can decrease to levels where chemical synaptic transmission is abolished. Moreover, several laboratories have shown the development of synchronized epileptiform activity in hippocampal slices when synaptic transmission is blocked with a low calcium artificial cerebrospinal fluid solution (ACSF) (Jefferys & Haas, 1982; Konnerth et al. 1984). This ‘non-synaptic’ epileptiform activity is characterized by negative shifts (field bursts) in the extracellular field potential that propagate slowly across the CA1 pyramidal cell layer and are always accompanied by a transient increase in extracellular potassium (Yaari et al. 1986).
Several electrical and non-synaptic chemical interactions have been proposed to facilitate the propagation of field bursts in the hippocampus (Jefferys, 1995). Both ephaptic and electrotonic interactions are enhanced during perfusion with low-Ca2+ medium (Perez-Velazquez et al. 1994; Ghai et al. 2000) and have been shown to facilitate the local synchronization of cell firing in response to antidromic stimulation (Taylor & Dudek, 1984). Furthermore, agents that antagonize gap junctions (Bikson et al. 1999) and field effects (Gluckman et al. 1996; Lian et al. 2001) have been shown to annihilate non-synaptic epileptiform bursting. Alternatively, it has been suggested that the slow diffusion of small molecules through (glial) gap junctions could facilitate burst propagation (Charles et al. 1993). CA1 astrocytes are characterized by an extremely high degree of cell-to-cell coupling and form a syncytium of hundreds of cells (D'Ambrosio et al. 1998). Since axonal and dendritic projections can extend along the entire length of the CA1 region (Brown & Zador, 1983), these processes represent a fourth possible mechanism of propagation. Lastly, it has been proposed that the diffusion of an extracellular potassium ‘wave’, or some other excitatory agent, could facilitate non-synaptic burst propagation (Yaari et al. 1986). Several recent studies have supported the potassium hypothesis (Jensen & Yaari, 1997; Bikson et al. 1999); however, since previously it was not possible to separate these different non-synaptic interactions, the role of extracellular ionic diffusion was not clear.
To determine which of these mechanisms underlies the propagation of low-Ca2+ field bursts, we tested the ability of spontaneous bursts to cross a mechanical lesion. Such a lesion would be expected to destroy all gap junction connections between neurons or glia as well as cut any processes that run along the propagation pathway. The ability of field effects and extracellular potassium waves to cross the lesion was similarly examined. The results of this study show, for the first time, that changes in extracellular ionic activity, most probably potassium, are sufficient to propagate neuronal activity and synchronize epileptic neuronal populations.
METHODS
Preparation of hippocampal slices
All experiments were performed in the CA1 pyramidal cell region of hippocampal brain slices prepared from Sprague-Dawley rats (175–250 g). The experimental protocol was reviewed and approved by the Institution Animal Care and Use Committee. Rats were anaesthetized using ethyl ether and decapitated. The brain was rapidly removed and one hemisphere glued to the stage of a Vibroslicer (Vibroslice, Campden). Slicing was carried out in cold (3–4 °C) oxygenated sucrose-based artificial cerebrospinal fluid (ACSF) consisting of (mm): sucrose 220, KCl 3, NaH2PO4 1.25, MgSO4 2, NaHCO3 26, CaCl2 2, and dextrose 10. Sucrose-based slicing medium has been shown to increase cell viability in vitro (Aghajanian & Rasmussen, 1989). The resulting 350 μm thick slices were immediately transferred to a holding chamber containing ‘normal’ ACSF consisting of (mm): NaCl 124, KCl 3.75, KH2PO4 1.25, CaCl2 2, MgSO4 2, NaHCO3 26, and dextrose 10, held at room temperature and bubbled with 95 % O2-5 % CO2.
After > 1 h of recovery, a slice was transferred to a standard interface recording chamber with ‘normal’ ACSF at 35 ± 0.5 °C, and a warmed, humidified 95 % O2-5 % CO2 vapour maintained over the exposed surface of the slice. After 10 min, slices were perfused with low calcium ACSF (low Ca2+) consisting of (mm): NaCl 124, KCl 5.25, KH2PO4 1.25, CaCl2 0.2, MgSO4 1.5, NaHCO3 26, and dextrose 10.
Electrical recording, lesioning and data analysis
Extracellular recordings of field potentials were obtained using glass micropipettes (2–5 MΩ) filled with 150 mm NaCl. Potassium-selective microelectrodes were constructed using established methods described elsewhere (Amman, 1986; Ghai et al. 2000). We used N,N-dimethyltrimethylsilylamine (Fluka Chemicals) to silanize the electrode tips and the Fluka 60398 potassium-selective membrane solution, which contains the potassium ionophore valinomycin. The potassium-selective microelectrodes were filled with 150 mm KCl. Electrodes were calibrated in 0.1, 1, 10 and 100 mm KCl using the separate solution method (Amman, 1986). Only electrodes of 95 % Nernstian slope and τ < 200 ms (where τ represents 10 % to 90 % rise time) were used. Electrodes were at least 1000-fold selective over sodium. During potassium measurement the voltage recorded by a field electrode placed in the somatic layer (within 50 μm of the potassium-selective electrode) was subtracted to provide a true potassium measurement. KCl was ejected by a pneumatic picropump (WPI) via a microelectrode (3 μm) filled with 100 mm KCl. Pressure pulses of 200 ms ranged between 1 and 5 bar (100–500 kPa). We used an ejection protocol sufficient to raise potassium 2 mm to trigger low-Ca2+ events. Antidromic monopolar pulses (200 μA, 200 μs) were delivered using tungsten electrodes positioned in the alveus.
Hippocampal slices were lesioned with a scalpel blade. Lesions were made through the entire thickness and across the entire width of the slice. Recordings were obtained at least 0.25–2 h after the lesioning or movement of the slice to exclude any transient activity. Cross-covariance analysis was used to evaluate the level of synchronization between two signals. The cross-covariance is similar to the cross-correlation, but with the mean value of the data subtracted. Data analysed were 2 min in duration. Results are reported as maximum cross-covariance value ± standard deviation; n = number of slices.
Optical imaging with voltage-sensitive dyes
Optical images were obtained using established methods described elsewhere (Colom & Saggau, 1994). Briefly, prior to recording, slices were transferred to a static chamber containing 200 μm RH414 (Molecular Probes) for 15 min. Slices were then transferred to a submerged recording chamber on an inverted microscope (Nikon). Light generated by a tungsten source was band-pass filtered and focused onto the slice through a × 10 objective. The emitted fluorescence was projected onto a photodiode array (C4675, Hamamatsu, Japan) consisting of 256 detectors arranged in a square array. Optical signals from individual photodiodes were current to voltage converted, amplified and filtered (Yale LBC, Argo Transdata, CT, USA) before being multiplexed and digitized (12 bits) at 1 kHz (Dap 3400a, Microstar Labs, WA, USA) onto a computer (Dimension XPS H266, Dell) running custom data acquisition and signal processing software. Because of the long time of optical recording and hence extended light exposure, weak AC coupling was applied to each analog channel to compensate for the constant drift due to dye bleaching (Colom & Saggau, 1994). Controls were performed to ensure that the source of the optical signals was extrinsic voltage-sensitive dye fluorescence rather than intrinsic signal due to light scattering.
RESULTS
Propagation of low-Ca2+ field bursts across an intact slice
Prolonged incubation (20–60 min) in low-Ca2+ solution resulted in the development of spontaneous epileptiform activity in the CA1 region of the hippocampus (Fig. 1). Previous studies have shown that spontaneous bursts usually initiate in the CA1a (near the entorhinal cortex) and can propagate the entire length of the CA1 region (Yaari et al. 1983; Haas & Jefferys, 1984). We quantified the propagation reliability using synchrony analysis. The synchronization between two sites was determined by evaluating the maximum cross-covariance of spontaneous activity measured by two field electrodes. The electrodes were positioned at sequentially greater distances along the CA1 pyramidal cell layer (Fig. 1A and B). Propagation was extremely reliable (cross-covariance > 0.7) over distances less than 500 μm. At distances > 700 μm the level of synchronization decreased (Fig. 1C, n = 10). Therefore, in subsequent experiments employing two field electrodes, the recording sites were always 300–400 μm apart.
Figure 1. Propagation reliability of non-synaptic bursts across slice quantified by correlation analysis.

A and B, left, during spontaneous bursting, two field electrodes were positioned at sequentially greater distances along the CA1 pyramidal cell layer. Right, the correlation at each separation distance was determined by the maximum value of cross-covariance (y-axes). C, summary of peak cross-covariance at 6 distances from 10 slices.
Synchronization of low-Ca2+ field bursts across a lesion
The role of mechanical connections (i.e. glial gap junctions, neuronal gap junctions and cell projections) in burst propagation was determined by lesioning a slice as illustrated in Fig. 2A. As above, cross-covariance analysis was used to quantify the degree of synchronization measured by two field electrodes positioned in the CA1 pyramidal cell layer. Prior to lesioning, spontaneous activity recorded by field electrodes separated by 400 μm was highly correlated (Fig. 2Aa, cross-covariance 0.81 ± 0.15, n = 11). Activity remained synchronized after a cut was made between the two electrodes, dividing the CA1 regions into approximately equal halves (Fig. 2Ab, cross-covariance 0.78 ± 0.10, n = 11). Separation of the two halves by ∼300 μm disrupted synchronization (Fig. 2Ac, cross-covariance 0.22 ± 0.09, n = 11). Each section, however, continued to generate spontaneous activity independently. Synchronization was restored when the two halves were moved back together (Fig. 2Ad, cross-covariance 0.80 ± 0.10, n = 11). The mismatch of the pyramidal cell layer (n = 3) was found to destroy synchrony significantly even for displacements as small as the width of the cell layer (about 60 μm).
Figure 2. Synchronization of spontaneous non-synaptic activity across a mechanical lesion.

In this and subsequent figures, the position of two field electrodes on the slice is illustrated schematically (•). The top voltage trace corresponds to the right electrode, and the bottom trace corresponds to the left one. The location of a mechanical lesion is shown with a vertical line. Synchronization was quantified using cross-covariance analysis. Aa, prior to lesioning, activity was highly synchronized between two sites on a slice. Ab, after a mechanical lesion was made between the two field electrodes dividing the slice into halves, activity remained synchronized between the two sites. Ac, separation of the halves by ∼300 μm disrupted synchronization. Ad, movement of the halves back to their original position restored synchronization. In another experiment the synchronization of spontaneous non-synaptic activity between two different slices was studied. Two slices were mechanically lesioned and one half from each slice was transferred to a single recording chamber. Ba, prior to the alignment of the two halves, each slice generated spontaneous non-synaptic activity that was uncorrelated. Bb, positioning of the two halves such that the cut ends were touching and pyramidal cell layers aligned resulted in the synchronization of spontaneous activity between each slice.
Spontaneous low-Ca2+ bursting was monitored simultaneously in two hippocampal halves from different slices (Fig. 2B). When the two halves were separated, bursting activity in each slice was not synchronized (Fig. 2Ba, cross-covariance 0.25 ± 0.07, n = 3). Positioning of the two slices such that the cut ends were touching and the pyramidal cell layers were aligned increased synchronization (Fig. 2Bb, cross-covariance 0.75 ± 0.16, n = 3). The cross-covariance curve does not have successive peaks, indicating that the synchronized activity had a large variance in the interburst interval.
The maximum covariance for aligned lesioned slices and aligned slices from different animals (cross-covariance 0.77 ± 0.15, n = 14) was not significantly different from intact slice controls (cross-covariance 0.81 ± 0.15, n = 11) (P > 0.05). Once synchronization was induced by aligning lesioned slices, bursting remained synchronized for the duration of the experiment (up to 4 h). When halves with different isolated burst rates were aligned, the resulting synchronized burst frequency was usually between both isolated rates and closer to the faster one. These results indicate that gap junctions or cell projections along the path of the activity are not required for the propagation of non-synaptic epileptiform activity. Furthermore, non-synaptic mechanisms can synchronize the bursting of two independently oscillating networks.
Potassium diffusion across a lesion
Extracellular potassium diffusion has been suggested to underly the propagation and synchronization of non-synaptic activity (Konnerth et al. 1984). Each low-Ca2+ event is accompanied by a transient increase in extracellular potassium (Fig. 3Aa). By passive diffusion along the pyramidal cell layer, this potassium ‘wave’ can facilitate burst propagation (Yaari et al. 1986). To determine if these potassium transients could diffuse across a cut, mechanical lesions were made across the CA1 region, and potassium concentration was measured inside and around the cleft during spontaneous bursting (n = 6). Potassium transients were always observed in the cleft under conditions that promoted synchrony across the lesion (Fig. 3Ab). The peak potassium concentration change in the cleft was 1.19 ± 0.17 mm or 53 % control (2.25 ± 0.27 mm, determined by averaging the changes on either side of the lesion). When the slices were separated sufficiently to suppress synchronization, no potassium transients were detected in the centre of the cleft (Fig. 3Ac). Thus, synchronization across a cut was always correlated with potassium transients in the lesion.
Figure 3. Potassium transients across a mechanic lesion and disruption of diffusion by a barrier.

Aa, simultaneous potassium (top) and field (bottom) measurements were obtained using field and potassium-selective microelectrodes positioned in the CA1 pyramidal cell layer of slices perfused with low-Ca2+ media. Each spontaneous low-Ca2+ burst was accompanied by a transient increase in extracellular potassium. Ab, slices were lesioned as before. The field and potassium-selective electrodes were moved across the CA1 pyramidal cell layer and bursting was monitored at the 5 sites shown schematically (separated by 100 μm). Site 3 was always inside the lesion. Prior to half separation, field burst and potassium transient were recorded around and in the mechanical lesion. Ac, after half separation by ∼300 μm potassium transient failed to invade the centre of the mechanical lesion. Ba, two sites across the lesion were highly synchronized. Bb, after a thin film was positioned in the gap, the activities on both sides of the lesion became unsynchronized.
We blocked the transportation of ions between the two halves by inserting a plastic film (∼80 μm) into the lesion. Well-synchronized activities (Fig. 3Ba, cross-covariance 0.72 ± 0.13, n = 3) became uncorrelated when the film was positioned in the gap of the lesion (Fig. 3Bb, cross-covariance 0.25 ± 0.07, n = 3). Synchrony redeveloped when the film was removed (data not shown).
Propagation of induced burst across a lesion
Consistent with previous studies (Yaari et al. 1986) we found that local pressure ejection of small amounts of KCl in the CA1 stratum pyramidale evoked a low-Ca2+ field burst. K+ pulses were delivered during the interval of two events to distinguish the evoked response from the spontaneous activity. The K+-induced activity was recorded by two field electrodes (Fig. 4A, n = 4). After lesioning, the locally evoked event propagated through the gap and across the other half of the slice (Fig. 4B, n = 4). These data show that an event generated independently in one half of the hippocampus can propagate across the lesion and induce an event in the other half.
Figure 4. Induction of low Ca2+ activity across a mechanical lesion by KCl pressure ejection.
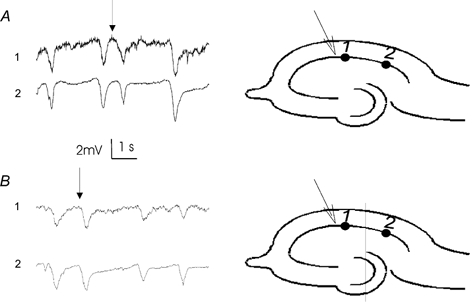
Two recording electrodes were positioned around 300 μm apart. A KCl pressure-ejecting electrode was placed within 50 μm of one. The original spontaneous activity was very periodic. A brief pressure pulse was applied during the interval of the events (indicated by an arrow). A, the evoked activity was recorded by two recording electrodes before the lesion (monitored by field electrodes 1 and 2). B, after a mechanical lesion was made, KCl ejected on one side of the slice evoked a local event that propagated to the other side of the lesion.
Electric field effects across a lesion
During perfusion with low-Ca2+ media, electrical stimulation of the alveus activates pyramidal cells antidromically and induces robust ‘afterdischarges’. In contrast with low-Ca2+ spontaneous bursts, whose slow time course is consistent with potassium diffusion, afterdischarges are considered to be too fast to be modulated by extracellular potassium transients (Jefferys, 1995). Instead, field effects and gap junction coupling are thought to synchronize these fast events. We designed an experiment to determine if a large field generated on one side of a lesion could be detected or recruit neurons to fire on the other side of a cut. As shown schematically in Fig. 5A, the depolarization of neuron a, on the left side, generates an electrical field across the lesion and along neuron b, on the other side. A field electrode located next to neuron b should detect this field as well as any subsequent activity generated by neuron b if it is excited by this field. Prior to lesioning, antidromic stimulation at one end of the hippocampus evoked afterdischarges (n = 5) across the entire pyramidal cell layer (Fig. 5Ba). After lesioning, stimulation still resulted in a large evoked response on the proximal side of the cut (Fig. 5Bb). If field effects were effective across the lesion, we would expect to directly detect the field generated by this discharge on the distal side of the lesion, as well as a population spike generated in response to this field by cells on the distal half. An electrode on the distal side of the lesion, however, failed to detect any activity in response to stimulation (Fig. 5Bb). The possibility of cell damage on the distal side was ruled out by moving the stimulation electrode to the distal side. This result indicates that ephaptic interactions (even from a very robust evoked signal) are not sufficient to synchronize neuronal activity across a lesion.
Figure 5. Field effects through a mechanical lesion.

A, schematic diagram shows two pyramidal cells separated by a lesion. Cell b, on the right, can be depolarized by the large electric field generated by cell a, on the left. Ba, antidromic stimulation of the alveus (arrow) was used to evoke robust afterdischarges in the CA1 pyramidal cell layer (monitored by field electrodes 1 and 2). Prior to lesioning, an evoked discharge propagated across the entire slice. b, after lesioning, stimulation still evoked a discharge on the proximal side of the cut; however, no endogenous response was detected across the lesion.
Spatial dynamics of non-synaptic propagation
The propagation of low-Ca2+ activity was studied using optical imaging with voltage-sensitive dyes. Voltage-sensitive dyes bind to cell membranes and fluoresce with intensity directly proportional to the trans-membrane voltage (Colom & Saggau, 1994). To our knowledge, this is the first demonstration that non-synaptic activity can be imaged with dyes. Spontaneous low-Ca2+ bursts, initiating at either side of the hippocampus successfully propagated across the lesion (Fig. 6). Activity propagated across the mechanical lesion without significant attenuation or additional delays, suggesting that burst synchronization mechanisms were intact. The slight hyperpolarization observed in the dendritic region was most probably generated by AC coupling. The velocity of propagation was measured in 10 slices before and after lesioning. In each case, the velocity of propagation across the intact slice (ranging from 0.68 ± 0.25 mm s−1 to 2.82 ± 0.82 mm s−1) was not significantly different (P > 0.05) from the velocity across a mechanical lesion.
Figure 6. Optical imaging of non-synaptic burst propagation across lesioned slice.

Spontaneous low-Ca2+ epileptiform activity was induced in a slice stained with voltage sensitive dye and a mechanical lesion was made across the hippocampus. The area around the lesion was imaged with a photodiode array. A, schematic diagram showing area of slice projected onto photodiode array and locations of sample photo-diodes (squares), field electrode (circle) and lesion (line). B, sample optical (top two) and field (bottom) recordings during a spontaneous low-Ca2+ burst. Vertical bar shows grey-scale code used to generate spatial map in C. C, grey-scale map of propagation of low-Ca2+ event across CA1 pyramidal cell layer (0.1 s per frame).
DISCUSSION
Role of field effects, electrotonic coupling and cell projections
A distinction is made between the synchronization of single action potentials and the synchronization (propagation) of spontaneous low-Ca2+ bursts across the hippocampus. While it has been demonstrated that ephaptic and electrotonic interactions can contribute to the former (Taylor & Dudek, 1984), the results of the present study show that these interactions are not required for burst propagation. Field effects and gap junctions can recruit individual neurons to synchronize with a population, but it has not been demonstrated that these interactions can facilitate the synchronization of an independently bursting population of neurons. Furthermore, the slow propagation velocity of low-Ca2+ bursts is inconsistent with the speed of ephaptic and electrotonic interactions, or with the velocity expected if propagation was facilitated by cell projections or gap junctions. Rather, it is more likely that these non-synaptic interactions contribute to burst initiation by enhancing neuronal excitability (Bikson et al. 1999).
Extracellular diffusion, K+ wave
The failure of ephaptic interactions to cross the lesion can be explained by the small size of field effects and their initially fast decay over distance (1/r2). In contrast, the concentration of small molecules in the extracellular space would decay by diffusion with a rate in the order of exp(-r2) (Grattarola & Massobrio, 1998). In low-calcium preparations, the potassium transient reaches a maximum value at the cell layer and drops quickly to ∼0.1–0.5 mm in the dendrite (Yaari et al. 1986). This concentration was presumably not large enough to propagate and synchronize the activity across a cut, which accounted for the loss of synchrony when pyramidal cell layers of two halves were not well aligned.
Addition of post-synaptic GABA and glutamate receptor antagonists does not block spontaneous low-Ca2+ activity (Jefferys & Haas, 1982), suggesting that the diffusion of neurotransmitters does not affect propagation. While small changes in extracellular Cl− and Na+ activity (Yaari et al. 1983; Haas & Jefferys, 1984) are known to accompany bursting, these fluctuations would exert a relatively small influence on cell excitability (Hille, 1992). In contrast, agents that interfere with potassium movement annihilate activity (Bikson et al. 1999), and relatively small changes in extracellular K+ concentration can exert a profound influence on neuronal excitability.
It has been hypothesized that an individual burst is initiated when intense neuronal firing results in a local increase in extracellular potassium (Yaari et al. 1986). This increase in [K+]o would diffuse and in turn depolarize neighbouring cells, which would be induced to fire. Thus, non-synaptic bursts can propagate as neuronal firing contributes in a feed forward manner to an extracellular potassium wave. Several earlier findings supported this hypothesis. (1) Injection of a small amount of potassium has been found sufficient to induce a burst (Heinemann et al. 1992). (2) In certain cases, a potassium concentration increase precedes the initiation of a field burst (Ghai et al. 2000). (3) The speed of burst propagation is consistent with the diffusion of potassium through the extracellular space (Konnerth et al. 1986).
In the present study, the ionic diffusion hypothesis for low-Ca2+ bursting was proven by demonstrating that low-Ca2+ bursts could be synchronized across a mechanical lesion, ruling out a contribution from gap junctions or cell projections. The associated potassium waves could diffuse across a lesion, while field effects could not. Furthermore, the interruption of propagation by interference with a piece of impermeable membrane and the demonstration that a low-Ca2+ burst initiated on one side could propagate across a lesion confirm that the synchronization observed across the cut was a result of propagation across the lesion.
Some attenuation in peak potassium concentration change was measured in the lesion. However, because neurons bathed in low-Ca2+ medium are close to threshold (Jefferys & Haas, 1982), even small changes in extracellular potassium activity would be sufficient to trigger action potentials. Lastly, optical imaging with voltage-sensitive dyes revealed that non-synaptic bursts were characterized by an extremely gradual onset and large space constant. This is in contrast to images of synaptically propagated activity (Colom & Saggau, 1994), which showed that activity in the focus site terminated before activity in the projection site reached its peak. Thus, the dynamics of low-Ca2+ activity are consistent with the slow diffusion of an extracellular potassium wave.
Non-synaptic communication in the brain
Non-synaptic interactions (i.e. gap junctions, ephaptic transmission and ion fluctuations) have been proposed to play a critical role in the modulation of both normal and pathological brain function (Jefferys, 1995). It is well established that local changes in extracellular ionic activity can lead to profound changes in cell and network function (Hille, 1992). The results of this study directly demonstrate, for the first time, that endogenously induced ionic transients can diffuse from a focus in the CNS and modulate activity in neighbouring regions, even when those regions are not mechanically connected. Furthermore, this diffusion can synchronize bursting in independent oscillators and thus promote seizure initiation.
Acknowledgments
We thank Dan Leventhal, Philip J. Hahn, Dr Ken Gustafson and Jian Chang for reading the manuscript and providing a number of suggestions. This work is supported by a Whitaker Foundation Development Award and a National Science Foundation Grant IBN93-19591.
References
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- Amman D. Ion-Selective Microelectrodes: Principles, Design and Application. Berlin Heidelberg New York Tokyo: Springer-Verlag; 1986. [Google Scholar]
- Bikson M, Ghai RS, Baraban SC, Durand DM. Modulation of burst frequency, duration, and amplitude in the zero-Ca2+ model of epileptiform activity. Journal of Neurophysiology. 1999;82:2262–2270. doi: 10.1152/jn.1999.82.5.2262. [DOI] [PubMed] [Google Scholar]
- Brown TH, Zador AM. Hippocampus. In: Shepherd GM, editor. Neurobiology. New York: Oxford University Press; 1983. p. 611. [Google Scholar]
- Charles AC, Dirksen ER, Merrill JE, Sanderson MJ. Mechanisms of intercellular calcium signaling in glial cells studied with dantrolene and thapsigargin. Glia. 1993;7:134–145. doi: 10.1002/glia.440070203. [DOI] [PubMed] [Google Scholar]
- Colom LV, Saggau P. Spontaneous interictal-like activity originates in multiple areas of the CA2-CA3 region of hippocampal slices. Journal of Neurophysiology. 1994;71:1574–1585. doi: 10.1152/jn.1994.71.4.1574. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Research. 1998;786:64–79. doi: 10.1016/s0006-8993(97)01412-1. [DOI] [PubMed] [Google Scholar]
- Ghai RS, Bikson M, Durand DM. Effects of applied electric fields on low-calcium epileptiform activity in the CA1 region of rat hippocampal slices. Journal of Neurophysiology. 2000;84:274–280. doi: 10.1152/jn.2000.84.1.274. [DOI] [PubMed] [Google Scholar]
- Gluckman BJ, Neel EJ, Netoff TI, Ditto L, Spano ML, Schiff SJ. Electric field suppression of epileptiform activity in hippocampal slices. Journal of Neurophysiology. 1996;76:4202–4205. doi: 10.1152/jn.1996.76.6.4202. [DOI] [PubMed] [Google Scholar]
- Grattarola M, Massobrio G, et al. Bioelectronics Handbook. New York San Francisco Washington DC: McGraw-Hill; 1998. [Google Scholar]
- Haas HL, Jefferys JGR. Low-calcium field burst discharges of CA1 pyramidal neurons in rat hippocampal slices. Journal of Physiology. 1984;354:185–201. doi: 10.1113/jphysiol.1984.sp015371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Research Supplement. 1992;7:273–280. [PubMed] [Google Scholar]
- Heinemann U, Lux HD, Gutnick MJ. Extracellular free calcium and potassium during paroxysmal activity in the cerebral cortex of the cat. Experimental Brain Research. 1977;27:237–243. doi: 10.1007/BF00235500. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland MA USA: Sinauer Associates; 1992. [Google Scholar]
- Jefferys JGR. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiological Reviews. 1995;75:689–783. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- Jefferys JGR, Haas HL. Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature. 1982;300:448–450. doi: 10.1038/300448a0. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Yaari Y. Role of intrinsic burst firing, potassium accumulation, and electrical coupling in the elevated potassium model of hippocampal epilepsy. Journal of Neurophysiology. 1997;77:1224–1233. doi: 10.1152/jn.1997.77.3.1224. [DOI] [PubMed] [Google Scholar]
- Konnerth A, Heinemann U, Yaari Y. Slow transmission of neural activity in hippocampal area CA1 in absence of active chemical synapses. Nature. 1984;307:69–71. doi: 10.1038/307069a0. [DOI] [PubMed] [Google Scholar]
- Konnerth A, Heinemann U, Yaari Y. Nonsynaptic epileptogenesis in the mammalian hippocampus in vitro. I. Development of seizurelike activity in low extracellular calcium. Journal of Neurophysiology. 1986;56:409–423. doi: 10.1152/jn.1986.56.2.409. [DOI] [PubMed] [Google Scholar]
- Lian J, Shuai J, Hahn P, Durand DM. Nonlinear dynamic properties of low calcium-induced epileptiform activity. Brain Research. 2001;890:246–254. doi: 10.1016/s0006-8993(00)03166-8. [DOI] [PubMed] [Google Scholar]
- Perez-Velazquez JL, Valiante TA, Carlen PL. Modulation of gap junctional mechanisms during calcium-free induced field burst activity: a possible role for electrotonic coupling in epileptogenesis. Journal of Neuroscience. 1994;14:4308–4317. doi: 10.1523/JNEUROSCI.14-07-04308.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumain R, Menini C, Heinemann U, Louvel J, Silva-Barrat C. Chemical synaptic transmission is not necessary for epileptic seizures to persist in the baboon Papio papio. Experimental Neurology. 1985;89:250–258. doi: 10.1016/0014-4886(85)90280-8. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Dudek FE. Excitation of hippocampal pyramidal cells by an electrical field effect. Journal of Neurophysiology. 1984;52:126–142. doi: 10.1152/jn.1984.52.1.126. [DOI] [PubMed] [Google Scholar]
- Yaari Y, Konnerth A, Heinemann U. Spontaneous epileptiform activity of CA1 hippocampal neurons in low extracellular calcium solutions. Experimental Brain Research. 1983;51:153–156. doi: 10.1007/BF00236813. [DOI] [PubMed] [Google Scholar]
- Yaari Y, Konnerth A, Heinemann U. Nonsynaptic epileptogenesis in the mammalian hippocampus in vitro. II. Role of extracellular potassium. Journal of Neurophysiology. 1986;56:424–438. doi: 10.1152/jn.1986.56.2.424. [DOI] [PubMed] [Google Scholar]


